Abstract
Background
Physical activity is important to maintain and promote health. This is of particular interest in patients with congenital heart disease (CHD) where acquired heart disease should be prevented. The World Health Organization (WHO) recommends a minimum of 2.5 h/week of physical activity exceeding 3 metabolic equivalents (METS) to achieve positive health effects. It is unknown whether physical activity levels (PAL) in adult CHD patients differ by country of origin.
Methods
3896 adults with CHD recruited from 15 countries over 5 continents completed self-reported instruments, including the Health Behaviour Scale (HBS-CHD), within the APPROACH-IS project. For each patient, we calculated whether WHO recommendations were achieved or not. Associated factors were investigated using Generalized Linear Mixed Models.
Results
On average, 31% reached the WHO recommendations but with a great variation between geographical areas (India: 10%–Norway: 53%). Predictors for physical activity level in line with the WHO recommendations, with country of residence as random effect, were male sex (OR 1.78, 95%CI 1.52–2.08), NYHA-class I (OR 3.10, 95%CI 1.71–5.62) and less complex disease (OR 1.46, 95%CI 1.16–1.83). In contrast, older age (OR 0.97, 95%CI 0.96–0.98), lower educational level (OR 0.41, 95%CI 0.26–0.64) and being unemployed (OR 0.57, 95%CI 0.42–0.77) were negatively associated with reaching WHO recommendations.
Conclusions
A significant proportion of patients with CHD did not reach the WHO physical activity recommendations. There was a large variation in physical activity level by country of origin. Based on identified predictors, vulnerable patients may be identified and offered specific behavioral interventions.
Abbreviations: APPROACH-IS, assessment of patterns of patient-reported outcomes in adults with congenital heart disease — international study; CHD, congenital heart disease; CI, confidence interval; HBS-CHD, health behaviour scale; METS, metabolic equivalents; NYHA, New York Heart Association (class); OR, odds ratio; PAL, physical activity level; PRO, patient-reported outcomes; WHO, World Health Organization
Keywords: Adult congenital heart disease, Physical activity level, Patient-reported outcome, Health-behaviour scale, Physical activity recommendation, Metabolic equivalent
1. Introduction
Due to improvements in the treatment and management of congenital heart disease (CHD), most children with congenital heart disease are expected to reach adulthood and the population of adults with CHD continues to grow [1,2]. However, the risk of complications increases as patients grow older [3]. With increasing age, there is also the risk of acquired heart disease, especially in those with traditional risk factors for cardiovascular disease such as hypertension, diabetes, and hyperlipidaemia [4,5]. In an adult CHD population, prevention of acquired heart disease is especially important given the risks associated with re-intervention [6,7] and pre-existing limitations in physical capacity [8].
A physically active lifestyle has the potential to modify cardiovascular risk factors and promote general health [[9], [10], [11]]. Most patients with CHD experience some degree of limitations in aerobic capacity, most pronounced in those with complex heart lesions [8]. This may pose barriers for being physically active. However, studies have suggested that adults as well as children with CHD are physically active on the same level as healthy subjects [12,13]. Nevertheless, approximately one-half to three-quarters of both patients and healthy subjects do not reach the World Health Organization (WHO) recommendations of 2.5 h per week of physical activity of 3 metabolic equivalents (METS) or more [12,14].
Several patient-related factors are potentially associated with low physical activity level (PAL) in patients with CHD, such as reduced aerobic capacity [8,15], impaired muscle function [[16], [17], [18]], self-concept [19], self-efficacy [20], parental overprotection [21], and restriction recommendations by their cardiologists [22]. In general, physical activity level may also be affected by external factors such as seasonal variation [[23], [24], [25]], socio-economic and local environmental factors [[26], [27], [28], [29]], and by country of origin [30]. These findings raise the question whether the degree of physical activity level also varies in different countries in patients with CHD. In the present study, physical activity level was analyzed in a large international cohort of adults with CHD, including geographical variation in physical activity level and general predictors of physical activity level in this particular population.
2. Methods
2.1. Patients and procedure
In total, 4028 adults with CHD from 15 countries in 5 continents participated in the cross-sectional study APPROACH-IS (Assessment of Patterns of Patient-Reported Outcomes in Adults with Congenital Heart disease — International Study) [31,32] on Patient-Reported Outcomes (PRO). Data were collected from April 2013 to March 2015. Informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki. The rationale, design and methodology of APPROACH-IS have been published previously [33].
Patients included in the study met the following criteria: (i) diagnosis of CHD, defined as a structural abnormality of the heart or intra-thoracic great vessels, that was present at birth and had actual or potential functional significance [34]; (ii) 18 years of age or older; (iii) diagnosis established before adolescence (i.e. before 10 years of age); (iv) continued follow-up at a CHD center or included in a national/regional register; and (v) physical, cognitive, and language capabilities necessary to complete self-report questionnaires. Exclusion criteria were prior heart transplantation or primary pulmonary hypertension [33]. The complexity of the congenital heart disease was based on the Bethestha classification [35].
2.2. Measurements
Socio-demographic variables were patient-reported. The self-report questionnaires in APPROACH-IS were administered to eligible patients by surface mail or in clinic during an outpatient visit. The questionnaires have been validated and reliability-tested and measure PROs within different PRO domains, including health behaviour. Data regarding the participants' medical background, such as CHD diagnosis and disease complexity [35], were added to the APPROACH-IS database by a member of the local research team and based on chart review [33].
The Health Behaviour Scale (HBS-CHD), including data on alcohol consumption, tobacco use and physical activity [36], was used to measure physical activity level. The instrument was translated into Chinese, French, German, Hindi, Italian, Japanese, Norwegian, Spanish, Swedish, and Tamil. The questionnaire has a good to excellent content validity and responsiveness [36]. The validity across different languages has not been tested. The HBS-CHD included questions regarding extremely and moderately demanding physical activity during a 7-day week, also including sports during school hours (the latter was relevant for a minority of the studied patients). The number of hours per week spent at an activity ≥3METS and ≥6METS was summarized. Based on the current WHO recommendations on physical activity for promoting health in adults aged 18–64 (i.e 150 min/week spent ≥3METS or 75 min/week spent ≥6METS or an equivalent combination of both), participants were dichotomized into two categories: high physical activity level (reaching WHO recommendations) and low physical activity level (not reaching WHO recommendations) based on their participation in physical exercise.
2.3. Statistical analyses
All analyses were performed using SPSS 23 (IBM, Armonk, NY, USA). Data were assessed for normality. Differences in means were tested with Student's t-test and ratios with chi2-test. The null hypothesis was rejected for p-values < 0.05.
The association between patient-specific variables, being age, sex, educational level, employment status, marital status, functional class and disease complexity, versus physical activity level was estimated through generalized linear mixed models that is a form of multilevel logistic regression. We applied a two-level structure in which patients were nested within countries. Hence, all patient characteristics available, which have been used in prior APPROACH-IS reports [31] were used as fixed effects. Country was used as random effect. Generalized linear mixed models do not result in a normally interpretable R2 statistics. Therefore, we computed the pseudo R2 using the method described by Nakagawa & Schielzeth [37].
For the physical activity level, full data on 3896 patients was available. For the predictors, missing values occurred in 0.0 to 2.2% of the subjects. Altogether, full data on patient characteristics was available for 3727 (95.7%) of the patients. Therefore, multiple imputation was not used and only patients for whom full data was available for the variables under study were included in the generalized linear mixed models.
3. Results
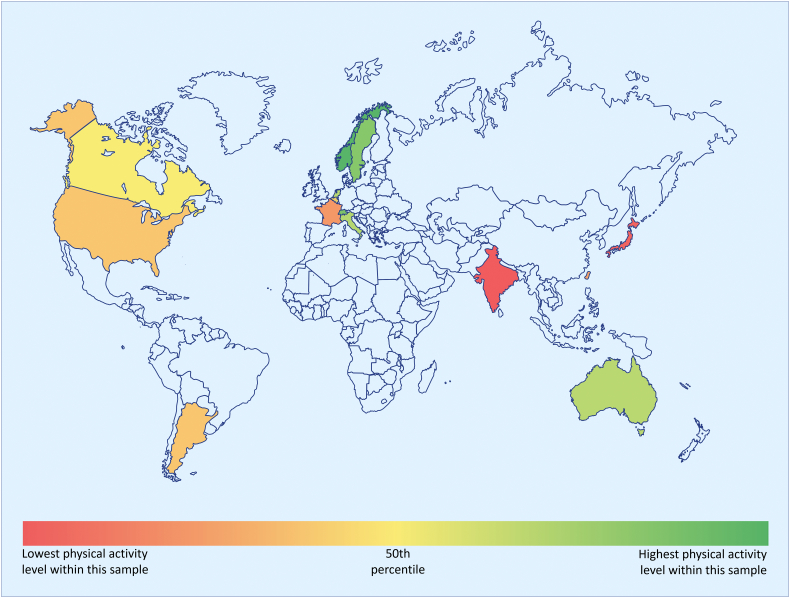
Out of 4028 participants, 3896 had data on physical activity level. 1217 (31%) reached WHO recommendations on physical activity level. The proportion of patients reaching recommended physical activity level varied among countries (p < 0.001), with the lowest proportions in India (10%) and Japan (11%) and highest in Norway (53%), Switzerland (47%), and Sweden (46%). However, the variation was large, also between adjacent countries e.g. France (19%) and Switzerland (Table 1, Fig. 1).
Table 1.
Distribution of PAL in the different countries.
| Country | All patients |
High PAL |
Low PAL |
p |
|---|---|---|---|---|
| n = 3896 | n = 1217 | n = 2679 | ||
| Norway | 172 (4) | 91 (53) | 81 (47) | <0.001 |
| Switzerland | 270 (7) | 128 (47) | 142 (53) | |
| Sweden | 463 (12) | 213 (46) | 250 (54) | |
| The Netherlands | 250 (6) | 99 (40) | 151 (60) | |
| Italy | 60 (2) | 24 (40) | 36 (60) | |
| Australia | 130 (3) | 51 (39) | 79 (61) | |
| Canada | 504 (13) | 157 (31) | 347 (69) | |
| Belgium | 263 (7) | 76 (29) | 187 (71) | |
| Malta | 118 (3) | 31 (26) | 87 (74) | |
| USA | 726 (19) | 190 (26) | 536 (74) | |
| Argentina | 168 (4) | 44 (26) | 124 (74) | |
| Taiwan | 248 (6) | 51 (21) | 197 (79) | |
| France | 88 (2) | 17 (19) | 71 (81) | |
| Japan | 237 (6) | 26 (11) | 211 (89) | |
| India | 199 (5) | 19 (10) | 180 (91) |
Data shown as n (%). p-Value represents comparison in physical activity levels between countries.
n = number; PAL = physical activity level.
Fig. 1.
Comparison of PAL between countries.
PAL in adults with CHD was measured using the Health Behaviour Scale (HBS-CHD) and the proportion reaching WHO recommendations on PAL was calculated for each country. There was a great inter-country variation in the proportion of CHD patients reaching recommended PAL. In this heat map, countries included in the study are marked in color. Shades of green denote a higher proportion of CHD patients reaching recommended PAL as compared with shades of red.
CHD = congenital heart disease, PAL = physical activity level, WHO = World Health Organization.
More men than women reached WHO recommendations on physical activity level (37% vs. 26%, p < 0.001). Patients with a high physical activity level were younger (32 vs. 36 years, p < 0.001), had less complex heart lesions (35% among patients with simple lesions vs. 26% among patients with complex lesions), and higher educational levels (39% of those with a university degree vs. 16% of those who did not finish high school). Employment status was also associated with physical activity level. Of full-time students, 41% reached WHO recommendations on physical activity level vs. 14% of those who were homemakers or retired. There was an association between self-reported limitations and low physical activity level, with 40% of those who reported no limitations meeting WHO recommendations on physical activity level compared to 10% among those with severe limitations (Table 2).
Table 2.
Overview of patient data.
| Variables | Data points |
All patients |
High PAL |
Low PAL |
p | |
|---|---|---|---|---|---|---|
| (n) | n = 3896 | n = 1217 | n = 2679 | |||
| Sex | 3880 | <0.001 | ||||
| Female | n (%) | 2035 (52) | 524 (26) | 1511 (74) | ||
| Male | n (%) | 1845 (48) | 685 (37) | 1160 (63) | ||
| Age years | Mean ± SD | 3890 | 35 ± 13 | 32 ± 11 | 36 ± 13 | <0.001 |
| Education | 3860 | <0.001 | ||||
| Less than high school | n (%) | 204 (5) | 32 (16) | 172 (84) | ||
| High school | n (%) | 1663 (43) | 483 (29) | 1180 (71) | ||
| College degree | n (%) | 824 (21) | 241 (29) | 583 (71) | ||
| University degree | n (%) | 1169 (30) | 454 (39) | 715 (61) | ||
| Work situation | 3874 | <0.001 | ||||
| Part-time or full-time work | n (%) | 2493 (64) | 861 (35) | 1632 (66) | ||
| Homemaker or retired | n (%) | 299 (8) | 42 (14) | 257 (86) | ||
| Job seeking, unemployed, or disabled | n (%) | 489 (13) | 79 (16) | 410 (84) | ||
| Full-time student | n (%) | 319 (8) | 130 (41) | 189 (59) | ||
| Other | n (%) | 274 (7) | 99 (36) | 175 (64) | ||
| Marital status | 3878 | 0.048 | ||||
| Never married | n (%) | 1716 (44) | 567 (33) | 1149 (67) | ||
| Divorced or widowed | n (%) | 192 (5) | 46 (24) | 146 (76) | ||
| Married or living with partner | n (%) | 1964 (51) | 598 (30) | 1366 (70) | ||
| Other | n (%) | 6 (0) | 2 (33) | 4 (67) | ||
| Limitations in physical functioning | 3811 | <0.001 | ||||
| Not limited during physical activities | n (%) | 2052 (54) | 829 (40) | 1223 (60) | ||
| Slightly limited during physical activities | n (%) | 1334 (35) | 323 (24) | 1011 (76) | ||
| Considerably limited during physical activities | n (%) | 277 (7) | 30 (11) | 247 (89) | ||
| Unable to be physically active without experiencing discomfort | n (%) | 148 (4) | 14 (10) | 134 (91) | ||
| Complexity of heart defect | 3896 | <0.001 | ||||
| Simple | n (%) | 1003 (26) | 354 (35) | 649 (65) | ||
| Moderate | n (%) | 1891 (49) | 598 (32) | 1293 (68) | ||
| Complex | n (%) | 1002 (26) | 265 (26) | 737 (74) |
p-Values represent comparisons of physical activity levels between different patient variables.
n = number; PAL = physical activity level.
In a multilevel logistic model with geographical area as a random factor, male sex (OR 1.78, 95% CI 1.52–2.08), NYHA I (OR 3.10, 95% CI 1.71–5.62) and less complex disease (OR 1.46, 95% CI 1.16–1.83) were positively associated with reaching WHO recommendations, whereas higher age (OR 0.97, 95% CI 0.96–0.98), lower educational level (0.41, 95% CI 0.26–0.64) and being unemployed (OR 0.57, 95% CI 0.42–0.77) were negatively associated. Marital status was not associated with physical activity level (Table 3). The pseudo R2 for this multilevel model was 0.464.
Table 3.
Multivariable generalized linear mixed models with patient characteristics as predictors of PAL (n = 3727).
| Variables | PAL | p |
|---|---|---|
| Sex | ||
| Female | # | |
| Male | 1.78 (1.52–2.08) | <0.001 |
| Age years | 0.97 (0.96–0.98) | <0.001 |
| Education | ||
| Less than high school | 0.41 (0.26–0.64) | <0.001 |
| High school | 0.59 (0.49–0.71) | <0.001 |
| College degree | 0.70 (0.57–0.87) | <0.05 |
| University degree | # | |
| Work situation | ||
| Part-time or full-time work | # | |
| Homemaker or retired | 0.76 (0.51–1.12) | N.S. |
| Job seeking, unemployed, or disabled | 0.57 (0.42–0.77) | <0.001 |
| Full-time student | 1.19 (0.90–1.58) | N.S. |
| Other | 1.13 (0.83–1.53) | N.S. |
| Marital status | ||
| Never married | 1.06 (0.88–1.27) | N.S. |
| Divorced or widowed | 1.01 (0.69–1.49) | N.S. |
| Married or living with partner | # | |
| Other | 0.55 (0.05–6.19) | N.S. |
| Limitations in physical functioning | ||
| Not limited during physical activities | 3.10 (1.71–5.62) | <0.001 |
| Slightly limited during physical activities | 1.76 (0.97–3.21) | N.S. |
| Considerably limited during physical activities | 0.85 (0.42–1.71) | N.S. |
| Unable to be physically active without experiencing discomfort | # | |
| Complexity of heart defect | ||
| Simple | 1.46 (1.16–1.83) | <0.05 |
| Moderate | 1.25 (1.03–1.52) | <0.05 |
| Complex | # |
Data presented as Odds Ratios (Confidence Intervals).
N.S. = non-significant; PAL = physical activity level.
Reference category.
4. Discussion
In the present study, we found that in a globally recruited sample, approximately one third of adults with CHD reached the WHO recommendations on physical activity to maintain or promote health. However, large geographical variations from 10% to slightly above 50% of the population reaching the current recommendations were seen. In a multilevel logistic regression model with geographical area as random effect, sex, age, educational level, employment status, complexity of heart lesions, and self-reported NYHA class were associated with reaching WHO recommendations on physical activity level. This knowledge may help in detecting vulnerable patients and thereby offer specific behavioral interventions.
The reasons for the large variation in physical activity level between different geographical regions are not clear. Factors such as climate, cultural variations, infrastructure and socioeconomic factors may be of importance. With a few exceptions, our studied population reached the recommendations on physical activity level to a similar extent as the reference general population in their respective countries [30,[38], [39], [40]].
In our study we demonstrated that men were more likely to be sufficiently active, which is in line with previous studies on adults with CHD [12,14] as well as the general population [41]. However, there are also conflicting reports on adults with CHD [42] where gender was not associated with physical activity level. The difference in physical activity level between the sexes persisted when adjusted for geographical area. Given this, health care providers should not only ensure activity recommendations are provided for all patients but additional efforts should be made to educate their female CHD patients about the importance of physicial activity and the potential long-term benefits.
In this study, the odds for reaching the WHO recommendations on physical activity level decreased by 3% each year of life. It is known that physical activity level decreases with age in the general population [41] and others have reported consistent results in adults with congenital heart disease [42]. A reduction in exercise capacity, which is found in the general population [43] as well as in adults with CHD [8], was a possible explanatory mechanism for lower physical activity level with increasing age. Our data supported that a decreased physical activity level due to increasing age, even at relatively young ages, is a global phenomenon in adults with CHD. These observations underscore the importance of addressing physical activity level in the management of older patients with CHD.
We found that the complexity of CHD was associated with physical activity level. This finding contrasts with previous reports [12,42,44]. In two previous studies, a slightly different definition of complexity was applied with two groups of complexity instead of three as used in this present investigation [35]. In our study, we showed that patients with simple and moderate lesions had higher physical activity level than patients with lesions of severe complexity. However, we noted that differences were modest and the point estimates were fairly similar for both groups. It may be that patients with lesions of severe complexity in our present study represented more severely limited patients compared with the previous studies. It may also be that the large sample size in this study allowed for detection of smaller differences between groups. Nevertheless, the complexity of the CHD lesion should be considered when giving advice on physical activity to patients with CHD [45,46].
The self-reported NYHA class was strongly associated with physical activity level with almost three times higher odds of reaching recommendations on physical activity level for patients in NYHA class I in contrast to the higher NYHA classes (III and IV). For NYHA class II, the point estimates were in line with NYHA class I but did not reach statistical significance. Our results were in agreement with a previous study that reported higher activity levels for patients in NYHA class I [47]. It was not surprising that patients without limitations are more active than those with limitations for physical activity. For patients describing themselves as physically limited, this is a very strong indicator of having a low physical activity level. These patients are at potential risk of developing complications related to low physical activity and may thus be trapped in a vicious cycle. Patients with higher NYHA classes should be assessed carefully regarding actual physical activity level and offered targeted advice and rehabilitation measures [45].
As in the general population [[48], [49], [50]], higher educational levels were associated with higher physical activity level among patients with CHD. Educational level is likely associated with employment status, which was also associated with high physical activity level in the current study. Both educational level and employment status are possible to modify and caregivers can support these efforts with discussions beginning in early adolesence on future education, vocation and employment.
Exercise training in adults with congenital heart disease has been proved safe in several trials. There are also recommendations for exercise training that can be applied on an individual basis, taking in account factors such as arrhythmia, arterial saturation and ventricular function [46].
4.1. Study limitations
The present study population only captured patients with CHD and reference data were lacking. While a reference population were not feasible for the current study, the current methods allowed for identification of those with CHD who may be more or less likely to reach WHO recommendations on physical activity level. Only self-reported instruments were used which have inherent limitations regarding under- or overestimation, recall bias, and social desirability bias. On the other hand, only validated instruments were used and our sample was large, which hopefully allowed for a valid output. We did not have access to data on medication. Some common drugs in cardiovascular therapeutics, e.g. beta-blockers, may affect physical performance and thereby also potentially the physical activity level. Our sample was not population based and not randomly selected. However, the large sample of adults with CHD and the multicenter recruitment of patients hopefully allowed for valid conclusions based on the present data.
5. Conclusions
Almost 70% of adults with CHD did not reach the WHO recommended physical activity level. There was a large variation between countries in the proportion of patients that reached recommended physical activity level, from 10% to slightly above 50%. Given the proportion of patients not reaching recommended physical activity level, many are at potential risk for developing long-term complications related to a low physical activity level. Therefore, issues regarding physical activity level should be encouraged and discussed in all consultations in adults with congenital heart disease. Furthermore, the identified predictors of physical activity level may help to identify vulnerable patients and thereby allow for targeted interventions.
Funding
This work was supported by the Research Fund — KU Leuven, Leuven, Belgium (OT/11/033); by the Swedish Heart-Lung Foundation, Sweden (20130607); by the University of Gothenburg Centre for Person-centred Care, Gothenburg, Sweden; and by the Cardiac Children's Foundation, Taiwan (CCF2013_02). Furthermore, this work was endorsed by and conducted in collaboration with the International Society for Adult Congenital Heart Disease (ISACHD).
Declarations of interest
None.
References
- 1.Marelli A.J., Mackie A.S., Ionescu-Ittu R., Rahme E., Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 2.Moons P., Bovijn L., Budts W., Belmans A., Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122(22):2264–2272. doi: 10.1161/CIRCULATIONAHA.110.946343. [DOI] [PubMed] [Google Scholar]
- 3.Schultz A.H., Wernovsky G. Late outcomes in patients with surgically treated congenital heart disease. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2005:145–156. doi: 10.1053/j.pcsu.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Moons P., Van Deyk K., Dedroog D., Troost E., Budts W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur. J Cardiovasc. Prevent. Rehabil. 2006;13(4):612–616. doi: 10.1097/01.hjr.0000197472.81694.2b. [DOI] [PubMed] [Google Scholar]
- 5.Lui G.K., Rogers I.S., Ding V.Y., Hedlin H.K., MacMillen K., Maron D.J. Risk estimates for atherosclerotic cardiovascular disease in adults with congenital heart disease. Am. J. Cardiol. 2017;119(1):112–118. doi: 10.1016/j.amjcard.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holst K.A., Dearani J.A., Burkhart H.M., Connolly H.M., Warnes C.A., Li Z. Risk factors and early outcomes of multiple reoperations in adults with congenital heart disease. Ann. Thorac. Surg. 2011;92(1):122–128. doi: 10.1016/j.athoracsur.2011.03.102. (discussion 9-30) [DOI] [PubMed] [Google Scholar]
- 7.Jacobs J.P., Mavroudis C., Quintessenza J.A., Chai P.J., Pasquali S.K., Hill K.D. Reoperations for pediatric and congenital heart disease: an analysis of the Society of Thoracic Surgeons (STS) congenital heart surgery database. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2014;17(1):2–8. doi: 10.1053/j.pcsu.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempny A., Dimopoulos K., Uebing A., Moceri P., Swan L., Gatzoulis M.A. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life—single centre experience and review of published data. Eur. Heart J. 2012;33(11):1386–1396. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 9.Smith S.C., Jr., Jackson R., Pearson T.A., Fuster V., Yusuf S., Faergeman O. Principles for national and regional guidelines on cardiovascular disease prevention: a scientific statement from the World Heart and Stroke Forum. Circulation. 2004;109(25):3112–3121. doi: 10.1161/01.CIR.0000133427.35111.67. [DOI] [PubMed] [Google Scholar]
- 10.Heran B.S., Chen J.M., Ebrahim S., Moxham T., Oldridge N., Rees K. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2011;7 doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naci H., Ioannidis J.P. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandberg C., Pomeroy J., Thilén U., Gradmark A., Wadell K., Johansson B. Habitual physical activity in adults with congenital heart disease compared with age- and sex-matched controls. Can. J. Cardiol. 2016;32(4):547–553. doi: 10.1016/j.cjca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Stone N., Obeid J., Dillenburg R., Milenkovic J., MacDonald M.J., Timmons B.W. Objectively measured physical activity levels of young children with congenital heart disease. Cardiol. Young. 2015;25(3):520–525. doi: 10.1017/S1047951114000298. [DOI] [PubMed] [Google Scholar]
- 14.Muller J., Hess J., Hager A. Daily physical activity in adults with congenital heart disease is positively correlated with exercise capacity but not with quality of life. Clin. Res. Cardiol. 2012;101(1):55–61. doi: 10.1007/s00392-011-0364-6. [DOI] [PubMed] [Google Scholar]
- 15.Diller G.P., Dimopoulos K., Okonko D., Li W., Babu-Narayan S.V., Broberg C.S. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112(6):828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 16.Sandberg C., Thilén U., Wadell K., Johansson B. Adults with complex congenital heart disease have impaired skeletal muscle function and reduced confidence in performing exercise training. Eur. J. Prev. Cardiol. 2015;22(12):1523–1530. doi: 10.1177/2047487314543076. [DOI] [PubMed] [Google Scholar]
- 17.Kröönström L.A., Johansson L., Zetterstrom A.K., Dellborg M., Eriksson P., Cider A. Muscle function in adults with congenital heart disease. Int. J. Cardiol. 2014;170(3):358–363. doi: 10.1016/j.ijcard.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Greutmann M., Le T.L., Tobler D., Biaggi P., Oechslin E.N., Silversides C.K. Generalised muscle weakness in young adults with congenital heart disease. Heart. 2011;97(14):1164–1168. doi: 10.1136/hrt.2010.213579. [DOI] [PubMed] [Google Scholar]
- 19.Chen C.W., Su W.J., Wang J.K., Yang H.L., Chiang Y.T., Moons P. Physical self-concept and its link to cardiopulmonary exercise tolerance among adolescents with mild congenital heart disease. Eur. J. Cardiovasc. Nurs. 2015;14(3):206–213. doi: 10.1177/1474515114521926. [DOI] [PubMed] [Google Scholar]
- 20.Ray T.D., Henry K. Self-efficacy and physical activity in children with congenital heart disease: is there a relationship? J. Spec. Pediatr. Nurs. 2011;16(2):105–112. doi: 10.1111/j.1744-6155.2011.00282.x. [DOI] [PubMed] [Google Scholar]
- 21.Reybrouck T., Mertens L. Physical performance and physical activity in grown-up congenital heart disease. Eur. J. Cardiovasc. Prevent. Rehabil. 2005;12(5):498–502. doi: 10.1097/01.hjr.0000176510.84165.eb. [DOI] [PubMed] [Google Scholar]
- 22.Swan L., Hillis W.S. Exercise prescription in adults with congenital heart disease: a long way to go. Heart. 2000;83(6):685–687. doi: 10.1136/heart.83.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gracia-Marco L., Ortega F.B., Ruiz J.R., Williams C.A., Hagstromer M., Manios Y. Seasonal variation in physical activity and sedentary time in different European regions. The HELENA study. J. Sports Sci. 2013;31(16):1831–1840. doi: 10.1080/02640414.2013.803595. [DOI] [PubMed] [Google Scholar]
- 24.Pivarnik J.M., Reeves M.J., Rafferty A.P. Seasonal variation in adult leisure-time physical activity. Med. Sci. Sports Exerc. 2003;35(6):1004–1008. doi: 10.1249/01.MSS.0000069747.55950.B1. [DOI] [PubMed] [Google Scholar]
- 25.Buchowski M.S., Choi L., Majchrzak K.M., Acra S., Mathews C.E., Chen K.Y. Seasonal changes in amount and patterns of physical activity in women. J. Phys. Act. Health. 2009;6(2):252–261. doi: 10.1123/jpah.6.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks S.E., Housemann R.A., Brownson R.C. Differential correlates of physical activity in urban and rural adults of various socioeconomic backgrounds in the United States. J. Epidemiol. Community Health. 2003;57(1):29–35. doi: 10.1136/jech.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamphuis C.B., Van Lenthe F.J., Giskes K., Huisman M., Brug J., Mackenbach J.P. Socioeconomic status, environmental and individual factors, and sports participation. Med. Sci. Sports Exerc. 2008;40(1):71–81. doi: 10.1249/mss.0b013e318158e467. [DOI] [PubMed] [Google Scholar]
- 28.Attard S.M., Howard A.G., Herring A.H., Zhang B., Du S., Aiello A.E. Differential associations of urbanicity and income with physical activity in adults in urbanizing China: findings from the population-based China Health and Nutrition Survey 1991–2009. Int. J. Behav. Nutr. Phys. Act. 2015;12:152. doi: 10.1186/s12966-015-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergman P., Grjibovski A.M., Hagströmer M., Bauman A., Sjöström M. Adherence to physical activity recommendations and the influence of socio-demographic correlates — a population-based cross-sectional study. BMC Public Health. 2008;8:367. doi: 10.1186/1471-2458-8-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjöström M., Oja P., Hagströmer M., Smith B.J., Bauman A. Health-enhancing physical activity across European Union countries: the Eurobarometer study. J. Public Health. 2006;14(5):291–300. [Google Scholar]
- 31.Apers S., Kovacs A.H., Luyckx K., Thomet C., Budts W., Enomoto J. Quality of life of adults with congenital heart disease in 15 countries: evaluating country-specific characteristics. J. Am. Coll. Cardiol. 2016;67(19):2237–2245. doi: 10.1016/j.jacc.2016.03.477. [DOI] [PubMed] [Google Scholar]
- 32.Moons P., Kovacs A.H., Luyckx K., Thomet C., Budts W., Enomoto J. Patient-reported outcomes in adults with congenital heart disease: inter-country variation, standard of living and healthcare system factors. Int. J. Cardiol. 2017 doi: 10.1016/j.ijcard.2017.10.064. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Apers S., Kovacs A.H., Luyckx K., Alday L., Berghammer M., Budts W. Assessment of Patterns of Patient-Reported Outcomes in Adults with Congenital Heart disease — International Study (APPROACH-IS): rationale, design, and methods. Int. J. Cardiol. 2015;179:334–342. doi: 10.1016/j.ijcard.2014.11.084. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell S.C., Korones S.B., Berendes H.W. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43(3):323–332. doi: 10.1161/01.cir.43.3.323. [DOI] [PubMed] [Google Scholar]
- 35.Warnes C.A., Williams R.G., Bashore T.M., Child J.S., Connolly H.M., Dearani J.A. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008;52(23):e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Goossens E., Luyckx K., Mommen N., Gewillig M., Budts W., Zupancic N. Health risk behaviors in adolescents and emerging adults with congenital heart disease: psychometric properties of the Health Behavior Scale-Congenital Heart Disease. Eur. J. Cardiovasc. Nurs. 2013;12(6):544–557. doi: 10.1177/1474515113475934. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa S., Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013;4(2):133–142. [Google Scholar]
- 38.Sisson S.B., Katzmarzyk P.T. International prevalence of physical activity in youth and adults. Obes. Rev. 2008;9(6):606–614. doi: 10.1111/j.1467-789X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 39.Caruana M., Grech V. Lifestyle habits among adult congenital heart disease patients in Malta. Congenit. Heart Dis. 2016;11(4):332–340. doi: 10.1111/chd.12366. [DOI] [PubMed] [Google Scholar]
- 40.Poggio R., Seron P., Calandrelli M., Ponzo J., Mores N., Matta M.G. Prevalence, patterns, and correlates of physical activity among the adult population in Latin America: cross-sectional results from the CESCAS I study. Glob. Heart. 2016;11(1):81–88.e1. doi: 10.1016/j.gheart.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagströmer M., Oja P., Sjöström M. Physical activity and inactivity in an adult population assessed by accelerometry. Med. Sci. Sports Exerc. 2007;39(9):1502–1508. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- 42.Bay A., Dellborg M., Berghammer M., Sandberg C., Engstrom G., Moons P. Patient reported outcomes are associated with physical activity level in adults with congenital heart disease. Int. J. Cardiol. 2017;243:174–179. doi: 10.1016/j.ijcard.2017.03.137. [DOI] [PubMed] [Google Scholar]
- 43.Fleg J.L., O'Connor F., Gerstenblith G., Becker L.C., Clulow J., Schulman S.P. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J. Appl. Physiol. 1995;78(3):890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 44.Muller J., Amberger T., Berg A., Goeder D., Remmele J., Oberhoffer R. Physical activity in adults with congenital heart disease and associations with functional outcomes. Heart. 2017;103(14):1117–1121. doi: 10.1136/heartjnl-2016-310828. [DOI] [PubMed] [Google Scholar]
- 45.Chaix M.A., Marcotte F., Dore A., Mongeon F.P., Mondesert B., Mercier L.A. Risks and benefits of exercise training in adults with congenital heart disease. Can. J. Cardiol. 2016;32(4):459–466. doi: 10.1016/j.cjca.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Budts W., Börjesson M., Chessa M., van Buuren F., Trigo Trindade P., Corrado D. Physical activity in adolescents and adults with congenital heart defects: individualized exercise prescription. Eur. Heart J. 2013;34(47):3669–3674. doi: 10.1093/eurheartj/eht433. [DOI] [PubMed] [Google Scholar]
- 47.Dua J.S., Cooper A.R., Fox K.R., Graham Stuart A. Physical activity levels in adults with congenital heart disease. Eur. J. Cardiovasc. Prevent. Rehabil. 2007;14(2):287–293. doi: 10.1097/HJR.0b013e32808621b9. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan M.S., Newsom J.T., McFarland B.H., Lu L. Demographic and psychosocial correlates of physical activity in late life. Am. J. Prev. Med. 2001;21(4):306–312. doi: 10.1016/s0749-3797(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 49.Droomers M., Schrijvers C.T., van de Mheen H., Mackenbach J.P. Educational differences in leisure-time physical inactivity: a descriptive and explanatory study. Social Sci. Med. 1998;47(11):1665–1676. doi: 10.1016/s0277-9536(98)00272-x. [DOI] [PubMed] [Google Scholar]
- 50.Jones D.A., Ainsworth B.E., Croft J.B., Macera C.A., Lloyd E.E., Yusuf H.R. Moderate leisure-time physical activity: who is meeting the public health recommendations? A national cross-sectional study. Arch. Fam. Med. 1998;7(3):285–289. doi: 10.1001/archfami.7.3.285. [DOI] [PubMed] [Google Scholar]