Abstract
Integrin alpha4/beta7 on circulating lymphocytes identifies them as gut-tropic, and can be targeted by the humanized antibody vedolizumab to treat inflammatory bowel disease (IBD). We found lymphocytes expressing alpha4/beta7 were significantly more responsive to the pro-inflammatory cytokines IL-6, IL-7, and IL-21, and less responsive to the regulatory T cell (Treg)- supporting cytokine IL-2. Alpha4/beta7 was expressed by a smaller percent of FOXP3+Helios+ thymically-derived Tregs (tTregs) than FOXP3+Helios- peripherally-derived Tregs (pTregs) or FOXP3- effector T cells. Integrin alpha4/beta7+ CD4 T cells were also rare among cells expressing the Th2 marker CRTh2, but enriched in cells bearing the circulating T follicular helper cell marker CXCR5. Thus the effect of this anti-integrin therapy on the mucosal immune system may be more qualitative than quantitative, and selectively replace pro-inflammatory effector cells with Tregs and Th2 cells to facilitate immune tolerance in the mucosa without globally depleting lymphocytes from the intestinal mucosa.
Keywords: Integrin, Vedolizumab, FOXP3, Helios, Stat, Crohn’s disease, Treg, Th1, Th2, cTFH, IL-2, IL-6, IL-7, IL-21
1. Introduction
Blockade of integrins with humanized monoclonal antibodies, such as natalizumab and vedolizumab, has offered a novel therapeutic strategy for the treatment of immune-mediated diseases such as multiple sclerosis[1-6] and inflammatory bowel disease (IBD)[7-14]. In the pathogenesis of the latter, the integrin heterodimer α4β7 facilitates lymphocyte migration to the gut by docking circulating lymphocytes to the addressin MAdCAM-1 on the endothelial cells of blood vessels under the intestinal mucosa [15-17], allowing immune cells to subsequently undergo diapedesis into the intestinal lamina propria and cause inflammation. By blocking the integrin α4β7, anti-integrin monoclonal antibody therapy is thought to reduce lymphocyte migration to the gut. However, long-term use of vedolizumab has been associated with minimal intestinal infection risk[18,19], suggesting that this agent does not severely deplete protective lymphocytes from the intestinal mucosa. Indeed, vedolizumab responders show only a modest decrease in colon mucosal T cells within the first 3 months of therapy, comparable to that seen with infliximab[20]. Thus the clinical benefit of this agent may reflect a greater effect on the quality than the quantity of immune cells present in the gut, perhaps selectively excluding only pathogenic immune cells.
Existing pharmacodynamics data on vedolizumab has focused largely on effector/memory CD4+ T cells[9,10,12], a minority subset of which express α4β7 at high levels[16]. However, integrin α4β7 is also expressed on CD8 and naive T cells[16], as well as other lymphocytes, such as B or NK lymphocytes[21]. Furthermore, effector/memory CD4+T cells are a heterogeneous population, within which reside multiple subpopulations with distinct pro- and anti-inflammatory properties[22]. It is unknown if or how α4β7 expression varies between these immune cell populations, in healthy or IBD patients, although anti-inflammatory CD4+, CD25+ regulatory T cells (Tregs) express less α4 and β7 integrins than CD4+CD25- T cells[23].
We evaluated the baseline expression patterns of α4β7 on a wide variety of circulating lymphocytes from IBD patients with no prior anti-integrin therapy. We found α4β7 expression on at least a subset of nearly every immune cell population, although the size of this α4β7+ fraction varied significantly between immune cell types, with little expression seen on CCR4+/CRTh2+ cells resembling Th2 cells[24]. Furthermore, we found α4β7+ cells to more often make pro-inflammatory cytokines, and be more responsive to pro-inflammatory cytokines, in vitro. Conversely, Helios+, FOXP3+, CD4+ T cells, believed to be thymically-derived regulatory T cells (tTregs)[25, 26], rarely expressed α4β7, despite being well-represented in the intestinal mucosa [27, 28], suggesting an alternate mechanism for their recruitment to the gut, as has been described in mice[29]. Taken together, these data suggest that vedolizumab may selectively block the intestinal migration of pathogenic, pro-inflammatory T cells, while sparing anti-inflammatory cells, and thus skewing the mix of lymphocytes present in the gut to be less prone to inflammation.
2. Materials and Methods:
2.1. Subjects and samples
Frozen PBMC samples were obtained for these studies from participants in the Benaroya Research Institute (BRI) Immune Mediated Disease Registry and Repository. For figure 1 A-E, healthy control subjects (n= 35) were selected based on the absence of autoimmune disease or any family history of autoimmunity. For subsequent figures, another 25 such healthy controls were selected with an age and gender distribution similar to the following disease cohorts, also selected for this study: Patients with Crohn’s disease (n = 40), or Multiple sclerosis (n=40, all but one with relapsing/remitting phenotype) on no glucocorticoids at the time of draw. Characteristics of these diseased study participants are listed in supplementary Table 1. All but one Crohn’s patient received no biologics or immunomodulators within 3 months prior to sampling. All but one MS patient received no interferon beta or other MS therapy within 3 months of sampling. Samples from a separate cohort of 25 additional inflammatory bowel disease (IBD) patients, likewise consented, were used for additional immunophenotyping studies, as described in results. Clinical details of these patient cohorts are listed in supplementary table 2. All experiments were performed in a blinded manner. The research protocols were approved by the Institutional Review Board at BRI (#07109-136).
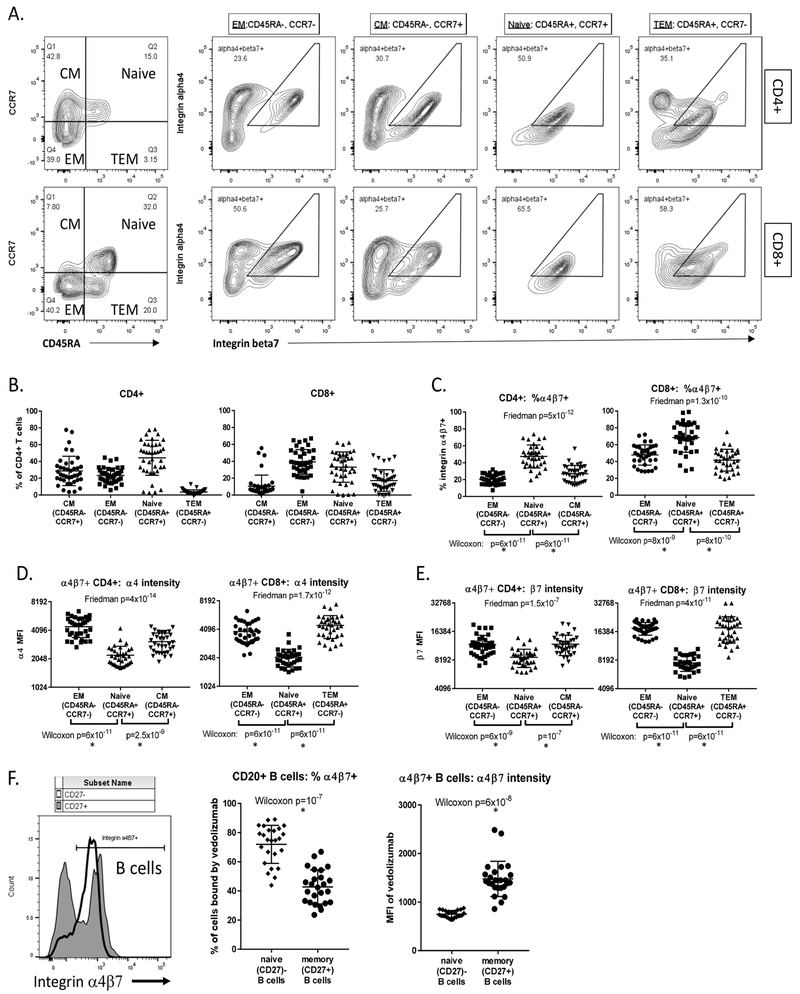
Figure 1:
Antigen-experienced B and T cells have a distinct pattern of α4β7 expression: CD4 and CD8 T cells were divided into naïve, central memory (CM), effector/memory (EM) and terminal effector memory (TEM) populations based upon CD45RA and CCR7 expression as shown, and coexpression of integrin α4 and β7 chains was measured in each population (A). The fraction of CD4 and CD8 cells falling into each of these four subpopulations from 35 healthy control subjects is shown (B). Omitting the largely nonexistent CD4 TEM and CD8 CM populations, the fraction of each subpopulation expressing integrin α4β7 is shown (C), as is the average expression level for integrin α4 (D) and β7 (E) of cells contained therein. In a separate cohort of 25 healthy controls, integrin α4β7 expression was detected with labeled vedolizumab on naïve (CD27-) and memory (CD27+) B cells, with the fraction of these populations expressing α4β7 (middle panel) and average expression level of α4β7 on each cell therein (right panel) shown (F). P-values are shown for three-way paired comparisons using Friedman’s test, or two-way paired analyses using the Wilcoxon matched pairs signed-rank test. Asterisks beneath the latter denote statistical significance after Bonferroni correction for multiple comparisons.
2.2. Flow Cytometry
PBMC were thawed and stained with panels of fluorphor conjugated antibodies. For intranuclear staining with Helios and FOXP3 antibodies, cells were first stained extracellularly, then fixed and permeabilized according to instructions with a FOXP3 staining kit (eBiosciences, Cat 00-5523-00). For phospho-Stat staining, thawed cells were rested at 37° C for 60 min in X-Vivo 15 media in the presence of vedolizumab that had been labeled with biotin with a kit (Molecular Probes cat. # B30010, or InVitrogen ct. # F6347) for detection of cells expressing integrin α4β7. Cells were then stained with a Zombie UV fixable viability dye (BioLegend, Cat 423108), and then incubated with 100 IU/mL Il-2, 2 ng/mL IL-6, 50 pg/mL IL-7, 50 ng/mL IL-21, or no cytokine for 10 minutes, with the exception of IL-21, where cells were incubated for 15 minutes, and then immediately fixed with Fixation Buffer I (BD, Cat 557870) for 15 minutes at 37°C. Cells were then washed and permeabilized on ice with Perm Buffer III (BD, Cat 558050) for 30 minutes, washed, and subsequently stained with a panel of antibodies containing both fluorophore-conjugated streptavidin and antibodies to a given phospho-Stat, as well as cell surface markers (supplementary table 3). These cytokine concentrations and incubation times had previously been determined to cause sub-maximal Stat phosphorylation in healthy donor T cells. Cell fluorescence was measured with a Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software (FlowJo LLC) by a blinded investigator. Gating strategies are detailed in supplementary figure 1.
2.3. Statistical Analyses
Data analyses were performed with Excel (Microsoft) and GraphPad Prism (GraphPad Software, Inc.) software as described in figure legends. For two-way comparisons, a Mann-whitney U test (for unpaired data) or Wilcoxon matched pairs signed-rank test (for paired data) was used. For three or four-way comparisons, a Kruskal-Wallis H test (for unpaired data) or Friedman test (for paired data) was used. If the latter revealed significant differences between patient cohorts, independent two way comparisons were performed between the Crohn’s cohort and each other cohort by Mann-Whitney test, with the p-value threshold corrected for multiple comparisons by a Bonferroni factor. This statistical testing plan was reviewed by an independent statistician for appropriateness (see Acknowledgements).
2.3. Ethical Considerations
All subjects consented to participate in a research biorepository, according to a protocol approved by the institutional review board (IRB) of the Benaroya Research Institute (BRI) and Virginia Mason Medical Center (VMMC). Use of their deidentified samples for these studies by researchers blinded to any protected health information (PHI) was likewise specifically reviewed and approved by the BRI/VMMC IRB.
3. Results
3.1. α4β7 integrin is expressed broadly across naïve and at high levels on a subset of antigen-experienced lymphocyte populations
PBMC from 35 healthy control subjects were stained for CD45RA and CCR7, to divide them into Naïve (CD45RA+/CCR7+), central memory (CM: CD45RA−/CCR7−), effector/memory (EM: CD45RA−, CCR7−) and terminal effector/memory (TEM: CD45RA+, CCR7−) populations[30] (figure 1A, left panels). Few to no TEM and CM cells thus defined were evident among CD4 and CD8 T cells, respectively (figure 1B). Antibodies to integrin α4 and β7 chains were used to identify cells in each population coexpressing integrin α4β7 (figure 1A). A higher fraction of naïve than antigen-experienced (such as EM) T cell populations expressed α4β7 (figure 1C). However, the level of α4 (figure 1D) and β7 (figure 1E) expressed on each α4β7+ cell was lower on average among naïve cells.
In PBMC from a separate cohort (detailed in supplementary table 1), CD27 expression was used to define antigen-experienced, memory B cells, while either its absence or the presence of surface IgD defined naïve B cells[31]. In this experiment, α4β7 expression was detected with a labeled version of vedolizumab. As with T cells, we found in B cells that a majority of naïve (CD27−) cells expressed α4β7 (figure 1B), but at modest levels (MFI) per cell, while, a distinct minority of antigen-experienced memory (CD27+) B cells expressed high levels of α4β7, (figure 1F).
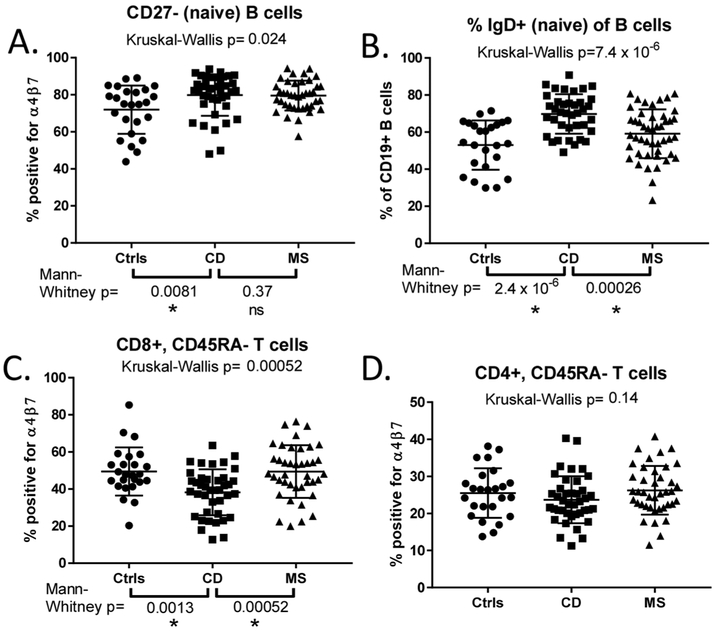
This pattern of α4β7 expression was also present in Crohn’s and MS cohorts. However we found a greater fraction of naïve (CD27−) B cells to be α4β7+ in CD than control specimens (p=0.0081 by Mann-Whitney U test), with CD and MS showing a similar frequency of α4β7 expression among naïve B cells (figure 2A). In CD, α4β7 expression by these naïve B cells decreased with increasing clinical disease activity based on the Harvey-Bradshaw index (HBI) (Pearson’s r2=0.44, p=0.0001) and, to a lesser extent, by serum C reactive protein (CRP) (r2=0.18, p=0.01) (supplementary figure 2A, B), suggesting gut sequestration. B cell integrin expression did not correlate with the estimated expanded disability status scale (EDSS) in MS (data not shown). More circulating B cells were naïve (IgD+) in CD than either MS (p=0.00026) or healthy control cohorts (p<0.0001, figure 2B) but this percentage did not correlate significantly with any clinical characteristics.
Figure 2:
More naïve B cells and fewer antigen-experienced CD8 T cells express α4β7 in Crohn’s patients than in healthy controls: α4β7 expression by naïve B cells (A) and antigen-experienced CD8+ (C) and CD4+ (D) T cells is compared between healthy controls (ctrls) and patients with Crohn’s disease (CD) or multiple sclerosis (MS). In (B), the fraction of B cells expressing IgD (a marker of B cell naïveté) is compared. P-values shown above plots reflect comparisons made by Kruskal-Wallis H testing. If the latter was significant (p<0.05), additional two-way testing was performed between the CD cohort and each of the other two cohorts by Mann-Whitney U testing. Asterisks under p-values denote their significance after adjusting for multiple comparisons, “ns” denotes non- significance.
No differences in α4β7 expression were seen in the CD45RA+ T cell populations between each group, but fewer circulating CD45RA− effector/memory CD8+cells bound vedolizumab in CD than in control (p=0.0013) or MS specimens (p=0.00052), suggesting these cells may constitutively be sequestered from the blood to the inflamed intestinal mucosa (figure 2C). By contrast, there were no differences between CD, MS, and control specimens in the percent of antigen-experienced, CD45RA− CD4+ T cells expressing α4β7 (figure 2D), and integrin expression by T cells did not correlate with CD or MS activity (data not shown).
3.2. CD4+ T cell subpopulations differ in α4β7 expression
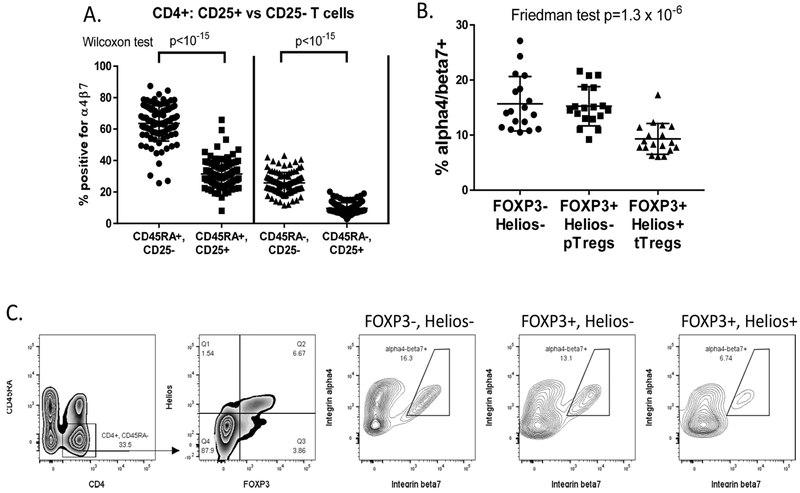
The CD4 T cell population is composed of multiple distinct lineages that are each characterized by unique transcription factors and cell surface markers. Among both naïve and experienced CD4+ T cells are FOXP3+ regulatory T cells (Tregs), which are heavily enriched within their CD4+CD25+ subpopulations[32]. Consistent with a prior report showing less integrin α4 and β7 chain expression in CD25+ CD4+ T cells[23], we found that the CD25+ populations of both naïve and experienced CD4+ T cells contained significantly fewer vedolizumab-binding, α4β7+ cells than their CD25− counterparts (figure 3A), suggesting that Tregs express less α4 and/or β7 than other T cells. Integrin expression on CD25+ T cells was not different between the control, CD and MS cohorts (data not shown).
Figure 3:
Regulatory T cells (Tregs) express less α4β7: (A) Pooling all 3 cohorts from figure 2, naïve (CD45RA+)and antigen-experienced (CD45RA-) populations of CD4T cells were each gated by FACS into CD25+ and CD25− subsets, and the percent of each expressing detectable α4β7 was compared by Wilcoxon matched pairs signed-rank test. (B) In a separate cohort of 18 IBD patients, CD4+, CD45RA- cells, gated by FACS from lymphocyte singlets, were divided into FOXP3+, Helios+ thymically-derived Tregs (tTregs), FOXP3+, Helios-peripherally-derived Tregs (pTregs)and FOXP3-, Helios-conventional T cells, and the percent of each population with detectable α4β7 was compared by Friedman test, demonstrating reduced expression exclusively in the tTreg population. (C) FACS gating strategy from lymphocyte singlets and representative α4β7 expression in each T cell subset is shown.
To confirm and further define the pattern of integrin α4β7 expression on Tregs, PBMC from an additional cohort of 18 IBD patients were stained intranuclearly with antibodies to the Treg-specific transcription factor FOXP3, as well as the transcription factor Helios which is expressed in thymically-derived Tregs (tTregs), but not in those induced in the periphery (pTregs)[25]. The frequency of these Treg populations in the blood of IBD patients was similar to what we have previously found[33], and have previously published in comparison to healthy controls[27]. We found that, among CD45RA− antigen-experienced CD4+ T cells, FOXP3+, Helios+tTregs, but not FOXP3+, Helios- pTregs, demonstrate significantly less coexpression of α4 and β7 than FOXP3-effector T cells (figure 3B, C).
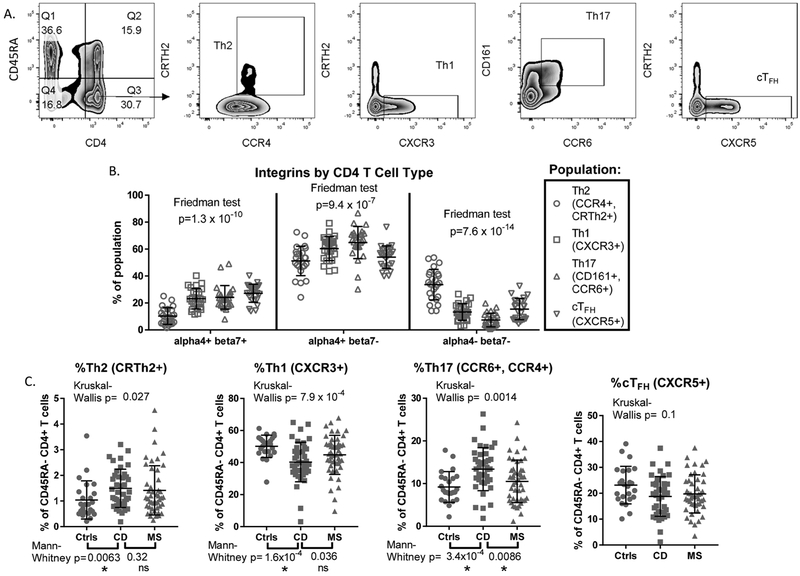
Effector CD4+ T cells are themselves divided into functional categories based upon their expression of cytokines, which in turn are tightly correlated with their expression of cell surface receptors. Using the latter, we evaluated the α4β7 expression of CD45RA− CD4+ T cells in Th1 (CXCR3+, CRTh2−)[34], Th17 (CCR6+, CD161+)[35], Th2 (CCR4+, CRTh2+)[24] and cTFH (circulating follicular helper, CXCR5+, CRTh2−)[36] populations from the PBMC of the 25 IBD patients in supplementary table 2 (figure 4A), the frequency of which, among CD45RA− CD4+ T cells, is shown in supplementary figure 5. We found significant differences in the percent of each subset expressing α4β7, with the highest mean expression among cTFH cells (figure 4B, left panel). By contrast, Th2 cells rarely expressed α4β7, and were more commonly negative for both integrin chains than were other CD4+ T cell populations (figure 4B, middle panel). A substantial fraction of all populations, but especially Th17 cells, expressed the α4 chain without integrin β7 (figure 4B, right panel), presumably being paired with the β 1 chain instead, as the only other known binding partner for α4[37, 38]. Comparisons of receptor expression patterns between CD and healthy control cohorts revealed there to be significantly fewer Th1 and more Th2, and a nonsignificant trend towards fewer cTFH cells, in the circulation of CD patients (Figure 4C), suggesting that more frequent α4β7 expression on Th1 and cTFH cells may be sequestering them to the gut from the peripheral blood in IBD, while selectively sparing Th2 cells. However, we also observed significantly more Th17 cells in the blood of Crohn’s patients (Figure 4C), despite their seldom being α4-negative (Figure 4B, middle panel).
Figure 4:
CD4 T cell subsets express different patterns of integrin expression: CD4+, CD45RA-antigen-experienced T cells, gated by FACS from the blood lymphocyte singlets of 25 IBD patients (described in supplemental table 2), were stained for surface receptors associated with Th1 (CXCR3+), Th2 (CRTh2+, CCR4+), Th17 (CD161+, CCR6+) and cTFH (CXCR5+) subsets, representative gating strategy of which is shown in (A). (B) The percent of each subset expressing both α4 and β7, just α4 (presumably with integrin β 1), or neither integrin were compared by paired Friedman test. (C) The percent of antigen-experienced (CD45RA-) CD4+ T cells resembling Th1, Th2, Th17 or cTFH cells by receptor expression were compared between Crohn’s disease (CD) and either healthy (Ctrl) or multiple sclerosis (MS) cohorts by Kruskal-Wallis H test. If the latter was significant (p<0.05), additional two-way testing was performed between the CD cohort and each of the other two cohorts by Mann-Whitney U testing. Asterisks under p-values denote their significance after adjusting for multiple comparisons. “ns” denotes non- significance.
3.3. Lymphocytes differ in their responsiveness to cytokines based on expression of integrin α4β7
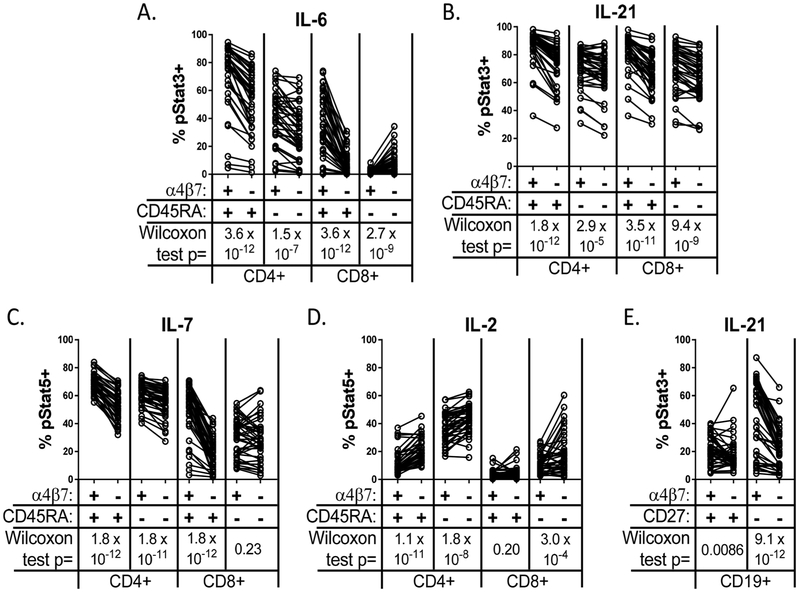
To determine if the gut-homing lymphocytes targeted by vedolizumab differ from lymphocytes which do not express α4β7 with respect to their responses to immune cytokines, we stimulated PBMC from CD patients with IL-2, IL-6, IL-7, or IL-21 in vitro, and assessed the level of phosphorylated signal transducing proteins Stat3 (in the case of IL-6 and IL-21) or Stat5 (for IL-2 or IL-7), a representative example of which is in supplementary figure 6. CD4+ T cells with α4β7 were significantly more likely than those without to phosphorylate Stat molecules in response to the pro-inflammatory cytokines IL-6, IL-7, and IL-21, regardless of whether cells were naïve (CD45RA+) or experienced (CD45RA−) (p<10−4 by Wilcoxon matched pairs signed-rank test in each case) (figure 5A-C). CD45RA+ (naïve plus terminal effector/memory) CD8+ T cells were likewise more IL-6, IL-7 and IL-21-responsive if α4β7+(p<10−10 in each case) (figure 5A-C). α4β7+ CD45RA− (effector/memory) CD8+ T cells were also more responsive to IL-21 (p=9.4 × 10−9), but not IL-7, and the few CD45RA− CD8+ T cells that responded to IL-6 were restricted to the α4β7- population (p=2.7 × 10−9) (figure 5A-C). B cells showed no response to IL-2, 6, or 7 (data not shown) but, like T cells, showed more IL-21-responsiveness among naïve (CD27−) B cells with than without α4β7 (p=9.1×10−12) (figure 5E). This trend was less evident in memory (CD27+) B cells (P=0.0086).
Figure 5:
Integrin α4β7+ lymphocytes show increased response to IL-6, 7, and 21, decreased response to IL-2. PBMC from 40 Crohn’s patients were stimulated in vitro with the indicated cytokines and then stained intracellularly for phospho Stat3 for IL-6 (A) and IL-21 (B and E) or phospho Stat5 for IL-7 (C) and IL-2 (D), and then gated into CD45RA+ or CD45RA− CD4 and CD8 T cells. (E) CD19+ B cells gated from the above PBMC following stimulation with IL-21 were likewise gated into naïve (CD27−) and memory (CD27+) populations and staining for phospho Stat3 is shown. In each condition, the percent of cells staining positive (ie: above the top 1-2% of unstimulated cells) for phosphorylated Stat in response to the indicated cytokine is compared by Wilcoxon matched pairs signed-rank test between the indicated lymphocytes with (α4β7+) or without (α4β7−) surface integrin α4β7, as detected by vedolizumab-binding.
In contrast to these pro-inflammatory cytokines, IL-2 produced less response among α4β7+ than α4β7- naïve and experienced CD4+(p<10−7) and CD45RA− (effector/memory) CD8+ T cells (p=0.0003, figure 5D). Cells expressing the IL-2 receptor alpha chain, CD25, are less frequent among integrin α4β7+ cells (figure 3A), which could result in a lower IL-2 response. However, this difference between the IL-2 response of α4β7+ and α4β7- T cells was present among both CD25+ and CD25− CD4 T cells in Crohn’s disease (supplementary figure 3A). Furthermore, the above differences in cytokine-responsiveness between α4β7+ and α4β7- lymphocytes were not unique to Crohn’s disease, but were also observed in MS patients and/or healthy controls (supplementary figure 3A-E). For IL-2, 6, and 7, correlations were seen between cytokine responsiveness and respective cytokine receptor alpha chain expression in most T cell populations (supplementary figure 4).
4. Discussion:
The results of this study show significant differences in the phenotype of circulating lymphocytes with or without the gut-homing integrin heterodimer α4β7 known to be bound by the therapeutic antibody vedolizumab. Likewise, we have confirmed that the pattern with which this heterodimer is expressed differs consistently between antigen-experienced and naïve B and T cells, with a greater fraction of the naïve cells expressing α4β7, but at a lower level per positive cell than experienced lymphocytes, as shown previously[16]. The presence of a gut-homing integrin on naïve T cells is counterintuitive, given how rare CD45RO−/CD45RA+ T cells are in the normal intestinal mucosa[28, 39]. Thus, perhaps in health, MAdCAM-1 levels on intestinal endothelial cells are only high enough to attract the most highly α4β7- positive minority subpopulation of experienced CD45RA−/RO+ T cells or CD27+/IgD− B cells to the gut, which thus competitively exclude naïve cells. As MAdCAM-1 becomes upregulated with inflammation, this competitive exclusion would become less complete, resulting in the observation that a significantly higher fraction of intestinal lamina propria T cells are CD45RA+ in IBD, particularly at sites of inflammation[28, 40]. If so, therapies which block α4β7 or MAdCAM-1 may alter this competition, to preferentially exclude naïve cells from the intestinal mucosa and thus restrict the clonal diversity of mucosal lymphocytes.
Similarly, vedolizumab may preferentially exclude pro-inflammatory cells from the intestines. CD4+, CD25+, FOXP3+ Tregs are known to express less α4β7 than effector T cells[23], and yet paradoxically represent a greater fraction of CD4+ T cells in the mucosa than in the peripheral blood[27]. While Tregs are more proliferative than FOXP3-negative T cells, based upon expression of Ki67[27, 32], and could thus have simply expanded within the intestinal mucosa after arrival, analyses of mucosal T cell receptors (TCRs) have shown a similar diversity in the clonality of FOXP3+ and FOXP3− populations, arguing against local Treg clonal expansion[28]. Similarly, only modest overlap in TCR repertoire exists between intestinal FOXP3− and FOXP3+ (particularly Helios+) cells to suggests that the plethora of Tregs seen in the mucosa are predominantly derived from cells which arrived from the blood as FOXP3−, but then up-regulated FOXP3 upon activation[28]. The FOXP3+, Helios+”tTreg” population expresses α4β7 significantly less frequently than FOXP3− T cells, suggesting that tTregs have an alternative mechanism for accessing the gut, which is independent of α4β7, and thus immune to vedolizumab. Indeed mice Tregs uniquely use GPR15 to access the intestines[29]. While GPR15 does not appear to perform this function in human Tregs[41], it is conceivable that an analogous system exists, in which case, by blocking the majority of T cells from entering the gut but selectively sparing tTregs, vedolizumab would be expected to eventually shift the ratio of Tregs to effector T cells in the mucosa in favor of Tregs, and thus suppress local inflammation. While we have not seen this effect at the mucosa with blockade of integrin α4 alone via natalizumab[42], the effect of the α4β7-specific vedolizumab on Treg (and particularly Helios+ tTreg) frequency in the mucosa has yet to be evaluated.
In addition to finding differences in α4β7 expression between CD4+ Tregs and effector T cells, we have found differences between subsets of effector T cells themselves. As defined by surface receptor expression patterns, the Th1 and Th17 phenotypes most associated with Crohn’s disease[43] were significantly more likely to express α4β7 than Th2 cells, more commonly associated with allergic or atopic conditions[44]. Thus, it is possible that vedolizumab could selectively exclude Th1 and Th17 cells from the mucosa to increase the fraction of Th2 cells therein, as we postulate for Tregs, above. Indeed, the GPR15 molecule which recruits Tregs to the gut in mice is preferentially expressed on Th2 cells in humans[41]. However, in large safety analyses, vedolizumab use has not been associated with Th2-driven eosinophilic gastroenteritis or food allergies[8,10,18,19]. The most highly α4β7+ population were the circulating T follicular helper (cTFH) cells, which support germinal center formation and B cell development in secondary lymphoid organs and produce copious IL-21[45]. While much less has been reported concerning the role of cTFH cells than Th1 or Th17 in IBD, it is possible that cTFH are particularly susceptible to vedolizumab blockade, which would then undermine the organization of mucosal lymphoid follicles in IBD. It will be interesting to see how vedolizumab affects histological architecture with mucosal healing in future studies.
We found significant differences between the cytokine responsiveness of circulating lymphocytes with versus without α4β7 on their surface. In general, α4β7+ cell populations were more responsive to the pro-inflammatory cytokines IL-6, IL-7, and IL-21, particularly among naïve and terminal effector/memory cells. This suggests that vedolizumab specifically targets those cells most sensitive to pro-inflammatory signals. An enhanced response to IL-21 among α4β7+ naïve B cells may enhance their maturation, resulting in inflammatory cytokine production and increased T cell costimulation. In contrast, α4β7+ naïve CD4+ and effector/memory CD8+ cells were significantly less responsive to IL-2, despite the IL-2 signal sharing a receptor chain (γc, CD132) with and using many of the same signal transduction proteins (PI3K, Jakl, Jak3, Stat5) as IL-7[46]. While IL-2 is a potent T cell growth factor, mice lacking IL-2 or its receptor paradoxically develop autoimmunity, including IBD, reflecting a critical role for IL-2 in gut immunoregulation[47, 48]. Indeed, IL-2 is essential for development of FOXP3+ Tregs, a population without which mice likewise develop autoimmunity[49] and humans develop severe intestinal inflammation[50-53]. Thus, our data demonstrates that vedolizumab targets lymphocytes most sensitive to some pro-inflammatory cytokines while selectively sparing the T cells (particularly naïve CD4+ T cells) most responsive to the Treg - supporting cytokine IL-2.
Our results reflect the heterogeneity of lymphocyte subsets, which in turn highlights the potential targets of anti-integrin therapy in IBD. By targeting some immune cell subsets more than others, vedolizumab may alter not only the quantity, but also the quality of immune cells migrating to the GI tract. It may not simply reduce the total lymphocyte count therein, but selectively prevent some lymphocytes from entering while permitting others to do so, and thus alter the mix of lymphocyte subpopulations present in the intestinal mucosa to favor immunoregulation. The data presented in this study was not obtained from anti-integrin recipients, and thus does not reveal the effect of vedolizumab on the peripheral immune system, which we have recently described in a separate report[33]. However, it does reveal strikingly significant differences between circulating lymphocytes with and without the gut-horning integrin α4β7, which may ultimately prove useful biomarkers for predicting responsiveness to anti-integrin therapy in untreated patients.
Supplementary Material
Acknowledgements:
We would like to thank Kassidy Benoscek and Sylvia Posso for subject recruitment and phlebotomy, Thien Son Nguyen for specimen processing and storage, and Bertrand Haas, Ph.D., for statistical review of the manuscript.
Funding:
This work was supported by the National Institutes of Health U01 AI101990 (7/01/2012-6/30-2017, J.H.B. PI) and a grant from the Digestive Disease Institute at Virginia Mason Medical Center (1/01/2015-12/31/2015, J.D.L., PI).
Sources of Support
NIH/NIAID: U01AI101990 (Buckner)
Digestive Disease Institute at Virginia Mason Medical Center, internal research grant
Abbreviations
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- MS
multiple sclerosis
- Treg
regulatory T cell
- tTreg
thymically-derived Treg
- pTreg
peripherally-derived Treg
- MFI
mean fluorescence intensity
- HBI
Harvey-Bradshaw index (of Crohn’s disease severity)
- EDSS
expanded disability status scale (of multiple sclerosis severity)
- PBMC
peripheral blood mononuclear cells
- Stat
signal transducing activator of transcription
- Th1
type 1 helper T cell
- Th2
type 2 helper T cell
- Th17
type 17 helper T cell
- cTFH
circulating T follicular helper cell
- IL
interleukin
- CRP
C reactive protein
- TCR
T cell receptor
Footnotes
Conflict of Interest:
J.D.L. has given a paid educational seminar to and received research funding from Takeda Pharmaceuticals, manufacturer of vedolizumab. All other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Dalton CM, et al. , Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. J Neurol, 2004. 251 (4): p. 407–13. [DOI] [PubMed] [Google Scholar]
- 2.Miller DH, et al. , A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med, 2003. 348(1): p. 15–23. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor P, et al. , Relapse rates and enhancing lesions in a phase II trial of natalizumab in multiple sclerosis. Mult Scler, 2005. 11(5): p. 568–72. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor PW, et al. , Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology, 2004. 62(11): p. 2038–43. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, et al. , A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med, 2006. 354(9): p. 899–910. [DOI] [PubMed] [Google Scholar]
- 6.Tubridy N, et al. , The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology, 1999. 53 (3): p. 466–72. [DOI] [PubMed] [Google Scholar]
- 7.Sands BE, et al. , Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology, 2014. 147 (3): p. 618–627. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, et al. , Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med, 2013. 369 (8): p. 711–721. [DOI] [PubMed] [Google Scholar]
- 9.Parikh A, et al. , Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm. Bowel. Dis, 2012. 18(8): p. 1470–1479. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, et al. , Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med, 2013. 369(8): p. 699–710. [DOI] [PubMed] [Google Scholar]
- 11.Feagan BG, et al. , Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N. Engl. J. Med, 2005. 352(24): p. 2499–2507. [DOI] [PubMed] [Google Scholar]
- 12.Feagan BG, et al. , Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin. Gastroenterol. Hepatol, 2008. 6(12): p. 1370–1377. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, et al. , Natalizumab for active Crohn’s disease. N Engl J Med, 2003. 348(1): p. 24–32. [DOI] [PubMed] [Google Scholar]
- 14.Gordon FH, et al. , A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology, 2001. 121 (2): p. 268–74. [DOI] [PubMed] [Google Scholar]
- 15.Berlin C, et al. , Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell, 1993. 74(1): p. 185–95. [DOI] [PubMed] [Google Scholar]
- 16.Erle DJ, et al. , Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol, 1994. 153(2): p. 517–28. [PubMed] [Google Scholar]
- 17.Souza HS, et al. , Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut, 1999. 45 (6): p. 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombel JF, et al. , The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulai PS, et al. , The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol, 2016. 111 (8): p. 1147–55. [DOI] [PubMed] [Google Scholar]
- 20.Arijs I, et al. , Effect of vedolizumab (anti-alpha4beta7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Soler D, et al. , The binding specificity and selective antagonism of vedolizumab, an anti- alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther, 2009. 330 (3): p. 864–75. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu Rev Immunol, 2016. 34: p. 317–34. [DOI] [PubMed] [Google Scholar]
- 23.Hirahara K, et al. , The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol, 2006. 177 (7): p. 4488–4494. [DOI] [PubMed] [Google Scholar]
- 24.Cosmi L, et al. , CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol, 2000. 30 (10): p. 2972–9. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, et al. , Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol, 2010. 184 (7): p. 3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevach EM and Thornton AM, tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev, 2014. 259(1): p. 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord JD, et al. , Human Blood and Mucosal Regulatory T Cells Express Activation Markers and Inhibitory Receptors in Inflammatory Bowel Disease. PLoS One, 2015. 10 (8): p. e0136485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord J, et al. , T-cell receptor sequencing reveals the clonal diversity and overlap of colonic effector and FOXP3+ T cells in ulcerative colitis. Inflamm Bowel Dis, 2015. 21 (1): p. 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SV, et al. , GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science, 2013. 340(6139): p. 1456–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, et al. , Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature, 1999. 401(6754): p. 708–12. [DOI] [PubMed] [Google Scholar]
- 31.Agematsu K, Memory B cells and CD27. Histol Histopathol, 2000. 15(2): p. 573–6. [DOI] [PubMed] [Google Scholar]
- 32.Miyara M, et al. , Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity, 2009. 30 (6): p. 899–911. [DOI] [PubMed] [Google Scholar]
- 33.Boden EK, et al. , Identification of Candidate Biomarkers Associated with Response to Vedolizumab in Inflammatory Bowel Disease. Dig Dis Sci, 2018. [DOI] [PubMed] [Google Scholar]
- 34.Bonecchi R, et al. , Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med, 1998. 187 (1): p. 129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosmi L, et al. , Human interleukin 17-producing cells originate from a CD161+CD4+ Tcell precursor. J. Exp. Med, 2008. 205(8): p. 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chtanova T, et al. , T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol, 2004. 173 (1): p. 68–78. [DOI] [PubMed] [Google Scholar]
- 37.van der Flier A and Sonnenberg A, Function and interactions of integrins. Cell Tissue Res, 2001. 305(3): p. 285–98. [DOI] [PubMed] [Google Scholar]
- 38.Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell, 2002. 110(6): p. 673–87. [DOI] [PubMed] [Google Scholar]
- 39.Sathaliyawala T, et al. , Distribution and compartmentalization of human circulating and tissue- resident memory T cell subsets. Immunity, 2013. 38(1): p. 187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horjus Talabur Horje CS, et al. , naïve T cells in the gut of newly diagnosed, untreated adult patients with inflammatory bowel disease. Inflamm Bowel Dis, 2014. 20(11): p. 1902–9. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen LP, et al. , Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat. Immunol, 2015. 16(2): p. 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurmaeva E, et al. , T cell-associated alpha4beta7 but not alpha4beta1 integrin is required for the induction and perpetuation of chronic colitis. Mucosal. Immunol, 2014. 7 (6): p. 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raza A, et al. , Th17 cells: interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev Clin Immunol, 2012. 8(2): p. 161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Prete G, Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy, 1992. 47(5): p. 450–5. [DOI] [PubMed] [Google Scholar]
- 45.Crotty S, T follicular helper cell differentiation, function, and roles in disease. Immunity, 2014. 41(4): p. 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Santo JP, Kuhn R, and Muller W, Common cytokine receptor gamma chain (gamma c)- dependent cytokines: understanding in vivo functions by gene targeting. Immunol Rev, 1995. 148: p. 19–34. [DOI] [PubMed] [Google Scholar]
- 47.Sadlack B, et al. , Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell, 1993. 75(2): p. 253–261. [DOI] [PubMed] [Google Scholar]
- 48.Willerford DM, et al. , Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity, 1995. 3(4): p. 521–30. [DOI] [PubMed] [Google Scholar]
- 49.Lyon MF, et al. , The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A, 1990. 87 (7): p. 2433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett CL, et al. , The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet, 2001. 27 (1): p. 20–21. [DOI] [PubMed] [Google Scholar]
- 51.Brunkow ME, et al. , Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet, 2001. 27 (1): p. 68–73. [DOI] [PubMed] [Google Scholar]
- 52.Patel DD, Escape from tolerance in the human X-linked autoimmunity-allergic disregulation syndrome and the Scurfy mouse. J. Clin. Invest, 2001. 107 (2): p. 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wildin RS, et al. , X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet, 2001. 27(1): p. 18–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.