Abstract
The trillions of synaptic connections within the human brain are shaped by experience and neuronal activity both of which underlie synaptic plasticity and ultimately learning and memory. G protein-coupled receptors (GPCRs) play key roles in synaptic plasticity by strengthening or weakening synapses and/or shaping dendritic spines. While most studies of synaptic plasticity have focused on cell surface receptors and their downstream signaling partners, emerging data point to a critical new role for the very same receptors to signal from inside the cell. Intracellular receptors have been localized to nuclear, endoplasmic reticulum, lysosomes and mitochondria. From these intracellular positions, such receptors may couple to different signaling systems, display unique desensitization patterns and/or show distinct patterns of subcellular distribution. Intracellular GPCRs can be activated at the cell surface, endocytosed and transported to an intracellular site or simply activated in situ by de novo ligand synthesis, diffusion of permeable ligands or active transport of nonpermeable ligands. Current findings reinforce the notion that intracellular GPCRs play a dynamic role in synaptic plasticity and learning and memory. As new intracellular GPCR roles are defined, the need to selectively tailor agonists and/or antagonists to both intracellular and cell surface receptors may lead to the development of more effective therapeutic tools.
Keywords: GPCR, mGlu5, FXS, CB1, CB2, GPER, mAchR, AT1R, AT2R, P2Y1, P2Y2, MT1, 5-HTR4
Graphical Abstract
INTRODUCTION
The strengthening or weakening of synapses coupled with the formation or alteration of dendritic spines is thought to underlie synaptic plasticity and ultimately learning and memory. Not surprisingly, GPCRs, which play critical roles in every cellular process, also play key roles shaping how neurons respond to synaptic input which is necessary to learn new skills and generate new behaviors. Just as evidence links GPCRs to normal synaptogenesis, spine morphogenesis, and learning and memory, emerging data have also associated numerous GPCRs to pathophysiological roles in various neurodevelopmental disorders that affect learning and memory (e.g. Fragile X, Autism Spectrum Disorders, schizophrenia, ADHD). Therefore understanding how GPCRs responding to environmental stimuli achieve the necessary changes in synaptic function to learn new tasks and form new memories remains at the cutting edge of this field.
As an important site of learning and memory, the hippocampus has been extensively characterized in terms of the molecules and regulatory cues used in changing synaptic strength. Expression profiling studies (1) indicate that ~300 GPCRs are expressed in the hippocampus and that at least 20 of these receptors are known to play active roles in synaptic plasticity (2). Some like the cannabinoid CB1 receptor mediate presynaptic plasticity whereas others such as the metabotropic glutamate receptor 5 (mGlu5) primarily modulate postsynaptic processes including increases (potentiation) or decreases (depression) in synaptic strength. Given the importance of these and other GPCRs in the development of normal synaptic plasticity as well as in disorders of synaptogenesis, it’s not surprising that their G protein-dependent and independent (e.g. β-arrestin) signaling pathways have been extensively characterized, at least from their classical position on the plasma membrane.
In the last decade however, emerging data show that many GPCRs also signal from inside the cell. For example, GPCRs have been found on the endoplasmic reticulum (ER) where they are synthesized, folded, modified, and assembled, as well as in sorting vesicles on their way to the cell surface, or on endosomes that have just come off the membrane. Certain intracellular membranes may even serve as alternate destinations or even the preferred location for a number of GPCRs where they may couple to different signaling systems and exhibit distinct patterns of subcellular distribution (3–8). Some of the first examples of intracellular GPCRs include the ocular albinism I (OA1) GPCR, or GPR143 (9, 10) which localizes to melanosomes and late endosomes/lysosomes in pigmented and non-pigmented cells (11); the prostaglandin EP3 and EP4 receptors which signal from nuclei in many tissues including the brain (12), and mGlu5 receptors which also signals from neuronal nuclei as well as from ER membranes (13). In addition, GPCRs have been found on vesicles, mitochondria (14), outer and inner nuclear membranes (3, 8, 15, 16), and even within the nucleoplasm on nuclear bodies and/or nuclear invaginations (17–19). Since so many of these receptors are also found in the brain in neurons, astrocytes and microglia, the question becomes do they play a role in processes such as synaptic plasticity and if so, what are the long terms consequences of receptor activation and how might that be different from signaling pathways activated by cell surface receptors? Many of the pioneering studies on intracellular GPCRs have been performed in peripheral systems, thus here we summarize that larger body of data so as to put more recent studies on CNS receptors in context. Where sufficient evidence exists, we have included information on GPCRs functioning on intracellular membranes such as endosomes and mitochondria (7, 20), particularly in the CNS. Finally we highlight several CNS receptors which play a role in synaptic plasticity from inside the cell.
Intracellular GPCRs
Nuclear GPCRs
GPCRs have always been found within the cell, including in the ER where they are synthesized and assembled, or in vesicles on their way to the cell surface, or on endosomes that have just come off the membrane. Previously, GPCRs in these locations were not thought to be functional but rather were considered as receptors on route to the plasma membrane, desensitized, sequestered receptors or receptors on their way for lysosomal destruction. GPCRs were also found on the nucleus. Amongst the first described were peptide receptors which were abundantly expressed both on the plasma membrane and the nucleus (21, 22). For many of these GPCRs, ligand stimulation triggers internalization of the entire receptor and subsequent trafficking to the nucleus (Fig. 1). For example, both coagulation factor II (thrombin) receptor-like 1 (F2rl1) and platelet-activating factor receptor (Ptafr) appear to internalize with their ligands bound to the receptor (16, 23). The oxytocin receptor also moves to the nuclear membrane after ligand binding (24, 25). Alternatively, peptide ligands can directly activate their cognate nuclear receptor via unknown mechanisms; ligand application shows radiolabeled colocalization of nuclear receptors followed by appropriate functional outcomes. For instance, application of radiolabeled gonadotropin-releasing hormone (GnRH) can be found in the nucleus along with the nuclear GnRH receptor where it triggers histone acetylation and phosphorylation within minutes (26).
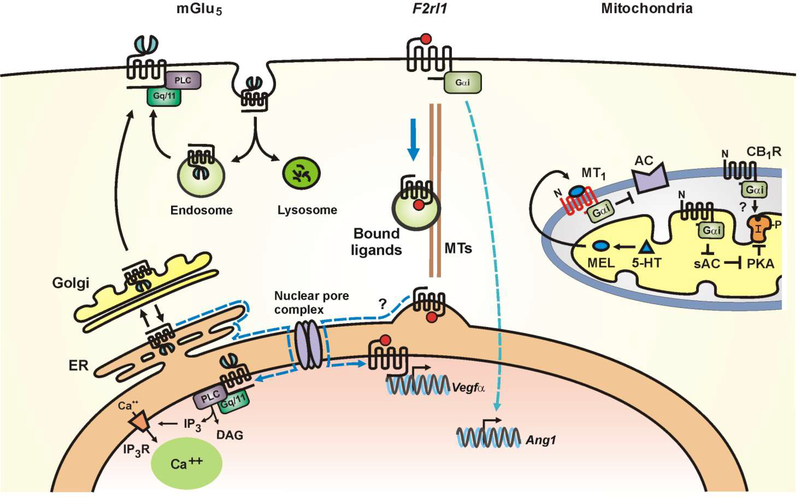
Fig. 1.
Schematic representation of various intracellular GPCRs. Left, proposed model of mGlu5 receptors trafficking in neurons in which >90% of mGlu5 traffics through the Golgi (27). Subsequently, 15–40% goes to the cell surface where it undergoes a cycle of constitutive endocytosis and recycling (27, 122). Alternatively, 60–85% of mGlu5 is retrogradely trafficked back to the endoplasmic reticulum (ER) and then, via lateral diffusion (dotted blue line), reaches the nuclear membrane (27, 123). Middle, ligand bound F2rll can translocate from the retinal ganglion cell surface to the nucleus along microtubules (MTs); nuclear F2rl1 activates vascular endothelial growth factor (Vegfα) expression. In contrast, signaling from cell surface F2rl1 results in the angiogenic gene, Angl expression (16). Right, a growing number of GPCRs have been localized to the outer (MT1, CB1 receptors) and inner mitochondrial membranes (AT2 receptor). Studies show that serotonin (5-HT) is converted into melatonin (MEL) within the mitochondrial matrix; MEL diffuses freely across membranes to activate MT1 receptors in the outer mitochondrial membrane (52). CB1 receptors have also been described in the outer mitochondrial membrane (124) although its downstream signaling machinery is primarily located within the matrix. The exact orientation of mitochondrial GPCRs and their signal transduction pathways is largely unknown. Other abbreviations include AC, adenylyl cyclase; sAC, soluble adenylyl cyclase; “I”, IP3R, inositol trisphosphate receptors; DAG, diacylglycerol; PLC, phospholipase C; Complex I; “P”, phosphorylation site on Complex I; black arrows indicate enhancement of activity; black bars indicate reduction of activity; αi inhibitory subunit of Gi protein; N, amino terminus; and PKA, protein kinase A.
Trafficking:
There appear to be diverse mechanisms by which nuclear GPCRs arrive at this destination. For example, certain GPCRs such as the apelin, angiotensin AT1, α1A and α1B adenosine and bradykinin B2 receptors use canonical nuclear localization signals (NLS), i.e. short stretches of basic amino acids, that are subsequently recognized by specific members of the karyopherin superfamily for nuclear import (4). Other GPCRs like the Ptafr traffic to the nucleus via a process involving the small GTPase, Rab11a, and importin-5 (23). Thus there is no one preferred pathway that is involved in this process nor do all nuclear GPCRs contain canonical NLS sequences. Some like mGlu5 receptors contain unidentified targeting sequences which are critical for the receptor’s nuclear localization (27). Interestingly, some receptors trafficked from the cell surface are not associated with nuclear membranes but rather appear within the nucleoplasm itself. These include the apelin receptor, chemokine receptor 2 (CCR2), arginine vasopressin receptor1α, sphingosine 1-phosphate receptor 1(S1P1), oxytocin receptor, and Cysteine (C)-X-C receptor 4 (CXCR4) (17, 24–32). For those GPCRs trafficked directly to the nuclear membrane, a simple diffusion-retention model has been proposed since the outer nuclear membrane is contiguous with the ER (33, 34). The diffusion-retention model suggests that proteins synthesized in the ER or retrogradely transported there, rapidly diffuse along the outer nuclear membrane before passing through peripheral channels located between the nuclear pore complex and the pore membrane to become tethered on the inner nuclear membrane via interactions with nuclear lamins or chromatin (35, 36). For example, the mGlu5 nuclear trafficking motif interacts with chromatin via a basic region (pI >9.8) which may promote its nuclear retention (27). Most recently, it was reported that VPAC1, a class B GPCR shared by pituitary adenylate cyclase activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP), was trafficked to the nuclear membrane via palmitoylation of its most N-terminal cysteine (Cys37) in the extracellular domain (37). Thus, nuclear GPCRs arrive at their destination via many different signals and types of processes (Fig. 1).
Ligand Activation of Nuclear Receptors:
Intracellular GPCRs can be activated at their subcellular location in a variety of ways (Fig. 2). Ligands can enter cells via diffusion or be made in situ, endocytosed, and/or transported through channels or pores (15, 22, 38). Since ligand binding sites would be within the vesicle or luminal region of the ER or nucleus, extracellular ligands would have to cross both the plasma membrane as well as the intracellular membrane to activate intracellular GPCRs (13). A highly permeable ligand might freely cross such membranes, whereas a less permeable, charged ligand might require an active transport process. Using mGlu5 as an example, at least two uptake systems are responsible for transporting glutamate into a neuron: the sodium-dependent excitatory amino acid transporters and the cystine/glutamate exchanger (39, 40). Conditions that block either type of transporter reduce agonist uptake in cortical, hippocampal and striatal neurons (39, 40, 41). We and others (42) have used microinjection of soluble caged ligands followed by uncaging via restricted photoactivation to directly demonstrate activation of intracellular receptors (42, 43).
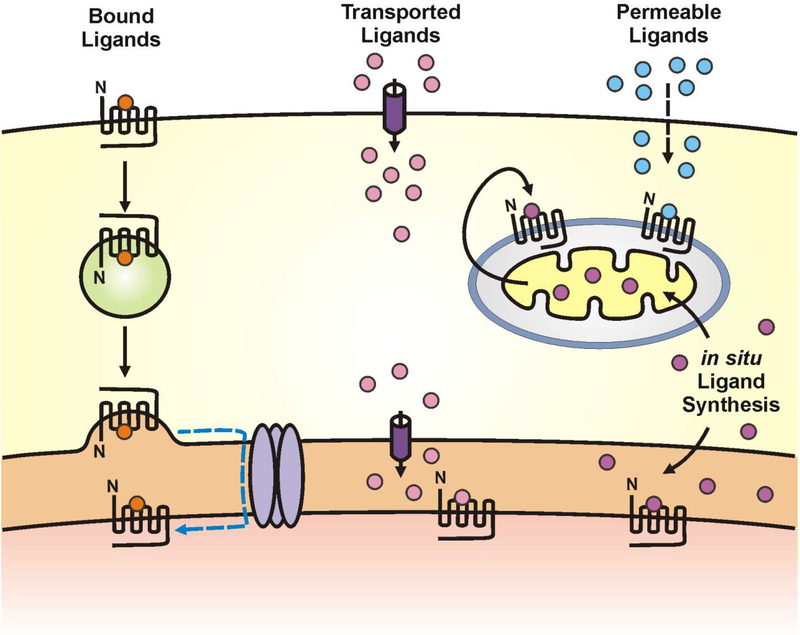
Fig. 2.
Ligand activation of intracellular GPCRs. Intracellular GPCRs can be activated via receptor-bound ligands (left) that can be internalized with a given GPCR such as F2rl1 receptors (16). Alternatively, channels, transporters or exchangers (middle) can transport specific ligands across the plasma membrane and even intracellular membranes to activate receptors whose ligand binding domain faces the endoplasmic reticulum (ER) or nuclear lumen such as glutamate in the case of mGlu5 or organic cation transporter 3 (OCT3) for norepinephrine activation of α1-adrenergic receptor (α1-AR) (3, 125). Permeable ligands such as endocannabinoids can freely diffuse across cell membranes to activate their corresponding receptor (right). Ligands can also be synthesized within the cell (lower right) and either diffuse or be trafficked to a given cellular compartment.
In contrast to ligand transport, ligands can also be made in situ via localized biosynthetic machinery. For example, a large number of GPCRs such as the prostaglandin, platelet-activating factor, and lysophosphatidic acid (LPA) receptors, whose ligands are bioactive lipids derived from membrane hydrolysis, are also located on nuclear membranes (44). As ligand-generating enzymes are present on nuclear membranes and because such ligands readily diffuse through lipid bilayers, Prostaglandin E2 (PGE2), platelet-activating factor, and LPA can easily activate their cognate receptors. Alternatively, many GPCRs exhibit constitutive ligand–independent activity that might allow nuclear receptors to function (22). For example, proteins like Homer1a can lead to agonist-independent mGlu5 receptor activation (45). Agonist-independent activation of the PACAP1 receptor also occurs due to a close association with the insulin-like growth factor 1 receptor and subsequent transactivation by Src (46). Thus as long as a ligand is either made in situ or transported to the site of action, an intracellular receptor can be activated (3, 15, 47; Fig. 2).
Mitochondrial GPCRs
Akin to the novelty of GPCRs being found on nuclear membranes, emerging studies are now pointing to an ever-growing list of GPCRs associated with mitochondria. For example, the angiotensin I and II receptors (AT1R, and AT2R) have been reported both in the nucleus and the mitochondria of several cell types. In mitochondria, AT2Rs have been co-localized with the ligand Ang II on the inner mitochondrial membrane where activation results in nitric oxide formation and suppression of respiration in various cell types including neurons (48). Interestingly, levels of mitochondrial receptor expression can vary depending upon the cell type; in monocytes for example, there is a 40-fold difference in AT2R in mitochondria versus on the cell surface (48). Moreover, with age AT2R decreases whereas AT1R becomes more abundant in mitochondria (48).
Other mitochondrial GPCRs include the purine, P2Y1 and P2Y2, receptors (49), 5hydroxytrptamine (5-HT4) receptor (50), melatonin MT1 receptors (51, 52) and cannabinoid CB1 receptors (53). The latter are thought to play a role in synaptic plasticity described in more detail below. The purine receptors were among the first GPCRs to be localized to mitochondria where they contribute to the regulation of mitochondrial Ca2+ uptake. Specifically, activation of P2Y1 stimulates mitochondrial Ca2+ uptake whereas activation of P2Y2 inhibits this process in hepatocytes (49). In cardiomyocytes, activation of 5HT4 receptors also decreases mitochondrial Ca2+ uptake and in turn, respiratory chain activity and ATP production (50). Melatonin, as a small, lipophilic ligand, is found in high levels in mitochondria where it can activate MT1 receptors. Very recent work shows that mitochondria can synthesize melatonin within the matrix where upon release it activates MT1 receptors on the outer mitochondrial membrane (52). Mitochondrial MT1 signal-transduction activates Gαi and blocks adenylate cyclase activity leading to the inhibition of stress-induced cytochrome c release and caspase activation (52). As further evidence of the importance of mitochondrial MT1, its targeted overexpression inhibited neuronal death resulting from hypoxic/ischemic injury (52). Taken together, there is increasing evidence that mitochondrial GPCRs play important roles in many processes previously thought to be mediated by plasma membrane receptors.
Endosomal GPCRs
Desensitization and endosomal internalization of GPCRs is a well-known mechanism to regulate receptor number via degradation and/or resensitization. A large body of data has now shown that internalization of receptor/G protein/β-arrestin complexes can lead to stable complexes generating sustained endosomal signals (54). Although initially arrestin-mediated signaling focused on the mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 (MAPK/ERK1/2) cascade, many other signaling moieties can interact with the receptor/G protein/β-arrestin complex such as protein kinase B (Akt), p38MAPK, c-Jun amino-terminal kinases (JNKs), and activators of transcription (STATs) (55). In turn, these proteins mediate downstream functions such as growth, cell survival, apoptosis, contractility, cell migration and cytoskeletal reorganization (55). Many drug discovery teams are searching for distinct ligands that can modulate β-arrestin, G-protein-independent processes versus G-protein-dependent pathways (56, 57).
Internalized endosomal GPCRs can also trigger G protein-dependent signaling. For example, conformation-specific single-domain antibodies (nanobodies) have been used to directly assess activation of the β2-adrenergic receptor. Using these tools, two activation states of the β2-adrenergic receptor were detected, first at the plasma membrane seconds after ligand application and then a second activation phase on the endosomes. The second, endosomal phase lasted long after the plasma membrane signals had diminished (58). Interestingly, although both the plasma membrane receptor and the endosomal receptor generated cyclic adenosine monophosphate (cAMP), the G protein-dependent response of the endosomal receptor induced unique cAMP-generated transcriptional responses vs those generated by the cell surface receptor (58). In addition to Gαs endosomal signaling (7, 20), Jensen et al. (59) recently demonstrated that internalized neurokinin, NK1R contributed to sustained endosomal signaling via Gαq. Endosomal NK1R/Gαq signaling but not cell surface NK1R induced cytosolic cAMP, protein kinase C (PKC) and nuclear ERK resulting in neuronal excitation and nociception; compounds that prevented internalization or blocked endosomal NK1R were effective in blocking pain transmission. It seems likely that as endosomal signaling pathways are further explored that additional Gα proteins will be discovered which also modulate unique signaling pathways from their position on the endosome.
ER membranes:
Given the pleiotropic roles the ER plays within a cell, it can be challenging to isolate a given, receptor-mediated function from interconnected membranes. Ultrastructure studies especially those using immunogold labeling are useful yet may simply reflect synthesis, folding and maturation. A number of GPCRs have clearly been localized on these membranes, however, and various technical strategies have shown functionality. One of the best described ER GPCRs is the G-protein-coupled estrogen receptor-1 (GPER), also known as GPR30, a novel estrogen receptor which in addition to the non-GPCR estrogen receptor mediates signaling in multiple cell types (60, 61). Interestingly, the majority of GPER is localized to the ER and Golgi apparatus in many cancer cells and peripheral cell types (62). In the brain, GPER has a widespread distribution in neurons as well as astrocytes (63). In either cell type, electron micrographs show most GPER is intracellular although in the hippocampus, some GPER is also localized at the cell surface in dendrites and spines (64). Since estrogen is a lipophilic compound it can easily slide through membranes to activate receptors in any membrane throughout the cell. Activated GPER appears to couple to Gαi/o and Gαs proteins that together with associated Gβγ subunits regulate many downstream effectors including phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt, ERK1/ 2, adenylyl cyclase, calcium mobilization, nitric oxide synthase (eNOS), and others (2, 65)
GPER is widely expressed in various cancer cell types and tumors where it promotes proliferation, migration, and invasion of cancer cells. However, in some studies, activated GPER inhibited the proliferation of estrogen receptor-negative breast cancer cells, ovarian cancer cells, and prostate cancer cells (65). GPER also influences lipid and glucose metabolism, inflammation and even further estrogen synthesis, actions which further enhance tumor growth and metastasis (65). These opposite and confusing effects may be due to different cell types, different GPER subcellular localization, different subcellular effector molecules and even unique stimuli affecting GPER function.
Another ER membrane GPCR is mGlu5. Although we have primarily characterized mGlu5 receptors on outer and inner nuclear membranes in the striatum, hippocampus and spinal cord dorsal horn neurons (3), ultrastructure studies have also shown large numbers of gold particles on ER membranes (13). Moreover, many earlier ultrastructural studies of various brain regions from the rat, mouse, and monkey have shown large amounts of intracellular receptors in dendrites (66), at the edge of asymmetric postsynaptic specializations and extrasynaptically along the plasma membrane (67–69). We used selective uncaging of glutamate in the presence of cell surface inhibitors to determine whether mGlu5 expressed on dendritic ER was functional. Only the region of the dendrite juxtaposed to the uncaging spot exhibited a change in fluorescence associated with downstream effector formation whereas proximal regions revealed no such fluorescent changes (41). Thus ER mGlu5 is not just undergoing maturation and processing but is also capable of sending signals. As described below, this same study showed that intracellular mGlu5 plays a necessary role in hippocampal long term depression (LTD) (41). It seems likely mGlu5 located on dendritic spine ER is the receptor responsible for the LTD effects (41). Taken together, these two examples of ER GPCR signaling further emphasize that studies investigating molecular mechanisms associated with either GPER or mGlu5’s subcellular distribution and downstream signaling molecules will be critical in the development of effective therapeutic agents targeting these key receptors.
Intracellular GPCRs in synaptic plasticity
GPCRs regulate key aspects of synaptic plasticity both presynaptically and postsynaptically. Presynaptically, various GPCRs affect presynaptic neurotransmitter release either positively or negatively (70, 71). Postsynaptically, GPCR signaling contributes to many processes including long term potentiation (LTP) and LTD, as well as morphogenetic changes associated with dendritic spine alteration and ultimately learning and memory (2, 72). Most of these processes have been explored from the perspective that a GPCR only signals from the cell surface. However, as in more peripheral model systems, it is clear that intracellular GPCRs can affect synaptic plasticity from inside the cell as well. As more intracellular GPCRs are described, this list will surely grow. For now we will highlight those GPCRs for which the best evidence exists for intracellular functions including mGlu5 receptors, M1 muscarinic acetylcholine receptors (mAChRs), CB1, CB2 receptors and GPER.
CB1 receptors:
In addition to its well-described localization on the presynaptic plasma membrane, CB1 receptors have also been localized to mitochondrial, endosomal, and lysosomal compartments (14, 73–76). Activation of these mitochondrial CB1 receptors suppresses respiration whereas blockade is associated with increased mitochondrial biogenesis, increased β-oxidation, and increased energy production (14). Short term consequences of mitochondrial CB1 receptor signaling include synaptic depression; long term consequences can include memory loss (77), metabolic defects and apoptosis (78). Current data suggest that mitochondrial CB1 receptors are coupled to Gαi which appears to inhibit soluble adenylyl cyclase within the mitochondrial matrix (77). Interestingly, cell surface, presynaptic CB1 receptors remain unchanged (77).
A second cannabinoid receptor, CB2, traditionally thought to mediate peripheral immune function, is also present in the brain including the prefrontal cortex and hippocampus (79). In both regions, CB2 receptors appear to be mostly neuronal, influencing excitatory synaptic transmission, plasticity, and long-term potentiation (80–82). Subcellular fractionation techniques, western blotting, binding assays and electrophysiology data from prefrontal cortex slices show that CB2 receptors are located intracellularly and that 2-arachidonoylglycerol (2-AG) activation results in inositol triphosphate 3 (IP3) receptor-dependent opening of Ca2+-activated chloride channels and decreased neuronal excitability (74, 83, 84). Thus both CB1 and CB2 receptors play a role inside the cell that contributes to synaptic plasticity.
mGlu5:
Besides presynaptic GPCRs modulating neurotransmitter release, postsynaptic receptors are also highly linked to learning and memory, including the Group 1 receptor, mGlu5. In the hippocampus, gene knock out studies show that LTP and LTD are impaired in mGlu5 null animals. This is in agreement with data showing mGlu5 plays a key role in the protein synthesisdependent phase of LTP (85–87) as well as LTD (88, 89). mGlu5 receptors are also highly expressed on the cell surface and intracellular membranes of hippocampal CA1 neurons where only intracellular mGlu5 activation triggered sustained Ca2+ responses in dendrites. Using an ex vivo slice approach, an important role for intracellular mGlu5 was also seen for electrically- and chemically-induced, protein-synthesis-dependent LTD but not for LTP in acute hippocampal slices (41; Fig. 3).
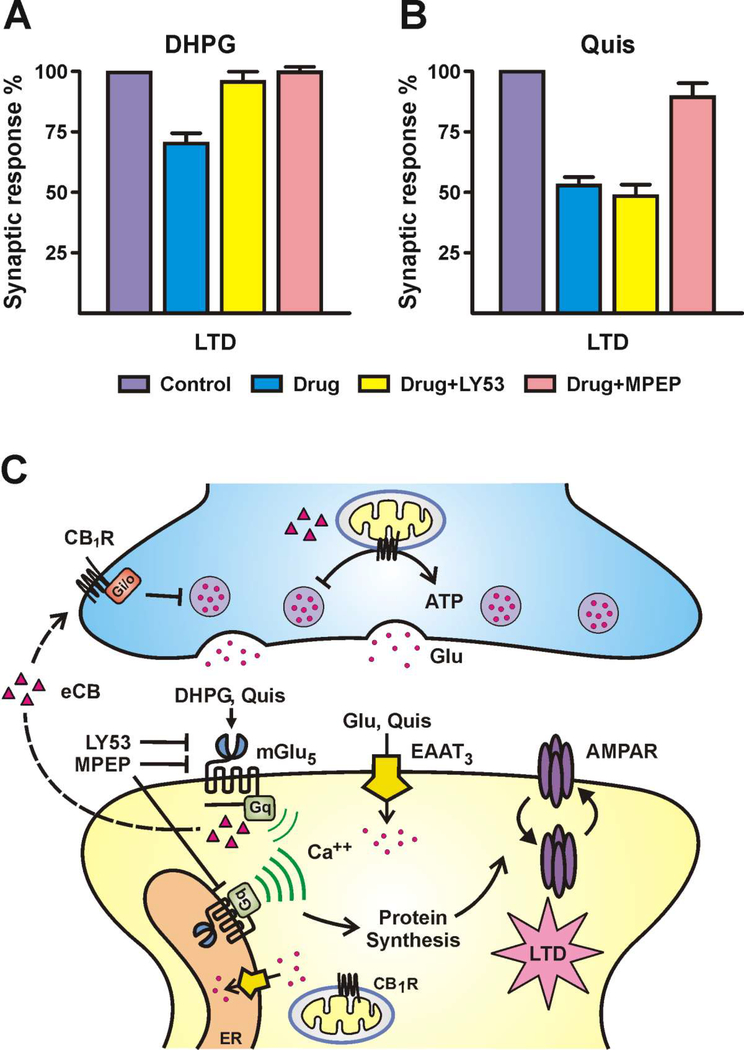
Fig. 3.
Pharmacological isolation demonstrates activation of intracellular mGlu5 receptors can mediate LTD. Information contained in the figure has been extrapolated from (41). Drug addition of DHPG (cell impermeant, non-transported agonist; A) or Quisqualate (Quis; cell impermeant, transported agonist; B) (both 10 μM) induces depressed synaptic responses in hippocampal slices (LTD) shown here at 90 min. DHPG but not Quis LTD is blocked by the impermeant antagonist LY393053 (LY53; 10 μM); whereas both are blocked by MPEP (10 μM) which is permeable. Quis is added in the presence of CNQX (10 μM), CPCCOEt (20 μM), and APV (100 μM) inhibitors to isolate mGlu5 activation; n = 5 for each experiment. C. Conceptual illustration based on information from (3, 41, 77, 112–114). Quis can be transported into the cells via excitatory amino acid transporter 3 (EAAT3). In the hippocampus activation of either cell surface or intracellular mGlu5 receptors leads to activation of Gαq, causing formation of IP3 via PLC and intracellular Ca2+ mobilization. mGlu5 activation triggers several signaling pathways ultimately modulating protein synthesis and AMPA receptor (AMPAR) internalization. Studies show that mGlu5 and CB1 can regulate each other’s function since mGlu5 activation generates endocannabinoids (eCBs; triangles) which can freely diffuse across membranes to the presynaptic terminal and/or mitochondrial CB1Rs decreasing presynaptic glutamate release and mitochondrial ATP production (114, 126). It is worth noting that activation of dendritic mGlu5 receptors leads to a sustained Ca2+ response in the hippocampus (41) in contrast to the cell surface response. This might account for the more pronounced Quis-mediated LTD effect (B) and possibly increased production of eCBs.
M1 mAChRs:
M1 mAChRs are also linked to LTP and LTD. For example, M1 knock out mice show severe deficits in hippocampal LTP, working memory and memory consolidation (92–93). Using silver-enhanced immunogold staining of both the cortex and the hippocampus, Yamasaki et al. (94) found that M1 mAChRs were primarily expressed in pyramidal cells where a large amount of the receptor was found intracellularly in association with the ER and Golgi. Subsequent studies revealed ER and Golgi-localized M1 receptors in the hippocampus and cortex of rats, mice and humans (95). As with mGlu5 receptors, pharmacological isolation was used to determine that while both cell surface and intracellular M1 mAChRs enhanced phosphoinositide turnover, only intracellular M1 mediated ERK1/2 activation (95). Importantly, carbachol-facilitated LTP was also differentially regulated in that only early (5–15 min) stages of potentiation were blocked by the M1 impermeant antagonist whereas late stages of potentiation (45–60 min; LTP) were blocked by a permeable antagonist as well as a MAPK blocker (95). Thus, just as intracellular mGlu5 is necessary for hippocampal LTD (41), intracellular M1 mAChRs are necessary for LTP (95). M1 mAChRs also induce LTD in hippocampal CA1 in a protein synthesis-, ERK1/2- and rapamycin (mTOR)-dependent fashion (96). Whether intracellular M1 mAChRs play a role in LTD remains unknown at present.
GPER:
Estrogens are known to influence a wide array of behaviors including hippocampal learning and memory in female rodents. Mechanisms underlying some of these behaviors are due to interactions between mGlu1 and the canonical estrogen receptors leading to estrogen E2induced phosphorylation of ERK1/2, PI3K, and Akt regulating local protein synthesis (97). GPER also plays a key role in promoting hippocampal memory formation since its agonist, G-1, enhances social recognition, object recognition and spatial memory in ovariectomized female mice, whereas G-15, the GPER antagonist, blocks these processes (98, 99). G-1 treatment of GPER also increases dendritic spine density in the CA1 hippocampus further underscoring its role in morphogenetic processes associated with learning and memory (100). Of interest here, ligand activation of GPER enhances memory consolidation by activating JNK, which, in turn, facilitates gene expression via transcription factors such as ATF2 (98). Although these studies have not directly shown whether GPER effects are due to cell surface and/or intracellular receptors, most GPER is found on intracellular membranes especially the ER (61). Thus, it seems likely that at least some percentage of intracellular GPER is regulating hippocampal learning and memory.
Intracellular GPCR signaling in disorders of synaptic plasticity
Fragile X syndrome (FXS):
Emerging evidence furthers the notion that GPCR localization also plays an important role in various disease conditions including disorders of synaptic plasticity. In part, this idea grew out of the discovery that the FXS gene product, the Fragile X mental retardation protein (FMRP), can act as a translational repressor of subsets of neuronal mRNAs, including ones involved in synaptic plasticity (101). A prominent hypothesis of FXS is that symptoms arise due to exaggerated signaling of mGlu5 which normally oppose the function of FMRP (102). Work in animal models showed that loss of FMRP enhanced mGlu5 signaling leading to the prediction that mGlu5 antagonists should restore normal synaptic balance and improve behavioral phenotypes (103). Consistent with this notion, several different mGlu5 inhibitors (MPEP, fenobam, CTEP) did indeed improve FXS-like phenotypes in various animal models (102, 104, 105). These findings prompted clinical trials with various mGlu5 negative allosteric modulators (106). Unfortunately, despite early promise, these trials were discontinued due to negative outcome results (107).
Although there can be many explanations for this conclusion (species differences; erroneous and/or inadequate disease modeling, paucity of clinically relevant outcome markers, tolerance, etc.), another possibility is differential inhibition of mGlu5 receptors on cell surface or intracellular membranes. For example, not only do drug candidates have unique chemical properties but different populations of neurons also have distinctive membrane constituents and lipophilic characteristics which might underlie differential receptor efficacy (108). One of the most dramatic examples of differential lipid composition in biological membranes is that of cholesterol which is heterogeneously distributed between cellular membranes and among different cell types (109). Recent studies show that the level of cholesterol in a membrane significantly increases or decreases the stability, ligand-binding properties and/or function of many different GPCRs including mGlu receptors (108). At least three different cholesterol binding motifs have been described in various GPCRs (110), suggesting a potential allosteric role for cholesterol in modulating GPCR functions. Inasmuch as cholesterol is enriched in the plasma membrane (20–25% of lipid molecules; 109) but is only 1% of ER membrane lipids (109), it is conceivable that membrane-specific cholesterol levels might differentially affect GPCR function at the cell surface versus ER or nuclear membranes. A drug that can readily access the brain parenchyma and easily modulate a cell surface GPCR may not have the same effect on an intracellular receptor due to membrane phospholipid composition. In addition to the other reasons the FXS clinical trials might have failed, we speculate that the drugs used for the Fragile X clinical trials may not have blocked all the mGlu5 necessary to achieve remediation of the disorder
Cognition:
Intracellular GPCRs also play key roles in responding to environmental stimuli and individual experiences that underlie synaptic change and normal brain function. As one of the most abundant GPCRs in the brain (111), CB1 receptors are especially important in mediating either physiological and/or pathological stimuli presynaptically, postsynaptically and from within the cell (112–114). As described above, pharmacological and genetic isolation of mitochondrial CB1 receptors have underscored their role in excitatory synaptic transmission as well as in memory performance (77). Specifically, activation of mitochondrial CB1 receptors induces memory impairment (amnesia) after training in a novel object recognition task whereas the same experiment done in animals that are unable to traffick CB1 receptors to the mitochondria shows no effect (77). The CB1-mediated memory impairment appears to be due to decreased protein kinase A (PKA)-dependent phosphorylation of complex I proteins, leading to decreased ATP and decreased mitochondrial respiration. Thus there appears to be a direct link between mitochondrial CB1 receptors, bioenergetics and higher brain function (114). The exact mechanisms underlying this process are still unclear, but dissecting the specific proteins/pathways involved in this process may generate new therapeutic tools for brain disorders leading to memory loss.
GPCR regulation of ATP production and respiration may be a more generalized phenomenon. For example, the mitochondrial GPCRs (AT1R, AT2R, P2Y1, P2Y2, 5-HT4, MT1) might also contribute to higher brain function via modulation of ATP levels in critical tissues at critical time points. Determining if and how mitochondrial receptors modulate respiration and other mitochondrial functions such as oxidative stress, fission/fusion, intracellular motility, apoptosis, and Ca2+ buffering, etc. may be critical in understanding how these receptors affect higher brain functions such as learning and memory.
Social Learning:
Estrogens are known to influence a wide variety of behaviors including social preferences, aggression, dominance, social recognition and social learning (62, 98, 115). Traditionally, these behaviors are thought to involve the canonical estrogen receptors, ERα and ERβ. However, not all such behaviors can be ascribed to ERα and ERβ. The discovery and characterization of GPER has led to the recognition that its wide expression in both the central and peripheral nervous system may mediate many of estrogen’s physiological and pathological functions. In support of this notion, social learning involves GPER, not ERα or ERβ (98). For example, studies investigating GPER-specific effects in the dorsal hippocampus showed that infusion of the GPER specific agonist, G-1, led to a dose and time-dependent improvement in social recognition and object recognition but not object placement learning in female rats (116). Using a similar paradigm, Kim et al., (117) also found that activation of GPER enhanced object recognition whereas GPER inhibition impaired memory. Interestingly, estrogen effects via ERα and ERβ resulted in ERK1/2 activation whereas Kim et al., found that GPER activation led to phosphorylation of JNK. Infusion of a JNK inhibitor blocked G-1 enhanced object recognition whereas ERK inhibition did not (117). Taken together these experiments underscore the diversity of signaling mechanisms associated with hippocampal memory formation and further emphasize that a single ligand, in this case estrogen, can affect many pathways independently to regulate learning and memory.
Other neuronal functions in which activation of GPER has been linked to positive outcomes include depression, pain, metabolic regulation (body weight, energy balance), and post ischemic stroke (62). Since most of the neurological studies have focused on targeting GPER per se, and because GPER-specific agonists and antagonists are permeable, it is unclear whether the observed neuronal effects are due to cell surface and/or intracellular GPER. Based on its widespread distribution and large body of data showing that the majority of GPER is within the cell, it seems likely that intracellular GPER participates in at least some of these behaviors. Improved pharmacological or genetic tools will help determine which pool of receptors is responsible for which behavioral outcome.
Summary
The wealth of new data demonstrating a plethora of receptors on almost every type of intracellular membrane (nuclei, ER, mitochondria, lysosomes, endosomes) represents a paradigm shift in GPCR research and opens the door for a host of new translational applications. Although for some GPCRs, receptor activation and/or inhibition may occur at the cell surface; for others, whether a ligand gets across a given cellular membrane may change its functional response. Although pharmacological isolation provides evidence of a given receptor’s in vivo physiological role, the development of genetically isolated animals in which receptors are targeted or excluded from a given intracellular membrane, would reinforce the role of an intracellular receptor. For example, Hebert-Chatelain et al. (77) discovered that removal of the first 22 amino acids at the N-terminus of CB1 receptors (DN22-CB1) prevents the receptor from going to or affecting mitochondrial processes such as respiration or mobility. Genetic isolation in vivo via viral re-expression of CB1 and DN22-CB1 in CB1 knock out animals revealed that only mitochondrial CB1 receptors mediated hippocampal synaptic transmission and memory formation. Joyal et al. used a similar approach to distinguish plasma-membrane from nuclear F2rl1 functions in vivo (5, 16). In these studies (5, 16) intravitreally injected viral constructs were targeted to either the cell surface or nuclei of retinal ganglion neurons of F2rl knock out mice. Plasma membrane-localized F2rl1 retinas exhibited increased Ang1, an angiogenic factor associated with vascular remodeling and maturation. In contrast, nuclear-localized Frl1showed increased Vegfa expression which is associated with neovascularization (5, 16). Injection of the native F2rl1 increased the expression of both angiogenic factors (5,16). Animals such as these can potentially serve as model systems for the development of drugs optimized for a desirable cell surface and/or intracellular response. In the latter case, the same key parameters associated with drug development for cell surface receptors such as efficacy, potency and specificity are still essential for intracellular GPCR drug design. However, strategies that can get a drug or a biomolecule into the cell and even to the appropriate organelle in the cytoplasm without perturbing its cell surface counterpart would be required (118). For example, to prevent NK1 receptor endosomal signaling, Jensen et al. (59) synthesized tripartite compounds composed of cholestanol to promote membrane insertion, a polyethylene linker for flexibility and a membrane impermeable NK1 receptor antagonist. This strategy successfully blocked further NK1 receptor endosomal signaling and promoted the desired behavioral response, in this case antinociception (59). Other new techniques include polymer-based nanocarriers, which can be tailored to display a given charge or combined with other biomolecules such as drugs, antibodies, proteins and oligonucleotides to deliver a particular compound to a particular intracellular location (119–121). Taken together these new tools and new strategies will allow an unprecedented ability to deliver therapeutics to every part of the cell.
As highlighted here, intracellular GPCRs have been linked to synaptogenesis, spine formation, learning and memory, cognition and behavior, as well as to pathophysiological roles in disorders such as FXS, Autism Spectrum Disorders, and depression. Therefore understanding how GPCRs responding to environmental stimuli achieve the necessary changes in synaptic function to learn new tasks and form new memories remains at the cutting edge of this field. Thus studies that investigate the molecular mechanisms that determine the subcellular distribution and signaling properties of a given GPCR are critical for developing effective pharmacological agents that target the chosen receptor.
Acknowledgment
This work was supported by National Institutes of Health (NINDS grant R21NS102783 and NIMH grant R21MH109019).
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- Akt
protein kinase B
- Arc
activity-regulated cytoskeletal-associated protein
- AT1R and AT2R
angiotensin I and II receptors
- ATF2
activating transcription factor 2
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- CB1
cannabinoid 1 receptors
- CCR2
chemokine receptor 2
- CXCR4
cysteine (C)-X-C Receptor 4
- egr-1
early growth response protein 1
- Elk-1
ETS domain-containing protein
- ER
endoplasmic reticulum
- ERα and ERβ
estrogen α and β receptors
- ERK
extracellular signal-regulated kinase
- F2rl1
coagulation factor II receptor-like 1
- FMRP
Fragile X mental retardation protein
- FXS
Fragile X syndrome
- GPER (GPR30)
G-protein-coupled estrogen receptor-1
- GPCRs
G protein-coupled receptors
- GnRH
gonadotropin-releasing hormone
- 5-HT4
5-hydroxytrptamine receptor 4
- IP3
Inositol trisphosphate
- LPA
lysophosphatidic acid
- LTD
long term depression
- LTP
long term potentiation
- JNKs
c-Jun amino-terminal kinases
- mAChRs
Muscarinic acetylcholine receptors
- MAPK
mitogen-activated protein kinase
- mGlu5
metabotropic glutamate receptor 5
- MT1
melatonin 1 receptors
- mTOR
mammalian target of rapamycin
- NK1R
neurokinin 1 receptor
- NLS
nuclear localization signals
- eNOS
nitric oxide synthase
- OA-1
ocular albinism I
- P2Y1, P2Y2
purine receptors
- PACAP
pituitary adenylate cyclase activating polypeptide
- PGE2
Prostaglandin E2
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- Ptafr
platelet-activating factor receptor
- S1P1
sphingosine 1-phosphate receptor 1
- STATs
activators of transcription
- VIP
vasoactive intestinal peptide
- VPAC1
vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptor
Footnotes
The authors declare no competing financial interest.
References
- 1.Regard JB, Sato IT, and Coughlin SR (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung CCY and Wong YH (2017) Role of G Protein-Coupled Receptors in the Regulation of Structural Plasticity and Cognitive Function. Molecules 24;22(7) doi: 10.3390/molecules22071239. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jong YI, Harmon SK, and O’Malley KL (2017) GPCR signalling from within the cell. Br J Pharmacol. September 5, 2017. doi: 10.1111/bph.14023. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branco AF and Allen BG (2015) G-protein-coupled receptor signaling in cardiac nuclear membranes. J Cardiovasc Pharmacol. 65, 101–109. Review. [DOI] [PubMed] [Google Scholar]
- 5.Joyal JS, Bhosle VK, and Chemtob S (2015) Subcellular G-protein coupled receptor signaling hints at greater therapeutic selectivity. Expert Opin Ther Targets 19, 717–721. Review. [DOI] [PubMed] [Google Scholar]
- 6.Campden R, Audet N, and Hébert TE (2015) Nuclear G protein signaling: new tricks for old dogs. J Cardiovasc Pharmacol 65,110–122. Review. [DOI] [PubMed] [Google Scholar]
- 7.Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M, and von Zastrow M (2017) Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol 13:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calebiro D, Godbole A, Lyga S, and Lohse MJ (2015) Trafficking and function of GPCRs in the endosomal compartment. Methods Mol Biol 1234, 97–211. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo E, Schiedel AC, and Manga P (2017) Interaction between G Protein-Coupled Receptor 143 and Tyrosinase: Implications for Understanding Ocular Albinism Type 1. J Invest Dermatol 137, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goshima Y, Nakamura F, Masukawa D, Chen S, and Koga M (2014) Cardiovascular actions of DOPA mediated by the gene product of ocular albinism 1. J Pharmacol Sci 126, 14–20. [DOI] [PubMed] [Google Scholar]
- 11.Schiaffno MV (2010) Signaling patyways in melanosome biogenesis and pathology. Int J Biochem Cell Biol. 42, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya M, Peri K, Ribeiro-da-Silva A, Almazan G, Shichi H, Hou X, Varma DR, and Chemtob S (1999) Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem 274, 15719–15724. [DOI] [PubMed] [Google Scholar]
- 13.O’Malley KL, Jong YJ, Gonchar Y, Burkhalter A, and Romano C (2003) Activation of metabotropic glutamate receptor on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem 278, 28210–28219. [DOI] [PubMed] [Google Scholar]
- 14.Bénard G, Massa F, Puente N, Lourenço J, Bellocchio L, Soria-Gómez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutiérrez S, Martín-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, López-Rodríguez ML, Grandes P, Rossignol R, and Marsicano G (2012) Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat Neurosci. 15, 558–564. [DOI] [PubMed] [Google Scholar]
- 15.Tadevosyan A, Vaniotis G, Allen BG, Hébert TE, and Nattel S (2012) G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol 590, 1313–1330. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z, Sapieha P, Hamel D, Sanchez M, Zaniolo K, St-Louis M, Ouellette J, Montoya-Zavala M, Zabeida A, Picard E, Hardy P, Bhosle V, Varma DR, Gobeil F Jr., Beauséjour C, Boileau C, Klein W, Hollenberg M, Ribeiro-da-Silva A, Andelfinger G, and Chemtob S (2014) Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med. 20, 1165–1173. [DOI] [PubMed] [Google Scholar]
- 17.Lee DK, Lança AJ, Cheng R, Nguyen T, Ji XD, Gobeil F Jr., Chemtob S, George SR, and O’Dowd BF (2004) Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem 279, 7901–7908. [DOI] [PubMed] [Google Scholar]
- 18.Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee MH, Luttrell L, and Ullian ME (2007) Identification of a putative nuclear localization sequence within ANG II AT(1A) receptor associated with nuclear activation. Am J Physiol Cell Physiol 292, C1398–1408. [DOI] [PubMed] [Google Scholar]
- 19.Wright CD, Wu SC, Dahl EF, Sazama AJ, and O’Connell TD (2012) Nuclear localization drives α1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal 24, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilardaga JP, Jean-Alphonse FG, and Gardella TJ (2014).Endosomal generation of cAMP in GPCR signaling. Nat Chem Bio 10, 700–706. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, and Barbaz D (2006) G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol 84, 287–297. Review. [DOI] [PubMed] [Google Scholar]
- 22.Boivin B, Vaniotis G, Allen BG, and Hébert TE (2008) G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res 28, 15–28. Review. [DOI] [PubMed] [Google Scholar]
- 23.Bhosle VK, Rivera JC, and Chemtob S (2017) New Insights into Mechanisms of Nuclear Translocation of G-protein Coupled Receptors. Small GTPases. January 26, 2017:1–10. doi: 10.1080/21541248.2017.1282402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Benedetto A, Sun L, Zambonin CG, Tamma R, Nico B, Calvano CD,Colaianni G4, Ji Y, Mori G, Grano M, Lu P, Colucci S, Yuen T, New MI, Zallone A, and Zaidi M(2014) Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc Natl Acad Sci U S A 111, 16502–16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinsey CG, Bussolati G, Bosco M, Kimura T, Pizzorno MC, Chernin MI, Cassoni P, and Novak JF (2007) Constitutive and ligand-induced nuclear localization of oxytocin receptor. J Cell Mol Med 11, 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Re M, Pampillo M, Savard M, Dubuc C, McArdle CA, Millar RP, Conn PM, Gobeil F Jr., Bhattacharya M, and Babwah AV (2010) The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One 5,e11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sergin I, Jong YI, Harmon SK, Kumar V, and O’Malley KL (2017) Sequences within the C Terminus of the Metabotropic Glutamate Receptor 5 (mGluR5) Are Responsible for Inner Nuclear Membrane Localization. J Biol Chem. 292, 3637–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verzijl D, Peters SL, and Alewijnse AE (2010) Sphingosine-1-phosphate receptors: zooming in on ligand-induced intracellular trafficking and its functional implications. Mol Cells 29, 99–104. [DOI] [PubMed] [Google Scholar]
- 29.Estrada R, Wang L, Jala VR, Lee JF, Lin CY, Gray RD, Haribabu B, and Lee MJ (2009). Ligand-induced nuclear translocation of S1P(1) receptors mediates Cyr61 and CTGF transcription in endothelial cells. Histochem Cell Biol 131, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Favre N, Camps M, Arod C, Chabert C, Rommel C, and Pasquali C (2008) Chemokine receptor CCR2 undergoes transportin1-dependent nuclear translocation. Proteomics. 8, 4560–4576. [DOI] [PubMed] [Google Scholar]
- 31.Don-Salu-Hewage AS, Chan SY, McAndrews KM, Chetram MA, Dawson MR, Bethea DA and Hinton CV (2013) Cysteine (C)-x-C receptor 4 undergoes transportin 1-dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS ONE 8, e57194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun RL, Huang CX, Bao JL, Jiang JY, Zhang B, Zhou SX, Cai WB, Wang H, Wang JF, and Zhang YL (2016) CC-Chemokine Ligand 2 (CCL2) Suppresses High Density Lipoprotein (HDL) Internalization and Cholesterol Efflux via CC-Chemokine Receptor 2 (CCR2) Induction and p42/44 Mitogen-activated Protein Kinase (MAPK) Activation in Human Endothelial Cells. J Biol Chem 291, 19532–19544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuleger N, Kerr AR, and Schirmer EC (2012) Many mechanisms, one entrance: membrane protein translocation into the nucleus. Cell Mol Life Sci 69, 2205–2216. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katta SS, Smoyer CJ, and Jaspersen SL (2014) Destination: inner nuclear membrane. Trends Cell Biol 24, 221–229. Review. [DOI] [PubMed] [Google Scholar]
- 35.Lusk CP, Blobel G, and King MC (2007) Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol 8, 414–420. Review. [DOI] [PubMed] [Google Scholar]
- 36.Zuleger N, Korfali N, and Schirmer EC (2008) Inner nuclear membrane protein transport is mediated by multiple mechanisms. Biochem Soc Trans. 36, 1373–1377. Review. [DOI] [PubMed] [Google Scholar]
- 37.Yu R, Liu H, Peng X, Cui Y, Song S, Wang L, Zhang H, Hong A, and Zhou T (2017) The palmitoylation of the N-terminal extracellular Cys37 mediates the nuclear translocation of VPAC1 contributing to its anti-apoptotic activity. Oncotarget. 8, 42728–42741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barlow CA, Laishram RS, and Anderson RA (2010) Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol 20, 25–35. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jong YJ, Kumar V, Kingston AE, Romano C, and O’Malley KL (2005). Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem 280, 30469–30480. [DOI] [PubMed] [Google Scholar]
- 40.Jong YJ, Schwetye KE, and O’Malley KL (2007) Nuclear localization of functional metabotropic glutamate receptor mGlu1 in HEK293 cells and cortical neurons: role in nuclear calcium mobilization and development. J Neurochem 101, 458–469. [DOI] [PubMed] [Google Scholar]
- 41.Purgert CA, Izumi Y, Jong YJ, Kumar V, Zorumski CF, and O’Malley KL (2014) Intracellular can mediate synaptic plasticity in the hippocampus. J Neurosci 34, 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadevosyan A, Létourneau M, Folch B, Doucet N, Villeneuve LR, Mamarbachi AM Pétrin D, Héber TE, Fournier A, Chatenet D, Allen BG, and Nattel S (2015) Photoreleasable ligands to study intracrine angiotensin II signalling. J Physiol. 593, 521–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jong YI and O’Malley KL (2017) Mechanisms Associated with Activation of Intracellular Metabotropic Glutamate Receptor, mGluR5. Neurochem Res. 42, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, Bkaily G, Peri K, Varma DR, Orvoine R, and Chemtob S (2006) Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol. 84, 377–391. [DOI] [PubMed] [Google Scholar]
- 45.Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, and Fagni L (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411, 962–965. [DOI] [PubMed] [Google Scholar]
- 46.Delcourt N, Thouvenot E, Chanrion B, Galéotti N, Jouin P, Bockaert J and Marin P (2007) ACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J 26, 1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaniotis G, Allen BG, and Hébert TE (2011) Nuclear GPCRs in cardiomyocytes: an insider’s view of β-adrenergic receptor signaling. Am J Physiol Heart Circ Physiol 301, H1754–H1764. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abadir PM, Walston JD, and Carey RM (2012) Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 38:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belous A, Wakata A, Knox CD, Nicoud IB, Pierce J, Anderson CD, Pinson CW, and Chari RS (2004) Mitochondrial P2Y-Like receptors link cytosolic adenosine nucleotides to mitochondrial calcium uptake. J Cell Biochem 92, 1062–1073. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Zhang H, Xu H, Guo D, Shi H, Li Y Zhang W, and Gu Y (2016) 5HTR3 and 5-HTR4 located on the mitochondrial membrane and functionally regulated mitochondrial functions. Sci Rep 6, 37336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gbahou F, Cecon E, Viault G, Gerbier R, Jean-Alphonse F, Karamitri A, Guillaumet G, Delagrange P, Friedlander RM, Vilardaga JP, Suzenet F, and Jockers R (2017) Design and validation of the first cell-impermeant melatonin receptor agonist. Br J Pharmacol 174, 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suofu YLW, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, Ferrando LM, Larkin TM, Sullivan M, Yablonska S, Wang J, Minnigh MB, Guillaumet G, Suzenet F, Richardson RM, Poloyac SM, Stolz DB, Jockers R, Witt-Enderby PA, Carlisle DL, Vilardaga JP, and Friedlander RM (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci U S A. 114, E7997–E8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busquets-Garcia A, Bains J, and Marsicano G (2018) CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology. 43, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bahouth SW and Nooh MM (2017) Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell Signal. 36, 42–55. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiter E, Ahn S, Shukla AK, and Lefkowitz RJ (2012). Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol Toxicol 52, 179–197. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rankovic Z, Brust TF, and Bohn LM (2016) Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg Med Chem Lett. 26, 241–250. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geppetti P, Veldhuis NA, Lieu T, and Bunnett NW (2015) G Protein-Coupled Receptors: Dynamic Machines for Signaling Pain and Itch. Neuron 88, 635–649. Review. [DOI] [PubMed] [Google Scholar]
- 58.Tsvetanova NG and von Zastrow M (2014) Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 10, 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen DD, Lieu T, Halls ML, Veldhuis NA, Imlach WL, Mai QN, Poole DP, Quach T, Aurelio L, Conner J, Herenbrink CK, Barlow N, Simpson JS, Scanlon MJ, Graham B, McCluskey A, Robinson PJ, Escriou V, Nassini R, Materazzi S, Geppetti P, Hicks GA, Christie MJ, Porter CJH, Canals M, and Bunnett NW (2017) Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med. May 31, 2017; 9(392) doi: 10.1126/scitranslmed.aal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas P, Pang Y, Filardo EJ, and Dong J (2005) “Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells,” Endocrinology, 146, 624–632. [DOI] [PubMed] [Google Scholar]
- 61.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, and Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630. [DOI] [PubMed] [Google Scholar]
- 62.Lu CL and Herndon C (2017) New roles for neuronal estrogen receptors. Neurogastroenterol Motil. July 2017; 29(7). doi: 10.1111/nmo.13121. Review. [DOI] [PubMed] [Google Scholar]
- 63.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, and Dun NJ (2007) Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 193, 311–321. [DOI] [PubMed] [Google Scholar]
- 64.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, and Milner TA (2015) G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 35, 2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filardo EJ (2017) A role for G-protein coupled estrogen receptor (GPER) in estrogen-induced carcinogenesis: Dysregulated glandular homeostasis, survival and metastasis. J Steroid Biochem Mol Biol. June 62017. doi: 10.1016/j.jsbmb.2017.05.005. Review [DOI] [PubMed] [Google Scholar]
- 66.Mitrano DA and Smith Y (2007) Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 500, 788–806. [DOI] [PubMed] [Google Scholar]
- 67.Lujan R, Nusser Z, Roberts JD, Shigemoto R, and Somogyi P (1996) Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 8, 1488–1500. [DOI] [PubMed] [Google Scholar]
- 68.Hubert GW, Paquet M, and Smith Y (2001) Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 21, 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitrano DA, Pare JF, and Smith Y (2010) Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J Comp Neurol. 518, 1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tedford HW and Zamponi GW (2006) Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 58, 837–862. Review. [DOI] [PubMed] [Google Scholar]
- 71.Betke KM and Wells CA (2012) Hamm HE. GPCR mediated regulation of synaptic transmission. Prog Neurobiol. 96, 304–321. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rojas A and Dingledine R (2013) Ionotropic glutamate receptors: regulation by Gprotein-coupled receptors. Mol Pharmacol. 83, 746–752. Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rozenfeld R and Devi LA (2008) Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 22, 2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brailoiu GC, Deliu E, Marcu J,. Hoffman NE, Console-Bram L, Zhao P, Madesh M, and Abood ME and Brailoiu E (2014) Differential activation of intracellular versus plasmalemmal CB2 cannabinoid receptors. Biochemistry 53, 4990–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hebert-Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R, Piazza PV, Benard G, Grandes P, and Marsicano G (2014). Cannabinoid control of brain bioenergetics: exploring the subcellular localization of the CB1. Mol Metab 3, 495– 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, and Horvath TL (2015) Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, BusquetsGarcia A, Pagano Zottola AC, Delamarre A, Cannich A, Vincent P, Varilh M, Robin LM, Terral G, García-Fernández MD, Colavita M, Mazier W, Drago F, Puente N, Reguero L, Elezgarai I, Dupuy JW, Cota D, Lopez-Rodriguez ML, Barreda-Gómez G, Massa F, Grandes P, Bénard G, and Marsicano G (2016) A cannabinoid link between mitochondria and memory. Nature 539, 555–559. [DOI] [PubMed] [Google Scholar]
- 78.Xu Z, Lv XA, Dai Q, Ge YQ, Xu J (2016) Acute upregulation of neuronal mitochondrial type-1 cannabinoid receptor and it’s role in metabolic defects and neuronal apoptosis after TBI. Mol Brain 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Onaivi ES, Ishiguro H, Gu S, and Liu QR (2012) CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 26, 92–103. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J and Li Y (2015) Chronic activation of CB2 cannabinoid receptors in the hippocampus increases excitatory synaptic transmission. J Physiol. 593, 871–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y and Kim J (2016) Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus 26, 275–281. [DOI] [PubMed] [Google Scholar]
- 82.Stempel AV, Stumpf A, Zhang HY, Özdoğan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Rácz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D (2016) Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 90, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, and Werkman TR (2012) Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A 109, 3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Currie S, Rainbow RD, Ewart MA, Kitson S, Pliego EH, Kane KA, and McCaron JG(2008) IP(3)R-mediated Ca(2+) release is modulated by anandamide in isolated cardiac nuclei. J Mol Cell Cardiol. 45: 804–811. [DOI] [PubMed] [Google Scholar]
- 85.Naie K and Manahan-Vaughan D (2004) Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 14, 189–198. [DOI] [PubMed] [Google Scholar]
- 86.Naie K and Manahan-Vaughan D (2005) Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-D-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur J Neurosci. 21, 411–421. [DOI] [PubMed] [Google Scholar]
- 87.Balschun D and Wetzel W (2002) Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 73, 375–380. [DOI] [PubMed] [Google Scholar]
- 88.Naie K and Manahan-Vaughan D (2005) Investigations of the protein synthesis dependency of mGluR-induced long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology 49 Suppl 1, 35–44. [DOI] [PubMed] [Google Scholar]
- 89.Naie K, Tsanov M, and Manahan-Vaughan D (2007) Group I metabotropic glutamate receptors enable two distinct forms of long-term depression in the rat dentate gyrus in vivo. Eur J Neurosci. 25, 3264–3275. [DOI] [PubMed] [Google Scholar]
- 90.Jong YJ, Kumar V, and O’Malley KL (2009) Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem. 284, 35827–35838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar V, Fahey PG, Jong YJ, Ramanan N, and O’Malley KL (2012) Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J Biol Chem. 287, 5412–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva, and A. J. (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 6, 51–58. [DOI] [PubMed] [Google Scholar]
- 93.Wess J, Eglen RM, and Gautam D (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 6, 721–733. Review. [DOI] [PubMed] [Google Scholar]
- 94.Yamasaki M, Matsui M, and Watanabe M (2010) Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci. 30, 4408–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anisuzzaman AS, Uwada J, Masuoka T, Yoshiki H, Nishio M, Ikegaya Y, Takahashi N, Matsuki N, Fujibayashi Y, Yonekura Y, Momiyama T, and Muramatsu I (2013) Novel contribution of cell surface and intracellular M1-muscarinic acetylcholine receptors to synaptic plasticity in hippocampus. J Neurochem. 126, 360–371. [DOI] [PubMed] [Google Scholar]
- 96.Volk LJ, Pfeiffer BE, Gibson JR, and Huber KM (2007) Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J. Neurosci. 27, 11624–11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boulware MI, Heisler JD, and Frick KM (2013) The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci. 33, 15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frick KM (2015) Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 74, 4–18. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hammond R, Nelson D, Kline E, and Gibbs RB (2012) Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 62, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gabor C, Lymer J, Phan A, and Choleris E (2015) Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol Behav. 149, 53–60. [DOI] [PubMed] [Google Scholar]
- 101.Garber KB, Visootsak J, and Warren ST (2008) Fragile X syndrome. Eur J Hum Genet. 16, 666–672. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pop AS, Levenga J, de Esch CE, Buijsen RA, Nieuwenhuizen IM, Li T, Isaacs A, Gasparini F, Oostra BA, and Willemsen R (2014) Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology (Berl) 231, 1227–1235. [DOI] [PubMed] [Google Scholar]
- 103.Bear MF, Dölen G, Osterwei l E, and Nagarajan N (2008) Fragile X: translation in action. Neuropsychopharmacology. 33, 84–7. Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lindemann L, Jaeschke G, Michalon A, Vieira E, Honer M, Spooren W, Porter R, Hartung T, Kolczewski S, Büttelmann B, Flament C, Diener C, Fischer C, Gatti S, Prinssen EP, Parrott N, Hoffmann G, and Wettstein JG (2011) CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther 339, 474–486. [DOI] [PubMed] [Google Scholar]
- 105.Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, and Lindemann L (2012) Chronic pharmacological inhibition corrects fragile X in adult mice. Neuron 74, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hagerman RJ, Des-Portes V, Gasparini F, Jacquemont S, and Gomez-Mancilla B (2014) Translating molecular advances in fragile X syndrome into therapy: a review. J Clin Psychiatry. 75, e294–307. [DOI] [PubMed] [Google Scholar]
- 107.Youssef EA, Berry-Kravis E, Czech C, Hagerman RJ, Hessl D, Wong CY, Rabbia M, Deptula D, John A, Kinch R, Drewitt P, Lindemann L, Marcinowski M, Langland R, Horn C, Fontoura P, Santarelli L, and Quiroz JA FragXis Study Group. (2017) Effect of the mGluR5-NAM Basimglurant on Behavior in Adolescents and Adults with Fragile X Syndrome in a Randomized, Double-Blind, Placebo-Controlled Trial: FragXis Phase 2 Results. Neuropsychopharmacology Aug 17, 2017. doi: 10.1038/npp.2017.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guixà-González R, Albasanz J, Rodriguez-Espigares IL, Pastor M, Sanz F, MartíSolano M, Manna M, Martinez-Seara H, Hildebrand PW, Martín M, and Selent J (2017) Membrane cholesterol access into a G-protein-coupled receptor. Nat Commun. 8, 14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ikonen E (2008) Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 9, 125–138. Review. [DOI] [PubMed] [Google Scholar]
- 110.Rouviere E, Arnarez C, Yang L, and Lyman E (2017) Identification of Two New Cholesterol Interaction Sites on the A2A Adenosine Receptor. Biophys J. 113, 2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marsicano G and Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 11, 4213–4225. [DOI] [PubMed] [Google Scholar]
- 112.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, and Watanabe M (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 89, 309–380. Review. [DOI] [PubMed] [Google Scholar]
- 113.Katona I and Freund TF (2012) Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 35, 529–558. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Djeungoue-Petga MA and Hebert-Chatelain E (2017) Linking Mitochondria and Synaptic Transmission: The CB1 Receptor. Bioessays. 2017. December;39(12) doi: 10.1002/bies.201700126. Review. [DOI] [PubMed] [Google Scholar]
- 115.Frick KM, Tuscher JJ, Koss WA, Kim J, and Taxier LR(2017) Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females. Physiol Behav. July 27,2017. doi: 10.1016/j.physbeh.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lymer J, Robinson A, Winters BD, and Choleris E (2017) Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology. 77, 131–140. [DOI] [PubMed] [Google Scholar]
- 117.Kim J, Szinte JS, Boulware MI, and Frick KM (2016) 17β-Estradiol and Agonism of G-protein-Coupled Estrogen Receptor Enhance Hippocampal Memory via Different Cell-Signaling Mechanisms. J Neurosci. 36, 3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stewart MP, Sharei A,, Ding X, Sahay G, Langer R, and Jensen KF(2016) In vitro and ex vivo strategies for intracellular delivery. Nature 538, 183–192. Review. [DOI] [PubMed] [Google Scholar]
- 119.Cohen O and Granek R (2014) Nucleus-targeted drug delivery: theoretical optimization of nanoparticles decoration for enhanced intracellular active transport. Nano Lett. 14, 2515–2521. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y, Yang Y, Yan L, Kwok SY, Li W, Wang Z, Zhu X, Zhu G, Zhang W, Chen X, and Shi P (2014) Poking cells for efficient vector-free intracellular delivery. Nat Commun. 5, 4466. doi: 10.1038/ncomms5466. [DOI] [PubMed] [Google Scholar]
- 121.Juliano RL (2016) The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 44, 6518–6548. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trivedi RR and Bhattacharyya S (2012) Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5). Biochem Biophys Res Commun. 427, 185–190. [DOI] [PubMed] [Google Scholar]
- 123.Vincent K, Cornea VM, Jong YJ, Laferrière A, Kumar N, Mickeviciute A, Fung JS, Bandegi P, Ribeiro-da-Silva A, O’Malley KL, and Coderre TJ (2016) Intracellular mGluR5 plays a critical role in neuropathic pain. Nat Commun.7, 10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harkany T and Horvath TL (2017) (S)Pot on mitochondria: cannabinoids disrupt cellular respiration to limit neuronal activity. Cell Metab. 25, 8–10. [DOI] [PubMed] [Google Scholar]
- 125.Wu SC and OʼConnell TD (2015) Nuclear compartmentalization of α1-adrenergic receptor signaling in adult cardiac myocytes. J Cardiovasc Pharmacol. 65, 91–100. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olmo IG, Ferreira-Vieira TH, and Ribeiro FM (2016) Dissecting the Signaling Pathways Involved in the Crosstalk between Metabotropic Glutamate 5 and Cannabinoid Type 1 Receptors. Mol Pharmacol. 90, 609–619. Review. [DOI] [PubMed] [Google Scholar]