Alcoholic hepatitis (AH) is the most severe form of alcoholic liver disease with 30-day mortality rate of up to 30%.[1] Corticosteroids are currently the only effective therapy but provide at best only short-term survival benefit.[2] Therefore, there is an urgent need to identify novel therapeutic targets for this lethal condition. Important pathways that have been the focus of treatment investigations include (1) bacterial and endotoxin translocation through disrupted gut barrier function (e.g. anti-lipopolysaccharides antibody, probiotics, and zinc) (2) hepatocellular apoptosis, necrosis, and injury (e.g. caspase inhibitor, and IL-22), and (3) innate immune system activation in the liver (e.g. IL-1 receptor antagonist).[3, 4]
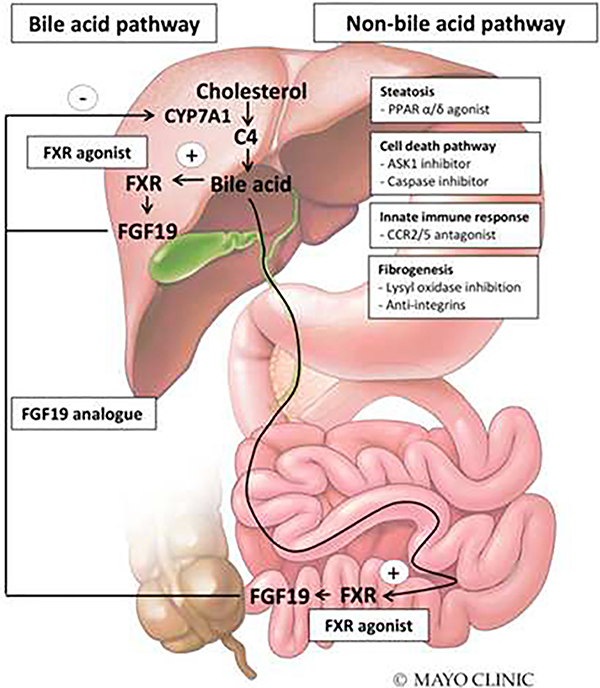
Recently, the bile acid pathway has become a major target for the treatment of primary biliary cholangitis (PBC) and non-alcoholic steatohepatitis (NASH).[5]Bile acids activate farsenoid X receptor (FXR) in the ileum and liver, leading to the production of fibroblast growth factor 19 (FGF19). FGF19 is an endocrine gastrointestinal hormone that suppresses the hepatocyte expression of CYP7A1, a rate-limiting enzyme in the synthesis of bile acids (Figure), thereby creating a negative feedback loop. Obeticholic acid (OCA), a semi-synthetic derivative of the chenodeoxycholic acid, has shown the most promising results in the treatment of NASH and PBC. OCA is a FXR agonist that protects hepatocytes against bile acid toxicity by inhibiting the synthesis of bile acids and upregulating bile acid transporters. In preclinical studies, OCA also improved steatosis, fibrosis and portal hypertension.[6] In the POISE trial, OCA was effective in reducing alkaline phosphatase, gamma-glutamyl transferase and aminotransferase levels in patients with PBC[7]. In the FLINT trial, OCA improved NAFLD activity score and ALT in patients with NASH.[8] Another therapeutic agent in the pipeline is NGM282, a FGF19 analogue. NGM282 can reduce liver fat content in patients with NASH[9] and a clinical trial of NGM282 in PBC is ongoing (NCT02135536). Following the positive findings in NASH and PBC and because cholestasis is a sentinel finding in AH, it is interesting to speculate whether bile acid pathway targets such as FXR agonist and FGF19 analogue could have beneficial effects in AH.
Figure.
Bile-acid and non-bile acid treatment targets of nonalcoholic hepatitis and cholestasis that can be opportunities in alcoholic hepatitis. Abbreviations: ASK1 = apoptosis signal-regulating kinase 1, C4 = 7α-Hydroxy-4-cholesten-3-one, CCR2/5 = C-C chemokine receptor type 2/5, FGF = fibroblast growth factor, FXR = farnesnoid X receptor, PPAR = peroxisome proliferator-activated receptor ©Mayo Foundation for Medical Education and Research. All rights reserved.
In the article by Brandl et al., the authors evaluated the relationship between bile acids, FGF19 and clinical outcomes in patients with AH compared to patients with alcohol use disorder and controls without known liver disease. The important findings were 1) total serum and conjugated bile acid levels were higher in AH compared to controls and correlated with FGF19 levels and AH severity (measured by MELD score); 2) FGF19 levels were increased and C4 levels (7-hydroxy-4-cholesten-3-one, a marker of de novo bile acid synthesis) were decreased in AH, suggesting that the negative feedback loop in the synthesis of bile acids was intact but not effective in lowering serum bile acid levels; 3) Liver tissue of patients with AH demonstrated an increase in FGF19 mRNA expression and stained positive for FGF19 suggesting the liver as a source of FGF19 production. Despite these novel findings, the authors did not demonstrate a correlation betweenFGF19 levels and survival in AH (although in the subgroup with very severe AH (MELD>30), FGF19 levels correlated modestly with 30-day survival).
Although this study provides insights on the interaction of bile acids and FGF19 in patients with AH, many questions still remain. First, it is unclear whether FGF19 has a protective effect on AH or it simply represents a marker of severity. FGF19 levels were 100 times greater in patients with AH compared to those with NASH or PBC.[7, 10] Furthermore, when compared to healthy controls, patients with NASH had decreased FGF19 and increased C4 levels while patients with AH had increased FGF19 and decreased C4.[11, 12] Thus, it is not clear whether further increments in FGF19 levels with FXR agonists or FGF19 analogues will be beneficial in AH. Second, the main source of FGF19 in patients with AH is still unknown. While an increase in hepatic FGF19 expression was observed in this study, the expression of FGF19 in the ileum was not assessed.
It’s worth noting that there is a safety concern regarding the use of OCA. There were 19 deaths reported in patients receiving OCA over a period of 13 months. This was thought to be due to incorrect dosing in setting of hepatic impairment. As a consequence, the FDA has issued a black box warning for PBC patients with Child B or C or patients with prior hepatic decompensation to take OCA once weekly rather than the standard once a day dosing recommended for PBC patients with normal liver function.[13] Moreover, the side effects of OCA include pruritus,[7]elevations of total cholesterol and low-density cholesterol, and lower levels of high-density cholesterol.[7–9] FGF19 may also advance the growth of hepatocellular carcinoma.[14] Newer agents, e.g. NGM282 (non-tumorigenic variant of FGF19 analogue) and tropifexor (non-bile acid compound FXR agonist) are being evaluated and it remains to be seen whether the newer compounds will have better safety profiles compared to OCA.
Current clinical trial activity in NASH dwarfs that of AH (149 clinical trials for NASH vs. 21 for AH/ASH-clinicaltrials.gov). Given the shared histological features of AH and NASH, bile acid independent targets investigated in NASH could be tested in AH.[15] For example, eleafibranor (GFT-505, peroxisome proliferator-activated receptor α/δ agonist) was found to reduce NAS score in patients with NAS score ≥4 in a post-hoc analysis of phase II clinical trials.[16] Selonsertib (GS-4997, apoptosis signal-regulating kinase 1 inhibitor) and Cenicriviroc (c-c chemokine receptor type 2 and 5 antagonist) were shown to reduce liver fibrosis in NASH patients on phase II clinical trials.[17, 18] Emricasan (IDN-6556, caspase inhibitor) is being tested in NASH (NCT02686762) although the trial in AH was terminated early due to potentially toxic drug levels in patients with severe hepatic impairment. Clearly, more resources are needed in AH research given the higher all-cause mortality compared to NASH.[19] Because moderate AH (generally considered by expert opinion as MELD score 11–20) shares greater similarities with NASH compared to severe AH, it may be the best initial target for some of these non-bile acid pathway therapies currently being investigated in NASH (Figure).
In conclusion, the bile acid pathway offers promising novel therapeutic targets for AH. Given the positive results in PBC and NASH, the results of the phase 2 clinical trial (NCT02039219) evaluating OCA in moderately severe AH are awaited with great expectation. Unfortunately, this study is currently on hold due to ongoing safety concerns as mentioned above. In addition to the bile acid pathway, there are many other potential targets (e.g. elafibranor, selonsertib and cenicriviroc etc.) being investigated in NASH that could also be tested in AH. Given the histopathological similarities between the two entities and the large clinical trial trial activity in the NASH space , hopefully effective treatments for AH soon follow.
Acknowledgments
Grant support: if any, we will communicate to Joël on Monday, May 7th
References
- 1.Maddrey WC, et al. , Corticosteroid therapy of alcoholic hepatitis. Gastroenterology, 1978. 75(2): p. 193–9. [PubMed] [Google Scholar]
- 2.Thursz MR, et al. , Prednisolone or Pentoxifylline for Alcoholic Hepatitis. New England Journal of Medicine, 2015. 372(17): p. 1619–1628. [DOI] [PubMed] [Google Scholar]
- 3.Singal AK, et al. , Alcoholic Hepatitis: Current Challenges and Future Directions. Clinical Gastroenterology and Hepatology, 2014. 12(4): p. 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrasek J, et al. , IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. The Journal of Clinical Investigation, 2012. 122(10): p. 3476–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhi H and Camilleri M, Modulating bile acid pathways and TGR5 receptors for treating liver and GI diseases. Curr Opin Pharmacol, 2017. 37: p. 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbeke L, et al. , Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology, 2014. 59(6): p. 2286–98. [DOI] [PubMed] [Google Scholar]
- 7.Nevens F, et al. , A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. New England Journal of Medicine, 2016. 375(7): p. 631–643. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, et al. , Farnesoid X nuclear receptor ligand obeticholic acid for noncirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. The Lancet, 2015. 385(9972): p. 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison SA, et al. , NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet, 2018. 391(10126): p. 1174–1185. [DOI] [PubMed] [Google Scholar]
- 10.Mouzaki M, et al. , Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One, 2016. 11(5): p. e0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri P, et al. , The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojcik M, et al. , A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. J Pediatr Endocrinol Metab, 2012. 25(11–12): p. 1089–93. [DOI] [PubMed] [Google Scholar]
- 13.FDA, FDA adds Boxed Warning to highlight correct dosing of Ocaliva (obeticholic acid) for patients with a rare chronic liver disease. 2018.
- 14.Ahn SM, et al. , Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology, 2014. 60(6): p. 1972–82. [DOI] [PubMed] [Google Scholar]
- 15.Greuter T, et al. , Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis. JCI Insight, 2017. 2(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratziu V, et al. , Elafibranor, an Agonist of the Peroxisome Proliferator−Activated Receptor−α and −δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology, 2016. 150(5): p. 1147–1159.e5. [DOI] [PubMed] [Google Scholar]
- 17.Loomba R, et al. , The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman SL, et al. , A randomized, placebo-controlled trial of cenicriviroc for treatment of non-alcoholic steatohepatitis with fibrosis. Hepatology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haflidadottir S, et al. , Long term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterology, 2014. 14(1): p. 166. [DOI] [PMC free article] [PubMed] [Google Scholar]