Summary
After encoding, memory traces are fragile and easily disrupted by new learning until they are stabilized through a process termed consolidation1, 2. However, a number of studies have suggested that consolidation does not make memory traces permanently stable. Results of these studies support the theory that retrieval of previously consolidated memory, termed reactivation, renders the memory traces labile again and subject to disruption by new learning unless they go through a further consolidation process, termed reconsolidation3, 4, 5, 6, 7, 8. However, it remains controversial whether reactivation and reconsolidation occur at a human behavior level9, 10, 11 and whether consolidation and reconsolidation have common mechanisms12, 13. Here, we found that reconsolidation does occur after reactivation in visual perceptual learning (VPL)14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, a type of skill learning, in humans. Moreover, changes in behavioral performance, as well as concentrations in the excitatory neurotransmitter glutamate (Glu) and in the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) as measured by magnetic resonance spectroscopy (MRS) in early visual areas exhibit similar time courses during consolidation and reconsolidation. These results indicate that reconsolidation after reactivation and consolidation in humans share common behavioral and neurochemical mechanisms.
In Study 1, we tested behaviorally whether VPL in humans undergoes reactivation and reconsolidation. Specifically, we examined whether reactivation, that is, a few blocks of performing the trained visual task, leads to two dynamic states; a fragile state shortly after reactivation and a reconsolidated state 3.5 hours after reactivation. If reactivation makes once consolidated VPL fragile, then a competing new VPL task, which follows immediately after reactivation, would disrupt, or interfere with the reactivated VPL. If the reactivated VPL becomes reconsolidated 3.5 hours after reactivation, the competing new VPL task should not interfere with the reactivated VPL.
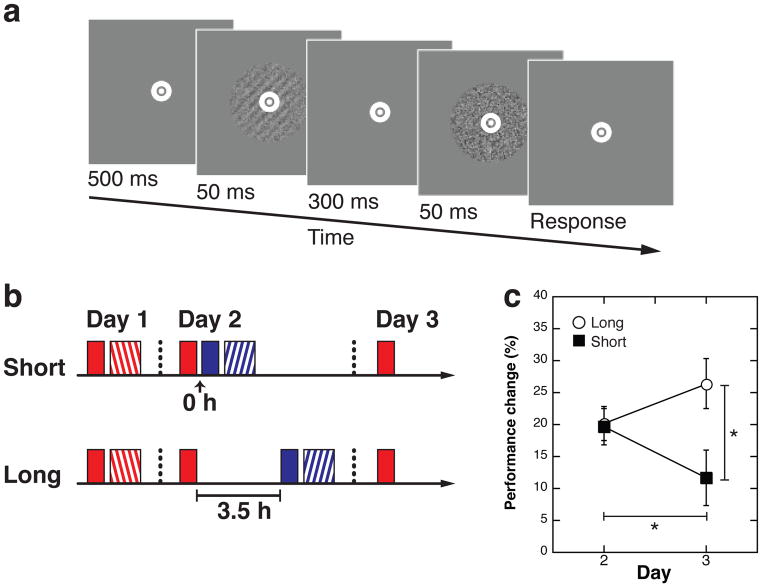
We conducted experiments with an orientation detection task (see Methods, Figure 1a). Two orientations (10 and 70 degree) were randomly assigned to Orientation A (first trained orientation) and B (second trained orientation) across participants. Our previous study showed that the learning of this task is consolidated after 3.5 hours24. There were two groups (Figure 1b), the Short interval (n=15) and Long interval groups (n=15). On Day 1, there were 16 blocks of training with Orientation A for both groups so that encoded VPL should be consolidated24 in both groups on Day 1. The only difference between the procedures with these two groups was the time interval between the offset of reactivation and the onset of the following test on Orientation B on Day 2.
Fig. 1.
Procedures and results of Study 1. (a) Orientation detection task. The bull’s eye fixation point is shown disproportionally larger for illustrative purposes. After the fixation point (500 ms), the Gabor orientation appeared either in the first or the second of two intervals, which were separated by a 300-ms blank. Participants reported in which interval the Gabor orientation was presented. (b) Experimental procedures of Study 1. There were the Short (n=15) and Long (n=15) interval groups. Red filled boxes represent three blocks of test on Orientation A. Red hatched boxes show 16 blocks of training on Orientation A. Blue filled boxes represent three blocks of test on Orientation B. Blue hatched boxes show 16 blocks of training on Orientation B. (c) Mean performance changes (± SEM) on Orientation A for Days 2 and 3 relative to Day 1 for both Long and Short interval groups. Black squares are for the Short interval group, and white circles are for the Long interval group. Asterisks (* p<0.05) indicate the results of posthoc tests of the ANOVA: a significant simple main effect of Group at Day 3 (p=0.017), and a significant simple main effect of Day for the Short group (p=0.047). See main text for details of the ANOVA results.
The results of Study 1 suggested that reactivated VPL was fragile immediately after reactivation, but was less fragile 3.5 hours after reactivation (Figure 1c). A two-way mixed ANOVA was applied to performance improvement (%) with factors Day (Day 2 vs. Day 3) and Group (Short vs. Long interval groups). Performance improvement on Day 2 is defined by [(threshold on the first day - threshold on the second day)/threshold on the first day × 100] (see Methods below) and performance improvement on Day 3 is defined by [(threshold on the first day - threshold on the third day)/threshold on the first day × 100]. If the fragility of reactivated VPL changes over time, a significant interaction should occur between Day and Group in the ANOVA. The results indicated a significant Day × Group interaction (F(1,28)=6.86, p=0.014, partial η2=0.197). Further post-hoc tests indicated a significant simple main effect of Group at Day 3 (F(1,28)=6.451, p=0.017, partial η2=0.187, 95% confidence interval of the difference (CI): 2.849–26.601), but not at Day 2 (F(1,28)=0.023, p=0.881). In addition, post-hoc analyses showed that performance improvements on Day 3 were significantly worse compared with Day 2 for the Short interval group (simple main effect of Day, F(1,28)=4.326, p=0.047, partial η2=0.134, 95% CI: 0.12–15.758), whereas no significant differences were found between Day 3 and Day 2 for the Long interval group (simple main effect of Day, (F(1,28)=2.638, p=0.116). See Supplementary Table 1 for the raw threshold values.
These results are in accord with the hypothesis that after reactivation VPL on Orientation A becomes fragile so that the following competing training on Orientation B interferes with the old VPL of Orientation A, whereas 3.5 hours later, reactivated VPL on Orientation A has become reconsolidated so that no interference occurs with the following competing training on Orientation B. While it has remained controversial whether or not reactivation and reconsolidation processes occur in humans, our results strongly suggest that reactivation and reconsolidation indeed do occur in humans with VPL.
Next, we conducted Study 2 in order to investigate whether underlying neurochemical mechanisms related to consolidation and reconsolidation are similar in the human visual cortex. Previous studies have shown that the plasticity of cortical regions is positively correlated with the concentration of Glu26, a major excitatory neurotransmitter, while being negatively correlated with the concentration of GABA, a chief inhibitory neurotransmitter27, 28. Moreover, a number of studies have indicated that the balance between excitatory and inhibitory signals determines the degree of plasticity29. In particular, our recent study24 demonstrates that a typical consolidation process in VPL is highly correlated with the ratio of the concentrations of Glu to GABA neurotransmitters (E/I ratio) in human early visual areas24 in strong association with psychophysical results: When VPL was in a plastic and therefore unstable state as indicated by significant interference with new learning immediately after encoding, the E/I ratio in early visual areas increased. However, within a few hours the E/I ratio returned to baseline levels, suggesting that VPL became stable and was not interfered with by new learning. In short, when a state of VPL is plastic and unstable, the E/I ratio in early visual areas is greater than baseline, but returns to baseline as VPL becomes consolidated.
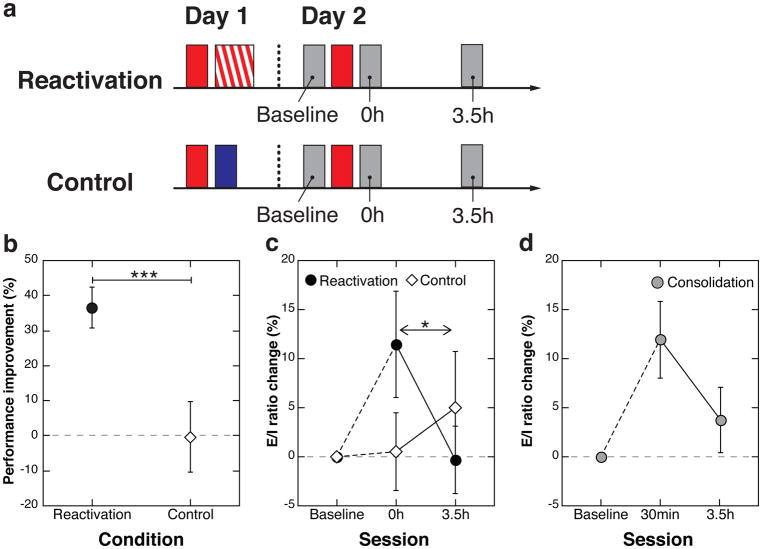
In Study 2, to address the above-mentioned question whether the neural mechanisms of reactivation and reconsolidation processes are similar or different from those of post-encoding and consolidation, we investigated the neurochemical dynamics underlying reconsolidation of VPL using MRS. There were 2 groups, the Reactivation and the Control groups. We measured and compared the E/I ratios in early visual areas in the Reactivation group (n=12) in which reactivation of consolidated learning occurred with those in the Control group (n=12) in which no reactivation occurred (see Figure 2a). The Reactivation group performed a total of 19 blocks (3 blocks for test and 16 blocks for training) on Orientation A on Day 1 in order to induce learning of Orientation A. On Day 2, three blocks on Orientation A were conducted to reactivate learning of Orientation A. In the Control group, on Day 1 there were three blocks of trials on Orientation A, followed by another three blocks of trials on Orientation B; this design assured that no learning of Orientation A could occur because of retrograde interference from Orientation B with Orientation A. The procedure on Day 2 in the Control group was identical to the Reactivation group. Importantly, the number of MRS scans as well as the intervals between the MRS measurements and the number of blocks were exactly the same between the two groups on Day 2. The difference in the experimental manipulation occurred only on Day 1.
Fig. 2.
Design and results of Study 2. (a) Design of Study 2. There were two groups, the Reactivation (n=12) and the Control (n=12) groups. Red filled boxes represent three test blocks on Orientation A and the red-hatched box represents 16 blocks of training on Orientation A. The blue filled box represents three test blocks on Orientation B. Gray boxes represent MRS measurements. “Baseline”, “0h”, and “3.5h” indicate MRS measurements performed before, immediately (0h) after and 3.5 hours after the test session on Orientation A. (b) Performance improvements (Mean ± SEM) in the two groups relative to Day 1. Asterisks indicate a significant main effect of Group (p=0.004, see main text for ANOVA results). (c) Mean E/I ratio changes (± SEM) in early visual areas for the two groups relative to the baseline session. The asterisk indicates a significant interaction between Group and Session (p=0.035, see main text for ANOVA results). (d) E/I Ratio changes (n=12, Mean ± SEM) in early visual areas after encoding, replotted from our previous study24. Note that the E/I ratio was measured 30 min after training in (d), whereas it was measured immediately after reactivation in (c). Although these measurement time-points were not exactly the same, in both cases significant interference was observed behaviorally (see the reference24 and Study 1 here). Thus, the E/I ratios at both time-points still reflect underlying neurochemical mechanisms in fragile states after training and after reactivation.
As predicted, learning of Orientation A occurred in the Reactivation group, whereas no such learning was found for the Control group. A one-way ANOVA with factor Group (Reactivation vs. Control) indicated a significant main effect of Group on performance improvement on Day 2 (Figure 2b; F(1,22)=10.208, p=0.004, partial η2=0.317, 95% CI: 12.93–60.81). The performance improvement on Day 2 was significantly larger than zero for the Reactivation group (one sample t-test, t(11)=6.282, p=5.99E-05, Cohen’s d=1.81, 95% CI: 23.72–49.32), but not for the Control group (one sample t-test, t(11)=−0.035, p=0.973).
Figure 2c shows that the E/I ratio in early visual areas increased in association with reactivation. If reactivation makes VPL unstable and fragile, the E/I ratio should be enhanced24 immediately after reactivation in the Reactivation group but not in the Control group. For the Reactivation group, the E/I ratio significantly increased by 11.45±5.40% (Mean ± SEM) immediately after reactivation compared with the E/I ratio before reactivation and returned to baseline 3.5 hours later. On the other hand, for the Control group, no significant changes in the E/I ratio occurred across the three time-points of MRS measurements. Confirming these differences between Reactivation and Control groups a two-way mixed ANOVA with factors Group (Reactivation vs. Control) and Session (0h vs. 3.5h) indicated that there was a significant interaction between Group and Session (F(1,22)=5.027, p=0.035, partial η2=0.186). In addition, there was a simple main effect of Session for the Reactivation group (F(1,22)=5.276, p=0.032, partial η2=0.193, 95% CI: 1.14–22.34), but not for the Control group (F(1,22)=0.763, p=0.392). As mentioned above, a higher E/I ratio indicates a greater degree of plasticity24. Thus, the present results are in accord with the hypothesis that reactivation of VPL leads to an increase in both plasticity and the E/I ratio in early visual areas. This increased plasticity and the E/I ratio taper off within a few hours of reconsolidation. This is strong evidence that reactivation and reconsolidation indeed occur in early visual areas at least in VPL. See Supplementary Figure 1 for normalized GABA and Glu concentrations for both groups.
Are the underlying mechanisms of reactivation and reconsolidation in VPL similar to those of post-encoding and consolidation? Figure 2d shows the E/I ratio changes in early visual areas in the post-encoding stage (n=12)24. The E/I ratio was found to be significantly increased by 11.97±3.91% (Mean ± SEM) 30 min after the end of training relative to pre-training and tapered off 3.5 hours after training24. These results indicate that the time course changes in the E/I ratio during reconsolidation after reactivation are similar to those during consolidation after encoding.
Is such similarity in neurochemical plasticity between reconsolidation and consolidation as indexed by the E/I ratios also observed when the concentrations of Glu and GABA in early visual areas are examined separately? In order to answer this question, we compared changes of each metabolite from the Reactivation group in the present study with the previous MRS data collected during consolidation24 and tested whether the concentration of each metabolite was significantly different between reconsolidation and consolidation. Since one of the assumptions for ANOVA, equality of error variances, was not satisfied (see Methods), we conducted a Mann-Whitney U test and compared the normalized metabolite (GABA and Glu) concentrations at each time-point (0h and after 3.5h) between reconsolidation and consolidation. We did not find any significant differences on either normalized metabolite at any time-point between consolidation and reconsolidation (GABA at 0h, U=93, p=0.242; GABA at 3.5h, U=76, p=0.843; Glu at 0h, U=103, p=0.078; Glu at 3.5h, U=79, p=0.713; all n=24, p-values are uncorrected). These results suggest that reconsolidation and consolidation processes are associated with similar changes in metabolite concentrations over time.
So far, the results from MRS measurements imply that the neural mechanisms underlying consolidation after post-encoding and reconsolidation after reactivation may be very similar. However, because it has been demonstrated that the measurements of Glu and GABA by MRS are of both intra- and extra-synaptic origin30, 31, 32, a similar time course for the E/I ratio and each metabolite does not necessarily demonstrate a similar neural mechanism. Therefore, we conducted additional behavioral experiments (Study 3) to test whether performance changes are significantly different in a specific time window following post-encoding (consolidation) and reactivation (reconsolidation).
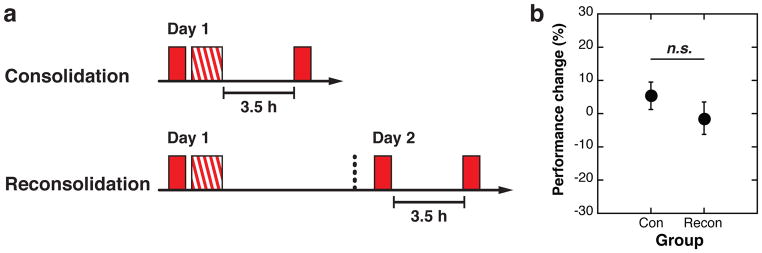
In Study 3, there were 2 groups (Figure 3a), the Consolidation group (n=15) and the Reconsolidation group (n=14). In the Consolidation group, participants trained on Orientation A for 16 blocks following a pre-test session (3 blocks), then performed a retest session 3.5 hours after the offset of training. All measurements were completed on Day 1 for the Consolidation group. Participants in the Reactivation group performed a pre-test session (3 blocks), which was followed by a training session (16 blocks) on Day 1. On the next day (Day 2), participants performed a reactivation session (3 blocks) and a retest session (3 blocks) 3.5 hours after the offset of reactivation.
Fig. 3.
Design and results of Study 3. (a) Design. There were two groups, the Consolidation (n=15) and the Reconsolidation (n=14) groups. Red filled boxes represent three test blocks on Orientation A and the red-hatched box indicates 16 blocks of training on Orientation A. (b) The mean (±S.E.) performance change 3.5h after encoding in the Consolidation (Con) group and the mean (±S.E.) performance change between reactivation and 3.5h after reactivation in the Reconsolidation (Recon) group. There was no significant difference in performance changes following the 3.5h intervals of consolidation and reconsolidation between the groups.
In the Consolidation group, VPL was expected to consolidate during the 3.5-hour interval following training on Day 1. In the Reconsolidation group, reactivated VPL was expected to reconsolidate during the 3.5-hour interval following reactivation on Day 2. If there are different neural mechanisms underlying consolidation and reconsolidation processes, then the performance changes over the 3.5-hour interval should be different between the Consolidation and Reconsolidation groups. However, the performance changes (see Methods) after the 3.5-hour interval were not significantly different between the Consolidation and Reconsolidation groups (paired t-test, t(27)=0.5811, p=0.2849). Therefore, these results together with our neurochemical findings are in accord with the hypothesis that consolidation and reconsolidation are similar processes, suggesting that both share a similar or common neural mechanism. Note that the performance during the reactivation in the Reconsolidation group showed a significant improvement in comparison to the pre-test (one sample t-test, t(13)=2.1937, p=0.047, d=0.59, 95% CI: 0.23–30.28), replicating the results of the Long interval condition in Study 1.
The results of behavioral and neurochemical changes provide important implications regarding reactivation and reconsolidation. First, although it has been controversial whether reactivation and reconsolidation of skill learning occurs in humans, our results show that reactivation and reconsolidation indeed occur in humans with VPL, a type of procedural learning.
Second, it has been also a matter of controversy whether or not the underlying neural mechanisms of reconsolidation after reactivation and consolidation after encoding in VPL are the same. The similarity in the time course changes in the E/I ratio and behavioral performance between reconsolidation and consolidation is in accord with the hypothesis that these two processes have similar or some common underlying mechanisms.
What do the time course changes in the E/I ratio in early visual areas in reconsolidation and consolidation processes reflect? A previous study has indicated that the E/I ratio is an index of the degree of plasticity in early visual areas24. Thus, the E/I ratio decreases from high after the end of training or reactivation to baseline levels observed 3.5 hour later, thereby reflecting that both consolidation and reconsolidation are associated with decreases in the degree of plasticity. These time course changes in the E/I ratio suggest that both consolidation and reconsolidation are driven by homeostasis in which once enhanced increased plasticity returns to baseline levels.
Importantly the E/I ratio does not seem to reflect sensory activation without relating to plasticity. First, a previous study showed that the E/I ratio was not increased after many trials that cause no learning24. Second, the results of the experiment in Study 2 of the present paper also showed that the performance of 3 test blocks, which were not associated with learning, did not increase the E/I ratio. These results together suggest that the E/I ratio is a reliable measure of plasticity.
Our experimental results suggest that reconsolidation occurs during a 3.5-hour interval following reactivation of the trained orientation detection task. However, it remains unclear to what extent these results can be generalized. In addition, these results do not necessarily indicate that the entire process of reconsolidation occurs within the 3.5-hour interval. For example, consolidation occurs during sleep33, 34 as well as during wakefulness. If reconsolidation has a similar mechanism to consolidation, sleep may be involved in reconsolidation. Further studies are needed to fully understand the temporal dynamics of plasticity changes associated with consolidation and reconsolidation.
Methods
Participants
A total of 84 healthy participants (Study 1, n = 30, mean ± SE = 20.8 ±0.4, 9 males and 21 females; Study 2, n = 24, mean ±SE = 21.7 ±0.5, 11 males and 13 females; Study 3, n = 29, mean ± SE = 25.7 ± 1.2, 5 males and 24 females) with no use of medication participated in the experiment. All participants had normal or corrected-to-normal vision. They kept regular wake and sleep patterns during experimental days. All participants were informed of the purpose and procedures of the experiment and gave written informed consent and their demographic information. All procedures were approved by the Institutional Review Board at Brown univerisity.
Gabor stimulus
For the behavioral experiments participants were presented with Gabor patches with one orientation (contrast = 100%, spatial frequency = 1 cycle/degree, sigma of Gaussian filter = 2.5 degree, random spatial phase). The center of the Gabor patches was positioned at the center of the display. The diameter of the Gabor patches was 4.5 degrees. A noise pattern was generated from a sinusoidal luminance distribution and was superimposed on the Gabor patches at a given signal-to-noise (S/N) ratio. For instance, in the case of a 20% S/N ratio, the noise pattern replaced 80% of the pixels of the Gabor patch.
Orientation detection task
Participants performed a 2-interval-forced-choice (2IFC) orientation detection task that was based on a previous study24. In one interval, a Gabor patch was presented with a certain S/N ratio. In the other interval, only a noise pattern (0% S/N ratio) was presented. The interval that included the Gabor patch was determined randomly. Throughout the task, participants were asked to fixate their eyes at the central white bull’s eye fixation point (diameter = 0.68 degrees). Each trial began with a 500-ms fixation period. After the fixation period, two intervals of stimuli were presented for 50-ms in order, separated by a 300-ms blank period. Participants were asked to determine in which interval (first or second) the Gabor patch appeared by pressing one of two buttons on the keypad. There was no feedback about the correctness of the response.
Threshold measurement
In the orientation detection task, each participant’s threshold S/N ratio in each block was determined by a 2-down 1-up staircase method in test sessions. This method yielded a 70.7% accuracy rate. There were three blocks per orientation. Within each block, S/N ratio started with 25% and was further adjusted with the step size, 0.05 log units. Each block terminated after 10 reversals. Typically, each block consisted of 40 trials and took approximately 1–2 minutes. We took the geometric mean of the last six reversals within a block as the threshold S/N ratio per block19. The first block served as practice and we took the geometric mean of the threshold S/N ratios across the remaining two blocks as a threshold S/N ratio for its assigned orientation.
Apparatus
We presented visual stimuli on a LCD display (1024 × 768 resolution, 60 Hz refresh rate) during the orientation detection task in Study 1 and on an MRI-compatible LCD display (1024 × 768 resolution, 60 Hz refresh rate) during MRS experiments in Study 2. Unlike Studies 1 and 2 in which an LCD monitor was used, in Study 3 a CRT display (1024 × 768 resolution, 60 Hz refresh rate) was used. Gamma correction was applied to the display in each study. All visual stimuli were created using Matlab and PsychToolbox 335.
Experimental design for Study 1
Experiments were conducted during daytime. There were two groups, the Short interval group (n=15) and the Long interval group (n=15). The difference between the groups was the time interval between the offset of reactivation and the onset of the following test. Participants were assigned to either the Short or Long interval groups in a counterbalanced manner.
Participants performed the orientation detection task (see above) using two orientations (10 and 70 degrees from the vertical) for two training sessions. Each of the two orientations were randomly assigned to Orientation A (first trained orientation) and B (second trained orientation) across participants.
The entire behavioral experiment consisted of three consecutive days. On Day 1, participants in both groups were given a brief test session consisting of three blocks of Orientation A. The purpose of the test session was to measure the initial threshold (see above) for Orientation A. After the test session, participants trained on Orientation A for 16 blocks.
On Day 2, participants in both groups performed a test session (three blocks) of Orientation A for the purpose of testing performance improvements from Day 1 and also for reactivating VPL of Orientation A. In the Short interval group, this test session was immediately followed by another test (less than a minute) and training sessions of Orientation B. Participants in the Long interval group performed the test and training sessions of Orientation B 3.5 hours after the test session of Orientation A.
On Day 3, both groups of participants performed a test session of Orientation A. By comparing the performance change on Orientation A between Day 2 and Day 3, we examined whether training of Orientation B interfered with reactivated VPL of Orientation A.
Experimental design for Study 2
There were two groups, the Reactivation (n=12) and the Control (n=12) groups. Participants were assigned to one of the groups in a counter-balanced manner. The only difference between the groups was the procedure on Day 1: In the Reactivation group, learning on the first trained orientation (Orientation A) was expected to occur and consolidate overnight, based on the findings in an earlier study24, whereas learning on Orientation A was not predicted for the Control group.
Study 2 consisted of 2 consecutive days. In the Reactivation group, on Day 1, participants performed a brief test session of three blocks on Orientation A in order to measure the initial thresholds (see above). Then, participants completed a training session on Orientation A consisting of 16 blocks. Participants performed this behavioral task inside an MRI simulator that looks identical to a real 3T MR scanner. On Day 2, there was a test session of three blocks inside the real 3T MR machine. It is important to note that this test session served to reactivate previously consolidated VPL of Orientation A. There were MRS scans (see below) immediately before, immediately after (less than a minute, corresponding to 0h in Figure 2a), and 3.5 hours after the test session.
In the Control group, the procedure on Day 1 was different from the Reactivation group, while the rest of the procedures were identical with the Reactivation group. On Day 1, after the initial three test blocks for Orientation A, participants were given another three test blocks for Orientation B.
In both groups, there were three magnetic resonance imaging (MRI) sessions to collect MRS data on Day 2. The first MRS scan served as baseline. The second MRS scan was conducted immediately after the test session (0h scan) and the last scan was conducted 3.5h after the test session (3.5h scan). The first and second MRS scans as well as the test session between the MRS scans were conducted sequentially while participants remained inside the scanner.
Experimental design for Study 3
There were two groups, the Consolidation (n=15) and the Reconsolidation (n=14) groups. Participants were assigned to one of the groups in a counter-balanced manner.
In the Consolidation group, the entire procedure of the experiment was completed within one day. Only Orientation A was used. Participants from the Consolidation group performed a pre-test for initial threshold measurements that consisted of three blocks. The pre-test was followed by 16 blocks of training. Three and a half hours after the offset of training, participants completed a retest session, which included three test blocks. Performance changes related to consolidation were calculated by the following ratio: [(threshold at pre-test - threshold at retest)/threshold at pre-test × 100].
In the Reconsolidation group, the experiment was conducted over the course of two days. Only orientation A was used. On Day 1, participants performed a pre-test session, consisting of 3 test blocks, and a training session, consisting of 16 blocks. On Day 2, there was a reactivation session, which included 3 test blocks. Three and a half hours after the offset of reactivation, participants completed a retest session, which again consisted of 3 test blocks. Performance during reactivation and retest was calculated by the following ratio: [(threshold at pre-test - threshold at reactivation or retest)/threshold at pre-test × 100]. Then, performance during reactivation was subtracted from performance during retest in order to calculate performance changes due to reconsolidation.
Magnetic resonance imaging data acquisition
Participants were scanned inside a 3T MR scanner (Siemens Trio/Prisma) with a 32-channel head coil at the Brown University MRI Research Facility.
First, for anatomical reconstruction, high-resolution T1-weighted MR images were acquired using a multi-echo magnetization-prepared rapid gradient echo (MEMPRAGE; 256 slices, voxel size = 1 × 1 × 1 mm, 0-mm slice gap, TR = 2530 ms, TE1 = 1.64 ms, TE2 = 3.5 ms, TE3 = 5.36ms, TE4 = 7.22 ms, flip angle = 7.0 degrees, FoV = 256 mm, bandwidth = 651 Hz/pixel).
Second, based on the anatomical images, a voxel placement for MRS acquisitions for early visual areas was conducted. We positioned a voxel (2 × 2 × 2 cm) manually along the calcarine sulci in the most posterior part of the occipital lobe bilaterally such that the voxel covered early visual areas while minimizing contamination from unnecessary tissues containing lipids. The voxel positioning was carefully replicated during the third MRS acquisition, as the first and second MRS scan were conducted consecutively while participants remained in the scanner. The mean overlap ratio in voxel positioning across scans was greater than 90%24.
Third, an automatic shimming was performed by a vendor-provided automated shim tool, then later manually for finer adjustments. The mean (± SEM) shim value (water linewidth) across three sessions was 14.93 ± 0.22 Hz.
Finally, we measured the concentration of GABA and Glu from the voxel. The GABA data were obtained using a MEGA-PRESS sequence (TR = 1500 ms, TE = 68 ms, number of average = 256, scan time = 774 sec) with double-banded pulses36, 37, 38, 39. Double-banded pulses were utilized to suppress water signal and edit the γ-CH2 resonance of GABA at 3 ppm. We subtracted the signals of alternate scans with the selective double-banded pulse applied at 4.7 and 7.5 ppm (‘Edit Off’) from those with the selective double-banded pulse applied at 1.9 and 4.7 ppm (‘Edit On’) to produce the final spectra. The Glu data were obtained by the PRESS sequence (TR = 3000 ms, TE = 30 ms, number of average = 128, scan time = 384 sec)40, 41. In both GABA and Glu sequences, a variable pulse power and optimized relaxation delays (VAPOR) technique was used for effective water suppression42. See Supplementary Figure 2 for exemplary spectra for PRESS and MEGA-PRESS sequences.
In an independent data set (n=3), the frequency drifts for the MEGA-PRESS sequence were measured24. The MEGA-PRESS sequence was conducted three times for each participant at similar time intervals as in Study 2. The mean (± SEM) frequency drifts for the GABA scans were 0.810 ± 0.034 Hz for the first MRS scan, 0.950 ± 0.212 Hz for the second MRS scan, and 0.854 ± 0.113 Hz for the third MRS scan. The mean value of within-participant standard deviations was 0.161 Hz24.
Fixation task
During MRS scans, participants performed a fixation task for which they fixated their eyes on the center of the screen and reported the color change of the center dot (0.34 degree in radius) by pressing a button on a key pad. The purpose of the central task was to keep participants’ fixation at the center of the screen and the level of attention and vigilance constant across the MRS scans. A fixation dot was placed on a gray disk and the color of the dot changed unpredictably from white ([R, G, B] = [255, 255, 255]) to faint pink ([R, G, B] = [255, 255–X, 255–X]) and returned to white 1.5 sec later. Initially, the color change X was set to 40 and controlled by a 2-down 1-up staircase method, based on a previous study24 that confirmed that all participants were able to clearly see the color change with this initial value. If participants pressed the button within 1.5 sec after a color change, this response was regarded a hit. However, if participants did not press the button within the 1.5 sec time interval, it was regarded as a miss. For each scan, we took the geometric mean of the last 6 trials’ color change values as a threshold for the degree of color change.
In the Reactivation group, the mean (±SE) color change values at the baseline, 0h and 3.5h were 16.34±2.86, 15.94±2.53, and 20.33±5.40 during the GABA scan, and 19.16±3.29, 17.04±2.10, and 16.20±2.48 for the Glu scan, respectively. In the Control group, the mean (±SE) color change values at the baseline, 0h and 3.5h were 18.61±1.67, 23.28±2.51, and 26.34±3.85 for the GABA scan, and 19.23±1.20, 22.27±2.48, and 20.48±1.95 for the Glu scan, respectively.
We tested whether there were any performance differences in the fixation task between groups, scans, or across sessions. To this aim, we conducted a three-way mixed ANOVA on the degree of color change with factors Group (Reactivation vs. Control), Scan (GABA vs. Glu; see below), and Session (baseline, 0h, and 3.5h). The results did not indicate any significant main effect of Group ((F(1,22)=2.042, p=0.167), Scan ((F(1,22)=0.955, p=0.339) or Session ((F(2,44)=0.917, Huynh-Feldt correction, epsilon=0.872, p=0.396). There was no significant three-way interaction (F(2,44)=0.003, Huynh-Feldt correction, epsilon=0.825, p=0.993), no significant two-way interactions between Session × Group (F(2,44)=1.048, Huynh-Feldt correction, epsilon=0.872, p=0.352), and Scan × Group (F(1,22)=0.828, p=0.373). Although there was a significant Session × Scan interaction (F(2,44)=4.164, Huynh-Feldt correction, epsilon=0.825, p=0.03), there was no significant simple main effect of Session for GABA (F(2,21)=2.616, p=0.097), or for Glu (F(2,21)=0.348, p=0.71), nor significant simple main effect of Scan during any of the sessions (baseline, F(1,22)=2.492, p=0.129; 0h, F(1,22)=0.003, p=0.959; 3.5h, F(1,22)=3.294, p=0.083). These results suggest that there were no significant differences in performance on the fixation task across groups, scans, or sessions.
MRS data analysis
LC-Model was used in all MRS data analysis43. Note that Glu and glutamine were separately fitted by LC-Model, and that the concentration of Glu was used for the calculation of E/I ratio changes. The Cramer-Rao Lower bounds (CRLB) show the reliability of quantification of GABA and glutamate. The mean (± SEM) CRLB% was 5.486 ± 0.194% for GABA scans across participants, and 4.847 ± 0.065% for Glu scans, respectively.
We normalized the amount of GABA and Glu by using the amount of N-Acetylaspartate (NAA), which is a standard reference resonance44 and taken from the Glu scan following the approach of a previous study24.
We calculated the E/I ratio change at each MRS session by using the equation:
Here, GABA(t) and Glu(t) represent the normalized concentrations of GABA and Glu by NAA, respectively, at a certain MRS session t (1 = baseline, 2 = 0h, 3 = 3.5h).
The NAA concentrations were not affected by group or session. A two-way mixed ANOVA with factors Session (baseline, 0h, 3.5h) and Group (Reactivation vs. Control) on NAA concentrations showed no significant main effect of Session (F(2,44)=0.864, p=0.429), no significant main effect of Group (F(1,22)=0.000, p=0.994), and no interaction between Session and Group (F(2,44)=1.169, p=0.320).
The average linewidth of NAA (± s.e.m) was 8.733 ± 0.165 Hz for GABA scans, 8.781 ± 0.181 Hz for Glu scans, 8.757 ± 0.122 Hz for all scans combined.
It is important to note that the overall E/I ratio patterns were maintained when GABA and Glu were normalized to another common control metabolite such as creatine, due to the fact that the contribution of control metabolite is cancelled out in the calculation of EI ratios.
Exclusion of participants
In addition to the total of 24 participants who participated in Study 2, we obtained 3 more participants’ MRS data but excluded their data from all analyses. The first participant exhibited (substantiated by self-report after the measurements) substantial head movements during the scan. The second participant exhibited extremely high CRLB%, such as 999%, while the CRLB% is recommended to be lower than 20%43. The third participant showed low signal to noise (S/N) value in LC-model fitting. This value seemed smaller than other values and was determined to be an outlier (Grubbs test, G=3.76, p=9.058E-05) in Study 2. In Study 3, there were originally 30 participants. However, we excluded one participant, because her performance deviated from those of the other subjects on Day 2 (Grubbs test, G=2.41, p=0.0495).
Statistics
Data collection and analyses were not conducted blindly by the investigators with respect to each participant’s group assignment. For all statistical tests, the two-tailed alpha level was set to 0.05. The sample size per group was estimated by prior similar experiments on neurochemical changes in early visual areas using MRS and VPL24. For the majority of analyses parametric statistical tests (e.g. t-tests, and ANOVA) were used, following confirmation of the normality of data using the Kolmogorov-Smirnov Test. For ANOVAs, we also tested whether equality of error variances of the dependent variable differed across groups by Levene’s Test. Significant differences in error variances between groups were only evident for the comparison of normalized metabolite (GABA and Glu) concentrations at each time-point (0h and after 3.5h) between reconsolidation and consolidation. Thus, we used a Mann-Whitney U test instead of an ANOVA. For repeated measures of ANOVA, Mauchly’s test of Sphericity was used to test the assumption of sphericity. We applied the Huynh-Feldt correction only when the sphericity was violated and report the estimated epsilon.
Data availability
The data that support the finding of this study are available from the corresponding author upon request.
Code availability
The computer codes are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
This work was supported by NIH (R01EY019466), NSF (BCS 1539717), and JSPS KAKENHI Grant Number 17H04789. The funding agencies had no role in the conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions: This work was conceived by JWB, SF, TW, and YS. JWB, KS, SF, and EGW collected and analyzed data, and JWB, KS, SF, EGW, MWG, TW and YS wrote the manuscript.
Financial and non-financial competing interests: The authors declare no competing interests.
References
- 1.Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 3.Dayan E, Laor-Maayany R, Censor N. Reward disrupts reactivated human skill memory. Sci Rep. 2016;6:28270. doi: 10.1038/srep28270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorkstrand J, et al. Disrupting Reconsolidation Attenuates Long-Term Fear Memory in the Human Amygdala and Facilitates Approach Behavior. Current biology: CB. 2016;26:2690–2695. doi: 10.1016/j.cub.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 8.Robertson EM. New insights in human memory interference and consolidation. Current biology: CB. 2012;22:R66–71. doi: 10.1016/j.cub.2011.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood NE, et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry research. 2015;225:31–39. doi: 10.1016/j.psychres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Bos MG, Beckers T, Kindt M. Noradrenergic blockade of memory reconsolidation: a failure to reduce conditioned fear responding. Front Behav Neurosci. 2014;8:412. doi: 10.3389/fnbeh.2014.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardwicke TE, Taqi M, Shanks DR. Postretrieval new learning does not reliably induce human memory updating via reconsolidation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:5206–5211. doi: 10.1073/pnas.1601440113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 13.Debiec J, Doyere V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 16.Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Q, Zhang P, Qiu J, Fang F. Perceptual Learning of Contrast Detection in the Human Lateral Geniculate Nucleus. Current biology: CB. 2016;26:3176–3182. doi: 10.1016/j.cub.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Amar-Halpert R, Laor-Maayany R, Nemni S, Rosenblatt J, Censor N. Memory reactivation improves visual perception. Nature neuroscience. 2017 doi: 10.1038/nn.4629. (in press) [DOI] [PubMed] [Google Scholar]
- 19.Xiao LQ, Zhang JY, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Current biology: CB. 2008;18:1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, Nanez JE, Sr, Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature neuroscience. 2002;5:1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- 22.Seitz AR, Watanabe T. Psychophysics: Is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- 23.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata K, et al. Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant. Nature neuroscience. 2017;20:470–475. doi: 10.1038/nn.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. 2011;334:1413–1415. doi: 10.1126/science.1212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolova S, Stark SM, Stark CE. 3T hippocampal glutamate-glutamine complex reflects verbal memory decline in aging. Neurobiology of aging. 2017;54:103–111. doi: 10.1016/j.neurobiolaging.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Stephenson MC, Morris PG, Jackson SR. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study. NeuroImage. 2014;99:237–243. doi: 10.1016/j.neuroimage.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Current biology: CB. 2011;21:480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensch TK. Critical period plasticity in local cortical circuits. Nature reviews Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 30.Stagg CJ. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. NeuroImage. 2014;86:19–27. doi: 10.1016/j.neuroimage.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Okubo Y, et al. Imaging extrasynaptic glutamate dynamics in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6526–6531. doi: 10.1073/pnas.0913154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers JF, Evans CJ, Kalk NJ, Edden RA, Lingford-Hughes AR. Measurement of GABA using J-difference edited 1H-MRS following modulation of synaptic GABA concentration with tiagabine. Synapse. 2014;68:355–362. doi: 10.1002/syn.21747. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Sasaki Y. Perceptual learning: toward a comprehensive theory. Annu Rev Psychol. 2015;66:197–221. doi: 10.1146/annurev-psych-010814-015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nature reviews Neuroscience. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 36.Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:18566–18573. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Rothman DL, Behar KL, Hetherington HP, Shulman RG. Homonuclear 1H double-resonance difference spectroscopy of the rat brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:6330–6334. doi: 10.1073/pnas.81.20.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic Action in the Autistic Brain. Current biology: CB. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Hancu I. Optimized glutamate detection at 3T. J Magn Reson Imaging. 2009;30:1155–1162. doi: 10.1002/jmri.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2008;60:964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- 42.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 44.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the finding of this study are available from the corresponding author upon request.