Abstract
A simple electrochemical assay to monitor the dispersion of Pseudomonas aeruginosa PA01 biofilm is described. Pyrolytic graphite (PG) electrodes were modified with P. aeruginosa PA01 using layer-by-layer (LbL) methods. The presence of the bacteria on the electrodes was directly monitored using square wave voltammetry (SWV) via the electrochemical reduction of electroactive phenazine compounds expressed by the bacteria, which indicate the presence of biofilm. Upon treatment of bacteria-modified electrodes with a 2-aminoimidazole (2-AI) derivative with known Pseudomonas anti-biofilm properties, the bacteria-related electrochemical reduction peaks decreased in a concentration dependent manner, indicating dispersal of the biofilm on the electrode surface. A similar 2-AI compound with negligible anti-biofilm activity was used as a comparative control and produced muted electrochemical results. Electrochemical responses mirrored previously established bioassay-derived half maximal inhibition concentration (IC50) and half maximal effective concentration (EC50) values.. Biofilm dispersal detection via the electrochemical response was validated by monitoring crystal violet absorbance after its release from electrode confined P. aeruginosa biofilm. Mass spectrometry data showing multiple redox active phenazine compounds are presented to provide insight into the surface reaction complexity. Overall, we present a very simple assay to monitor the anti-biofilm activity of compounds of interest.
Graphical abstract

1. Introduction
Antibiotic resistance due to bacterial biofilms is a major health concern. Biofilms are essentially a protective layer consisting of DNA, proteins, polysaccharides, and/or other macromolecules that act to physically block and/or remove foreign antibiotics from accessing the underlying bacteria[1–3]. Approximately 80% of human bacterial infections are biofilm related, the infections of which have been estimated to cause upwards of 500,000 deaths annually[2]. Biologically relevant biofilm-related infections are numerous and include Pseudomonas infections, methicillin resistant staphylococcus aureus (MRSA) infections[4], chronic infections in wounded soldiers[5], as well as bacterial plaques in dental patients [6,7] and on medical implants[8,9]. Pseudomonas aeruginosa is an opportunistic pathogen that is especially problematic in healthcare-related fields, often infecting immune-compromised individuals. For those with cystic fibrosis, this infection, and the resulting biofilm production in the lungs, is life threatening [1].
Several different compounds have been described that have the ability to inhibit biofilm formation through a variety of pathways[2,10,11]. One group of compounds that has shown significant anti-biofilm activity are derivatized 2-aminoimidazole (2-AI) compounds (Figure 1) [12,13]. These compounds were modeled after the oroidin family of marine alkaloids, natural products known to prevent biofouling in marine environments. Analogs of these secondary metabolites were shown to exhibit enhanced potentcy toward the dispersal of Pseudomonas aeruginosa PA01 biofilm as compared to oroidin itself [13]. Anti-biofilm activity was determined using the standard crystal violet reporter assay, a well-plate assay involving the spectrophotometric measure of crystal violet as an indirect biofilm readout [4].
Figure 1.
Structures of anti-biofilm compounds. a) Oroidin, b) RA13 with reverse amide and 2-aminoimidazole functional groups highlighted, and c) 2B3 control.
Electrochemical assays can potentially further accelerate the discovery of anti-biofilm agents, coupling the inexpensive instrumentation and rapid setup times of the 96-well format with faster analysis times and miniaturization benefits[14–16]. Several electrochemical sensor methods for biofilm monitoring have been described. Often they employ P. aeruginosa, as this bacteria produces electroactive phenazines that can be easily monitored[17]. As part of their biofilm phenotype, this family of bacteria produce pyocyanin and related derivatives, that exhibit reduction potentials around −0.25 V vs. SCE. Pyocyanin electrochemistry involves the transfer of two protons and two electrons at the electrode surface [17,18]. Novel electrochemical sensor arrays have been described to monitor P. aeruginosa electroactive products [17,19] and quorum sensing (QS) mechanisms[20]. Voltammetry [21–23] and impedance [24,25] techniques have been utilized to monitor the formation of various biofilms on electrode surfaces. For example, Goluch and co-workers developed an electrochemical microfluidic assay that monitored P. aeruginosa PA14 biofilm dispersal via the change in its pyocyanin reduction current as it was exposed to the antiobiotic colistin sulfate[26]. Sultana et al. showed that electrochemical treatment of P. aeruginosa persister cells could enhance the effectiveness of tobramycin[27].
It is this latter strategy that is of interest here. Many electrochemical bacterial assays primarily focus on detecting biofilm formation or identifying electrochemical changes to the bacteria over time. Monitoring small-molecule biofilm dispersal is often not performed[26]. We previously developed an electrochemical assay that looked at alginate disruption following antimicrobial peptide (AMP) exposure[28]. Alginate is the primary exopolysaccharide (EPS) product produced by P. aeruginosa[29,30]. We showed that alginate disruption by the AMP was related to anti-biofilm activity utilizing a facile layer-by-layer (LbL) electrode modification strategy [31,32] and validating the response with the crystal violet assay[28]. Here, we improve upon that initial proof-of-concept by immobilizing P. aeruginosa PA01 on the electrode in a similar fashion to monitor the electrochemical response upon exposure to a known anti-biofilm compound RA-13 previously developed and characterized by the Melander group[13].
Our general electrochemical approach is shown in Scheme 1. Immobilization of P. aeruginosa produces a significantly more facile electrochemical assay, as the bacteria produce electroactive phenazine derivatives, including pyocyanin and other related compounds that can be directly measured electrochemically[17,19,26,28,29,33]. Here, we used square wave voltammetry (SWV) to monitor the P. aeruginosa electrochemical response on the electrode, which is indicative of the presence and health of the biofilm on the electrode surface[26]. Overall, a simple electrochemical assay is presented that has the ability to evaluate the potential efficacy of anti-biofilm compounds utilizing minimal reagent in a very rapid time frame.
Scheme 1.
Overview of the P. aeruginosa electrochemical assay. I. Formation of the electrode using (i) layer by layer cationic (yellow) and anionic (blue) polymers and (ii) P. aeruginosa PA01 (orange) that can produce biofilm (aqua). Electroactive phenazines produced by the bacteria can be reduced or oxidized directly. II. After exposure to the anti-biofilm compounds (RA13 is shown) the biofilm is compromised, resulting in lower electrochemical currents.
2. Experimental
2.1. Electrochemical assay materials
Tris(hydroxymethyl)amino methane buffer (Tris), poly(diallyldimethylammonium) chloride (PDDA), and polystyrene sulfonate (PSS) were obtained from Sigma-Aldrich. Deionized (DI) water was from an Elga Purelab Ultra 18-MΩ system. All other materials were reagent grade and used as received.
2.2. Biological materials
Pseudomonas aeruginosa biofilm growth media (pirate media) was generated using 6 g Na2HPO4, 3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 4 g arginine, and 1 liter of DI water. The pH was adjusted to 7.4. Once autoclaved, 2 mL of 1 M MgSO4 and 0.1 mL of 1 M CaCl2 were added. Pyocyanin standard was from Sigma-Aldrich. All other compounds were reagent grade and used as received.
2.3. 2-aminoimidazole anti-biofilm compounds
The synthesis and biological assay characterization of the anti-biofilm compounds used in this study has been described previously[13,34]. Before use, the concentrated compounds (100 mM) were stored in DMSO at −20°C. The anti-biofilm compound, RA13 (shown in Figure 1, compound 9 from ref [34]), was previously shown to be very potent toward PA01 biofilm. The control, 2B3 (Scheme 1, compound 39 from ref [34]), had been shown to exhibit muted Pseudomonas anti-biofilm activity.
2.4. Electrode film assembly
2 mm-dia. pyrolytic graphite (PG) electrodes were abraded on fine grit carbide paper and sonicated in water and ethanol followed by drying under a stream of argon. Layer by layer (LbL) films were formed on the electrodes by exposing the electrode to 30 µL drops of the following solutions for 15 min. each: PDDA, 2 mg mL−1 in deionized water + 50 mM NaCl, PSS, 2 mg mL−1 in deionized water + 50 mM NaCl[31,32,35]. Electrodes were rinsed with deionized water between each layer. The layers were applied to form a PDDA/PSS/PDDA architecture on the electrode surface. The polymer-modified electrodes were then modified with P. aeruginosa (see below) to create a final film formation of PDDA/PSS/PDDA/PA01. After removal from the bacteria, the electrodes were rinsed with 10 mM Tris+10 mM NaCl pH 7.4 (electrochemical run buffer) and used immediately.
2.5. Electrode modification with P. aeruginosa
Pseudomonas aeruginosa PA01 was stored at −80°C in a glycerol stock. 5 mL pirate media was inoculated with the P. aeruginosa PA01 using a sterile loop and allowed to grow overnight at 37°C in an incubating orbital shaker. Following overnight growth, the green pyocyanin-containing biofilm could visibly be seen in the media. 100 µL of the bacteria solution was placed in an Eppendorf microcentrifuge tube followed by insertion of the polymer modified PG electrode and wrapped with parafilm. The electrodes were then placed in the incubating shaker at 37°C and 100 RPM for 6 hours.
2.6. Organic extraction
For mass spectrometry analysis, 1-mL overnight P. aeruginosa PA01 culture was filtered (Millapore 3K filter) followed by extracting 3× with 1-mL chloroform. The organic layer was collected and evaporated under argon. The extracted material was resuspended in MS-grade 50/50 methanol/0.1 % formic acid water.
2.7. Electrochemical experiments
All electrochemical measurements were performed using a CH Instruments (Austin, TX) 660A workstation. The modified electrodes were placed in an electrochemical cell containing 10 mL 10 mM Tris/10mM NaCl pH 7.4 (electrochemical run buffer) along with saturated calomel (SCE) reference electrode and a platinum wire as a counter electrode. Solutions were purged for approximately 10 mins. with Ar before the analysis.
SWV scans were taken initially before any exposure to the RA compounds. SWV scans were obtained at intermittent time periods with Ar purging in between. After a period (~5–10 minutes), the SWV stabilize, and the last acquired scan was the non-exposure (t = 0 s) baseline. The electrode was then removed from the electrochemical run buffer, rinsed briefly with the electrochemical buffer, and then exposed to 20 µL of the RA compound at desired concentration diluted in the same buffer. After the timed interval (15 – 600 s), the electrode was rinsed again, and placed back into the electrochemical analysis cell. The solution was again purged extensively with Ar before acquisition of SWV data. SWV was employed with the following parameters: potential scan range 0.5 to −0.2 V, step increment of 4 mV, amplitude of 25 mV, frequency 15 Hz, quiet time 2 s and sensitivity of 1×10−5 A.
2.8. Electrode Crystal Violet Assay
A series of electrodes were prepared as identically as described in sections 2.4–5. After exposure to P. aeruginosa PA01, the electrodes were rinsed with electrochemical run buffer before exposure to either buffer alone or buffer + 50 µM RA13 for 600 s. The electrodes were again rinsed in electrochemical run buffer folled by exposure to 50 µL 0.1% crystal violet in DI H2O for 600 s. The electrodes were rinsed in DI H2O. Electrodes were then inserted into 100 µL 35% acetic acid for 2 mins in an Eppendorf microfuge tube.
Spectra were acquired using a Varian Cary 300 Bio Spectrometer in double beam mode with acetic acid as the blank. The following parameters were employed: 800–400 nm scan range, 0.600 nm/s scan rate, 2.0 nm slit width.
2.9. Mass Spectrometry
Analysis of the overnight culture and pyocyanin standards was performed on a Micromass Q-ToF Micro with an electrospray ionization source. The following conditions were employed: ESI+ mode, 250°C soure, 150°C desolvation gas, collision energy 10V, Ar collision gas.
3. Results
3.1. P. aeruginosa stabilization
After exposure to the P. aeruginosa in the growth medium for several hours, the electrodes were removed and placed into the electrochemical run buffer. Upon exposure to a different environment, the bacteria undergo a period of stabilization, and this is seen in the SWV plots shown in Figure 2. The SWV consist of two reduction waves indicative of the presence of multiple electroactive compounds on the electrode surface at the onset of run buffer exposure. Over time, the more positive potential population becomes more prevalent, and both peaks stabilize and remain unchanging after 10–12 min. The arrows in the figure show the direction of peak growth for the two populations. The peak at approximately −0.25 V vs. SCE is consistent with the production of pyocyanin by the bacteria[26], while the more positive potential arises from an as of yet unidentified compound. Several reports have shown that P. aeruginosa produce a wide range of phenazine compounds with a differing electrochemical potentials, accounting for this second peak[17,33,36]. We turn our attention to the possibilities that give rise to this peak below, but it is worth noting that the emergence of this population is likely influenced by our choice of electrochemical environment, and it is stable and reproducible.
Figure 2.
SWV plots of PDDA/PSS/PDDA/PA01 modified electrode upon removal from P. aeruginosa PA01 growth media and insertion into electrochemical run buffer (10 mM Tris/10 mM NaCl pH 7.4). SWV were recorded before any exposure to RA13. Plots were recorded at denoted time points: 0s (black), 180 s (blue), 540 s (orange), 600 s (magenta), and 660 s (green).
Both species that give rise to the electroactive response are due to the P. aeruginosa bacteria on the electrode, not due to the other polymers present or some solution phase phenomenon. Figure S1 shows that the response is not from the underlying polymer layers and that all meaningful electrochemical responses are due to the bacteria. Additionally, Figure S2 shows that the LbL protocol and the added underlying polymer layers enhance the electrochemical signal acquired from the bacteria, presumably by facilitiating the initial attachment of the bacteria to the electrode surface.
3.2. SWV Response to RA13 Exposure on P. aeruginosa Modified Electrodes
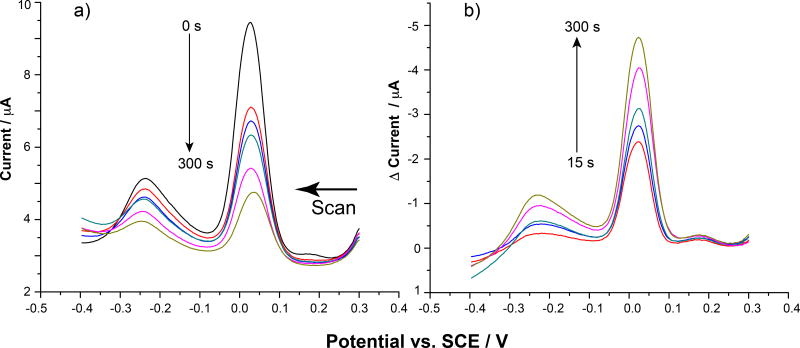
Figure 2 shows the P. aeruginosa voltammetry stabilization after insertion of the electrode into the electrochemical cell after 12 mins. The unchanging signals indicate that the bacteria is stable on the electrode surface. At this point, if allowed to remain in the buffered electrochemical cell, the response remains relatively unchanged over a prolonged period of time (data not shown). The last acquired SWV in an electrochemical analysis like that described in Figure 2 was set as the “baseline” (t = 0 s) time point for compound exposure comparisons, which is shown in Figure 3a. The electrode was then removed from the buffer and exposed to RA13 at various concentrations diluted in the same electrochemical run buffer for set periods of time up to 5 min. After each time point, the electrode was placed back into the cell to monitor the voltammetry. Representative raw data results for this process as the Pseudomonas-modified electrode was exposed to 50 µM RA13 are shown in Figure 3a.
Figure 3.
a) Raw SWV data showing the bacteria modified electrode response as it was exposed to 50 µM RA13 for increasing periods of time from 0s to 300 s (0 s = black, 15 s = red, 30 s = blue, 60 s = green, 180 s = pink, 300 s = mustard). b) Resulting plots after subtracting out the 0 s baseline (black plot in a)) from the timed runs. Colors are the same for a and b.
The plot shows that upon exposure to RA13, the current from bacteria-related redox species decreases dramatically over the 5 min analysis time. To account for inter-electrode differences, the relative current change was determined. To obtain this data, the 0 s plot was subtracted from the subsequent timed runs to obtain the background subtracted plots seen in Figures 3b and 4 [28,37,38]. The larger peaks in these differential plots indicate more change upon compound exposure. Also, it is important to note that the current change decreases over time for these analyses, but the differential result is plotted “upside down” to show a peak for ease in data handling and comparative purposes.
Figure 4.
SWV overlay showing the different background subtracted responses at different RA13 concentrations at 300 s exposure time. Orange is the 2B3 control response at the same time
3.3. RA13 Kinetic Data
The peak current decrease was shown to be dependent on RA13 concentration. This is shown in the SWV overlay plot in Figure 4 and the kinetic plots shown in Figure 5. The plots show that the peak current change and rate of decrease is dependent on the RA13 concentration as expected. Figure 4 also shows representative results for current change detected from exposure of the bacteria-modified electrode to the control compound 2B3. The data show that RA13 is much more active in causing changes to the Pseudomonas on the electrode as compared to 2B3. The control (50 µM) was utilized at concentrations ten times the lowest RA13 concentration, and the decrease in current was negligible as compared to the 5 µM RA13 runs.
Figure 5.
Percent decrease of the peak current at +0.03 V over the exposure time as a function of RA13 (or 2B3) concentration. Error bars represent s.d. for n = 3.
The initial slopes of the timed plots shown in Figure 5 allow the calculation of pseudo-first order rate constants for relative comparisons between the anti-biofilm compounds, which can then be contrasted to their previously reported IC50 (inhibition) or EC50 (established biofilm disruption) values. The pseudo-first order rate constant for RA13 was approximately 0.05 s−1 while for 2B3 it was 3 × 10−4 s−1. This nearly two-order of magnitude difference was also seen in the actual IC50 values for these compounds, which were ~ 0.7 µM for RA13 and >100 µM for 2B3 [34].
3.4. Crystal violet confirmation assay
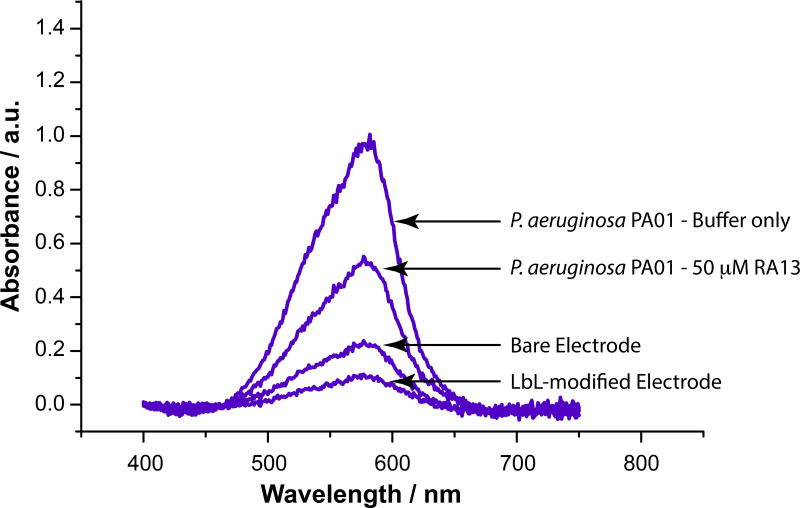
Figure 6 shows representative crystal violet absorbance spectra after exposure to and subsequent release from P. aeruginosa exposed electrodes and relevant control electrodes. Crystal violet is extensively used in anti-biolfilm assays using 96-well plates, as it accumulates in the produced biofilm and allows for colorimetric detection [39,40]. The first important aspect of this data is that a significantly higher 585 nm absorbance was observed when P. aeruginosa was exposed to the electrodes. This is consistent with the accumulation of crystal violet due to its aggregation into the bacterial biofilm that had formed on those electrode surface. Second, a statistically significant difference was observed in the average absorbance from the electrodes that were exposed to RA13 vs. those that were only exposed to buffer. The average (n = 5) 585 nm crystal violet absorbance from electrodes that were exposed to RA13 was 30% lower than those that were exposed to buffer alone (p < 0.05). This demonstrates that RA13 was effective in dispersing the biofilm on the electrode surface, which is consistent with the results of a larger crystal violet microtiter plate assay previously reported on this compound [2,4,13,34]. Controls showed that crystal violet accumulation was driven primarily by the presence of the bacteria and did not accumulate on the bare or LbL modified surfaces in as great amounts. Interestingly, the accumulation of crystal violet and its absorbance actually decreases upon polymer modification, which is likely due to the cationic repulsion of the crystal violet by the external PDDA layer on the electrode surface.
Figure 6.
Representative absorbance spectra from crystal violet released from the denoted electrodes.
3.5. Mass Spectrometry of P. aeruginosa growth medium
ESI-QTof-MS was utilized to identify compounds from the P. aeruginosa that may produce the two electrochemical peaks. P. aeruginosa produces a wide range of electroactive phenazine compounds, mainly pyocyanin. Figure S3 shows MS of organic extracted overnight growth medium as well as pyocyanin standard. The insets show the tandem MS and the fragmentation pattern for m/z 211 corresponding to [M+H]+ of pyocyanin[41]. Extracted P. aeruginosa showed several m/z that are as of yet unidentified, but in addition to a significant m/z 211 peak, the presence of m/z 225 is consistent with the [M+H]+ of phenazine-1-carboxylic acid, a precursor to pyocyanin[41]. The tandem MS for m/z 225 along with a proposed product ion fragmentation is showin in Figure 7. We turn our attention to the significance of this data below, but the presence of this compound demonstrates that multiple electroactive phenazine compounds are present due to the P. aeruginosa immobilized on the electrode.
Figure 7.
ESI-QTof MSMS of m/z 225 from P. aeruginosa extraction. Inset shows structure of phenazine-1-carboxylic acid that provides the observed m/z.
4. Discussion
Electrochemical SWV plots show that the primary electroactive compound initially produced by the immobilized P. aeruginosa exhibited a reduction potiential of −0.25 V vs. SCE, which is consistent with previously reported potentials for pyocyanin[17,26,33]. SWV of electrode-immobilized standard pyocyanin at identical buffer conditions resulted in a single reduction current peak at this potential, which is shown in Figure S4. However, two electroactive populations are initially present, and after stabilization in the electrochemical cell, the relative proportion of electroactive compounds produced by the bacteria changed. The production of an electroactive compound with a significantly positive shifted reduction potential was seen, representing the major electroactive contributor that these bacteria produce on the electrode under these conditions. The absolute identity of this compound is not known at this time; however, it may be related to a species involved in the bacterial production of pyocyanin, which results in a wide array of phenazine compounds shown to exhibit positively shifted reduction potentials compared to pyocyanin[17,26]. Mass spectrometry of the bacterial culture confirmed the presence of not only pyocyanin but also species consistent with other electroactive phenazines. The presence of m/z 197 is consistent with the presence of 1-hydroxyphenazine. The m/z 225 species and fragmentation are consistent with the presence of phenazine-1-carboxylic acid, which is the biosynthetic precursor to pyocyainin [35]. While this electroactive phenazine has been shown to exhibit more negative reduction potentials compared to pyocyanin[42], other substituted carboxylic acid phenazines, such as 5-methyl-phenazine-1-carboxylic acid, undergo reduction at more positive potentials[17,26]. We did not detect a significant presence of m/z consistent with this compound, but this may be due to our extraction procedures, and more investigation is necessary to unambiguously identify this compound.
Regardless, the MS data show the presence of numerous pyocyanin-related compounds. The emergence of an electroactive compound exhibiting more positive reduction potential was also detected by Goluch and co-workers in monitoring P. aeruginosa biofilm production[26]. Bosire et al. showed that the electrochemical environment is vitally important in dictating the production of electroactive phenazine compounds by Pseudomonas [36]. They showed that the production of phenazine-1-carboxylic acid could be mediated by the applied potential on the electrode. More interestingly, they showed that under certain conditions a redox species was generated by Pseudomonas that exhibited a positive shifted formal potential (Eo’ = +0.012 V vs. SCE, Saturated KCl). While the identity of the species exhibiting the positive shifted reduction potential was not identified, its presence was definitively assigned to the biofilm on the electrode surface. The unknown compound was only detected when the biofilm was established on the electrode surface, and not in solution [36]. The positive shifted reduction potential species seen in this study is likely similar to that described by these aforementioned reports. It is feasible that the electrode-immobilized P. aeruginosa alter their behavior as the environment changes from growth media to electrochemical buffer conditions and/or as the electrochemical environment equilbrates over the analysis time. This stress may alter pyocyanin and/or other redox mediator production, resulting in the detection of multiple electrochemically active compounds.
The production of these phenazine derivatives by P. aeruginosa is characteristic of the biofilm phenotype, and production of these compounds in vivo is indicative of virulence[2,19,26,29]. The current due to P. aeruginosa electroactive components is clear evidence that biofilm is present on the electrode. The electroactive species are not produced unless P. aeruginosa is present and actively producing biofilm. The detected electrochemical signal is consistent, remains relatively unchanged, and is reproducible before exposure to the anti-biofilm compounds, which indicates that the bacteria is stable and produces biofilm on the electrode surface. The decrease of both the +0.030 V and the −0.25 V vs. SCE reduction peaks upon exposure to RA13 indicates disruption of PA01 biofilm that had formed on the electrode surface. The natural amide functionality exhibited in 2B6 coupled with the decreased alkyl chain length was shown to diminish its activity toward P. aeruginosa [2,34].
Confirmation of biofilm dispersal from the electrode surface was provided by performing a crystal violet assay using PG electrodes. Crystal violet is known to partition into P. aeruginosa biofilms, and is used extensively in 96-well plate assays to monitor biofilm dispersal [39,40]. Crysal violet was detected from electrodes that were exposed to P. aeruginosa vs. controls. Crystal violet absorption into the films at much higher amounts after bacteria exposure is consistent with the presence of P. aeruginosa PA01 biofilm originally present on electrode surfaces to which it was exposed under the conditions described here. Subsequent exposure to RA13 resulted in a statistaically significant decrease in the amount of detected crystal violet, which is consistent with the dispersal of the biofilm from the surface facilitated by RA13 expsoure.
The MS data confirms the presence of pyocyanin and related compounds, which is consistent with the monitored redox potentials. These compounds are produced in conjunction with biofilm. The crystal violet assay confirmed the presence and dispersal of electrode-confined biofilm, and the data were obtained under identical electrode formation and RA13 exposure conditions as the electrochemical data. Therefore, the electrochemical SWV response decreases upon RA13 expsoure are due to changes in the electrode-confined biofilm over the monitored drug exposure time. Overall, all data are consistent with the electrochemical detection of biofilm dispersal due to RA13 exposure.
The claim of electrochemical detection of RA13-induced biofilm dispersion is further validated by confirmatory colorimetric assay experiments that were performed previously and established the anti-biofilm capability of the 2-AI family of compounds, and specifically RA13 [2,4,13,34]. Briefly, these experiments involve growing biofilm in a 96-well plate before exposure to the anti-biofilm compound of interest, in this case RA13. After exposure, the wells are rinsed before staining with crystal violet. Crystal violet binds within the biofilm remaining attached to the wells, which can be released into solution before recording the absorbance using a plate reader. The main drawbacks to this type of assay are the time involvement and amount of material needed for completion. For instance, this well-plate assay may take upwards of multiple days to complete and requires enough compound to fill each individual plate well. The electrochemcial assay described here provides real time activity measurements of these compounds toward biofilm dispersion in a relatively short time frame versus these established methods using a fraction of the volume of anti-biofilm compound. Concerning the time involvement, after the standard bacterial preparation, the experiments are completed over the course of five minutes, as compared to up to a few days to complete a standard assay.
5. Conclusions
The data presented here demonstrate that this simple electrochemical assay has the ability to distinguish the activity of established anti-biofilm compounds in real time. RA13 was previously identified as a potent dispersal agent of P. aeruginosa biofilms, while 2B3 was shown to have significantly muted responses toward biofilms. The electrochemical assay presented was able to distinguish this, and the decrease in the P. aeruginosa electrochemical peaks was due to concentration-dependent RA13 exposure. While the assay here focused on Pseudomonas due to the ease in using this from an electrochemical detection standpoint, we envision the benefits of this assay will extend to screening other bacteria and anti-biofilm compounds. Improvements and current research in our lab are focused on developing electrochemical detection schemes to utilize alternative bacteria and testing a wider array of compounds with anti-biofilm activities.
Supplementary Material
Acknowledgments
CM acknowleges support by National Institutes of Health (GM055769 and DE022350).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial Biofilms: A Common Cause of Persistent Infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Worthington RJ, Richards JJ, Melander C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 2012;10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009;4:273–278. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers SA, Huigens RW, Cavanagh J, Melander C. Synergistic Effects between Conventional Antibiotics and 2-Aminoimidazole-Derived Antibiofilm Agents. Antimicrob. Agents Ch. 2010;54:2112–2118. doi: 10.1128/AAC.01418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks RP, Abercrombie JJ, Wong RK, Leung KP. Antimicrobial peptides containing unnatural amino acid exhibit potent bactericidal activity against ESKAPE pathogens. Bioorg. Med. Chem. 2013;21:205–214. doi: 10.1016/j.bmc.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]
- 7.Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontal. 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 8.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Artif. Organs. 2005;28:1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 9.Ramage G, Martínez JP, López-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 10.Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 11.Batoni G, Maisetta G, Brancatisano FL, Esin S, Campa M. Use of antimicrobial peptides against microbial biofilms: advantages and limits. Curr. Med. Chem. 2011;18:256–279. doi: 10.2174/092986711794088399. [DOI] [PubMed] [Google Scholar]
- 12.Richards JJ, Huigens RW, III, Ballard TE, Basso A, Cavanagh J, Melander C. Inhibition and dispersion of proteobacterial biofilms. Chem. Commun. 2008:1698–1700. doi: 10.1039/b719802g. [DOI] [PubMed] [Google Scholar]
- 13.Richards JJ, Ballard TE, Melander C. Inhibition and dispersion of Pseudomonas aeruginosa biofilms with reverse amide 2-aminoimidazole oroidin analogues. Org. Biomol. Chem. 2008;6:1356–1363. doi: 10.1039/b719082d. [DOI] [PubMed] [Google Scholar]
- 14.Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2. John Wiley & Sons, Inc.; New York: 2001. [Google Scholar]
- 15.Hvastkovs EG, Buttry DA. Recent advances in electrochemical DNA hybridization sensors. Analyst. 2010;135:1817–1829. doi: 10.1039/c0an00113a. [DOI] [PubMed] [Google Scholar]
- 16.Hvastkovs EG, Schenkman JB, Rusling JF. Metabolic Toxicity Screening Using Electrochemiluminescence Arrays Coupled with Enzyme-DNA Biocolloid Reactors and Liquid Chromatography–Mass Spectrometry. Ann. Rev. Anal. Chem. 2012;5:79–105. doi: 10.1146/annurev.anchem.111808.073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellin DL, Sakhtah H, Rosenstein JK, Levine PM, Thimot J, Emmett K, et al. Integrated circuit-based electrochemical sensor for spatially resolved detection of redox-active metabolites in biofilms. Nat. Commun. 2014;5:1–10. doi: 10.1038/ncomms4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster TA, Sismaet HJ, Conte JL, Chan IJ, Goluch ED. Electrochemical detection of Pseudomonas aeruginosa in human fluid samples via pyocyanin. Biosensors and Bioelectronics. 2014;60:265–270. doi: 10.1016/j.bios.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Koley D, Ramsey MM, Bard AJ, Whiteley M. Discovery of a biofilm electrocline using real-time 3D metabolite analysis. Proceedings of the National Academy of Sciences. 2011;108:19996–20001. doi: 10.1073/pnas.1117298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connell JL, Kim J, Shear JB, Bard AJ, Whiteley M. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc. Nat. Acad. Sci. 2014;111:18255–18260. doi: 10.1073/pnas.1421211111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsili E, Rollefson JB, Baron DB, Hozalski RM, Bond DR. Microbial Biofilm Voltammetry: Direct Electrochemical Characterization of Catalytic Electrode-Attached Biofilms. Appl. Environ. Microbiol. 2008;74:7329–7337. doi: 10.1128/AEM.00177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Congdon RB, Feldberg AS, Ben-Yakar N, McGee D, Ober C, Sammakia B, et al. Early detection of Candida albicans biofilms at porous electrodes. Anal. Biochem. 2013;433:192–201. doi: 10.1016/j.ab.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Gamby J, Pailleret A, Clodic CB, Pradier C, Tribollet B. In situ detection and characterization of potable water biofilms on materials by microscopic, spectroscopic and electrochemistry methods. Electrochim. Acta. 2008;54:66–73. [Google Scholar]
- 24.Kim S, Yu G, Kim T, Shin K, Yoon J. Rapid bacterial detection with an interdigitated array electrode by electrochemical impedance spectroscopy. Electrochim. Acta. 2012;82:126–131. [Google Scholar]
- 25.Dheilly A, Linossier I, Darchen A, Hadjiev D, Corbel C, Alonso V. Monitoring of microbial adhesion and biofilm growth using electrochemical impedancemetry. Appl. Microbiol. Biotechnol. 2008;79:157–164. doi: 10.1007/s00253-008-1404-7. [DOI] [PubMed] [Google Scholar]
- 26.Webster TA, Sismaet HJ, Chan I-J, Goluch ED. Electrochemically monitoring the antibiotic susceptibility of Pseudomonas aeruginosa biofilms. Analyst. 2015;140:7195–7201. doi: 10.1039/c5an01358e. [DOI] [PubMed] [Google Scholar]
- 27.Sultana ST, Call DR, Beyenal H. Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility. npj Biofilms and Microbiomes. 2016;2:2. doi: 10.1038/s41522-016-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinogradov SM, Satterwhite-Warden JE, Hicks RP, Anderson E, Hvastkovs EG. Electrochemical Detection of Alginate Penetration in Immobilized Layer-by-Layer Films by Unnatural Amino Acid Containing Antimicrobial Peptides. Electrochim. Acta. 2015;186:245–252. [Google Scholar]
- 29.Ramsey DM, Wozniak DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005;56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 30.Stapper AP, Narasimhan G, Ohman DE, Barakat J, Hentzer M, Molin S, et al. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J. Med. Microbiol. 2004;53:679–690. doi: 10.1099/jmm.0.45539-0. [DOI] [PubMed] [Google Scholar]
- 31.Lvov YM, Lu Z, Schenkman JB, Zu X, Rusling JF. Direct Electrochemistry of Myoglobin and Cytochrome p450cam in Alternate Polyion Layer-by-Layer Films with DNA and Poly(styrenesulfonate) J. Am. Chem. Soc. 1998;120:4073–4080. [Google Scholar]
- 32.Rusling JF, Hvastkovs EG, Hull DO, Schenkman JB. Biochemical applications of ultrathin films of enzymes, polyions and DNA. Chem. Commun. 2008:141–154. doi: 10.1039/b709121b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang VB, Chua S, Cao B, Seviour T, Nesatyy VJ, Marsili E, et al. Engineering PQS Biosynthesis Pathway for Enhancement of Bioelectricity Production in Pseudomonas aeruginosa Microbial Fuel Cells. PLOS ONE. 2013;8:e63129. doi: 10.1371/journal.pone.0063129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballard T, Richards J, Wolfe A, Melander C. Synthesis and Antibiofilm Activity of a Second-Generation Reverse-Amide Oroidin Library: A Structure–Activity Relationship Study. Chemistry – A European Journal. 2008;14:10745–10761. doi: 10.1002/chem.200801419. [DOI] [PubMed] [Google Scholar]
- 35.Lvov Y, Ariga K, Ichinose I, Kunitake T. Assembly of Multicomponent Protein Films by Means of Electrostatic Layer-by-Layer Adsorption. J. Am. Chem. Soc. 1995;117:6117–6123. [Google Scholar]
- 36.Bosire EM, Rosenbaum MA. Electrochemical Potential Influences Phenazine Production, Electron Transfer and Consequently Electric Current Generation by Pseudomonas aeruginosa. Frontiers in Microbiology. 2017;8:892. doi: 10.3389/fmicb.2017.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hvastkovs EG, So M, Krishnan S, Bajrami B, Tarun M, Jansson I, et al. Electrochemiluminescent Arrays for Cytochrome P450-Activated Genotoxicity Screening. DNA Damage from Benzo[a]pyrene Metabolites. Anal. Chem. 2007;79:1897–1906. doi: 10.1021/ac061975q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satterwhite JE, Trumbo CM, Danell AS, Hvastkovs EG. Electrochemical study on the effects of epigenetic cytosine methylation on anti-benzo[a]pyrene diol epoxide damage at TP53 oligomers. Anal. Chem. 2013;85:1183–1191. doi: 10.1021/ac303077h. [DOI] [PubMed] [Google Scholar]
- 39.O'Toole A G. Microtiter Dish Biofilm Formation Assay. Journal of Visualized Experiments : JoVE. 2011:2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merritt JH, Kadouri DE, O'Toole A G. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2005;01(Unit–1B.1) doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional Analysis of Genes for Biosynthesis of Pyocyanin and Phenazine-1-Carboxamide from Pseudomonas aeruginosa PAO1. Journal of Bacteriology. 2001;183:6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hameed A, Pi H, Lin S, Lai W, Young L, Liu Y, et al. Direct Electrochemical Sensing of Phenazine-1-carboxylic Acid Secreted by Pseudomonas chlororaphis subsp. aureofaciens BCRC 11057T Using Disposable Screen-printed Carbon Electrode. Electroanalysis. 2016;28:846–853. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.