Abstract
Background:
Continued use of standardized, first-line ART containing NNRTIs and NRTIs may contribute to ongoing emergence of HIV drug resistance (HIVDR) in Namibia.
Methods:
A nationally representative cross-sectional survey was conducted during 2015–16 to estimate the prevalence of significant pretreatment HIV drug resistance (PDR) and viral load (VL) suppression rates 6–12 months after initiating standardized first-line ART. Consenting adult patients (≥18years) initiating ART were interviewed about prior antiretroviral drug (ARV) exposure and underwent resistance testing using dried blood spot samples. PDR was defined as mutations causing low-, intermediate- and high-level resistance to ARVs according to the 2014 WHO Surveillance of HIV Drug Resistance in Adults Initiating ART. The prevalence of PDR was described by patient characteristics, ARV exposure and VL results. Results were weighted to be nationally representative.
Results:
Successful genotyping was performed for 381 specimens; 144 (36.6%) specimens demonstrated HIVDR, of which 54 (12.7%) demonstrated PDR. Resistance to NNRTIs was most prevalent (11.9%). PDR was higher in patients with previous ARV exposure compared with no exposure (30.5% versus 9.6%) (prevalence ratio “ 3.17; P < 0.01).
Conclusions:
This survey demonstrated overall PDR at >10% among adults initiating ART in Namibia. Patients with prior ARV exposure had higher rates of PDR. Introducing a non-NNRTI-based regimen for first-line ART should be considered to maximize benefit of ART and minimize the emergence of HIVDR.
Introduction
Emergence of HIV drug resistance (HIVDR) is a threat to the global scale-up of ART for HIV infection. The WHO recommends that countries affected by HIV should implement strategies for HIVDR prevention to sustain the gains made with ART scale-up, improve the quality of life of patients and reduce costs of ART programmes, which includes surveillance of pretreatment HIV drug resistance (PDR) among patients initiating ART.1
HIVDR can be transmitted at the time of the initial infection or acquired owing to previous exposure to antiretroviral drugs (ARVs) [e.g. reported prevention of mother-to-child transmission of HIV (PMTCT), pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP) or previous ART use].2 Increased prevalence of PDR also can be associated with virological failure after ART initiation, which could contribute to further emergence of resistance.3–8 Knowledge of PDR at the population level can assist countries in choosing efficacious first-line ART, especially when resistance testing is not performed prior to ART initiation.
Currently, two NRTIs and one NNRTI constitute the preferred, standardized first-line ART in Namibia: emtricitabine or lamivudine plus tenofovir disoproxil fumarate plus efavirenz. Alternative medications that can be considered for substitutions in first-line ART include abacavir, zidovudine and nevirapine.9 Namibia has accelerated its efforts to achieve the UNAIDS 90–90-90 targets, including implementing a ‘test-and-start’ strategy and the WHO recommendations for HIVDR monitoring and surveillance.10 However, continued use of this standardized, first-line ART may contribute to ongoing selection of acquired drug resistance and PDR.11,12
This national survey had four main objectives: (i) estimate the proportion of ART initiators who had prior exposure to ARVs; (ii) estimate the overall national prevalence of PDR, regardless of ARV exposure; (iii) estimate the prevalence of PDR in individuals initiating ART with prior exposure to ARVs; and (iv) compare levels of PDR with patient demographic and clinical characteristics, including viral load (VL) suppression 6–12 months after ART initiation.
Methods
Survey design and sampling
A cross-sectional survey, based on the WHO PDR survey guide, was conducted between April and September 2015 and additional specimens were collected between March and May 2016.2 Patient eligibility criteria included: CD4 count of <500cells/mm3 (ART start criterion according to the National ART Guidelines at the time of the survey); age ≥18 years; and initiating ART for the first time or re-starting treatment after having interrupted it for >90 days.
Subjects were identified using a cross-sectional two-stage cluster sampling technique. The sampling frame was composed of 57 public health facilities that initiated >75 adult patients (≥18years of age) on ART during 2012–13, representing 93% of all adult patients during the study period. These facilities were stratified into six geographic zones, and in the first stage of sampling ART clinics were selected with probability proportional to the number of facilities in each zone, resulting in a total of 23 randomly selected ART clinics. In the second stage, eligible patients were recruited and enrolled in each selected clinic. The sampling weight for each patient was calculated as the inverse of the probability of selection adjusted for non-response. For variance estimation, a geographic zone with only one selected facility was combined with facilities in a neighbouring zone.
A target sample size was calculated at 437 patients (19 patients per clinic site). Probability of PDR prevalence was set at 10.0%. Additional sample size parameters included: PDR 95% CI (9.5%−10.5%); 20.0% genotyping failure rate; 25.0% pretreatment exposure rate to ARVs; a site-level variability via intra-class correlation of 0.01; and a design effect of 1.5.
Individuals initiating ART and fulfilling additional eligibility criteria were eligible for enrolment. Eligible patients provided informed consent, which explained the study purpose and procedures involved, potential risks and benefits, and the confidentiality policy for the information collected. The survey protocol was reviewed and approved by the Ministry of Health and Social Services in Namibia. The protocol was also reviewed according to CDC human research protection procedures. The anonymous testing at CDC was determined as non-human subjects research by the Office of the Associate Director for Science at the Center for Global Health, CDC, Atlanta, GA, USA.
Patients were sampled consecutively during the initial 6-month period (April to September 2015) and again from March to May 2016 to achieve the target sample size for estimation of the national prevalence of PDR. The study continued to administer the ARV exposure-screening questionnaire to all adult ART initiators at each site for the duration of the survey in order to generate a reliable baseline estimate of the national prevalence of prior ARV exposure history among adult patients initiating ART in Namibia.
Data and specimen collection
Demographic and clinical data were collected using interviews and chart abstraction by trained health workers. Age in completed years reported by patients was used in the analysis. Identifier information [patient survey ID, date of dried blood spot (DBS) collection, ART clinic name and the unique ART number of the participant] was used to merge epidemiological data with genotyping results.
Data on the first VL result after starting ART were collected for patients with documented testing performed up to 12 months after treatment initiation to allow for clinical and laboratory lag times (Namibia ART guidelines set the first VL test after ART initiation at 6 months). VL suppression was defined as VL <1000copies/mL.3 VL testing data were evaluated for any association with patient characteristics, prior ARV exposure history and PDR.
Consented patients provided a whole blood sample collected by the lancet finger-stick technique, which was used to prepare a DBS sample on Whatman 903 filter paper. An ART nurse or clinician recorded demographic and clinical data and collected the DBS sample (the Demographic and Clinical Data Collection Form for Patients Initiating ART is available as Supplementary data at JAC Online). Patient identifier information and the date and time of DBS collection were recorded for specimen tracking. The DBS sample was dried at room temperature for ≥4 h and packed in a single gas-impermeable, zipper-lock plastic bag containing desiccant packs. A humidity indicator card inside the bag monitored for excess moisture. Identification labels were placed on the patient’s recorded demographic data and in the plastic bag containing the DBS sample.
HIVDR genotyping
DBS specimens were batched daily and sent to the Namibia Institute of Pathology (NIP) laboratory in Windhoek. At NIP, the specimens were stored at –20°C until shipment to the WHO-designated laboratory at the US CDC (Atlanta, GA, USA). Shipment of DBS specimens was at ambient temperature, according to routine procedure, in zipper-lock plastic bags also containing the described desiccants and humidity indicators. HIVDR testing was conducted using the ATCC® HIV-1 genotyping kit.13 Sequences of the partial HIV polymerase gene covering the HIVDR mutation sites for protease and reverse transcriptase were generated with an ABI 3730 DNA Analyzer and processed using ReCall software designated for sequence editing and proofreading. Further analysis of sequence quality was performed using BioEdit to rule out possible sample contaminations based on the differences in genetic pairwise distances (2% cut-off). For some clinics, the initial DBS amplification rate was low, so additional specimens were collected between March and May 2016 to ensure the target sample size was achieved (final amplification rate, 75%).
HIVDR mutations were interpreted and classified according to the Stanford HIVDR Database (HIVdb) algorithm. Following the 2014 WHO Surveillance of HIV Drug Resistance in Adults Initiating ART, PDR was defined as any mutation predicted to cause low-, intermediate- and/or high-level resistance to nevirapine and efavirenz (NNRTIs), all NRTIs and darunavir/ritonavir, lopinavir/ritonavir and atazanavir/ritonavir (PIs). Susceptible or potential low-level HIVDR was not considered to be PDR.2 Drug resistance results were communicated with site clinicians to facilitate appropriate patient management.
Statistical analysis
Weighted estimates of the national prevalence of PDR were calculated with 95% CIs. χ2 tests were performed to evaluate associations between prevalence of PDR and age, sex, prior ARV exposure status, WHO clinical stage, CD4 count and the first VL results obtained within 12 months after initiating ART. For each association, a prevalence ratio with 95% CI was calculated.14 Statistical significance was assessed at the 0.05 level. All analyses were performed using Stata software version 14.2 (StataCorp, College Station, PA, USA), accounting for the two-stage cluster survey design and sampling weights.
Results
Unweighted results of patient screening, DBS specimen collection and genotyping
Overall, 851 patients (63.1% female) were screened for history of prior exposure to ARVs. A total of 507 DBS specimens were collected from patients screened for prior ARV exposure and 381 (75.1%) were successfully genotyped (87.2% of target sample size).
Weighted results for prior ARV exposure
Exposure history was known for 849/851 patientsand114 reported taking ARVs previously, leading to a weighted national estimate for prior ARV exposure of 12.0% (95% CI 7.6%−18.4%). Of the 114 patients reporting prior exposure, 90 reported that it was from taking ART, 23 reported from PMTCT and 1 from PEP, representing weighted rates of 74.0%, (95% CI 57.7%−85.7%), 25.5% (95% CI 14.1%−41.5%) and 0.5% (95% CI 0.1%−4.3%), respectively.
Weighted prevalence of any HIVDR mutations
For the 381 participants successfully genotyped, accounting for sampling weights, the median age of ART patients was 33 years, 61.1% were female and 15.2% had prior exposure to ARVs (Table 1). Any HIVDR mutation was identified in 144 (36.6%) genotyped specimens (95% CI 31.8%−41.8%). HIVDR occurred at similar rates in male (40.6%, 95% CI 32.0%−49.8%) and female patients (34.1%, 95% CI 25.5%−43.8%) (P = 0.39).
Table 1.
Demographic and clinical characteristics of patients with genotype results
| Characteristic | Patients | Weighted median or % | IQR/ 95% CI |
|---|---|---|---|
| Age (years), median (IQR) | 381 | 33 | 28–41 |
| Age group (years) | |||
| 18–29 | 132 | 37.3 | 25.4–51.0 |
| 30–39 | 145 | 37.0 | 30.2–44.3 |
| 40–49 | 66 | 19.4 | 11.7–30.4 |
| 50–86 | 38 | 6.3 | 3.1–12.7 |
| Sex | |||
| male | 137 | 38.9 | 33.9–44.1 |
| female | 244 | 61.1 | 55.9–66.1 |
| History of exposure to ARVs | |||
| yes | 69 | 15.2 | 8.2–26.5 |
| no | 311 | 84.8 | 73.5–91.8 |
| unknown | 1 | 0.3 | |
| WHO clinical stage at ART initiation | |||
| 1 or 2 | 328 | 90.6 | 80.6–95.7 |
| 3 or 4 | 51 | 9.4 | 4.3–19.4 |
| unknown | 2 | 0.5 | |
| Baseline CD4 count (cells/mm3) | |||
| <200 | 148 | 36.0 | 22.3–52.4 |
| ≥200 | 226 | 64.0 | 47.6–77.7 |
| unknown | 7 | 1.8 | |
| VL (viral copies/mL) 6–12 months after ART initiation | |||
| <1000 | 165 | 94.2 | 88.3–97.2 |
| ≥1000 | 13 | 5.8 | 2.8–11.7 |
| unknown | 203 | 53.3 | |
Weighted national prevalence of PDR
A sensitivity analysis compared the 70 additional, amplified DBS specimens, collected between March and May 2016, with the 311 samples originally collected in 2015. PDR rates were comparable for both groups (13.9% and 12.4%, respectively; P = 0.75). Combining 2015 and 2016 results, 381 patients with successful genotypes were evaluated and 54 (12.7%) had PDR detected (95% CI 9.2%−17.2%). The design effect was 1.24, with an intra-class correlation of 0.0155. PDR was observed at similar rates in males and females (11.5% versus 13.5%; P = 0.69) (Table 2). PDR mutations were three times more prevalent in patients with previous ARV exposure compared with patients with no prior exposure (30.5% versus 9.6%, prevalence ratio 3.17, 95% CI 1.92–5.23) (P< 0.01). Patients with baseline WHO HIV clinical disease stage 3 or 4 had almost twice the rate of PDR compared with patients with WHO stage 1 or 2 (21.1% versus 11.8%), but this difference was not significant (P = 0.19). PDR rates were similar in patients with baseline CD4 count <200cells/mm3 (12.8%) versus patients with baseline CD4 count ≥200cells/mm3 (11.9%) (P = 0.75). PDR rates in patients with an unsuppressed VL (≥1000 copies/mL) 6–12 months after initiating ART were slightly higher than in individuals with a suppressed VL, but this finding also was not significant(15.4% and 9.3%, respectively; P = 0.51).
Table 2.
Prevalence of PDR by demographic and clinical characteristics at ART initiation
| Characteristic | Patients | Positive PDR (weighted %) | Pa | Prevalence ratio (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 137 | 16(11.5) | 0.69 | 1.00 (referent) |
| female | 244 | 38(13.5) | 1.17(0.50–2.74) | |
| History of exposure to ARVs | ||||
| Yes | 69 | 24(30.5) | <0.01b | 3.17(1.92–5.23) |
| No | 311 | 30(9.6) | 1.00 (referent) | |
| WHO clinical stage | ||||
| 1 or 2 | 328 | 44(11.8) | 0.19 | 1.00 (referent) |
| 3 or4 | 51 | 10(21.1) | 1.79 (0.75–4.25) | |
| Baseline CD4 count (cells/mm3) | ||||
| <200 | 148 | 20(12.8) | 0.75 | 1.00 (referent) |
| ≥200 | 226 | 31 (11.9) | 0.93 (0.58–1.48) | |
| VL (viral copies/mL) 6–12 months after ART initiation | ||||
| <1000 | 165 | 18 (9.3) | 0.51 | 1.00 (referent) |
| ≥1000 | 13 | 2 (15.4) | 1.66 (0.34–8.07) | |
Pearson’s design-based test of the null hypothesis of no association between the characteristic and PDR.
Statistically significant.
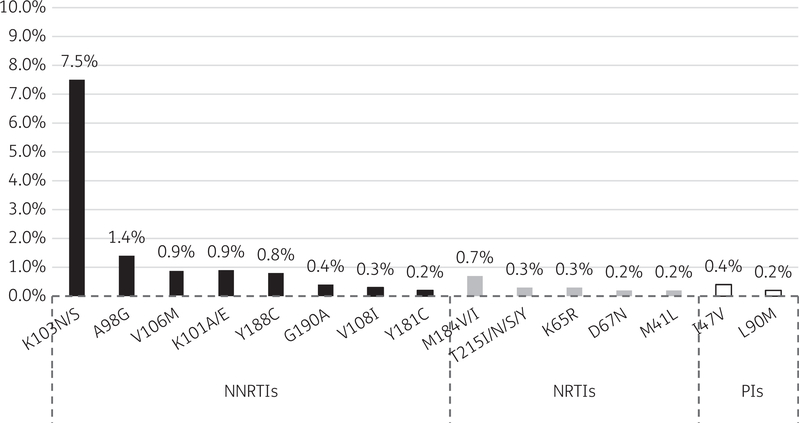
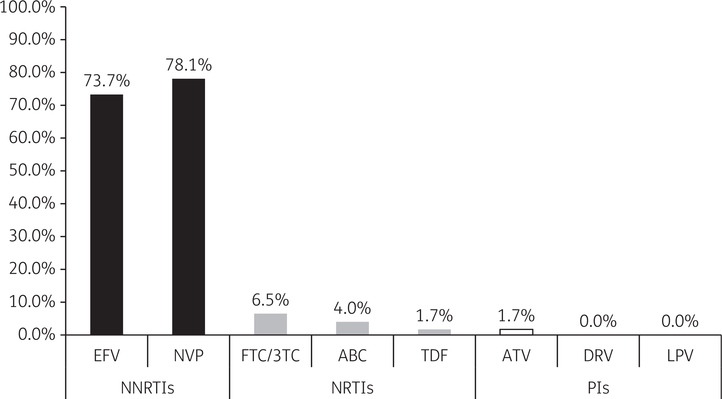
HIVDR mutations affecting NNRTIs, NRTIs and PIs defining PDR were observed in 11.9%, 1.0% and 0.6% of patients, respectively. PDR was observed in 54 patients and the most prevalent HIVDR mutations observed contributing to PDR are detailed in Figure 1. Evaluating PDR affecting individual ARVs demonstrated that resistance to nevirapine and efavirenz was most common (78.1% and 73.7%, respectively) (Figure 2). Overall, in patients with demonstrated PDR, 94% had at least one significant resistance mutation to EFV or NVP.
Figure 1.
Most prevalent HIVDR mutations contributing to PDR, by ARV drug class, among adult patients initiating ART in Namibia (n = 381).
Figure 2.
Prevalence of resistance to individual ARVs among adult patients with PDR in Namibia (n = 54). EFV, efavirenz; NVP, nevirapine; FTC/3TC, emtricitabine/lamivudine; ABC, abacavir; TDF, tenofovir disoproxil fumarate; ATV, atazanavir; DRV, darunavir; LPV, lopinavir.
Weighted VL suppression rates
VL test results done within 6–12 months after ART initiation were available for 178/381 (46.7%) patients with genotype results. A total of 13 (7.3%) patients failed to achieve VL suppression (median 102119 copies/mL, range 22711–558509). Of the 165 patients (92.7%) who achieved VL suppression, 155 had an undetectable VL (<40 copies/mL) and 10 had low-level viraemia (40–917 copies/mL). Patients with prior ARV exposure had a lower VL suppression rate (85.7%, 95% CI 65.4%−95.0%) compared with those without ARV exposure (95.8%, 95% CI 88.6%−98.5%), but the difference was not statistically significant (P = 0.11). No other patient characteristics, including PDR, were significantly associated with VL suppression.
Discussion
This survey demonstrated a moderate level of PDR among adults initiating ART in Namibia, consistent with a recent WHO report raising concern about PDR rates of ≥10% in 6 of 11 countries that conducted PDR surveys. This WHO report cited an unweighted overall PDR rate of 14.6% (95% CI 11.6%−18.2%) for Namibia, which is slightly higher than the weighted PDR rate of 12.7% observed in this study. The WHO report noted a high rate of PDR in patients with prior ARV exposure history in Namibia (36.2% with prior ARV exposure versus 9.9% for ARV-naive patients), which is similar to our weighted results (30.5% with previous ARV exposure versus 9.6% for ARV-naive patients).11
Findings from our study are consistent with a recently published meta-regression analysis of PDR before ARV initiation, which reported NNRTI PDR in southern Africa at 11.0%,15 similar to the 11.9% weighted NNRTI PDR for Namibia reported by this study. Additional studies conducted outside of Africa have reported overall PDR prevalence estimates ranging from 3.5%−15.5%.7,16,17
Similar to other studies, no relationship between PDR and gender, WHO clinical stage, CD4 count or VL suppression 6–12 months after starting ART was observed, suggesting these factors may not be relevant when evaluating for possible risk factors for PDR.16,17
The most common PDR mutations detected in Namibia were against NNRTIs, which is logical considering National ART Guidelines have included NNRTIs as part of the standard first-line ART regimen, and for PMTCT, for >10years. By mid-2017, Namibia had 217000 adult patients living with HIV (PLHIV), with a total of 158485 receiving ART in public health facilities.18 With a high level of ART coverage among PLHIV and a large percentage of these individuals on an NNRTI-based first-line treatment regimen, the risk of continued increase in PDR is substantial.
Overall, 94% of individuals with PDR in this study had at least one resistance mutation to an NNRTI, and PLHIV with prior ART exposure were 3-fold more likely to have PDR. These findings emphasize the need to quantify prior ARV exposure for all patients initiating ART in order to optimize virological response and to prevent further acquisition of NNRTI drug resistance in Namibia.
PDR mutations associated with efavirenz and nevirapine were observed at rates in this survey that were similar to other studies completed in SSA.6,19–22 Additionally, resistance to the second- generation NNRTIs rilpivirine and etravirine was observed. Multiple factors could explain the presence of resistance to these ARVs, including: cross-resistance driven by long-standing, population- level exposure to nevirapine and efavirenz;23 the presence of the E138A mutation as a spontaneous mutation in treatment-naive patients who are infected with HIV subtype C,24,25 and recent introduction of etravirine and rilpivirine in Namibia for use in salvage therapy regimens.9
This study has at least three limitations. First, the crosssectional design does not allow definitive inference on how the level of PDR is linked to prior exposure to ARV or whether PDR is secondary to acquired versus transmitted resistance. Secondly, because 27% of the DBS samples arising from all but one site could not be genotyped during the initial data collection period (April— September 2015) the target sample size was not achieved and this had the potential to affect survey results. Although routine procedures for preparation of DBS samples for early infant diagnosis were followed, the higher than expected overall genotyping amplification failure rate (25%) might be owing to the fact that the DBS samples were stored at −20°C (not −80°C) before shipment and that finger-prick whole blood samples were used to make DBS samples.26 In addition, data have shown that ~10% of patients initiating ART may have VL <1000copies/mL, which would make genotyping amplification less reliable.2 Nonetheless, repeat specimen collection resulted in a final amplification rate of 75% and a sensitivity analysis demonstrated that PDR rates were comparable, confirming that combining results from both groups of genotypes likely did not affect the estimated level of PDR reported in this study. Thirdly, VL data for our study were available for <50% of participants owing to challenges in linking clinical and laboratory electronic databases without unique patient identifiers. Nonetheless, a strong association between prior ARV exposure and presence of PDR was similar to what has been reported in other countries.15
This first PDR study in Namibia demonstrated a moderate level of PDR among adult patients initiating ART, and individuals with prior ARV exposure had a higher probability of having PDR. WHO recommends that countries demonstrating a national prevalence of PDR to NNRTIs of >10% consider transitioning to alternative drugs for patients initiating standardized first-line ART.4,27 For Namibia, transitioning to an alternative, non-NNRTI (e.g. based on a PI or an integrase strand transfer inhibitor) first- line regimen has the potential to maximize ART efficacy and minimize risk of HIVDR.
Acknowledgements
Unweighted results from this survey were previously presented as an abstract at the Ninth IAS Conference on HIV Science, Paris, France, 2017 (‘HIV drug resistance among adults initiating antiretroviral therapy in Namibia’; MOPEB0275).
Funding
This project has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of CDC and MoHSS cooperative agreement grant #1U2GGH001181–05.
Footnotes
Transparency declarations
None to declare.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Supplementary data
The Demographic and Clinical Data Collection Form for Patients Initiating ART is available as Supplementary data at JAC Online.
References
- 1.WHO. Global Strategy for the Surveillance and Monitoring of HIV Drug Resistance, 2012. Geneva, Switzerland: WHO, 2012. http://apps.who.int/iris/bitstream/handle/10665/77349/9789241504768_eng.pdf?sequence=1. [Google Scholar]
- 2.WHO. Surveillance of HIV Drug Resistance in Adults Initiating Antiretroviral Therapy (Pre-Treatment HIV Drug Resistance): Concept Note. Geneva, Switzerland: WHO, 2014. http://www.who.int/hiv/pub/drugresistance/pretreatment_drugresistance/en/. [Google Scholar]
- 3.Hamers RL, Sigaloff KC, Kityo C et al. Emerging HIV-1 drug resistance after roll-out of antiretroviral therapy in sub-Saharan Africa. Curr Opin HIV AIDS 2013; 8:19–26. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance: July 2017. Geneva, Switzerland: WHO, 2017. http://www.who.int/iris/handle/10665/255880. [Google Scholar]
- 5.Hamers RL, Schuurman R, Sigaloff KC et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multi-center cohort study. Lancet Infect Dis 2012; 2:307–17. [DOI] [PubMed] [Google Scholar]
- 6.Mungati M, Mhangara M, Gonese E et al. Pre-treatment drug resistance among patients initiating antiretroviral therapy (ART) in Zimbabwe: 2008–2010. BMC Res Notes 2016; 9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissio E, Barbas MG, Bouzas MB et al. Pre-treatment HIV-1 drug resistance in Argentina: results from a surveillance study performed according to WHO-proposed new methodology in 2014–15. J Antimicrob Chemother 2016; 72: 504–10. [DOI] [PubMed] [Google Scholar]
- 8.Tang MW, Shafer RW. HIV-1 antiretroviral resistance: scientific principles and clinical applications. Drugs 2012; 72: e1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namibia Ministry of Health and Human Services. National Guidelines for Antiretroviral Therapy, Fifth Edition 2016. Windhoek, Namibia: MoHSS, 2016. [Google Scholar]
- 10.Namibia Ministry of Health and Social Services HIV Drug Resistance Surveillance Strategy 2014. Windhoek, Namibia: MoHSS, 2014. [Google Scholar]
- 11.WHO. HIV Drug Resistance Report 2017. Geneva, Switzerland: WHO, 2017. http://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf?sequence=1. [Google Scholar]
- 12.Mulu A, Maier M, Liebert UG. Upward trends of acquired drug resistances in Ethiopian HIV-1C isolates: a decade longitudinal study. PLoS One 2017; 12: e0186619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Wagar N, Devos JR et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One 2011; 6: e28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenland S Introduction to regression models In: Rothman KJ, Greenland S, Lash TL , eds. Modern Epidemiology. 3rd edn. Philadelphia, PA, USA: Lippincott Williams & Wilkins, 2008; 381–417. [Google Scholar]
- 15.Gupta RK, Gregson J, Parkin N et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle- income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18:346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avila RS, Garcia-Morales C, Matias-Florentino M et al. Pretreatment HIV- drug resistance in Mexico and its impact on the effectiveness of first-line anti-retroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV 2016; 3:e579–91. [DOI] [PubMed] [Google Scholar]
- 17.Duy Pham Q, Thi Do N, Ngoc Le Y et al. Pretreatment HIV-1 drug resistance to first-line drugs: results from a baseline assessment of a large cohort initiating ART in Vietnam, 2009–10. J Antimicrob Chemother 2015; 70:941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namibia Ministry of Health and Social Services. ART PMIS Quarterly Report, April-June 2017 MoHSS, Windhoek, Namibia: Directorate of Tertiary Health Care and Clinical Support Services, 2017. [Google Scholar]
- 19.Seu L, Mulenga LB, Siwingwa M et al. Characterization of HIV drug resistance mutations among patients failing first-line antiretroviral therapy from a tertiary referral center in Lusaka, Zambia. J Med Virol 2015; 87: 1149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkins C, Ulenga N, Liu E et al. HIV virological failure and drug resistance in a cohort of Tanzanian HIV-infected adults. J Antimicrob Chemother 2016; 71:1966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowell CS, Maiga AI, Sylla M et al. High rates of baseline drug resistance and virologic failure among ART naive HIV-infected children in Mali. Pediatr Infect Dis J 2017; 36: e258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzaule SC, Osi SJ, Akinbiyi G et al. High prevalence of HIV drug resistance among newly diagnosed infants aged <18 months: results from a nationwide surveillance in Nigeria. J Acquir Immune Defic Syndr 2018; 77: e1–7. [DOI] [PubMed] [Google Scholar]
- 23.Neogi U, Shet A, Shamsundar R et al. Selection of nonnucleoside reverse transcriptase inhibitor-associated mutations in HIV-1 subtype C: evidence of etravirine cross-resistance. AIDS 2011; 25:1123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluis-Cremer N, Jordan M, Huber K et al. E138A in HIV-1 reverse transcriptase is more common in subtype C than B: implications for rilpivirine use in resource limited settings. Antiviral Res 2014; 107:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theys K, Van Laethem K, Gomes P et al. Sub-epidemics explain localized high prevalence of reduced susceptibility to rilpivirine in treatment-naive HIV-1-infected patients: subtype and geographic compartmentalization of baseline resistance mutations. AIDS Res Hum Retroviruses 2016; 32: 427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parry CM, Parkin N, Diallo K et al. Field study of dried blood spot specimens for HIV-1 drug resistance genotyping. J Clin Microbiol 2014; 52:2868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Transition to New Antiretrovirals in HIV Programmes, Policy Brief. Geneva, Switzerland: WHO, 2017. http://www.who.int/hiv/pub/toolkits/transition-to-new-arv/en/. [Google Scholar]