Abstract
People living with HIV (PLWH) are more likely to smoke compared to HIV-uninfected counterparts, but little is known about smoking behaviors in sub-Saharan Africa. To address this gap in knowledge, we characterized smoking cessation patterns among people living with HIV (PLWH) compared to HIV-uninfected individuals in rural Uganda. PLWH were at least 40 years of age and on antiretroviral therapy for at least three years, and HIV-uninfected individuals were recruited from the clinical catchment area. Our primary outcome of interest was smoking cessation, which was assessed using an adapted WHO STEPS smoking questionnaire. We fit Cox proportional hazards models to compare time to smoking cessation between PLWH pre-care, PLWH in care, and HIV-uninfected individuals. We found that, compared to HIV-uninfected individuals, PLWH in care were less likely to have ever smoked (40% vs. 49%, p=0.04). The combined sample of 267 ever-smokers had a median age of 56 (IQR 49–68), 56% (n=150) were male, and 26% (n=70) were current smokers. In time-to-event analyses, HIV-uninfected individuals and PLWH prior to clinic enrollment ceased smoking at similar rates (HR 0.8, 95% CI 0.5–1.2). However, after enrolling in HIV care, PLWH had a hazard of smoking cessation over twice that of HIV-uninfected individuals and three times that of PLWH prior to enrollment (HR 2.4, 95% CI 1.3–4.6, p=0.005 and HR 3.0, 95% CI 1.6–5.5, p=0.001, respectively). In summary, we observed high rates of smoking cessation among PLWH after engagement in HIV care in rural Uganda. While we hypothesize that greater access to primary care services and health counseling might contribute, future studies should better investigate the mechanism of this association.
Keywords: Africa, tobacco, primary care
Introduction
Smoking was responsible for over 6 million deaths globally in 2015 (GBD, 2016, 2017). People living with HIV (PLWH) are more likely to smoke compared to HIV-uninfected counterparts in high (Mamary, Bahrs, & Martinez, 2002; Mdodo et al., 2015) and low-income countries (Mdege, Shah, Ayo-Yusuf, Hakim, & Siddiqi, 2017). Smoking-related morbidity and mortality are particularly high among PLWH on antiretroviral therapy (ART) (Crothers et al., 2009), for whom smoking contributes to a greater decrement in life expectancy than HIV infection (Helleberg et al., 2013; Helleberg et al., 2015; Reddy et al., 2017; Reddy et al., 2016). For PLWH who quit smoking, health outcomes improve with continued cessation (Petoumenos et al., 2011).
Almost 70% of PLWH in the world live in sub-Saharan Africa (SSA) (UNAIDS, 2017), where smoking prevalence is increasing due to limited governmental legislation and expanding tobacco marketing campaigns (Blecher and Ross, 2013; Brathwaite, Addo, Smeeth, & Lock, 2015; Sreeramareddy, Pradhan, & Sin, 2014). Nearly 10% of the adult Ugandan population smokes (Kabwama et al., 2016). Tobacco control programs (WHO, 2003) can improve smoking behaviors, but require primary care access and/or developed health systems. In SSA, HIV care provides millions of people with access to routine medical care, and some have hypothesized that this additional access to care has positively impacted non-HIV health outcomes including blood pressure control and diabetes management (Feinstein et al., 2017; Manne-Goehler et al., 2017). Thus, the HIV care platform may provide a framework for smoking cessation interventions (Gupta and Bukhman, 2015; Niaura et al., 2000).
To address this gap in knowledge, we quantified smoking patterns in a clinic-based cohort of PLWH on stable ART and compared them to smoking patterns in a population-based, HIV-uninfected group residing in the clinic catchment area in rural Uganda. We hypothesized that PLWH who are engaged in HIV care would be less likely to smoke and more likely to quit smoking than HIV-uninfected controls.
Methods
We analyzed data from two cross-sectional samples. PLWH were enrolled using convenience sampling into the Ugandan Non-Communicable Diseases and Aging Cohort (UGANDAC; NCT02445079), a cohort study of older-aged PLWH on ART in rural Uganda. All UGANDAC study participants were at least 40 years old, had received ART for at least three years from the Mbarara Regional Referral Hospital HIV clinic (North et al., 2017) – which is one of the largest clinics in the region and provides much of the HIV care for PLWH in southwestern Uganda – and all lived within approximately 130 km of the HIV clinic. Eligible participants were approached for study participation after having voluntarily arrived at the HIV clinic for routine HIV-related care, and study enrollment had no impact on their medical care. Per clinic protocols, in-person, 1-on-1 smoking cessation counseling is provided by trained HIV testing counselors for all clinic patients who screen positive for smoking and are referred by treating physicians at their initial intake and/or quarterly follow-up clinic appointments. Counseling includes teaching on the health risks of smoking and advice on lifestyle modifications for successful smoking cessation, tailored to the needs of each patient. Written educational materials are not present due to resource constraints.
Data from HIV-uninfected comparators were collected during one of five health screening events in the Nyakabare parish, a cluster of 8 villages within the HIV clinic catchment area, located approximately 20 kilometers from the HIV clinic. Eligible participants were approached for research data collection after voluntarily arriving for a free health screening, and consent to allow data collection had no bearing on whether they could complete the health screening. Interested community members who were unable to travel to the health screening locations due to physical ailments were identified in pre-event informational meetings, and free transportation was arranged. We included HIV-negative participants if they were over 40 years of age and tested negative for HIV at the health fair.
Smoking behaviors were characterized in both cohorts with an adapted WHO STEPS questionnaire (WHO, 2017) (See Online Supplement). HIV testing was conducted according to Ugandan Ministry of Health guidelines (Uganda National Policy Guidelines for HIV Counseling and Testing, 2005). For PLWH, CD4+ T-cell count and HIV viral load within one year of study visits were obtained from clinical record abstraction. We defined virologic suppression as a viral load below the limit of assay detection (dried blood spot: <550 copies/μL; plasma: <40 copies/μL). Participants gave written informed consent in both the UGANDAC study and the health screening events, and study procedures for both studies were approved by the Partners Healthcare and Mbarara University of Science and Technology human studies ethics committees, as well as by the Ugandan National Council for Science and Technology.
Statistical Analysis
We compared demographic characteristics by HIV serostatus using chi-squared for categorical variables or Fisher’s exact tests for any variables with a cell size of less than 5 participants. To asses for selection bias, we compared characteristics of participants with and without complete smoking data, and between HIV-uninfected participants who did or did not attend the health screenings. Data from those who did not attend the health fairs were available from a population census collected the prior year. Our primary outcomes of interest were (1) cessation of smoking and (2) time from smoking initiation to cessation. The primary exposure of interest was HIV serostatus. We estimated the association between HIV serostatus and smoking cessation by constructing multivariable logistic regression models including all covariates with a p≤0.2 in unadjusted analyses. Age was explored as a non-linear covariate, and the joint statistical significance of the regression coefficients of categorical variables was tested using an F-type Wald test. We conducted a sensitivity analysis by excluding smoking duration from regression models to limit missingness. For our secondary outcome, we fit Cox proportional hazard models with robust standard errors to account for clustering within individuals. We considered HIV clinic enrollment as a time varying exposure by dividing smoking duration among PLWH into two observation periods using the stsplit command in Stata: time before and time after enrollment in HIV care, and compared each group separately to HIV-uninfected comparators. We tested proportionality of hazard estimates with log-log plots and assessed for time varying effects. Potential confounders in both the regression and time to event analyses included age, gender, household asset wealth (Filmer and Pritchett, 2001), education and medical comorbidities, with smoking duration additionally included as a covariate in the correlates of smoking cessation analysis. Data were analyzed with Stata 13 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Among 574 total participants in both cohorts (154 PLWH and 420 HIV-uninfected), 267 (47%) ever smoked (Table 1). Ever-smoking was less prevalent among PLWH (40%) than HIV-uninfected participants (49%, p=0.045). Among ever-smokers, PLWH were more commonly men (72% vs. 52%, p<0.001) and had higher educational attainment (p=0.002). Most PLWH were virologically suppressed (88%), and 81% had CD4+ counts ≥350 cells/mm3 at last measurement. Nearly all PLWH (92%) were taking non-nucleoside reverse transcriptase inhibitor-based ART for a median of nine years (IQR 8–10).
Table 1:
Baseline Cohort Characteristics of Current or Former Smokers
| Total (n=267) | HIV+ (n=61) | HIV- (n=206) | p-value | |
|---|---|---|---|---|
| Age, years | 56 [49, 68] | 51 [49, 57] | 60 [49, 71] | <0.001 |
| Male | 150 (56) | 44 (72) | 107 (52) | <0.01 |
| 1+ Medical Comorbidity a | 72 (27) | 11 (18) | 61 (30) | 0.07 |
| Body Mass Index, kg/m2 | 0.29 | |||
| Underweight (<18.5) | 30 (11) | 6 (10) | 24 (12) | |
| Normal (18.5–24.9) | 170 (65) | 45 (74) | 125 (62) | |
| Overweight (25–29.9) | 41 (16) | 8 (13) | 33 (16) | |
| Obese (≥30) | 22 (8) | 2 (3) | 20 (10) | |
| Education level | 0.002 | |||
| No school | 92 (34) | 11 (18) | 81 (39) | |
| Any primary school | 136 (51) | 35 (57) | 101 (49) | |
| More than primary school | 39 (15) | 15 (25) | 24 (12) | |
| Farmer | 193 (72) | 38 (62) | 155 (75) | 0.05 |
| Smoking status | 0.10 | |||
| Current smoker | 70 (26) | 11 (18) | 59 (29) | |
| Former smoker | 197 (74) | 50 (82) | 147 (71) | |
| Smoking duration, years b | ||||
| Current smoker | 30 [22, 41] | 35 [22, 42] | 29 [21, 40] | 0.73 |
| Former smoker | 13 [5, 24] | 15 [6, 23] | 11 [4, 26] | 0.54 |
| HIV Cohort | ||||
| Duration in HIV care, years | 9 [8, 10] | |||
| Baseline HIV viral load, copies/μL | ||||
| Undetectable | 0 (0) | |||
| Detectable, <10,000 | 1 (2) | |||
| Detectable, ≥10,000 | 60 (98) | |||
| Last HIV viral load, copies/μL | ||||
| Undetectable | 51 (88) | |||
| Detectable, <10,000 | 6 (10) | |||
| Detectable, ≥10,000 | 1 (2) | |||
| Baseline CD4+ count, cells/μm3 | ||||
| <100 | 23 (38) | |||
| 100–349 | 37 (61) | |||
| 350–499 | 1 (2) | |||
| ≥500 | 0 (0) | |||
| Last CD4+ count, cells/μm3 | ||||
| <100 | 0 (0) | |||
| 100–349 | 12 (20) | |||
| 350–449 | 26 (43) | |||
| ≥500 | 23 (38) | |||
| Current antiretroviral regimen c | ||||
| TDF/3TC/EFV | 5 (8) | |||
| AZT/3TC/EFV | 13 (21) | |||
| AZT/3TC/NVP | 38 (62) | |||
| TDF/3TC/LPV/r | 5 (8) | |||
n (%) or median [IQR], unless otherwise noted
Comorbidities include self-reported history of hypertension, hyperlipidemia, diabetes mellitus, renal disease, cancer, chronic obstructive pulmonary disease or asthma.
Missing in 60 (19%) of study participants.
AZT = zidovudine; 3TC = lamivudine; EFV = efavirenz; NVP = nevirapine; TDF = tadalfil; LPV/r = lopinavir/ritonavir.
HIV-uninfected participants who attended the health screening were older (55 vs. 50, p<0.001) than those who did not attend. Smoking duration data were missing in 30% of HIV-uninfected participants. Those with complete smoking data were younger and were more educated than those with incomplete data (eTable 1).
Correlates of Smoking Cessation
In unadjusted analyses, there was no difference in likelihood of having quit smoking or smoking duration by HIV serostatus. Most PLWH (68%) quit smoking prior to entry into HIV care, at a median of 14 years (IQR 6–18) after smoking initiation. Those who quit after care entry did so within a median of 2 years (IQR 0–6). In multivariable logistic regression models adjusted for confounders, HIV serostatus was associated with a non-significant doubling of the odds of smoking cessation (AOR 2.6, 95% CI 0.9–7.4) (Table 2). Older individuals (AOR 1.2 per year, 95% CI 1.1–1.3), men (AOR 3.1, 95% CI 1.2–8.1) and those with ≥1 medical condition (AOR 4.1, 95% CI 1.1–15.1) were more likely to cease smoking. Longer smoking duration was associated with decreased odds of quitting (AOR 0.9 per year, 95% CI 0.8–0.9). Results were similar in models restricted to participants with complete smoking duration data.
Table 2:
Unadjusted and adjusted correlates of smoking cessation in rural Uganda
| Characteristics | Unadjusted | Adjusted† | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| HIV Serostatus | 1.8* | 0.9 – 3.7 | 2.6 | 0.9 – 7.4 |
| Age, per year | 1.04*** | 1.02 – 1.07 | 1.2*** | 1.1 – 1.3 |
| Male | 2.9*** | 1.7 – 5.1 | 3.1* | 1.2 – 8.1 |
| 1+ Comorbidities | 3.7** | 1.7 – 8.3 | 4.1* | 1.1 – 15.1 |
| Education | ||||
| None | REF | |||
| Any primary school | 0.5* | 0.2 – 0.9 | 0.5 | 0.2 – 1.6 |
| More than primary school | 0.9 | 0.4 – 2.4 | 1.0 | 0.2 – 5.1 |
| Asset Index | ||||
| Poorest | REF | |||
| Poorer | 1.3 | 0.7 – 2.5 | 1.1 | 0.3 – 3.6 |
| Richer | 2.6* | 1.1 – 6.1 | 2.5 | 0.6 – 9.2 |
| Richest | 2.1 | 0.9 – 5.0 | 1.8 | 0.4 – 8.4 |
| Smoking Duration, per year | 0.94*** | 0.92 – 0.96 | 0.9*** | 0.8 – 0.9 |
p < 0.05,
p < 0.01,
p < 0.001
n = 203
Time to Cessation
In time-to-event analyses, there was no difference in time to cessation between PLWH and HIV-uninfected participants prior to entry into HIV care (HR 0.8, 95% CI 0.5–1.2). However, the hazard of smoking cessation among PLWH after care entry was significantly higher than that for HIV-uninfected individuals (HR 2.4, 95% CI 1.3–4.6, p=0.005) and for PLWH prior to care entry (HR 3.0, 95% CI 1.6–5.5, p=0.001) (Figure 1, eTable 2). Asset index was the only covariate that violated proportionality assumptions (eTable 3). In analyses stratified by asset index, the comparative hazard of cessation for PLWH versus HIV-negatives was higher in the wealthier subsample (HR 4.0, 95% CI 1.6–9.9, p=0.003) as compared to the less wealthy subsample (HR 1.9, 95% CI 0.7–4.6, p=0.18) (eTable 2).
Figure 1:
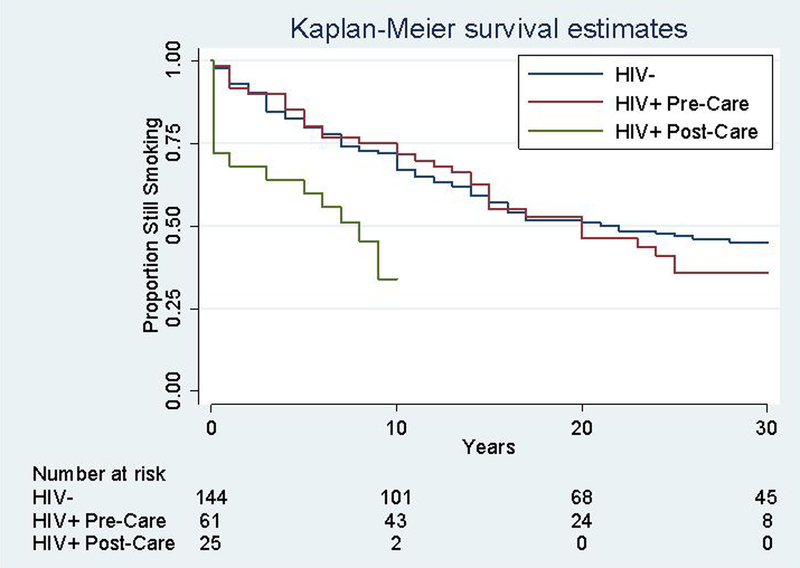
Kaplan-Meier curve of time-to-smoking cessation for PLWH pre- and post-HIV care engagement vs. HIV-uninfected participants.
Discussion
In rural Uganda, rates of smoking cessation among PLWH dramatically increase following engagement in HIV care, and are notably higher than cessation rates for population-based, HIV-uninfected comparators. Our study is among the first in SSA to compare smoking cessation patterns by HIV care engagement status, and lends support to the role of secondary health benefits afforded by HIV care programs.
Smoking prevalence was lower among PLWH than HIV-uninfected comparators in the current study, a finding that is consistent with data from SSA (Mutevedzi, Rodger, Kowal, Nyirenda, & Newell, 2013) but is contrary to much of the literature from other high-income settings (Mdege, et al., 2017; Mdodo, et al., 2015). The lower smoking prevalence among PLWH in our cohort may be due to the focus on PLWH in long-term care, who therefore have had repeated interactions with the healthcare system, while much of the data on HIV and smoking prevalence is focused on HIV cohorts that include participants at various stages along the HIV care continuum (Crothers, et al., 2009; Mdege, et al., 2017).
Several potentially complementary mechanisms may explain why engaging in HIV care may be associated with smoking behaviors. Firstly, in-person cessation counseling, which increases successful smoking cessation (Amiya et al., 2011; Berg et al., 2014; Chaiton et al., 2016; Louwagie, Okuyemi, & Ayo-Yusuf, 2014; Pacek, Rass, & Johnson, 2017; Ramon et al., 2013), is included as part of routine health counseling during initial enrollment at the Mbarara HIV clinic. Similar effects have been noted in the U.S., with one study showing a higher likelihood of smoking cessation among PLWH engaged in HIV care compared to the general population (Mdodo, et al., 2015). If these relationships are corroborated, our data support the expansion of smoking cessation programs both within HIV clinics and to other primary care delivery systems in the region.
Additional contributors to the observed phenomenon of smoking cessation among PLWH in clinical care could include changing social norms within social networks of PLWH (Christakis and Fowler, 2008) and changing perceived smoking norms (Perkins et al., 2017). For example, increasing media portrayal of binge drinking as socially undesirable contributed to decreasing binge drinking prevalence among youth in a longitudinal cohort study (Yanovitzky and Stryker, 2001). Alternatively, PLWH who present with co-infections or other severe comorbidities might have increased health-consciousness and/or be more apt to discontinue unhealthy behaviors (Kruse et al., 2014). Lastly, the advent of widespread ART has increased life expectancy among PLWH close to that of the HIV-uninfected community (Bor, Herbst, Newell, & Bärnighausen, 2013; Samji et al., 2013), which is known to change personal risk versus benefit calculations by attenuating the subjective mortality expectations of PLWH engaging in care (Baranov and Kohler, 2018) or by modifying the competing mortality risks among PLWH engaged in care, for whom HIV may no longer be the most proximal health threat (Ganz, 2000). PLWH on ART, who can now reasonably expect to live a long life, may be more apt to stop smoking because they no longer interpret non-ART related health behaviors as futile.
Although time to smoking cessation decreased significantly after enrollment in care, the majority (68%) of PLWH who quit smoking did so before engaging in HIV care. Two potential hypotheses might explain this observation. PLWH who have enrolled in HIV care may be inherently more health-conscious and thus more likely to have quit smoking. Since we did not observe a group of PLWH not in care, we cannot test this hypothesis in this analysis. A diagnosis of HIV infection itself could also trigger increased health consciousness. This theory is supported by a study of PLWH in South Africa that found a higher odds of smoking cessation among PLWH versus uninfected individuals who participated in a smoking cessation program (Louwagie and Ayo-Yusuf, 2015). Alternatively, since PLWH in SSA present to care at more advanced stages of disease in comparison to PLWH in developed countries (Auld, 2017; Siedner et al., 2014), they may have quit smoking because they feel worse as their disease advances (Halpern and Warner, 1994; Wilkes and Evans, 1999).
Our study provides key data that can be used to identify predictors of smoking cessation for public health policy (Mohiuddin et al., 2007; Rahmanian et al., 2011; Rigotti, Munafo, & Stead, 2007). Notably, we found that, in addition to enrollment in HIV care, men and older individuals were more likely to cease smoking, which is consistent with prior literature (Abdullah, Driezen, Quah, Nargis, & Fong, 2015; Hymowitz et al., 1997; Lee and Kahende, 2007; Li et al., 2010). Such data can be used to target cessation interventions to populations in SSA with increasing tobacco consumption, and prevent smoking-related health outcomes.
The main strength of this analysis is the inclusion of a population-based, HIV-uninfected comparator group, which allows effect estimation by HIV serostatus. We additionally characterized smoking behaviors with a standardized, validated questionnaire, allowing for comparisons across cohorts. There are also several limitations. First, the cross-sectional design prevents assumptions regarding causality. Second, self-reported smoking data are subject to misclassification (Stuber, Galea, & Link, 2008), although some (Caraballo, Giovino, Pechacek, & Mowery, 2001; Vartiainen, Seppala, Lillsunde, & Puska, 2002) but not all (Kruse, et al., 2014) studies have shown correlation between self-report and objective measures of tobacco use. Third, data regarding cessation counseling among HIV-uninfected participants were not collected. Data on time of HIV diagnosis were not available, preventing analysis of the impact of HIV diagnosis on smoking behaviors. This analysis was also limited to one clinic, and the relationship between counseling and smoking cessation may differ based on resources and consistency of smoking cessation activities in HIV clinics. Missing data on smoking initiation and/or cessation times may have biased our results if smoking duration differed among those with missing data. Additionally, among HIV-uninfected participants, health screening attendees may not be representative of the larger population, which may have biased our results if attendance was associated with differences in smoking behaviors. Although analyses accounted for demographic differences among study participants, unmeasured regional differences in cultural beliefs and/or practices throughout Uganda may have introduced bias into our estimates. Finally, this cohort was limited to older, rural Ugandans, so results are generalizable only to similar populations.
In conclusion, PLWH in rural Uganda who receive HIV care quit smoking faster than they do prior to engaging in care, and faster than HIV uninfected individuals. While further work is required to corroborate these findings and explore underlying mechanisms, this study suggests that integrating smoking cessation counseling into routine clinical care may decrease smoking among PLWH in SSA. Given the increased smoking prevalence and heightened risk of smoking-related morbidity and mortality among PLWH in SSA (Crothers, et al., 2009; Mdege, et al., 2017), broadening the integration of tobacco control interventions into existing HIV and general primary care could substantially improve health in the region.
Supplementary Material
Acknowledgements
We thank the Uganda Non-Communicable Diseases and Aging Cohort and HopeNet Cohort study participants who made this study possible; and Ruth Sentongo, Sheila Abaasabyoona, Zulaika Namboga, Doreen Kyomuhendo, Alan Babweteera, members of the HopeNet Study team and volunteers at the 2015 HopeNet Health Fair for research assistance. No endorsement of manuscript contents or conclusions should be inferred from these acknowledgements.
Funding
This work was supported by the U.S. National Institutes of Health (Grant #s: R21HL124712, R24AG044325, P30AI060354), the MGH Executive Committee on Research, and Friends of a Healthy Uganda. Additional salary support was provided by NIH Grants R01MH113494–01, K23MH099916 and T32HL116275. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic healthcare centers or the National Institutes of Health.
Footnotes
Disclosure Statement: All authors report no conflicts of interest.
References
- Abdullah AS, Driezen P, Quah AC, Nargis N, & Fong GT (2015). Predictors of smoking cessation behavior among Bangladeshi adults: findings from ITC Bangladesh survey. Tob Induc Dis, 13(1), p 23. doi:10.1186/s12971-015-0050-y Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26261450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiya RM, Poudel KC, Poudel-Tandukar K, Kobayashi J, Pandey BD, & Jimba M (2011). Physicians are a key to encouraging cessation of smoking among people living with HIV/AIDS: a cross-sectional study in the Kathmandu Valley, Nepal. BMC Public Health, 11, p 677. doi:10.1186/1471-2458-11-677 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21878132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld AF (2017). Trends in Prevalence of Advanced HIV Disease at Antiretroviral Therapy Enrollment—10 Countries, 2004–2015. MMWR. Morbidity and Mortality Weekly Report, 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov V, & Kohler HP (2018). The impact of AIDS treatment on savings and human capital investment in Malawi. Am Econ J: Appl Econ, 10(1), pp. 266–306. [Google Scholar]
- Berg CJ, Nehl EJ, Wang X, Ding Y, He N, Johnson BA, & Wong FY (2014). Healthcare provider intervention on smoking and quit attempts among HIV-positive versus HIV-negative MSM smokers in Chengdu, China. AIDS Care, 26(9), pp. 1201–1207. doi:10.1080/09540121.2014.892565 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24601710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher E, & Ross H (2013). Tobacco use in Africa: Tobacco control through prevention. American Cancer Society [Google Scholar]
- Bor J, Herbst AJ, Newell M-L, & Bärnighausen T (2013). Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science, 339(6122), pp. 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brathwaite R, Addo J, Smeeth L, & Lock K (2015). A Systematic Review of Tobacco Smoking Prevalence and Description of Tobacco Control Strategies in Sub-Saharan African Countries; 2007 to 2014. PLoS One, 10(7), p e0132401. doi:10.1371/journal.pone.0132401 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26162085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo RS, Giovino GA, Pechacek TF, & Mowery PD (2001). Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol, 153(8), pp. 807–814. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11296155 [DOI] [PubMed] [Google Scholar]
- Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, & Schwartz R (2016). Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ open, 6(6)doi:10.1136/bmjopen-2016-011045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis NA, & Fowler JH (2008). The collective dynamics of smoking in a large social network. N Engl J Med, 358(21), pp. 2249–2258. doi:10.1056/NEJMsa0706154 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18499567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Goetz MB, . . . Justice AC (2009). Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev, 21(3 Suppl), pp. 40–53. doi:10.1521/aeap.2009.21.3_supp.40 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19537953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein MJ, Kim JH, Bibangambah P, Sentongo R, Martin JN, Tsai AC, . . . Siedner MJ (2017). Ideal Cardiovascular Health and Carotid Atherosclerosis in a Mixed Cohort of HIV-Infected and Uninfected Ugandans. AIDS Res Hum Retroviruses, 33(1), pp. 49–56. doi:10.1089/AID.2016.0104 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27476547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer D, & Pritchett LH (2001). Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography, 38(1), pp. 115–132. [DOI] [PubMed] [Google Scholar]
- Ganz ML (2000). The relationship between external threats and smoking in central Harlem. Am J Public Health, 90(3), pp. 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD. (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), pp. 1659–1724. doi:10.1016/S0140-6736(16)31679-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27733284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD. (2017). Global Burden of Disease Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancetdoi:10.1016/S0140-6736(17)30819-X Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28390697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, & Bukhman G (2015). Leveraging the lessons learned from HIV/AIDS for coordinated chronic care delivery in resource-poor settings. Healthc (Amst), 3(4), pp. 215–220. doi:10.1016/j.hjdsi.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Halpern MT, & Warner KE (1994). Differences in former smokers’ beliefs and health status following smoking cessation. Am J Prev Med, 10(1), pp. 31–37. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8172729 [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, . . . Obel N (2013). Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis, 56(5), pp. 727–734. doi:10.1093/cid/cis933 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23254417 [DOI] [PubMed] [Google Scholar]
- Helleberg M, May MT, Ingle SM, Dabis F, Reiss P, Fatkenheuer G, . . . Obel N (2015). Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS, 29(2), pp. 221–229. doi:10.1097/QAD.0000000000000540 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25426809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, & Hartwell TD (1997). Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control, 6 Suppl 2, pp. S57–62. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9583654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabwama SN, Ndyanabangi S, Mutungi G, Wesonga R, Bahendeka SK, & Guwatudde D (2016). Tobacco use and associated factors among Adults in Uganda: Findings from a nationwide survey. Tob Induc Dis, 14, p 27. doi:10.1186/s12971-016-0093-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27524959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse GR, Bangsberg DR, Hahn JA, Haberer JE, Hunt PW, Muzoora C, . . . Rigotti NA (2014). Tobacco use among adults initiating treatment for HIV infection in rural Uganda. AIDS Behav, 18(7), pp. 1381–1389. doi:10.1007/s10461-014-0737-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24638166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, & Kahende J (2007). Factors associated with successful smoking cessation in the United States, 2000. Am J Public Health, 97(8), pp. 1503–1509. doi:10.2105/AJPH.2005.083527 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17600268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Borland R, Yong HH, Fong GT, Bansal-Travers M, Quah AC, . . . Fotuhi O (2010). Predictors of smoking cessation among adult smokers in Malaysia and Thailand: findings from the International Tobacco Control Southeast Asia Survey. Nicotine Tob Res, 12 Suppl, pp. S34–44. doi:10.1093/ntr/ntq030 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20889478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie GM, & Ayo-Yusuf OA (2015). Predictors of tobacco smoking abstinence among tuberculosis patients in South Africa. J Behav Med, 38(3), pp. 472–482. doi:10.1007/s10865-015-9620-y Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25655663 [DOI] [PubMed] [Google Scholar]
- Louwagie GM, Okuyemi KS, & Ayo-Yusuf OA (2014). Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction, 109(11), pp. 1942–1952. doi:10.1111/add.12671 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24962451 [DOI] [PubMed] [Google Scholar]
- Mamary EM, Bahrs D, & Martinez S (2002). Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDS, 16(1), pp. 39–42. doi:10.1089/108729102753429389 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11839217 [DOI] [PubMed] [Google Scholar]
- Manne-Goehler J, Montana L, Gómez-Olivé FX, Rohr J, Harling G, Wagner RG, . . . Gaziano, T. A. (2017). The ART advantage: healthcare utilization for diabetes and hypertension in rural South Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes, Publish Ahead of Printdoi:10.1097/qai.0000000000001445 Retrieved from http://journals.lww.com/jaids/Fulltext/publishahead/The_ART_advantage___healthcare_utilization_for.96949.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, & Siddiqi K (2017). Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health, 5(6), pp. e578–e592. doi:10.1016/S2214-109X(17)30170-5 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28495263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, & Skarbinski J (2015). Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med, 162(5), pp. 335–344. doi:10.7326/M14-0954 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25732274 [DOI] [PubMed] [Google Scholar]
- Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, & Hilleman DE (2007). INtensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease*. Chest, 131(2), pp. 446–452. doi:10.1378/chest.06-1587 Retrieved from http://dx.doi.org/10.1378/chest.06-1587 [DOI] [PubMed] [Google Scholar]
- Mutevedzi PC, Rodger AJ, Kowal P, Nyirenda M, & Newell ML (2013). Decreased chronic morbidity but elevated HIV associated cytokine levels in HIV-infected older adults receiving HIV treatment: benefit of enhanced access to care? PLoS One, 8(10), p e77379. doi:10.1371/journal.pone.0077379 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24143226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, & Abrams DB (2000). Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis, 31(3), pp. 808–812. doi:10.1086/314048 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11017836 [DOI] [PubMed] [Google Scholar]
- North CM, Allen JG, Okello S, Sentongo R, Kakuhikire B, Ryan ET, . . . Siedner MJ. (2017). HIV Infection, Pulmonary Tuberculosis, and COPD in Rural Uganda: A Cross-Sectional Study. Lungdoi:10.1007/s00408-017-0080-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29260309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Rass O, & Johnson MW (2017). Positive smoking cessation-related interactions with HIV care providers increase the likelihood of interest in cessation among HIV-positive cigarette smokers. AIDS Care, 29(10), pp. 1309–1314. doi:10.1080/09540121.2017.1330532 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28535687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins JM, Nyakato VN, Kakuhikire B, Mbabazi PK, Perkins HW, Tsai AC, . . . Bangsberg DR (2017). Actual Versus Perceived HIV Testing Norms, and Personal HIV Testing Uptake: A Cross-Sectional, Population-Based Study in Rural Uganda. AIDS Behav doi:10.1007/s10461-017-1691-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoumenos K, Worm S, Reiss P, de Wit S, d’Arminio Monforte A, Sabin C, . . . Group DADS (2011). Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*). HIV Med, 12(7), pp. 412–421. doi:10.1111/j.1468-1293.2010.00901.x Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21251183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, & Diaz P (2011). Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc, 8(3), pp. 313–319. doi:10.1513/pats.201009-058WR Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21653534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon JM, Nerin I, Comino A, Pinet C, Abella F, Carreras JM, . . . Masuet-Aumatell C (2013). A multicentre randomized trial of combined individual and telephone counselling for smoking cessation. Prev Med, 57(3), pp. 183–188. doi:10.1016/j.ypmed.2013.05.014 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23732247 [DOI] [PubMed] [Google Scholar]
- Reddy KP, Kong CY, Hyle EP, Baggett TP, Huang M, Parker RA, . . . Walensky RP. (2017). Lung Cancer Mortality Associated With Smoking and Smoking Cessation Among People Living With HIV in the United States. JAMA Intern Med, 177(11), pp. 1613–1621. doi:10.1001/jamainternmed.2017.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA, . . . Walensky RP (2016). Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study. J Infect Dis, 214(11), pp. 1672–1681. doi:10.1093/infdis/jiw430 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27815384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti N, Munafo MR, & Stead LF (2007). Interventions for smoking cessation in hospitalised patients. The Cochrane Library; [DOI] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, . . . Gill MJ (2013). Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One, 8(12), p e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, & Tsai AC (2014). Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clinical Infectious Diseases, 60(7), pp. 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeramareddy CT, Pradhan PM, & Sin S (2014). Prevalence, distribution, and social determinants of tobacco use in 30 sub-Saharan African countries. BMC medicine, 12(1), p 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber J, Galea S, & Link BG (2008). Smoking and the emergence of a stigmatized social status. Social science & medicine, 67(3), pp. 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda National Policy Guidelines for HIV Counseling and Testing. (2005). http://library.health.go.ug/publications/leadership-and-governance-governance/policy-documents/uganda-national-policy-guidelin-0
- UNAIDS. (2017). Fact sheet—Latest statistics on the status of the AIDS epidemic.
- Vartiainen E, Seppala T, Lillsunde P, & Puska P (2002). Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health, 56(3), pp. 167–170. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11854334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2003). WHO Framework Convention on Tobacco Control. Retrieved Date Accessed, November 3, 2017 from http://apps.who.int/iris/bitstream/10665/42811/1/9241591013.pdf?ua=1.
- WHO. (2017). The STEPS Instrument and Support Materials. Retrieved Date Accessed, November 13, 2017 from http://www.who.int/chp/steps/riskfactor/en/.
- Wilkes S, & Evans A (1999). A cross-sectional study comparing the motivation for smoking cessation in apparently healthy patients who smoke to those who smoke and have ischaemic heart disease, hypertension or diabetes. Family practice, 16(6), pp. 608–610. [DOI] [PubMed] [Google Scholar]
- Yanovitzky I, & Stryker J (2001). Mass media, social norms, and health promotion efforts: A longitudinal study of media effects on youth binge drinking. Communication Research, 28(2), pp. 208–239. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


