Abstract
The development of a novel coagulation factor VIII (FVIII) expression cassette with an enhanced activity for gene therapy of hemophilia A (HA) is essential. The biological properties of several non-human FVIII sequences, such as porcine and canine, have been evaluated. Here, we compared the activity level of rat FVIII (rFVIII) and human FVIII (hFVIII) by using single-chain and dual-chain strategies in 293 T cells and the HA mice. In both in vitro and hydrodynamic injection studies, the activity of rFVIII detected by the activated partial thromboplastin time assay was higher than that of hFVIII both by single-chain (~2.96-fold and ~1.72-fold, respectively) and dual-chain (~7.69-fold and ~2.35-fold, respectively). Moreover, the dual chain exerted a potentially higher delivery efficacy compared with the single chain (~4.96-fold and ~2.99-fold, respectively). The blood loss of HA mice administrated with rFVIII was less than those with hFVIII. AAV-delivered rFVIII and hFVIII also exerted longterm therapeutic effects on HA mice and caused a transient ALT elevation. These data might help to the development of novel, optimized FVIII expression cassettes based on the amino acid difference between rFVIII and hFVIII. These data indicate that the dual-chain strategy would likely enhance the delivery efficiency of the AAV-mediated FVIII gene therapy.
Keywords: Hemophilia A, Rat factor VIII, Human factor VIII, Dual-chain strategy, Single-chain strategy
1. Introduction
Coagulation factor VIII (FVIII) is an essential plasma glycoprotein that plays a critical role in normal hemostasis [1]. Deficiencies or defects in FVIII are responsible for hemophilia A (HA), an X-linked, recessive bleeding disorder [2] that affects approximately 1 in 5000 males [3]. HA is characterized by spontaneous and prolonged bleeding in joints, muscles, and internal organs [2]. Currently, the most widely accepted treatment for this hereditary disease is the life-long administration of either plasma-derived or recombinant FVIII concentrates [4]. However, some disadvantages come with this treatment [5], including the high cost of FVIII replacement therapy, the inconvenience of frequent and repetitive intravenous infusion, the risk of pathogen transmission, and the development of neutralizing antibodies (inhibitors) in up to 20%–30% of severe patients [6].
Gene therapy has the therapeutic potential to correct FVIII deficiency in a long-term manner in HA [7–11], and as little as 2% of normal FVIII activity could lead to substantial improvement in bleeding episodes [12]. In a recent clinical trial, nine subjects with severe HA reportedly received treatment with adeno-associated viral (AAV) serotype 5 containing the codon-optimized B domain-deleted FVIII (FVIIIBDD) expression cassette at doses ranging from 6 × 1012 vg/kg to 6 × 1013 vg/kg, and six of seven participants who received a high dose achieved a sustained normalization of FVIII activity level over a period of one year [13].
Despite these remarkable approaches, a more efficient gene-based therapy for HA is still highly desired. One strategy for improving the therapeutic effects of HA is to develop a high-activity FVIII, which may lead to a better coagulation effect and the reduced risks that arise from high-titer viral vectors [14–16]. Codon-optimized FVIII cDNA [17,18] and some variants of FVIII [19–23] have exhibited an enhanced FVIII activity. FVIII proteins from other species (such as porcine and canine) have been shown to be more efficient in expression or secretion than those from human. Porcine FVIII (pFVIII) was shown to secrete more efficiently than human FVIII (hFVIII) by 10- to 100-fold [24,25]. In 2009, studies revealed that B domain-deleted canine factor VIII (cFVIII-BDD) exhibits a higher activity than hFVIII-BDD by 3-fold and a more stability than activated hFVIII-BDD [26]. Furthermore, researchers evaluated the biological differences of canine and human FVIII in AAV-mediated gene delivery and found that the secretion of canine HC was 5- to 30-fold higher than that of hHC [27]. However, activity level comparison of hFVIII and rat FVIII (rFVIII) has not yet been evaluated.
The insufficient expression of transgenic FVIII [12] owing to the AAV packaging restriction [28] is another challenge for HA gene therapy. The AAV vector is one of the most promising vehicles for gene delivery because of the safety and efficiency of its use. However, AAV-mediated gene therapy for HA has been hampered by the oversize of the FVIII cDNA [28]. In contrast to the 1.5 kb of FIX cDNA, the full size of FVIII is 7.0 kb. Even the B domain-deleted (BDD) FVIII (~4.3 kb) alone is close to the ~4.7 kb limit for the packaging capacity of AAV, which leaves little space for regulatory elements such as promoters, poly A sequences, and introns [28]. Splitting the FVIII coding sequences into two separate AAV vectors could at least partially circumvent the packaging limitation of AAV [27,29–32]. However, the main disadvantage of this strategy is chain imbalance, which means that HC secretion is inefficient, LC secretion is redundant, and the redundantly synthesized LC that is not in association with HC induces host cell destabilization and apoptosis [33]. Up to now, the evaluation of FVIII delivery efficacy by single chains and dual chains has not been intensively explored.
In this study, we compared and evaluated the activity levels of rat and human FVIIIs in transfected cells and in HA mice and examined the efficiency of AAV delivery by single-chain strategy and dual-chain strategy. In addition, the long-term therapeutic effects of rFVIII and hFVIII delivered by AAV in HA mice in vivo were observed.
2. Materials and methods
2.1. FVIII expression plasmids
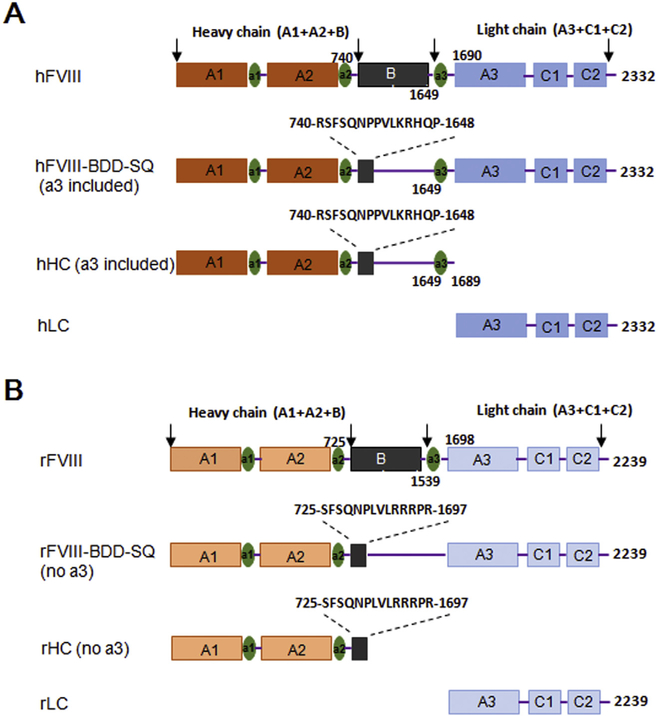
Expression cassettes of hFVIII-BDD-SQ, rFVIII-BDD-SQ, human FVIII heavy chain (hHC), human FVIII light chain (hLC), rat FVIII heavy chain (rHC), and rat FVIII light chain (rLC) were constructed into AAV expression vectors with a CB promoter (CMV enhancer with human β-actin promoter, ubiquitous expression). Furthermore, hHC, hLC, rHC, and rLC were cloned into AAV vectors containing ApoE-hAAT promoter (liver-specific apolipoprotein E enhancer and the human alpha1-antitrypsin promoter) for AAV virus production. The rFVIII (rHC and rFVIII-BDD-SQ) sequences lacked acidic-region-3 (a3), and the hFVIII (hHC and hFVIII-BDD-SQ) sequences included a3 in construct (Fig. 1). The AAV expression vectors containing CB and the ApoE-hAAT promoter were gifts from Prof. Weidong Xiao, the hFVIII sequence was from Prof. Guowei Zhang, and the rHC and rLC expression cassettes were synthesized by BioSune Biotechnology Co. Ltd. (Shanghai, China) according to the reference sequence from NCBI (NM_183331.1). For the in vitro and hydrodynamic injection experiments, we placed the FVIII cassettes under the control of a CB promoter. We employed the ApoE-hAAT promoter for AAV virus production.
Fig. 1.
Schematic representation of the FVIII constructs. (A) Schematics of human FVIII (hFVIII), B domain-deleted single chain human FVIII (hFVIII-BDD-SQ), hFVIII heavy chain (hHC), and hFVIII light chain (hLC). (B) Schematics of rat FVIII (rFVIII), B domain-deleted single chain rat FVIII (rFVIII-BDD-SQ), rFVIII heavy chain (rHC), and rFVIII light chain (rLC).
2.2. 293 T cells culture and transfection
293 T cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS, Moregate, Australia and New Zealand), penicillin (100 U/mL), and streptomycin (Invitrogen, Carlsbad, CA) (100 μg/mL) at 37 °C in a moisturized environment supplied with 5% CO2. Transfection was performed using a Polyjet kit (Signagen, Rockville, MD) following the manufacturer’s instructions. After transfection, the cells were grown for 8–12 h in DMEM medium with 10% FBS to minimize cell death. Then, the medium was replaced with F10 medium (HyClone, Logan, UT) with 2% inactivated FBS for another 48 h before the medium was collected. The activity level of the secreted FVIII in medium was measured by using the activated partial thromboplastin time (aPTT) assay.
2.3. rAAV vector preparation
The AAV serotype 8 (AAV8) was applied to mediate FVIII delivery to mouse livers. Each of the rAAV expression vectors (hHC, hLC, rHC, and rLC with ApoE-hAAT promoter), an AAV8 helper plasmid, and an adenovirus helper plasmid were co-transfected into 293 T cells at a ratio of 1:1:1 to create the AAV package. After three days of transfection, the cells were harvested. The AAV virus was purified by two rounds in a cesium chloride-gradient ultracentrifuge [30,34]. Then, buffer exchange was performed by using phosphate-buffered saline with 5% D-sorbitol. After that, the purified viruses were pooled and stored at −80 °C.
2.4. Animal procedures
Mice bearing a S129 genetic background were used in this study. HA mice with exons 16–19 of the mouse FVIII gene-targeted knocked out were obtained from the Shanghai Research Center for Model Organisms. All mice were housed in a specific pathogen-free environment (21 ± 1 °C) with humidity (55% ± 10%) and a 12-h light/12-h dark cycle with free access to water and chow diet. All experimental procedures were approved by the Studies Ethics Committee of Ruijin Hospital affiliated with the Shanghai Jiao Tong University School of Medicine. All animals were handled in accordance with the Institutional Animal Care and Use Committee guidelines.
The six- to eight-week old mice were pre-warmed with light to let the tail vein fill well and were hydrodynamically injected via the tail vein in 7–8 s with 2.0 mL saline containing 150 μg column-purified plasmids (including FVIII single chain or HC and LC mixtures at three ratios of 1:1, 2:1, and 4:1). The mice were allowed to recover from the injection stress before being returned to the cages. Blood was collected at 48 h after hydrodynamic injection by eye bleeding and anti-coagulated using sodium citrate at a final concentration of 0.38% (wt/vol). The blood samples were then centrifuged at 3000 rpm for 15 min at 4 °C. The plasmas were then collected and used for FVIII activity detection. To evaluate blood loss, we clipped the mouse tail at 1.5 mm diameter. Six hours after tail clipping, the blood of the mice was collected by eye bleeding and anti-coagulated using sodium citrate. Hemoglobin (Hb) concentration was measured using the blood routine examination.
For AAV virus transduction, six- to eight-week old HA mice were injected with 200 μl AAV virus containing FVIII diluted in saline. The blood was collected by eye bleeding every two weeks post virus administration. The plasmas were then collected and stored in −80 °C until FVIII activity detection.
2.5. Quantitative analysis of FVIII activity
The FVIII activity in medium and plasma was measured by using the aPTT assay following the manufacturer’s instructions. The FVIII-Deficient plasma and STA-PTT were purchased from STAGO (Diagnostica Stago S.A.S., France). Pooled normal human plasma (NHP) was used as the standard. The FVIII activity in HA mice was calculated by using normal mouse plasma as the standard and labeled as the activity relative to normal mouse FVIII activity.
2.6. Thromboelastography (TEG) assay
Blood was collected at 54 h after hydrodynamically injecting the mice by eye bleeding and anti-coagulating the blood using sodium citrate at a final concentration of 0.38% (wt/vol). The samples for the TEG assay had to be tested within 2 h. The kaolin-activated TEG reagents and cuvettes were purchased from Haemonetics (Heamoscope Corporation, Niles, USA), and the TEG assay was performed in a TEG 5000 device according to the manufacturer’s instructions. Briefly, 400 μl of citrated blood was added to the designated kaolin vial and mixed gently. Then, a 340 μl aliquot was transferred from each kaolin vial to a 37 °C pre-warmed cuvette preloaded with 20 μl CaCl2 (0.2 mol/l) [35]. The parameter of “reaction time” represents clot formation time.
2.7. ELISA for alanine aminotransferase (ALT)
An enzyme-linked immunosorbent assay (ELISA) for mouse alanine aminotransferase (ALT) was conducted with the mouse ALT ELISA Kit (Suer, Shanghai, China).
2.8. Statistical analysis
One-tailed Student’s t-test and one-way ANOVA with Bonferroni multiple comparison post-test were used in this study. Analysis was performed in GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Results are expressed as mean ± S.E. A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. The activity level of rFVIII was higher than that of hFVIII in transfected 293 T cells
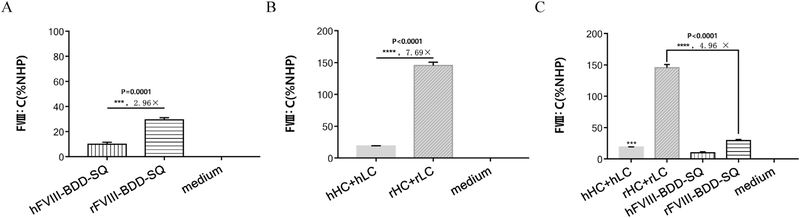
Primarily, the coagulation activity of rFVIII and hFVIII delivered by single chain and dual-chain strategies were evaluated. The expression plasmids of hFVIII-BDD-SQ and rFVIII-BDD-SQ (Fig. 1) were transfected into 293 T cells, and the FVIII activity level was detected at 48 h after transfection. The activity of rFVIII in single chain was higher than that of hFVIII by about 2.96-fold (Fig. 2A, S1A). The plasmids encoding hHC, hLC, rHC, and rLC (Fig. 1) were paired with a ratio of HCs:LCs at 1:1 and co-transfected into 293 T cells. The coagulation activity of rHC + rLC was higher than that of hHC + hLC by about 7.69-fold (Fig. 2B, S1B). In addition, we compared the delivery efficiency of rFVIII and hFVIII by using the single chain and dual-chain strategies. Results showed that the activity of rFVIII and hFVIII delivered by dual chain was higher than that by single chain. The activity of rHC + rLC was higher than that of rFVIII-BDD-SQ by about 4.96-fold (p < 0.0001) (Fig. 2C, S1C). The activity level of hHC + hLC was also greater than that of hFVIII-BDD-SQ (p = 0.0001) (Fig. 2C, S1C). These data indicate that the activity level of rFVIII is higher than that of hFVIII either by single chain or by dual chain modes, and that the coagulation activity level of rFVIII and hFVIII delivered by dual chain is higher than that by single chain.
Fig. 2.
Comparison the activity of rat FVIII (rFVIII) and human FVIII (hFVIII). (A) Expression plasmids of hFVIII-BDD-SQ and rFVIII-BDD-SQ were transfected into 293 T cells by Polyjet, and the FVIII activity was detected 48 h post-transfection by the activated partial thromboplastin time (aPTT) assay (n = 3, ***p =0.0001). (B) Expression plasmids hHC, hLC, rHC, and rLC were co-transfected into 293 T cells with combinations of hHC + hLC and rHC +rLC with the ratio of HC:LC at 1:1. FVIII activity was detected 48 h post-transfection by the aPTT assay (n = 3, ****p < 0.0001). The serial-diluted pooled normal human plasma (NHP) was employed as the standard. (C) Comparison of the activities of rFVIII and hFVIII by single-chain and dual-chain strategy (n =3, ***p =0.0001, ****p < 0.0001).
3.2. Chain imbalance in the dual-chain strategy
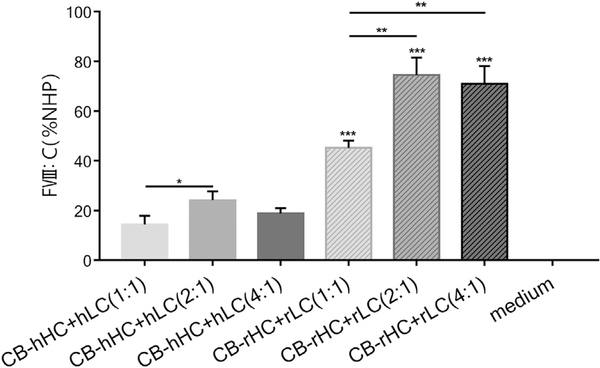
Since the FVIII LC is secreted more efficiently than the HC, the chain imbalance in the dual-chain strategy attracted great attention [33]. To solve the problem, we tried to enhance the ratio of HCs in the mixture of HCs and LCs. The total amount of AAV plasmids were kept constant (1 μg/well), but the ratio of HCs to LCs was set at 1:1, 2:1, and 4:1. The plasmids were co-transfected into 293 T cells with combinations of hHC + hLC and rHC + rLC, and the FVIII activity was measured by aPTT assay 48 h after transfection. Results indicated that the activity level of rHC + rLC was significantly higher than that of the corresponding hHC + hLC for all three ratios (p < 0.001, Fig. 3, Fig. S2), which was in accordance with the results from Fig. 2. Furthermore, among the three ratios in this system, the activity level of rHC + rLC at the ratios 2:1 and 4:1 were significantly higher than that of 1:1 (p < 0.01, Fig. 3, Fig. S2). rHC + rLC at the ratio of 2:1 exhibited the highest rFVIII activity level, which was greatly higher than that of 1:1 (p < 0.05) and slightly higher than that of 4:1 (no significant difference, p > 0.05). Similar results from different ratios were also observed in hHC + hLC combination. The FVIII activity level of the HC and LC combination at three ratios from two different species both reached the peak value at the ratio of 2:1 (HC:LC). This result implies that the chain imbalance should be paid attention to since the ratios of HC and LC are related to delivery efficiency. In the present study, the ratios 2:1 and 4:1 were better than 1:1.
Fig. 3.
Measurement of FVIII activity levels of the three ratios of the HC and LC combination in transfected 293 T cells. The expression plasmids driven by the CB promoter were co-transfected into 293 T cells with combinations of hHC + hLC and rHC + rLC by dual-chain strategy. The ratios of HC:LC were 1:1, 2:1, and 4:1. Then, FVIII activity was detected 48 h post-transfection by the aPTT assay. NHP was used as the standard (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001).
3.3. rFVIII activity level was higher than that of hFVIII in hydrodynamically injected HA mice
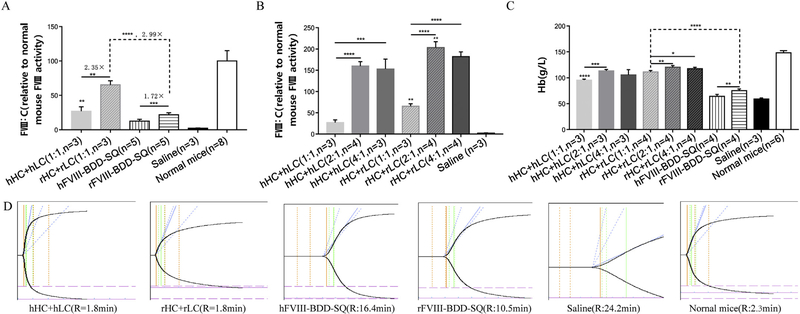
To determine whether or not the activity level of rFVIII was higher than that of hFVIII in vivo, we hydrodynamically injected the plasmids containing the CB promoter and FVIII in single chain (FVIII-BDD-SQ) and dual chain (HC + LC) into HA mice. FVIII activity was measured by aPTT assay, and pooled NHP was used as the standard. The FVIII activity in the plasma of hydrodynamically injected HA mice was normalized to the FVIII activity of normal mice (referred to as 100%) (Fig. 4A, S3A). The results indicated that the activity level of rFVIII was greater than that of hFVIII (p < 0.01) in both single chain (~1.72-fold) and dual chain (~2.35-fold) (Fig. 4A, S3A). Furthermore, with regard to the two delivery strategies, the activity level of rFVIII in the dual-chain group (rHC + rLC) was significantly higher than that in the single-chain group (rFVIII-BDD-SQ) by approximately 2.99-fold (p < 0.0001). A similar trend where the dual chain-mediated FVIII activity level was higher than that of the single chain was also observed in hFVIII groups (p < 0.01). These results were quite coincident with those from the in vitro transfection of 293 T cells.
Fig. 4.
Measurement of FVIII (rFVIII and hFVIII) activity levels in hydrodynamically injected HA mice. The expression plasmids of rFVIII and hFVIII were hydrodynamically injected into HA mice by single-chain and dual-chain strategy. A total of 150 μg plasmids in 2 mL of saline was delivered into each HA mouse. Plasma was collected 48 h after injection. (A) The FVIII activity levels from the single chain and dual chain were detected by aPTT (**p < 0.01, ***p < 0.001, ****p < 0.0001). (B) The FVIII activity level from the three ratios of the HC and LC combination were detected by aPTT (****p < 0.0001). (C) Measurement of the hemostatic activity of FVIII by detecting the amount of blood loss based on the remaining hemoglobin (Hb) concentration in the blood routine examination 6 h after tail clipping. *p < 0,05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (D) TEG data from one representative mouse in each group. FVIII:C (relative to normal mouse FVIII activity) is the activity of hydrodynamically injected HA mice calibrated by the activity of WT mice. R value represents the integrative action of coagulation factors.
We also detected chain imbalance in hydrodynamically injected HA mice by using the three ratios of 1:1, 2:1, and 4:1 of the HC and LC combination with a constant total amount of plasmids (150 μg) in each group. HC and LC plasmids with CB promoters were applied. In both rHC + rLC and hHC + hLC groups, the similar results displayed that the activity level at the ratios 2:1 and 4:1 were greatly higher than that of 1:1 (p < 0.001, Fig. 4B, S3B). The highest coagulation activity level of rHC + rLC and hHC + hLC was detected at the ratio 2:1, which was significantly higher than that of the ratio 1:1 (p < 0.0001) (Fig. 4B, S3B). The activity levels of rHC + rLC and hHC + hLC at 4:1 were still greatly higher than that of 1:1 (p < 0.0001 and p < 0.001, respectively) (Fig. 4B, S3B). The data from the hydrodynamic injection confirmed the fact that chain imbalance indeed existed in HA mice in vivo and that the optimal ratios were seemingly 2:1 and 4:1, which was consistent with the in vitro results.
In addition, we estimated the hemostatic activity of FVIII by detecting the amount of blood loss based on the remaining Hb concentration in the blood routine examination at 6 h after tail clipping. Three mice were used in each group. As shown in Fig. 4C, the remaining Hb of HA mice that received the hydrodynamic injection of rFVIII-BDD-SQ (75 ± 3.65 g/l, n = 4) was significantly higher than that of hFVIII-BDD-SQ (64 ± 4.16 g/l, n = 4) (p < 0.01) (Fig. 4C). Similarly, the remaining Hb of HA mice administrated rHC + rLC (1:1) (111.3 ± 2.63 g/L, n = 4) was higher than those administrated hHC + hLC (1:1) (95.33 ± 2.08 g/L, n = 3) (p < 0.001). Furthermore, we compared the remaining Hb concentrations by dual chainwith that by single chain-delivered FVIII and found that the Hb concentrations delivered by rHC + rLC (1:1) and hHC + hLC (1:1) were significantly higher than that corresponding by rFVIII-BDD-SQ and hFVIII-BDD-SQ (p < 0.0001) (Fig. 4C). The results of the chain imbalance in the rHC + rLC group revealed that the Hb concentration at the ratios 2:1 (120.3 ± 3.5 g/l, n = 4) and 4:1 (117.5 ± 2.64 g/l, n = 4) were higher than that of 1:1 (111.3 ± 2.63 g/L, n = 4) (p < 0.01 and p < 0.05, Fig. 4C). Similar results were observed in the different ratios of the hHC + hLC group. hHC + hLC at the ratio of 2:1 exhibited the highest Hb concentration, which was greatly higher than that of 1:1 (p < 0.001) and slightly higher than that of 4:1 (no significant difference). The data showed that the ratio 2:1 exerted the highest Hb concentration both in hHC + hLC (113.3 ± 2.52 g/L, n = 3) and in rHC + rLC (120.3 ± 3.5 g/L, n = 4), which was greatly higher than that of the ratio 1:1 (p < 0.001 and p < 0.01) (Fig. 4C) and slightly higher than that of the ratio 4:1. And the two highest remaining Hb concentration from rHC + rLC (2:1) (120.3 ± 3.5 g/L, n = 4) and hHC + hLC (2:1) (113.3 ± 2.52 g/L, n = 3) were near that from the normal mice (148 ± 4.29 g/L, n = 6) (Fig. 4C), which indicates that the dual chain-delivered FVIII could almost correct the bleeding phenotype of HA mice.
To further evaluate the coagulation activity of the FVIII in clot formation, the TEG assay was performed. The R value represents the clotting time from the beginning to when the waveform reaches 2 mm above baseline and was employed to assess the procoagulation activity of FVIII in each hydrodynamically injected HA mouse. In the single-chain groups, the R value of the rFVIII group (10.5 min) was shorter than that of the hFVIII group (16.4 min), which suggests that the coagulation activity of rFVIII is higher than that of hFVIII. In the dual-chain groups, the R values were both 1.8 min in the rHC + rLC group and the hHC + hLC group, respectively. These results were slightly inconsistent with that of the single-chain groups since hypercoagulability was observed in the two groups, and the R value was too short to discern the exact difference. Meanwhile, the coagulation activity of the FVIII from the dual-chain strategy surpassed that from the single-chain strategy (Fig. 4C). The saline control was 24.2 min, scarcely exhibiting coagulation activity. The normal mouse control was 2.3 min, exhibiting full coagulation activity. The results from the TEG assay were basically consistent with that from the aPTT assay, which demonstrates that the activity of rFVIII is higher than that of hFVIII either by single-chain strategy or dual-chain strategy, and that the dual-chain strategy is potentially more efficient than the single-chain strategy in delivering FVIII in vivo.
3.4. AAV-delivered rFVIII and hFVIII had long-term therapeutic effects in HA mice in vivo
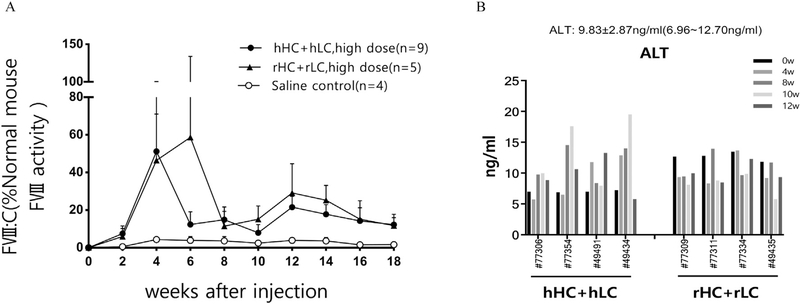
Although the rFVIII exhibited a higher activity level than hFVIII in vitro or in hydrodynamically injected HA mice, the in vivo effects needed to be evaluated. AAV viruses with ApoE-hAAT-controlled hHC, hLC, rHC, and rLC were packaged through triple plasmid co-transfection and purified by two rounds in a cesium chloride gradient ultracentrifuge. The viruses containing HC and LC were packaged and purified separately and mixed at a ratio of 1:1 for infusion. The AAV viruses were injected into HA mice through the tail vein. Results revealed that AAV-delivered FVIII (rHC + rLC and hHC + hLC) in a dual-chain strategy administered at a high dose (2 × 1013 vg/kg) exerted long-term therapeutic effects in HA mice for up to 18 weeks (Fig. 5A, Table S1). In general, AAV-delivered rHC + rLC exhibited equal or slightly higher FVIII activity level than those treated with the hHC + hLC from 4 to 16 weeks after treatment (Fig. 5A, Table S1). In addition, we noticed that the expression level of FVIII fell almost to baseline and then increase with time. To understand the possible causes, the mouse plasma ALT was measured. A mild, transient elevation of ALT level was exhibited in the mice who received the high-dose AAV virus injection at about 8th week (Fig. 5B), this indicates a temporary damage of the function of mouse liver. When the ALT level decreased gradually, the level of FVIII increased accordingly. These data demonstrate that AAV-delivered FVIII in a dual-chain strategy could have long-term therapeutic effects on HA mice in vivo after a one-time injection of the AAV virus, and rFVIII could also exert a relatively higher activity level than hFVIII.
Fig. 5.
Long-term therapeutic effects in HA mice injected with AAV-delivered FVIII in vivo. AAV-delivered hHC + hLC and rHC + rLC exert long-term therapeutic effects on HA mice. AAV viruses carrying hHC + hLC and rHC + rLC were injected into HA mice through the tail vein at a high dose (2 × 1013 vg/kg) in 200μl. (A) Mouse blood was collected every two weeks, and FVIII activity was measured by using aPTT assays from 0 to 18 weeks in all mice. (B) The examination of mouse alanine aminotransferase (ALT). The ALT level calculated according to the data from the 8 mice injected by AAV-delivered hHC +hLC and rHC + rLC at 0 week was used as normal control in this study.
3.5. Incorporation of the rFVIII sequences altered the hFVIII activity
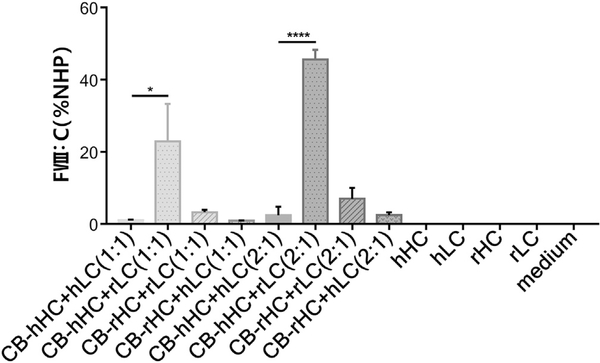
Since the acquired coagulation activity level of rFVIII is greater than that of hFVIII in both in vitro and in vivo settings, we sought to screen the sequence of rFVIII which may potentially enhance the activity of hFVIII. Based on the hypothesis that the rHC or rLC could play roles in increasing the activity level of hFVIII, we replaced the hHC and hLC with the corresponding rHC and rLC, respectively. The coagulation activity of rat and human hybrid-FVIII was measured. Results showed that coagulation activity of hHC + rLC was higher than that of hHC + hLC at the comparable expression level regardless of the ratio of HC to LC (n = 3, p < 0.05 and p < 0.0001, respectively) (Fig. 6). Data indicated that the sequences of rFVIII (rLC) combined with hFVIII (hHC) indeed enhanced the activity level of hFVIII.
Fig. 6.
The substitution of the rFVIII sequences in hFVIII led to an increased coagulation activity. The expression plasmids of HC (CB-hHC, CB-rHC) were co-transfected into 293 T cells with LC (CB-hLC, CB-rLC). The total amount of the plasmids of each group was 1 μg and the ratios of HC:LC were 1:1 and 2:1. The activity of FVIII (hHC +hLC, hHC +rLC, rHC +hLC, rHC +rLC) was detected at 48 h post-transfection by the aPTT assay. NHP (normal human plasms) was used as the standard (n =3, *p < 0.05, ****p < 0.0001).
4. Discussion
The most common treatment for HA is the frequent intravenous infusion of FVIII (from plasma or recombinant protein), which is expensive and inconvenient [36,37]. Most hemophilia patients worldwide still cannot access this treatment [4]. By contrast, gene therapy is expected to cure HA by maintaining a continuous expression of FVIII following delivery of a therapeutic gene into hemophilic patients [38]. HA might be considered a “low-hanging fruit” for gene therapy because a very small increment of FVIII levels (> 2% of normal) in plasma could significantly improve bleeding tendency from severe to moderate, eliminating most spontaneous bleeds [12]. The relatively higher activity of FVIII and the lower dose of AAV vectors are supposed to increase therapeutic effects with the reduced immune response to either AAV capsids or FVIII.
Previous studies revealed that FVIII from other species, as therapeutic molecules, could correct the bleeding phenotype of HA mice and potentially exert higher activity than hFVIII [24–26,39–41]. pFVIII is secreted 10- to 100-fold more efficiently than hFVIII [24], and the A1 domain of the HC and the A3 domain of the LC of the pFVIII are responsible for the enhanced secretion of protein [25]. Zhu et al. [42] demonstrated that hybrid human/porcine FVIII (hp-FVIII) confers a high expression level via enhanced secretion. Likewise, cFVIII also displays a 3- to 7-fold higher activity than hFVIII [27]. Wang et al. [27] evaluated the different biological properties of canine FVIII heavy chain (cHC) and light chain (cLC) by comparing them to hHC and hLC and demonstrated that cHC + hLC exhibited an approximately 18-fold increase in coagulation activity than hHC + hLC delivery by AAV vectors; furthermore, cLC improved the specific activity of FVIII by 2- to 3-fold compared with hLC. Our results indicate that the activity level of rFVIII is higher than that of hFVIII both in single-chain strategy (~2.96-fold) and in dual-chain strategy (~7.69-fold). Notably, the rFVIII (rHC and rFVIII-BDD-SQ) sequence lacked a3, unlike the hFVIII (hHC and hFVIII-BDD-SQ) sequence in our constructs (Fig. 1). Previous studies demonstrated that a3-included HCHL (HC1690) exhibits higher coagulation activity compared to HC745 (a3 not included), pointing to the fact that a3 has the potential to enhance the activity of FVIII [43,44]. In the present study, we found that the activity level of rFVIII lacking a3 was still greater than that of hFVIII with the a3 (Figs. 2, 3, 4, and 5), which implies that rFVIII with a3 might exert further higher activity level than hFVIII with a3.
The delivery efficiencies of single chain and dual chain AAV vectors are still short of systemic research up to now. In this study, we demonstrated that the dual-chain strategy was potentially more efficient in delivering FVIII than the single-chain strategy both in vitro and in vivo. Since the size of B domain-deleted FVIII (FVIII-BDD) was quite close to the AAV package capacity limitation, FVIII-BDD delivery in single-chain strategy maintained a relatively low package efficacy. This result is ascribed from the use of a short liver-specific promoter (but not the larger promoter) and the lack of regulatory elements like enhancers that are necessary for more efficient FVIII expression. Even though several groups took efforts to achieve the fairly high titers of packaging of > 5 kb of DNA, the high proportion of empty capsids due to the large size suggests inefficient packaging [45]. For the dual-chain strategy, the small sizes of HC and LC were sufficient to accommodate the AAV vector, making the package of AAV vectors more efficient and enabling the inclusion of large liver-specific promoters and regulatory elements. Coincidentally that, a study found that the liver bio-distribution of AAV8-mediated transgenic FVIII of dual chain (42.20 copies/cell) was higher than that of single chain (2.70 copies/cell) four months after injection by the tail vein at a dose of 1 × 1011 gc/vector/mouse [45], suggesting that the dual-chain strategy is likely to superior to the single-chain strategy in reaching a high gene copy number in the liver [45].
On the selection of the AAV serotype, several studies demonstrated that AAV8 exerts relatively higher delivery efficiency to the liver than other serotypes [46]. Thus, we chose to use AAV8 in the present study. Moreover, the dual-vector approach has been successfully extended to the AAV8 serotype [30]. The chain imbalance issue of the dual-chain strategy attracts attention [47]. To elevate the secretion of HC, we increased the ratio of the HC plasmids in the mixture of HC and LC. In the present study, we explored several ratios of HC:LC to compare FVIII expression efficiency. In transfected 293 T cells (Fig. 3) and in hydrodynamically injected HA mice (Fig. 4), we observed that the ratios 2:1 and 4:1 (HC:LC) exhibited potentially higher activity of FVIII compared with 1:1, suggesting that increasing the ratio of HC could improve the efficiency of the dual-chain strategy. This result was basically consistent with another study that showed that 4:1 (HC:LC) was the better choice for FVIII expression by dual-chain strategy [27].
AAV-delivered FVIII (rHC + rLC and hHC + hLC) exerted long-term therapeutic effects in HA mice for up to 18 weeks (Fig. 5A, Table S1). However, the FVIII activity almost fall to the baseline at about 8th week and then increased with time. In fact, previous studies also showed the similar phenomenon [13,48,49]. The fall of the expression level of FIX or FVIII was attributed to a transient elevation of the level of alanine aminotransferase (ALT), indicating a temporary damage of liver cells. When the ALT level decreased gradually, the expression level of FIX or FVIII increased accordingly [13,48,49]. In this study, a mild, transient ALT elevation was also observed in the mice at about 8 to 10 weeks, followed with a gradual decrease of ALT (Fig. 5B). These data could thus explain why the FVIII level falls almost to baseline and then increases with time. Our results are basically consistent with the previously published data [13,48,49].
In summary, the present data demonstrates that the coagulation activity level of rFVIII is greater than that of hFVIII in vitro and in vivo, providing clues for developing optimized FVIII expression cassettes based on the amino acid difference between rat and human FVIII. In addition, we also revealed that the dual-chain strategy is potentially more efficient than the single-chain strategy in delivering an oversized gene such as the FVIII, suggesting that the dual-chain strategy will likely become another promising approach for AAV-mediated FVIII gene therapy of HA in the future.
Supplementary Material
Acknowledgements
We thank all the laboratory members for the helpful discussion. This study was financially supported by the National Key Basic Research Program of China (2013CB966800), National Natural Science Foundation of China (81670127, 81101721, 81123005, 81170531), and grants from the Science and Technology Commission of Shanghai Municipality (16PJ1406100, 16ZR1421000), the Novo Nordisk Hemophilia Research Fund in China, and Zhejiang Provincial Natural Science Foundation of China (LY17H080004).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bcmd.2018.09.004.
Compliance with ethics guidelines
The authors declare that no conflict of interest exists. All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- [1].Mazurkiewicz-Pisarek A, Plucienniczak G, Ciach T, Plucienniczak A, The factor VIII protein and its function, Acta Biochim. Pol 63 (2016) 11–16. [DOI] [PubMed] [Google Scholar]
- [2].Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, Ludlam CA, Mahlangu JN, Mulder K, Poon MC, Street A, Guidelines for the management of hemophilia, Haemophilia 19 (2013) e1–47. [DOI] [PubMed] [Google Scholar]
- [3].Mannucci PM, Tuddenham EG, The hemophilias–from royal genes to gene therapy, N. Engl. J. Med 344 (2001) 1773–1779. [DOI] [PubMed] [Google Scholar]
- [4].Peyvandi F, Garagiola I, Young G, The past and future of haemophilia: diagnosis, treatments, and its complications, Lancet 388 (2016) 187–197. [DOI] [PubMed] [Google Scholar]
- [5].Chuah MK, Collen D, Vandendriessche T, Preclinical and clinical gene therapy for haemophilia, Haemophilia 10 (Suppl. 4) (2004) 119–125. [DOI] [PubMed] [Google Scholar]
- [6].Lai JD, Lillicrap D, Factor VIII inhibitors: advances in basic and translational science, Int. J. Lab. Hematol. 39 (Suppl. 1) (2017) 6–13. [DOI] [PubMed] [Google Scholar]
- [7].Chuah MK, Collen D, VandendDriessche T, Gene therapy for hemophilia, J. Gene Med 3 (2001) 3–20. [DOI] [PubMed] [Google Scholar]
- [8].Sarkar R, Xiao W, Kazazian HH Jr., A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype, J. Thromb. Haemost 1 (2003) 220–226. [DOI] [PubMed] [Google Scholar]
- [9].Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, Kazazian HH Jr., Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice, Hum. Gene Ther 17 (2006) 427–439. [DOI] [PubMed] [Google Scholar]
- [10].Connelly S, Mount J, Mauser A, Gardner JM, Kaleko M, McClelland A, Lothrop CD Jr., Complete short-term correction of canine hemophilia A by in vivo gene therapy, Blood 88 (1996) 3846–3853. [PubMed] [Google Scholar]
- [11].VandendDriessche T, Vanslembrouck V, Goovaerts I, Zwinnen H, Vanderhaeghen ML, Collen D, Chuah MK, Long-term expression of human coagulation factor VIII and correction of hemophilia A after in vivo retroviral gene transfer in factor VIII-deficient mice, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 10379–10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nathwani AC, Davidoff AM, Tuddenham EGD, Gene therapy for hemophilia, Hematol. Oncol. Clin. North Am 31 (2017) 853–868. [DOI] [PubMed] [Google Scholar]
- [13].Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, Yu H, Vettermann C, Pierce GF, Wong WY, Pasi KJ, AAV5-factor VIII gene transfer in severe hemophilia A, N. Engl. J. Med 377 (2017) 2519–2530. [DOI] [PubMed] [Google Scholar]
- [14].Spencer HT, Riley BE, Doering CB, State of the art: gene therapy of haemophilia, Haemophilia 22 (Suppl. 5) (2016) 66–71. [DOI] [PubMed] [Google Scholar]
- [15].Lheriteau E, Davidoff AM, Nathwani AC, Haemophilia gene therapy: Progress and challenges, Blood Rev. 29 (2015) 321–328. [DOI] [PubMed] [Google Scholar]
- [16].Nienhuis AW, Nathwani AC, Davidoff AM, Gene therapy for hemophilia, Mol. Ther 25 (2017) 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ward NJ, Buckley SM, Waddington SN, Vandendriessche T, Chuah MK, Nathwani AC, McIntosh J, Tuddenham EG, Kinnon C, Thrasher AJ, McVey JH, Codon optimization of human factor VIII cDNAs leads to high-level expression, Blood 117 (2011) 798–807. [DOI] [PubMed] [Google Scholar]
- [18].McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, Patel N, Tuddenham EG, Christophe OD, McVey JH, Waddington S, Nienhuis AW, Gray JT, Fagone P, Mingozzi F, Zhou SZ, High KA, Cancio M, Ng CY, Zhou J, Morton CL, Davidoff AM, Nathwani AC, Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant, Blood 121 (2013) 3335–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Radtke KP, Griffin JH, Riceberg J, Gale AJ, Disulfide bond-stabilized factor VIII has prolonged factor VIIIa activity and improved potency in whole blood clotting assays, J. Thromb. Haemost 5 (2007) 102–108. [DOI] [PubMed] [Google Scholar]
- [20].Gale AJ, Radtke KP, Cunningham MA, Chamberlain D, Pellequer JL, Griffin JH, Intrinsic stability and functional properties of disulfide bond-stabilized coagulation factor VIIIa variants, J. Thromb. Haemost 4 (2006) 1315–1322. [DOI] [PubMed] [Google Scholar]
- [21].Nguyen GN, George LA, Siner JI, Davidson RJ, Zander CB, Zheng XL, Arruda VR, Camire RM, Sabatino DE, Novel factor VIII variants with a modified furin cleavage site improve the efficacy of gene therapy for hemophilia A, J. Thromb. Haemost 15 (2017) 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pipe SW, Kaufman RJ, Characterization of a genetically engineered inactivationresistant coagulation factor VIIIa, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 11851–11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siner JI, Iacobelli NP, Sabatino DE, Ivanciu L, Zhou S, Poncz M, Camire RM, Arruda VR, Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype, Blood 121 (2013) 4396–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P, High level expression of recombinant porcine coagulation factor VIII, J. Biol. Chem 277 (2002) 38345–38349. [DOI] [PubMed] [Google Scholar]
- [25].Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P, Identification of porcine coagulation factor VIII domains responsible for high level expression via enhanced secretion, J. Biol. Chem 279 (2004) 6546–6552. [DOI] [PubMed] [Google Scholar]
- [26].Sabatino DE, Freguia CF, Toso R, Santos A, Merricks EP, Kazazian HH Jr., Nichols TC, Camire RM, Arruda VR, Recombinant canine B-domain-deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model, Blood 114 (2009) 4562–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Q, Dong B, Firrman J, Wu W, Roberts S, Moore AR, Liu LS, Chin MP, Diao Y, Kost J, Xiao W, Evaluation of the biological differences of canine and human factor VIII in gene delivery: implications in human hemophilia treatment, Gene Ther. 23 (2016) 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nathwani AC, Nienhuis AW, Davidoff AM, Current status of gene therapy for hemophilia, Curr. Hematol. Rep 2 (2003) 319–327. [PubMed] [Google Scholar]
- [29].Mah C, Sarkar R, Zolotukhin I, Schleissing M, Xiao X, Kazazian HH, Byrne BJ, Dual vectors expressing murine factor VIII result in sustained correction of hemophilia A mice, Hum. Gene Ther 14 (2003) 143–152. [DOI] [PubMed] [Google Scholar]
- [30].Wang Q, Dong B, Firrman J, Roberts S, Moore AR, Cao W, Diao Y, Kapranov P, Xu R, Xiao W, Efficient production of dual recombinant adeno-associated viral vectors for factor VIII delivery, Hum. Gene Ther. Methods 25 (2014) 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burton M, Nakai H, Colosi P, Cunningham J, Mitchell R, Couto L, Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 12725–12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhu F, Liu Z, Wang X, Miao J, Qu H, Chi X, Inter-chain disulfide bond improved protein trans-splicing increases plasma coagulation activity in C57BL/6 mice following portal vein FVIII gene delivery by dual vectors, Sci. China Life Sci 56 (2013) 262–267. [DOI] [PubMed] [Google Scholar]
- [33].Scallan CD, Liu T, Parker AE, Patarroyo-White SL, Chen H, Jiang H, Vargas J, Nagy D, Powell SK, Wright JF, Sarkar R, Kazazian HH, McClelland A, Couto LB, Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII, Blood 102 (2003) 3919–3926. [DOI] [PubMed] [Google Scholar]
- [34].Dong B, Nakai H, Xiao W, Characterization of genome integrity for oversized recombinant AAV vector, Mol. Ther 18 (2010) 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou J, Ding Q, Chen Y, Ouyang Q, Jiang L, Dai J, Lu Y, Wu X, Liang Q, Wang H, Wang X, Clinical features and molecular basis of 102 Chinese patients with congenital dysfibrinogenemia, Blood Cells Mol. Dis 55 (2015) 308–315. [DOI] [PubMed] [Google Scholar]
- [36].Laffan M, New products for the treatment of haemophilia, Br. J. Haematol 172 (2016) 23–31. [DOI] [PubMed] [Google Scholar]
- [37].Mahdi AJ, Obaji SG, Collins PW, Role of enhanced half-life factor VIII and IX in the treatment of haemophilia, Br. J. Haematol 169 (2015) 768–776. [DOI] [PubMed] [Google Scholar]
- [38].Hough C, Lillicrap D, Gene therapy for hemophilia: an imperative to succeed, J. Thromb. Haemost 3 (2005) 1195–1205. [DOI] [PubMed] [Google Scholar]
- [39].Dooriss KL, Denning G, Gangadharan B, Javazon EH, McCarty DA, Spencer HT, Doering CB, Comparison of factor VIII transgenes bioengineered for improved expression in gene therapy of hemophilia A, Hum. Gene Ther 20 (2009) 465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sabatino DE, Lange AM, Altynova ES, Sarkar R, Zhou S, Merricks EP, Franck HG, Nichols TC, Arruda VR, Kazazian HH Jr., Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors, Mol. Ther 19 (2011) 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zakas PM, Gangadharan B, Almeida-Porada G, Porada CD, Spencer HT, Doering CB, Development and characterization of recombinant ovine coagulation factor VIII, PLoS One 7 (2012) e49481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu FX, Liu ZL, Qu HG, Chi XY, Enhancing effect of porcine coagulation factor VIII A1 and A3 domains on secretion of post-translationally spliced human/porcine hybrid coagulation factor VIII, Acta Phys. Sin 62 (4) (2010) 373–381. [PubMed] [Google Scholar]
- [43].Chen L, Zhu F, Li J, Lu H, Jiang H, Sarkar R, Arruda VR, Wang J, Zhao J, Pierce GF, Ding Q, Wang X, Wang H, Pipe SW, Liu XQ, Xiao X, Camire RM, Xiao W, The enhancing effects of the light chain on heavy chain secretion in split delivery of factor VIII gene, Mol. Ther 15 (2007) 1856–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen L, Lu H, Wang J, Sarkar R, Yang X, Wang H, High KA, Xiao W, Enhanced factor VIII heavy chain for gene therapy of hemophilia A, Mol. Ther 17 (2009) 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, Wilson JM, Kazazian HH Jr., Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype, Blood 103 (2004) 1253–1260. [DOI] [PubMed] [Google Scholar]
- [46].Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, Powell S, Keller T, McMurray M, Labelle A, Nagy D, Vargas JA, Zhou S, Couto LB, Pierce GF, Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs, Blood 108 (2006) 107–115. [DOI] [PubMed] [Google Scholar]
- [47].Scallan CD, Lillicrap D, Jiang H, Qian X, Patarroyo-White SL, Parker AE, Liu T, Vargas J, Nagy D, Powell SK, Wright JF, Turner PV, Tinlin SJ, Webster SE, McClelland A, Couto LB, Sustained phenotypic correction of canine hemophilia A using an adeno-associated viral vector, Blood 102 (2003) 2031–2037. [DOI] [PubMed] [Google Scholar]
- [48].George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, Cuker A, Sullivan LM, Majumdar S, Teitel J, McGuinn CE, Ragni MV, Luk AY, Hui D, Wright JF, Chen Y, Liu Y, Wachtel K, Winters A, Tiefenbacher S, Arruda VR, van der Loo JCM, Zelenaia O, Takefman D, Carr ME, Couto LB, Anguela XM, High KA, Hemophilia B gene therapy with a high-specific-activity factor IX variant, N. Engl. J. Med 377 (2017) 2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM, Adenovirus-associated virus vector-mediated gene transfer in hemophilia B, N. Engl. J. Med 365 (2011) 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.