Graphical abstract
Keywords: Thyroid surgery, Graves’ disease, Neural monitoring, Recurrent laryngeal nerve, Morbidity
Highlights
-
•
Risk of RLN palsy during thyroidectomy for Graves’ disease is high.
-
•
I-IONM and C-IONM are feasible and useful during thyroidectomy for Graves’ disease.
-
•
Proportion of complete thyroidectomy was significantly higher using IONM technology.
Abstract
We evaluate the role of intraoperative neuromonitoring (IONM) in thyroidectomy performed for Graves’ disease (GD) with an emphasis on recurrent laryngeal nerve (RLN) management and completeness of resection. The study is a retrospective series comprising 55 thyroidectomy (control group) versus 82 procedures with intermittent IONM (I-IONM) and 72 by means of continuous IONM (C-IONM). In the control group the laryngeal nerves have been identified by visualization solely. In the I-IONM group both vagal nerve (VN) and RLN have been localized and monitored during thyroid resection. C-IONM was achieved with a vagal stimulation probe. I-IONM group had shorter operating times (P = 0.032). RLN morbidity, meaning palsy rate, was 2.7% in the C-IONM group, 3.6% in I-IONM and 5.4% in the control group (P = 0.058). The proportion of complete procedures (total or near total resections) were significantly higher using monitoring technology (P = 0.049). Persistent positive serum TBII values were found in 25 (45%), 25 (30%) and 20 (27%) patients at 12 months in the control, I-IONM and C-IONM groups respectively (P = 0,04). IONM is an effective technology in GD patients.
Introduction
Recurrent laryngeal nerve injury (RLNi) during thyroidectomy relates to multiple patient- and/or surgeon-related risk factors [1]. The risk is higher for thyroid cancer, Graves’ disease (GD), re-operation, mediastinal goiter, low volume experience and in cases of failure to identify the RLN [1]. Intraoperative neuromonitoring (IONM) has been proposed as a complementary procedure to standard visual identification of the laryngeal nerves during thyroid surgery. Authors have shown that IONM allows a reliable intraoperative identification and monitoring of the laryngeal nerves [2], [3], [4]. The continuous intraoperative neural monitoring (C-IONM) technique is increasingly acknowledged as a useful tool to recognize impending nerve injury and to abort the related maneuver to prevent nerve injury during thyroid surgery [2], [3], [4], [5], [6]. C-IONM provides valuable real-time information, which is really useful during complex thyroid surgeries especially in the setting of unusual anatomic variants [2], [3], [4], [5], [6]. The aim of this retrospective study is to compare intermittent and continuous IONM of the RLN with the sole visualization during thyroidectomy performed for GD.
Materials and methods
Design
Retrospective cohort study of patients undergoing thyroid surgery for GD. Data were collected from prospectively maintained departmental clinical medical charts database. The database is maintained with quality assurance by a database specialist and searchable through International Classification of Disease codes, Current Procedural Terminology, and faculty names.
Setting
Setting was a University Hospital.
Participants
Internal review board approved retrospective records analysis in all GD patients who had received thyroid surgery over a 5-year period (2009–2014) (#Jl-prot-2008-5). All patients routinely signed an informed consent before surgery for prospective data base collection. Participants were assured of anonymity. Eligible patients with GD were assessed for the study. This study included patients referred by endocrinologists for surgical management of GD. The study included patients undergoing first time open/transcervical thyroid surgery. Patients with hyperthyroidism due to GD underwent surgery because of their poor response to antithyroid drugs, need for high doses of antithyroid drugs, recurrence upon treatment discontinuation, and poor patient compliance [7]. Patients with preoperative vocal fold paralysis, pregnancy or lactation, American Society of Anesthesiologists fitness grade IV, and inability to comply with the follow-up were excluded from this study. Endoscopic thyroidectomy procedures were excluded. Patients were followed pre- and postoperatively in collaboration with the Division of Endocrinology [7].
Preoperative assessment
Patients were clinically and biochemically euthyroid following antithyroid treatment with thiamazole prior to surgery [7]. Laboratory tests included measurement of serum free tri-iodothyronine (fT3; reference range 0.22–6.78 pmol/l), free thyroxine (fT4; reference range 12–30 pmol/l) and thyroid-stimulating hormone (TSH, thyrotropin; reference range 0.4–4.2 munits/l). Serum thyrotropin- binding inhibitory immunoglobulin (TBII) levels were measured. A TBII value above 1.5 units/l was defined as positive [7]. High-resolution Doppler ultrasonography of the neck with both 7.5- and 12-MHz linear-array transducers was performed during an outpatient visit before admission.
Operative technique
Thyroid surgery was performed by 3 experienced endocrine surgeons. All operations were carried out under general anaesthesia. A central, traverse cervical Kocher incision was made along the skin crease between the cricoid cartilage and the sternal notch. Instruments adopted for dissection and control hemostasis included anatomical pliers, knife blades, mosquitos, monopolar and bipolar scalpel and vessel sealing system. RLN identification, exposure and neural monitoring was offered in all procedures. Efforts to identify 4 parathyroid glands was professed in all cases. Any inadvertently removed parathyroid gland was autotransplanted into the sternocleidomastoid muscle. Efforts were made to identify and remove the entire pyramidal thyroid lobe. A 15Fr Jackson-Pratt drain was placed routinely. Postoperative management included application of a non-compressing dressing for 8–12 days. Closed-suction drains were typically removed when postoperative encounter collection was less than 50 cc/24hrs. Stitches were removed 5–8 days postoperative at the discretion of the surgeon. Antibiotic prophylaxis was not routinely used.
Nerve monitoring
In the control group, the RLN was routinely identified solely by visual detection.
In the I-IONM group, the laryngeal nerves are identified and dissected by visualization plus nerve monitoring. A non-invasive monitoring system [nerve integrity monitor (NIM) Response 2.0 sub sequentially 3.0 System, Medtronic Xomed, Jacksonville, Florida] was used for nerve monitoring. RLN is identified, mapped and stimulated in the surgical field via the application of a sterile single-use pulse-generated monopolar stimulator probe (n.8225101, Medtronic Xomed). Stimulation level is set between 0.5 and 3.0 mA (mean 1), 30 Hz frequency, four stimulation per second, stimulation duration 100 ls and impedance\5 kX [8]. We routinely set the probe to deliver an electric current of 2–3 mA intensity for both vagal nerve (VN) and RLN identification, while 1–0.5 mA for RLN and related branches confirmation [9]. Thus, the RLN is mapped out in the inferior paratracheal region through probe stimulation and visually identified early via directed dissection based on the previous neural mapping. In the IONM group, the vagus nerve and the RLN have been stimulated before, during and after thyroid resection after a complete hemostasis according to Chiang’s recommendations [10]. The VN is directly stimulated by the application of the stimulator on the carotid sheath without dissection (stimulation probe intensity, 2–3 mA). Moreover, the stimulator is continuously used during (monitoring) the RLN and/-or vagus dissection of the tracheo-esophageal groove, for neurophysiologic confirmation of a visually intact nerve. The identification of an intact nerve is confirmed through a series of audible and quantitative signals generated by the machine and by analyzing and comparing the EMG amplitudes of both VN and RLN stimulation before and after thyroid resection [11], [12], [13], [14].
C-IONM group consisted in the application of the Automatic Periodic Stimulating (APS, Medtronic, Jacksonville, Florida, USA) accessory. The APS electrode was wet before to facilitate its sliding onto the VN. We proceed with the median approaches to the VN, i.e. in between the sternothyroid and thyroid gland. The APS was positioned gently on the VN after opening the carotid sheet by a 2 cm pouch. Careful 360° dissection of the VN with Maryland forceps is required. To prevent VN thermal injuries, energy based devices were avoided. The APS electrodes were then repose gently on the VN. During VN dissection and after the C-IONM electrode was placed, the VN was stimulated repeatedly by means of the intermitted stimulating probe, proximally and distally to the location of APS, to verify whether the dissection or electrode placement determined VN injury. After connecting the APS electrode with the monitor system, baselines for the latency and amplitude of the evoked response were calibrated automatically to serve as control data. Stimulation frequency for C-IONM was set for every second, thus evaluating the entire RLN constantly. The amplitude and latency waveforms were displayed separately, and an upper limit threshold for the latency (+10%) and a lower limit threshold for amplitude (−50%) were depicted as separate alarm lines. In addition, acoustic and optical signals alerted the surgeon when a preset threshold had been crossed or when the electrode had become dislodged. Attempts were to keep the surgical field dry with a swab to avoid any artifacts and\or signal shunting and\or dispersion. Removal of APS was performed by gently open and pull out the pins.
Follow-up
Clinical, biochemical, ophthalmological and ultrasonographic follow-up was undertaken at 1, 3, 6, 9 and 12 months after surgery, and then annually for 5 years. Biochemical evaluation consisted of measurement of serum concentrations of TSH, fT3 and fT4 at each visit, and serum TBII levels yearly [7]. Ophthalmological examination was carried out once a year with assessment of the severity of ophthalmopathy and the activity of the eye disease. All patients received postoperative levothyroxine treatment. The levothyroxine dose was adjusted to keep the serum TSH concentration within the reference range of 0.4–4.2 munits/l. The total serum calcium level (reference range 2.05–2.55 mmol/l) was measured 24 h after surgery and medical treatment was initiated if the concentration was below 2 mmol/l.
Outcomes measured
This retrospective study has been conducted to determine the outcomes of thyroidectomy for GD performed with intermittent IONM (I-IONM group), compared to continuous nerve monitoring (C-IONM group) and conventional technique without the use of neuromonitoring (control group). The following RLN morbidities have been assessed: transient or definitive laryngeal nerve injury, uni- or bilateral. RLN paralysis rates were calculated for nerves at risk (NAR). Pre- and postoperative follow-up included vocal fold mobility check performed via laryngoscopy at 24–48 h before and in a range of 1–2 days after the surgical procedure by an independent laryngologist. Any reduction in the vocal cord movement has been recorded as postoperative vocal fold paralysis. For those patients with documented postoperative VF palsy, repeated examinations were performed periodically at 1, 2, 4, 6, and 12 months after the operation until a full functional recovery had been confirmed usually.
Theory/calculation
The study was not powered for any outcome. There was no randomization per group. The distribution by group depended on the availability in the operating room of the I-IONM or C-IONM accessories. The non-availability of the I-IONM or C-IONM was contingent on: economic (annual resources completed), lack of stocks, non-procurement, temporal criteria (C-IONM is applied in our Department since 2011). All data for continuous variables have been expressed as median and range (unless otherwise specified). All patients’ data has been collected with a dedicated electronic Microsoft Office Access Data Base (Microsoft Corp, Redmond, Wash). The use of this database for clinical research has been approved by our institutional review board. The primary outcome measure has been RLN morbidity. Statistical analysis has been performed using SPSS, release 15.0 for Windows (SPSS Inc, Chicago-Ill, USA). The level of significance has been set at P less than 0.05.
Results
Data collection analysis
The distribution by subgroup depended on the resources in the operating room of the neuromonitoring accessories (Table 1). On the basis of selection and exclusion criteria, 209 patients constituted the review (Fig. 1). This series included 79 men and 130 women, aged 12–58 years (median 40 years). Overall, 80 (38.2%) patients underwent surgery because of their poor response to antithyroid drugs, 34 (16.3%) large goitre with compression, 34 (16.3%) ophthalmopathy, 21 (10%) need for higher doses, 29 (14%) recurrence upon treatment discontinuation, 3 (1.4%) poor patient compliance, 8 (3.8%) suspicious thyroid nodule (the reasons for the surgery can be multiple). The un-monitored, intermitted group and C-IONM group composed of 55, 82 and 72 patients respectively. Complete follow-up was available for all patients. The number of NAR were 110, 164, 144 per group.
Table 1.
Non-availability of IONM and/or C-IONM accessories. The subgroup distribution depended on the resources in the operating room of the neuromonitoring accessories (2009–2014).
| Non-availability | Limited annual budget (N, %) |
Finite stocks (N, %) |
Non-procurement (N, %) |
|---|---|---|---|
| IONM (n.55) | 20 (36) | 20 (36) | 15 (27) |
| C-IONM* (n.40) | 25 (62.5) | 10 (25) | 5 (12.5) |
C-IONM was applied since 2011.
Fig. 1.
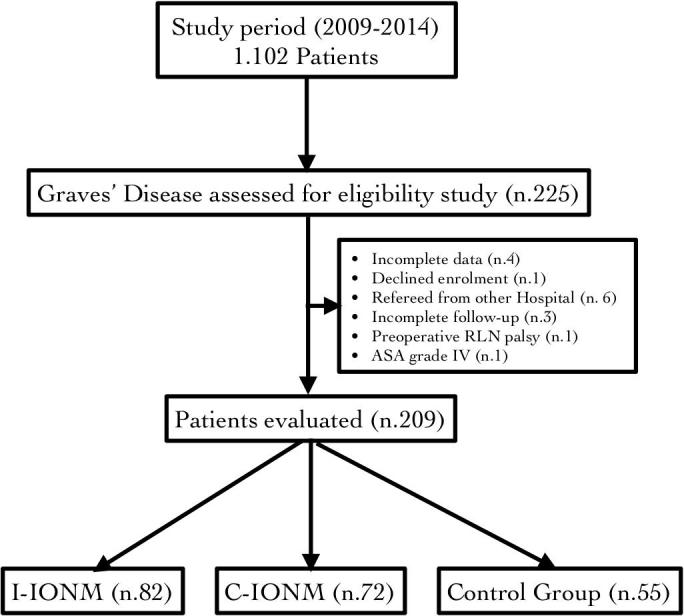
Flow-chart of retrospective patients selection process and analysis. GD was confirmed in 20.4% of overall surgeries (n.1.102). GD: Graves disease. I-IONM: intermitted neural monitoring group. C-IONM: continuous neural monitoring group. RLN: recurrent laryngeal nerve. ASA: American Society of Anesthesiologists (ASA) physical status classification system.
Comparative study for clinical and operative findings
Table 2 summarise the 3 groups regarding the distribution of epidemiological characteristics, the thyroid pathology, mean weight of the thyroid. There were no significant differences in baseline characteristics between the 3 groups. I-IONM and C-IONM procedures were performed successfully and there has been no instances of equipment malfunction. The I-IONM group had shorter operating times (P = 0.032). No mortality was observed. The comprehensive morbidity rate was 34%, 20% and 20.8% in the control, I-IONM and C-IONM group respectively (P = 0.052). The rate of transient RLN palsy was 2.7% in the C-IONM group, 3.6% in I-IONM and 5.4% in the control group (P = 0.058). One bilateral vocal cord paresis occurred in control group. EMG profiles data for RLN injuries are presented in Table 2. Mechanism of RLN injury is unknown for the control group. Mechanism of injury for the nerve monitoring groups was elucidated in 4/6 (66%) nerves in the I-IONM group, 3/4 (75%) in the C-IONM group respectively. There were 9 RLN traction injuries overall, and one compression lesion in the I-IONM group. All injuries were segmental lesions. One case of permanent RLN paralysis in control group and none when using monitoring technology. No difference in regard to hypoparathyroidism (Table 2).
Table 2.
Analysis for Demographics, Clinical, Laboratory and Operative Findings of the study participants.
| Variable^ (reference values) | Control Group N. 55 |
I-IONM N. 82 |
C-IONM N. 72 |
P† |
|---|---|---|---|---|
| Gender (M/F) | 1:1.6 | 1:1.7 | 1 :1.6 | 0.283 |
| Age at diagnosis (years) | 40 ± 5 (15–58) | 39 ± 3 (18–53) | 38 ± 3 (12–53) | 0.07 |
| BMI (Kg/m2) | 24.5 ± 3 (16.5–38) | 24.9 ± 4 (18–40) | 23.5 ± 3 (17–39) | 0.126 |
|
Smoking status Yes No |
10 (18) 45 (82) |
22 (26)60 (73) |
20 (27) 52 (72) |
0.189 |
|
Thyroid disease duration - <1 year - >1 year |
5 (9%) 50 (91%) |
8 (9%) 74 (91%) |
6 (8%) 66 (92%) |
0.17 |
| hTSH levels (0.55–4.78 mIU/l) | 2.62 ± 0.48 | 2.45 ± 0.72 | 2.35 ± 0.6 | 0.13 |
| Free T3 (2.3–4.2 pg/ml) | 3.05 ± 0.30 | 3.0 ± 0.38 | 2.9 ± 0.28 | 0.79 |
| Free T4 (0.76–1.4 ng/dl) | 1.93 ± 0.13 | 1.2 ± 0.1 | 1.93 ± 0.13 | 0.29 |
| hTg (3–40 ng/ml) | 19.3 ± 2.1 | 20.1 ± 0.9 | 25.2 ± 1.9 | 0.07 |
| Preoperative TBII level (units/l) | 11.2 ± 0.22 | 12.1 ± 0.1 | 10.1 ± 0.1 | 0.2 |
|
TBII levels at 12 months after surgery Positive Negative |
9 46 |
4 78 |
4 68 |
0.5 |
|
Procedure performed -Total thyroidectomy - Near total thyroidectomy - Subtotal thyroidectomy |
45 (81) 9 (16.3) 1 (1.8) |
77 (93) 5 (6) 0 (0) |
69 (95) 3 (4.1) 0 (0) |
0.049^^ |
| Duration of surgery (min) | 63 ± 5 (49–140) | 43 ± 4 (35–100) |
52 ± 4 (45–220) |
0.032 |
| Thyroid volume (grams) | 43.1 ± 10.8 (16–50) | 47.9 ± 8.8 (7–70) | 45.9 ± 7.8 (15–51) | 0.09 |
| Parathyroid found in pathological report | 3 (5,4) | 5 (6) | 3 (4.1) | 0.1 |
|
Histology Benign Malignant |
54 (98) 1 (1.8) |
81 (99) 1 (1.2) |
70 (96) 2 (2.7) |
0.17 |
| Hospital stay (days) | 2.6 ± 0.4(2–7) | 2.3 ± 0.3(1–5) | 2.4 ± 0.3(1–5) | 0.145 |
| Hypoparathyroidism transient | 10 (18) | 12 (14) | 12 (16) | 0.086 |
| Hypoparathyroidism definitive | 1 (1.8%) | 1 (1.2) | 0 | 0.57 |
|
RLNP transient (NAR = )* |
6/110 (5.4) | 6/164 (3.6) | 4/144 (2.7) | 0.058 |
|
RLNP definitive (NAR = )* |
1 (0.9) | – | – | – |
| Bilateral RLNP | 1 (1.8) | – | – | – |
| RLNP time recovery (months) | 2 (1–12) | 2 (1–4) | 2 (1–4) | 0.8 |
|
RLNP EMG amplitude data (μV) V1 V2 |
– – |
843 ± 120 50 ± 10 |
791 ± 55 68 ± 18 |
0.8 |
| Wound hematoma | 1 (1.8) | – | – | – |
| Bleeding ° | – | 1 (1.2) | – | |
| Seroma | 2 (3.6) | – | – | – |
| Wound infection | – | – | 1 (1.3) | – |
| Mortality | – | – | – | – |
| Overall morbidity (total ^) | 19/55 (34) | 17/82 (20) | 15/72 (20.8) | 0.052 |
Patients groups are described in the “Methods” section.
^Data are given as mean number, ± standard deviation (percentage, range) of patients.
Blood examinations were tested preoperatively, <1 months before surgery.
BMI: body mass index.
hTSH: human thyroid-stimulating hormone.
T3: triiodothyronine.
T4: thyroxine.
TBII level (units/l): thyrotropin-binding inhibitory immunoglobulin.
hTg: serum thyroglobulin.
RLNP: recurrent laryngeal nerve palsy.
EMG: electromyography.
* Numbers are give as number (percentage) of nerves at risk.
NAR: nerves at risk.
° Bleeding required re-intervention.
† Student’s t test.
^^ C-IONM + I-IONM vs. Control group.
Consequences and follow-up
Mean length of follow-up was 54 ± 5.4 months. The proportion of complete procedures (total or near total resections) was significantly in favour when using I-IONM or C-IONM (P = 0.049). In two patients with RLN injury of the I-IONM group, the identity of injured nerve was confirmed intraoperatively through a negative series of signals generated by the machine by stimulating both the right RLN and vagus nerve. These patients were scheduled for a total thyroidectomy. The procedure was stopped after the first side lobectomy. Postoperative follow- up of these cases included direct laryngoscopy performed at 48 h after the surgical procedure that confirmed a reduction in the movement of the respective right vocal folds. A repeat examination was performed at 1, 2, 4 months after the operation until full recovery of vocal cord function was confirmed after logopedy. Mean time recovery for nerve injuries is reported in the Table 2. Statistics revealed no significant differences for time recovery period per group analysis (Table 2). One patient was programmed for completion right thyroidectomy 3 months after the first operation (stage thyroidectomy). The second patient, after multidisciplinary discussion, was submitted for remnant ablation. Thus, 2/10 (20%) palsies occurred first side of resection. During the follow-up period, recurrent hyperthyroidism was diagnosed in 1 patient of control group (subtotal resection), none in the I-IONM or C-IONM group. There were no differences in serum TSH concentration between groups during the follow-up (data not shown). Persistent positive serum TBII values, related to residual thyroid tissue, were found in 25 (45%), 25 (30%) and 20 (27%) patients at 12 months in the control, I-IONM and C-IONM groups respectively (P = 0,04).
Discussion
This study has low level of evidence and recommendation according to evidence-based criteria [16], [17]. It is delicate to propose to patients to be enrolled into a prospective randomized trail which analyzes surgical technique or any accessory to it. Further studies should be performed in order to support and confirm our results. Perhaps, the power of the study is that both groups of patients performed routine pre- and postoperative laryngoscopy. Pre- operative and postoperative laryngoscopy allow for objective evaluation of RLN morbidity, and is important in improving outcomes of individual surgical performance, and quality control of the surgical practice [18], [19], [20], [21]. It is of even increasing importance while using IONM technology as there are some minimum requirements for optimal intraoperative neural monitoring which involve not only vagal nerve stimulation before and after dissection but also pre- and postoperative laryngoscopy [20]. Laryngeal examination is reference for IONM. Our clinical results demonstrate that monitoring is an effective technology in high-risk thyroid procedures such as GD. The overall RLN morbidity tended to be lower using IONM (P = 0.058). Barczynski in 2009 [4] prospectively demonstrated that the prevalence of transient RLN paresis was 2.9% lower in high-risk patients undergoing surgery for cancer thyrotoxicosis, and thyroiditis patients, who received nerve monitoring. A multivariate logistic regression analysis confirmed that the use of IONM decreased the rate of postoperative transient (P = 0.008) and permanent (P = 0.004) RLN palsies by factors of 0.58 and 0.30, respectively [3]. Early identification and exposure of RLN reduces the risk of RLN injury. IONM can locate the RLN before visual confirmation and is associated with a nerve identification rate near 100% [3], [4]. The identification of the laryngeal nerves can be difficult in patients undergoing operations for hyperthyroidism [1]. For this reason, the IONM can be used to detect the correct position of the RLN (before visual confirmation early at the beginning of the procedure), stimulating via the probe the overlying tissue of the tracheo-esophageal groove. Due to its small flexible non-traumatic tip, the stimulating probe itself has the function as a nerve hook-dissector instrument: once nerves are identified and mobilized, the surgeon can curve, shape and hook the nerves proximally with the probe tip to gently enhance the working space and the plain of dissection or to carefully ease the nerve from a coagulating point [11], [12], [13], [14], [15], even more with a continuous neurophysiologic confirmation of the nerve to allow complete and secure thyroid and lymph node resection. In this study, the proportion of complete procedures (total resections) was significantly in favour when using I-IONM or C-IONM (P = 0.049). Persistent positive serum TBII values were found in 45%, 30% and 27% patients at 12 months in the control, I-IONM and C-IONM groups respectively (P = 0,04). Recent consensus guidelines have recommended more extended surgery for GD, i.e., total thyroidectomies [19], [20], [21], [22], [23], [24], [25], [26], [27]. Most of the difficulties in performing a total thyroidectomy for GD occurs with the separation of the thyroid tissue in the region of Berry’s ligament [19]. This surgical step is a frequent source of accidental injury to the RLN and incompleteness of resection [9], [10]. Therefore, the surgeon requires vigilance, optimal exposure and continuous neurophysiologic confirmation of a visually intact nerve during dissection of the RLN at Berry’s ligament to allow complete and secure resection to achieve a true total thyroidectomy [18].
Conclusions
In conclusion, we evaluate the role of IONM for thyroidectomy performed in Graves’ disease (GD) with emphasis on RLN management and completeness of resection. RLN morbidity tended to be lower using monitoring technology: 2.7% in the C-IONM group, 3.6% in I-IONM and 5.4% in the control group (P = 0.058). The proportion of total or near total resections was significantly higher when using IONM (P = 0.049). Persistent positive serum TBII values were found in 45%, 30% and 27% patients at 12 months in the control, I-IONM and C-IONM groups respectively (P = 0,04).
Declarations
Ethics approval and consent to participate: The Institution's Ethical Board Committee approved the review
Consent to publish: Patients signed an informed consent before surgery for dataset use.
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests: All the authors declare that they have no competing interests.
Funding: No funding was received.
Authors' Contributions: All Authors equally contributed to conception, design, manuscript writing and final approval of manuscript.
Acknowledgements
Not applicable.
Contributor Information
Gianlorenzo Dionigi, Email: https://orcid.org/0000-0003-0864-6087, gdionigi@unime.it.
Hui Sun, Email: thyroidjl@163.com.
References
- 1.Lo C.Y., Kwok K.F., Yuen P.W. A prospective evaluation of recurrent laryngeal nerve paralysis during thyroidectomy. Arch Surg. 2000;135(2):204–207. doi: 10.1001/archsurg.135.2.204. [DOI] [PubMed] [Google Scholar]
- 2.Timmermann W., Hamelmann W.H., Thomusch O., Sekulla C., Grond S., Neumann H.J. Effectiveness and results of intraoperative neuromonitoring in thyroid surgery. Statement of the Interdisci- plinary Study Group on Intraoperative Neuromonitoring of Thyroid Surgery. Chirurg. 2004;75(9):916–922. doi: 10.1007/s00104-004-0858-0. [DOI] [PubMed] [Google Scholar]
- 3.Thomusch O., Sekulla C., Machens A., Neumann H.J., Timmermann W., Dralle H. Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg. 2004;389(6):499–503. doi: 10.1007/s00423-003-0444-9. [DOI] [PubMed] [Google Scholar]
- 4.Barczynski M., Konturek A., Cichon’ S. Randomized clini- cal trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg. 2009;96(3):240–246. doi: 10.1002/bjs.6417. [DOI] [PubMed] [Google Scholar]
- 5.Casella C., Fusco M. Thyroid cancer. Epidemiol Prev. 2004;28:88–91. [PubMed] [Google Scholar]
- 6.Davies L., Welch H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 7.Schlumberger M., Pacini F. 2nd edn. Editions Nucleon; Paris: 2003. Thyroid tumors. [Google Scholar]
- 8.Wu C.W., Lu I.C., Randolph G.W., Kuo W.R., Lee K.W., Chen C.L. Investigation of optimal intensity and safety of electrical nerve stimulation during intraoperative neuromonitor- ing of the recurrent laryngeal nerve: a prospective porcine model. Head Neck. 2010;32(10):1295–1301. doi: 10.1002/hed.21324. [DOI] [PubMed] [Google Scholar]
- 9.Serpell J.W., Yeung M.J., Grodski S. The motor fibers of the recurrent laryngeal nerve are located in the anterior extralaryn- geal branch. Ann Surg. 2009;249(4):648–652. doi: 10.1097/SLA.0b013e31819ed9a4. [DOI] [PubMed] [Google Scholar]
- 10.Chiang F.Y., Lee K.W., Chen H.C., Chen H.Y., Lu I.C., Kuo W.R. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid opera- tion. World J Surg. 2010;34(2):223–229. doi: 10.1007/s00268-009-0316-8. [DOI] [PubMed] [Google Scholar]
- 11.Dionigi G., Chiang F.Y., Rausei S., Wu C.W., Boni L., Lee K.W. Surgical anatomy and neurophysiology of the vagus nerve (VN) for standardised intraoperative neuromonitoring (IONM) of the inferior laryngeal nerve (ILN) during thyroidectomy. Langenbecks Arch Surg. 2010;395(7):893–899. doi: 10.1007/s00423-010-0693-3. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz K., Sekulla C., Schelle J., Schmeiss B., Brauckhoff M., Dralle H., German Neuromonitoring Study Group What are normal quantitative parameters of intraoperative neuromonitoring (IONM) in thyroid surgery? Langenbecks Arch Surg. 2010;395(7):901–909. doi: 10.1007/s00423-010-0691-5. [DOI] [PubMed] [Google Scholar]
- 13.Cernea C.R., Nishio S., Hojaij F.C. Identification of the external branch of the superior laryngeal nerve (EBSLN) in large goiters. Am J Otolaryngol. 1995;16(5):307–311. doi: 10.1016/0196-0709(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 14.Jonas J., Bahr R. Neuromonitoring of the external branch of the superior laryngeal nerve during thyroid surgery. Am J Surg. 2000;179(3):234–236. doi: 10.1016/s0002-9610(00)00308-1. [DOI] [PubMed] [Google Scholar]
- 15.Dionigi G. Energy based devices and recurrent laryngeal nerve injury: the need for safer instruments. Langenbecks Arch Surg. 2009;394(3):579–580. doi: 10.1007/s00423-008-0454-8. [DOI] [PubMed] [Google Scholar]
- 16.Sackett D.L. Rules of evidence and clinical recommenda- tions on the use of antithrombotic agents. Chest. 1989;95(2 Suppl):2S–4S. [PubMed] [Google Scholar]
- 17.Heinrich S., Schäfer M., Rousson V., Clavien P.A. Evidence- based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243(2):154–168. doi: 10.1097/01.sla.0000197334.58374.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph G.W. The importance of pre- and postoperative laryngeal examination for thyroid surgery. Thyroid. 2010;20(5):453–458. doi: 10.1089/thy.2010.1632. [DOI] [PubMed] [Google Scholar]
- 19.Steurer M., Passler C., Denk D.M., Schneider B., Niederle B., Bigenzahn W. Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of preoperative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope. 2002;112:124–133. doi: 10.1097/00005537-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Dionigi G. True incidence of recurrent laryngeal nerve injury: time to audit! Int J Clin Pract. 2010;64(4):523. doi: 10.1111/j.1742-1241.2009.02193.x. [DOI] [PubMed] [Google Scholar]
- 21.Dionigi G., Boni L., Rovera F., Rausei S., Castelnuovo P., Dionigi R. Postoperative laryngoscopy in thyroid surgery: proper timing to detect recurrent laryngeal nerve injury. Langenbecks Arch Surg. 2010;395(4):327–331. doi: 10.1007/s00423-009-0581-x. [DOI] [PubMed] [Google Scholar]
- 22.Masiello E., Veronesi G., Gallo D., Premoli P., Bianconi E., Rosetti S., Cusini C., Sabatino J., Ippolito S., Piantanida E., Tanda M.L., Chiovato L., Wiersinga W.M., Bartalena L. Antithyroid drug treatment for Graves' disease: baseline predictive models of relapse after treatment for a patient-tailored management. J Endocrinol Invest. 2018 doi: 10.1007/s40618-018-0918-9. [Epub ahead of print] PubMed PMID: 29946800. [DOI] [PubMed] [Google Scholar]
- 23.Bartalena L., Chiovato L., Vitti P. Management of hyperthyroidism due to Graves’ disease: frequently asked questions and answers (if any) J Endocrinol Invest. 2016 Oct;39(10):1105–1114. doi: 10.1007/s40618-016-0505-x. Epub 2016 Jun 18. Review. PubMed PMID: 27319009. [DOI] [PubMed] [Google Scholar]
- 24.Bartalena L., Baldeschi L., Boboridis K., Eckstein A., Kahaly G.J., Marcocci C., Perros P., Salvi M., Wiersinga W.M. European group on graves' orbitopathy (EUGOGO). The 2016 european thyroid association/european group on graves' orbitopathy guidelines for the management of graves' orbitopathy. Eur Thyroid J. 2016;5(1):9–26. doi: 10.1159/000443828. Epub 2016 Mar 2. PubMed PMID: 27099835; PubMed Central PMCID: PMC4836120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piantanida E., Lai A., Sassi L., Gallo D., Spreafico E., Tanda M.L., Bartalena L. Outcome prediction of treatment of graves' hyperthyroidism with antithyroid drugs. Horm Metab Res. 2015;47(10):767–772. doi: 10.1055/s-0035-1555759. Epub 2015 Jul 21. Review. PubMed PMID: 26197855. [DOI] [PubMed] [Google Scholar]
- 26.Bartalena L., Burch H.B., Burman K.D., Kahaly G.J. A 2013 European survey of clinical practice patterns in the management of Graves' disease. Clin Endocrinol (Oxf) 2016;84(1):115–120. doi: 10.1111/cen.12688. Epub 2015 Jan 9 PubMed PMID: 25581877. [DOI] [PubMed] [Google Scholar]
- 27.Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013;9(12):724–734. doi: 10.1038/nrendo.2013.193. Epub 2013 Oct 15. Review. PubMed PMID: 24126481. [DOI] [PubMed] [Google Scholar]


