Abstract
Background
There is a paucity of studies reporting long-term survival outcomes for HPV/p16 positive oropharyngeal squamous cell carcinoma (OPSCC). This study aims to compare long-term outcomes of advanced stage p16 positive and negative OPSCCs, treated by surgical and non-surgical modalities.
Methods
OPSCC patients from 1998 to 2012 were identified through a prospectively collected cancer registry. P16 immunohistochemistry was used as a surrogate marker for HPV-OPSCC. Overall survival (OS) and aspiration free survival (AFS) comparisons were made between patients treated with chemoradiation (CRT) versus primary surgery and radiation/chemoradiation (S+RT/CRT) at 5, 10 and 15 years post-treatment.
Results
A total of 319 patients were included. P16 positive patients and non-smokers had significantly higher long-term (5, 10 and 15-year) OS. Smokers and p16 negative patients treated with S+RT/CRT had improved long-term OS compared to patients who received CRT. Smokers and p16 negative patients had lower long-term AFS. Multivariate analysis showed improved OS was associated with p16 positivity (HR 0.42, 0.28–0.61) and surgery (HR 0.47, 0.32–0.69), whereas lower OS was associated with ECOG ≥ 2 (HR 2.46, 1.61–3.77), smoking (HR 2.37, 1.41–3.99) and higher stage (HR 1.68, 1.05–2.68).
Conclusions
In smokers and p16-negative OPSCC patients, primary surgery may be associated with improved long-term survival and dysphagia-related outcomes.
Abbreviations: ACR, Alberta Cancer Registry; CI, confidence interval; CRT, chemoradiation; CCI, Charleson comorbidity index; DSS, disease specific survival; ENE, extranodal/extracapsular extension; ECOG, European Consortium Oncology Group; G-tube, gastrostomy tube; HPV, human papillomavirus; HPV-OPSCC, human papillomavirus-related oropharyngeal squamous cell carcinoma; HR, hazard ratio; IHC, immunohistochemistry; IMRT, intensity modulated radiation therapy; LOHS, length of hospital stay; LVI, lymphovascular invasion; NCCN, National Comprehensive Cancer Network; OPSCC, oropharyngeal squamous cell carcinoma; OS, overall survival; PEG, percutaneous endoscopic gastrostomy; PNI, perineural invasion; RT, radiation therapy; S+RT/CRT, primary surgery with post-operative radiation or chemoradiation; VFSS, videofluoroscopic swallowing study
1. Background
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) is rising at an alarming rate due to an epidemic of oncogenic human papillomavirus (HPV) [1], [2], [3], [4]. In Canada, the incidence of HPV-related OPSCC (HPV-OPSCC) is expected to soon surpass cervical cancer [5] and epidemiological estimates suggest these rising trends will continue for several decades [3]. In contrast to HPV-negative OPSCC, HPV-OPSCC is more often diagnosed in young men without extensive tobacco or alcohol use [6], [7], [8]. HPV-OPSCC is associated with favorable treatment responses and high cure rates and consequently, there is an emerging population of long-term survivors with sequelae of cancer therapy that will continue to rise [9], [10].
Early studies suggested advanced stage OPSCC should be treated with either chemoradiation therapy (CRT) or primary surgery with adjuvant therapy [11], [12]. Randomized control trials have identified the improved outcomes of CRT as compared to RT alone. Several recent studies have suggested that patients with HPV-OPSCC may have improved outcomes with primary surgery as compared to CRT particularly in patients with a significant smoking history [13], [14], [15]. There remains a paucity of literature examining long-term survival outcomes for either treatment modality [16], though causes of death in patients with HPV-related and unrelated cancers appear to be quite different [17].
Survivorship refers to the health and life of a person with cancer post treatment until the time of their death. With improved survival outcomes, more patients are living with many complex needs. Up to 50% of head and neck cancer patients identify swallowing difficulties as the primary concern post treatment [18]. Dysphagia has been established as a dose-related toxicity of radiotherapy based treatments with altered swallowing secondary to edema, neuropathy and fibrosis [19]. Aspiration pneumonia remains an underdiagnosed entity in head and neck cancer patients treated with both CRT and surgery, and can lead to prolonged gastronomy tube (g-tube) dependence and death [20], [21], [22]. Severe swallowing dysfunction has been reported in patients who are more than 5 years post treatment for OPSCC. This suggests a progressive nature of dysphagia that could complicate long-term morbidity and mortality [20].
With rising rates of OPSCC, particularly in younger, otherwise healthy patients, survival alone is not enough and swallowing impairment and its impact on patients’ quality of life must also be considered as part of the treatment paradigm. This study aimed to identify long-term survival (>10 year) and treatment-related dysphagia outcomes for advanced stage OPSCC in patients treated with chemoradiation therapy or primary surgery.
2. Methods
2.1. Patients
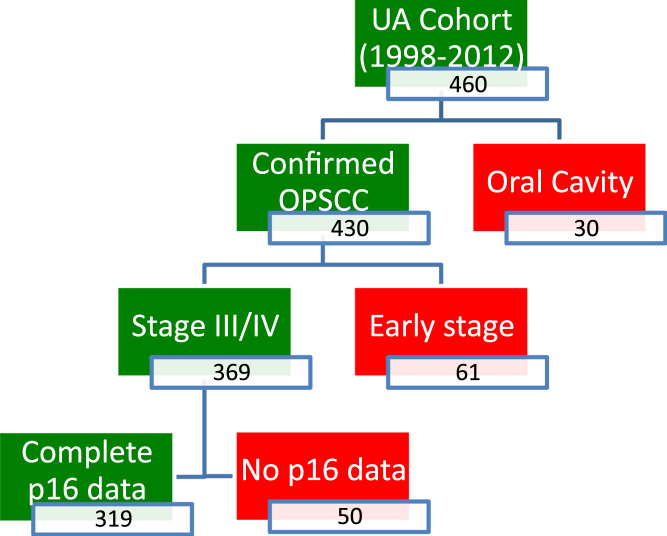
Consent to obtain patient data was obtained through the Health Research Ethics Board at the University of Alberta (Pro00016426 HREBA.CC-16–0829). Patients included in this study were initially identified through the Alberta Cancer Registry (ACR), a prospectively collected legally mandated database that includes all patients diagnosed with cancer in Alberta [14], [23]. Patients treated at the University of Alberta from 1998 to 2012 with a pathologic diagnosis of OPSCC and known p16 status were selected for inclusion in this study (Fig. 1). From 1998–2009, p16 status was obtained from a constructed tissue microarray as previously reported, using the accepted cutoff for p16 positivity as > 70% nuclear and cytoplasmic staining [14], [24], [25], [26]. From 2010–2012, p16 status was obtained from clinical pathology, using the same cutoff for p16 positivity, performed as standard of care for patients with OPSCC. Patients were excluded from the study if they did not receive treatment with intent-to-cure, lost to post-treatment follow-up or p16 status was not available. Further chart reviews were completed to construct a database used for further analysis including the following factors and variables: age, gender, smoking status (defined as positive with >10 pack years [27], dates of diagnosis and treatment, date of death, cause of death, date last known alive, gastrostomy tube (g-tube) dependence, video fluoroscopy swallowing study (VFSS) data, barium swallowing study data, post-operative length of hospital stay (LOHS), European Consortium Oncology Group (ECOG) status, Charleson Comorbidity Index (CCI), treatment type, radiation type and dose, chemotherapy type and dose, tumor subsite, clinical and pathologic stage according the AJCC 7th Edition [28], p16 status, extranodal/extracapsular extension (ECE), perineural invasion (PNI), lymphovascular invasion (LVI) and post-operative margin positivity. Patients were categorized into the following intent-to-treat subgroups for our analysis: 1) surgery and postoperative radiation/chemoradiation (S+RT/CRT), 2) chemoradiation +/- salvage surgery (CRT). Patients treated with primary surgery tumor extirpation was performed using a lip-splitting mandibulotomy [29], [30], [31] when required for access or non-robotic transoral approaches. Bilateral selective neck dissections were performed, with levels 2–4 and unilateral submandibular gland transfer [32] in N0 necks and 1–5 in node positive necks. All surgically treated patients were reconstructed with an adipofascial free flap, using a beavertail modification procedure for tongue base reconstruction [33], [34], [35] and soft palate reconstruction protocols [36]. If patients did not receive the full radiation and/or chemotherapy intended due to toxicity, they remained included in the respective treatment group. Prior to statistical analysis, database accuracy was reviewed independently by two oncologists and any inconsistencies found were resolved by consensus.
Fig. 1.
Summary of patients included in this study. Patients with no p16 data did not have adequate specimen for tissue microarray construction (1998–2009) or had an inconclusive result clinical pathology (2010–2012). UA, University of Alberta.
2.2. Statistical analysis
Survival time was calculated in years from time of pathologic diagnosis to date last known alive by follow-up or electronic medical records, or date of death using a right censoring method. Cause of death was determined by the ACR and chart reviews. Survival data was available for patients up to 18.79 years post treatment. However, in one subset of patients (p16 positive treated with CRT), the longest survival data available was 12.5 years post-treatment. Comparative analysis of survival between groups was therefore set at a maximum of 12.5 years. Aspiration free survival (AFS) was defined as the time of pathologic diagnosis up to last documented aspiration on barium and/or videofluoroscopic swallowing study (VFSS) as measured by a certified speech and language pathologist. The degree of aspiration of barium was classified as either 1) no or minimal aspiration, 2) mild to moderate aspiration and 3) moderate to severe aspiration. Information from VFSS was categorized as either 1) normal, 2) evidence of significant penetration and 3) evidence of significant aspiration (mild to severe). Data from barium studies and VFSS was combined to determine whether patients were aspirating (Supplementary table 2).
SPSS version 25.0 was used for all statistical analyses (SPSS Inc., Chicago, IL, USA). The Kaplan-Meir algorithm was used to estimate overall and disease specific survival, employing the Log-rank test (Mantel-Cox) to compare data [37]. The Cox proportional hazards model [38] was used to perform a multivariate analysis of factors and covariates, including age, sex, TNM staging, tumor subsite, treatment, smoking status, ECOG, ENE and p16 positivity. ANOVA, Student's t-test, Pearson's chi-square and the Kruskal-Wallis test were used to calculate differences between groups where appropriate. Statistical significance was defined as p < 0.05.
3. Results
3.1. Patient characteristics
A total of 460 patients who were treated and followed at the University of Alberta were identified for inclusion in this study (Fig. 1). Following further chart review, 30 patients were excluded as they were misclassified as oral cavity squamous cell carcinoma and 61 patients had early stage disease (defined by AJCC 7th edition staging). An additional 50 patients were excluded from 1998 to 2009 as p16 staining could not be performed due to lack of pathological material [14]. Kaplan-Meier analysis comparing these excluded patients to all other included patients showed no significant differences in survival (Supplemental figure 1).
A cohort of 319 patients with advanced stage OPSCC was included for comparative analyses in this study (Table 1). When comparing characteristics between S+RT/CRT and RT/CRT treatment groups, no statistically significant differences are present in terms of gender, tumor subsite, p16 positivity or 10 pack year smoking status. Patients treated with CRT had a more advanced age (61.4 vs 55.7 years, p = 0.001) at the time of diagnosis and higher percentage of 20 pack-year smokers (60.9 vs 46.7%, p = 0.04) but had a lower overall TNM stage (71.8 vs 83.1% stage IV, p = 0.022).
Table 1.
Characteristics of patients with advanced stage oropharyngeal squamous cell carcinoma included in this study.
| Demographics | S+RT/CRT (n = 202) | CRT (n = 117) | P |
|---|---|---|---|
| Age (mean, SD) | 55.7, 8.7 | 61.4, 11.1 | 0.001 |
| Gender (%M) | 82.2 | 77.8 | 0.379 |
| Tumor Subsite (%) | |||
| Tonsil | 58.9 | 42.7 | 0.43 |
| Base of Tongue | 30.2 | 42.3 | |
| Soft Palate | 2.9 | 0 | |
| Pharyngeal wall | 8.0 | 12.0 | |
| TNM Stage (% stage IV vs III) | 83.1 | 71.8 | 0.022 |
| Clinical T-Stage | |||
| T1 | 25.3 | 28.9 | 0.70 |
| T2 | 41.6 | 35.6 | |
| T3 | 20.7 | 20.7 | |
| T4 | 12.4 | 14.8 | |
| Clinical N-Stage | |||
| N0 | 10.1 | 11.0 | 0.79 |
| N1 | 15.0 | 16.2 | |
| N2 | 69.5 | 67.6 | |
| N3 | 5.4 | 5.2 | |
| p16 positive (%) | 63.3 | 53.8 | 0.099 |
| Tobacco smokers (%) | |||
| 10 pack years | 70.9 | 71.8 | 0.800 |
| 20 pack years | 46.7 | 60.9 | 0.04 |
Radiation type (conventional vs IMRT) was similar between patients treated with primary surgery vs CRT, but radiation dose administered was significantly lower post-operatively (Table 2). The dose of platinum-based chemotherapy administered was similar between S+RT/CRT and CRT groups.
Table 2.
Radiation and chemotherapy type and dosage for patients with advanced stage oropharyngeal squamous cell carcinoma included in this study.
| Treatments | S+RT/CRT (n = 202) | CRT (n = 90) | RT (n = 27) | p |
|---|---|---|---|---|
| Radiation Type (%)* | ||||
| Conventional | 23.2 | 21.2 | 48.0 | 0.226 |
| IMRT | 76.2 | 78.8 | 52.0 | |
| Radiation dose primary (mean Gy)* | 55.4 | 64.5 | 61.9 | < 0.001 |
| Radiation dose neck (mean Gy)* | 48.9 | 59.1 | 50.3 | < 0.001 |
| Chemotherapy dose (mg/m2)** | 88.0 | 85.4 | – | 0.714 |
Radiation doses shown for CRT and RT are shown but means for these groups combined (CRT/RT) are compared statistically to S+RT/CRT.
Chemotherapy dose for either cisplatin or carboplatin, comparing S+CRT and CRT groups.
3.2. Survival analysis
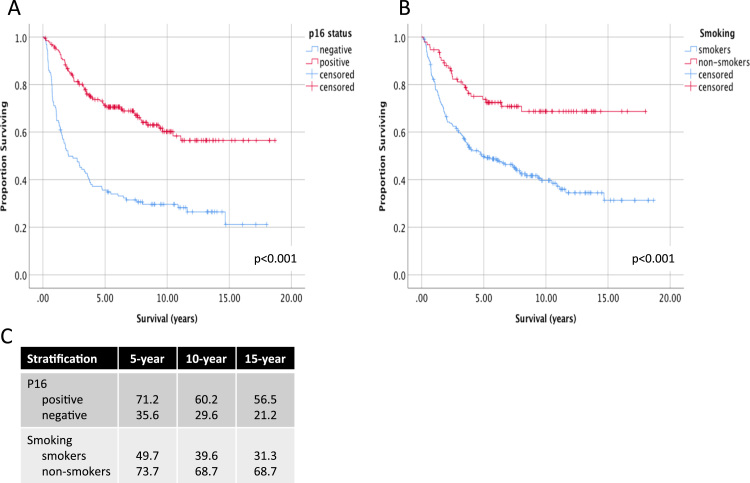
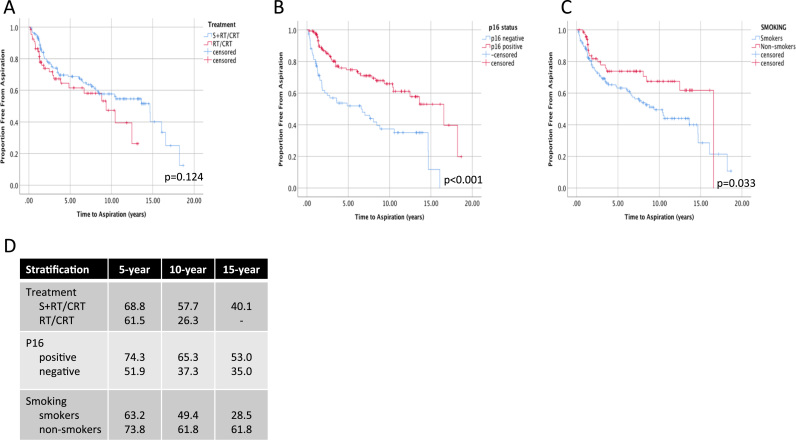
Stratification of OPSCC patients according to established predictors of survival, p16 status or smoking, showed significant differences in 10 and 15-year overall survival (OS) (Fig. 2). P16 positive patients had significantly higher OS compared than p16 negative patients (60.2% vs 29.6% at 10 years and 56.5 vs 21.2% at 15 years, p < 0.001). Non-smoking patients compared to smokers also had significantly higher long-term OS (68.7% vs 39.6% at 10 years and 68.7 vs 31.3% at 15 years, p < 0.001).
Fig. 2.
Long-term survival of patients with oropharyngeal cancer according to p16 and smoking status. Survival up to 18 years follow-up is shown for advanced stage OPSCC patients, stratified by A) p16 status and B) smoking. Patients are classified as smokers with a > 10 pack year tobacco smoking history. Log-rank p-values are show comparing differences pooled over strata. C) 5, 10 and 15-year estimates of overall survival shown for each stratum.
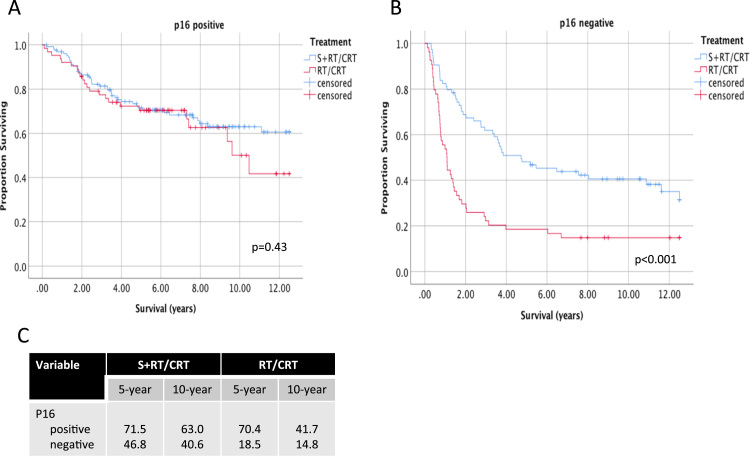
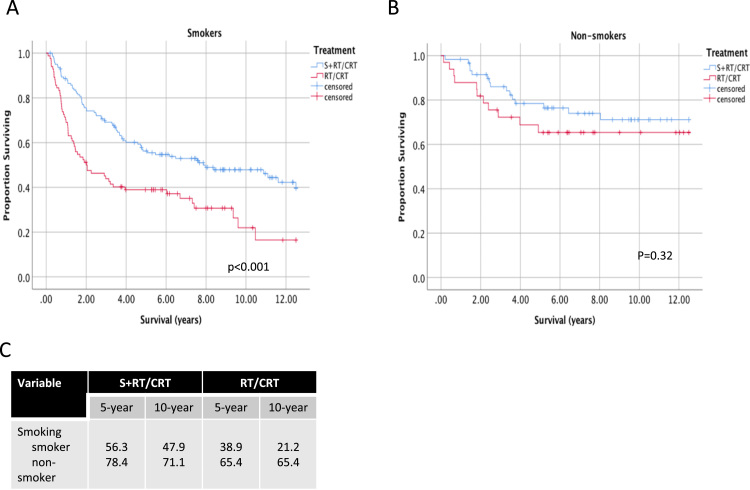
Long-term OS of OPSCC according to p16 and smoking status was further stratified according to treatment. In p16 positive patients, 5 and 10-year OS was similar between S+RT/CRT and CRT treatment groups (Fig. 3). Beyond 10 years, a trend for higher OS is seen for patients treated with S+RT/CRT. In p16 negative patients, 5 and 10-year OS was significantly higher is the S+RT/CRT treatment group (46.8% vs 18.5% at 5 years and 40.6% vs 14.8% at 10 years, p < 0.001). Primary surgery was also associated with higher OS in smokers (Fig. 4) (56.3% vs 38.9% at 5 years and 47.9 vs 21.9% at 10 years, p < 0.001). In non-smokers, no significant differences were seen in long-term OS between S+RT/CRT and CRT treatment groups.
Fig. 3.
Long-term survival of patients with oropharyngeal cancer according to treatment and p16 status. Survival up to 12.5 years follow-up is shown for advanced stage OPSCC patients, stratified according to treatment type in A) p16 positive and B) p16 negative patients. Log-rank p-values are show comparing pairwise differences between strata. C) 5 and 10-year estimates of overall survival shown for each stratum.
Fig. 4.
Long-term survival of patients with oropharyngeal cancer according to treatment and smoking status. Survival up to 12.5 years follow-up is shown for advanced stage OPSCC patients, stratified according to treatment type in A) smokers and B) non-smokers. Log-rank p-values are show comparing pairwise differences between strata. C) 5 and 10-year estimates of overall survival shown for each stratum.
Multivariate analyses of OS was performed to include age, gender, performance status, smoking, p16 status, TNM stage, treatment and aspiration (Table 3). Given a significant number of data points missing for aspiration (135/319), separate Cox regression models were performed to include either all 319 patients or those with aspiration data available, whereby aspiration is also a covariate. In both models, lower performance status (ECOG>2), and higher TNM stage were associated with a significantly higher risk of death, whereas P16 positivity and primary surgical treatment were associated with a greater than 50% reduction in risk of death. In a model that includes only patients with aspiration status available, aspiration was found to be a significant predictor of overall survival showing a reduced risk of death (HR=0.32, p = <0.001) in patients free from aspiration (Table 3). In a model that includes all patients, advanced age and smoking were associated with a significantly higher risk of death, but these covariates were not significant in a model that includes aspiration. This may suggest an interaction between aspiration and age and smoking.
Table 3.
Multivariate Cox Proportional Hazard Model of survival in 319 patients with advanced oropharyngeal squamous cell carcinoma.
|
Overall Survival, N = 319 (model without aspiration) |
||
|---|---|---|
| Covariate | Hazard Ratio (95% CI) | P |
| Age | 1.03 (1.01–1.05) | 0.007 |
| Gender (male vs female) | 1.12 (0.72–1.75) | 0.607 |
| ECOG (≥2 vs <1) | 2.46 (1.61–3.77) | < 0.001 |
| Smoking (vs non-smoker) | 2.37 (1.41–3.99) | 0.001 |
| P16 positive | 0.42 (0.28–0.61) | < 0.001 |
| TNM stage IV (vs III) | 1.68 (1.05–2.68) | 0.030 |
| Surgical treatment (vs RT/CRT) | 0.47 (0.32–0.69) | < 0.001 |
| Overall Survival N = 184 (model with aspiration data) | ||
| Covariate | Hazard Ratio (95% CI) | P |
| Age | 1.01 (0.98–1.03) | 0.591 |
| Gender (male vs female) | 0.73 (0.43–1.24) | 0.248 |
| ECOG (≥2 vs <1) | 2.1 (1.34–3.39) | 0.001 |
| Smoking (vs non-smoker) | 1.55 (0.089–2.69) | 0.118 |
| P16 positive | 0.47 (0.28–0.79) | 0.005 |
| Aspiration Free | 0.32 (0.19–0.53) | < 0.001 |
| TNM stage IV (vs III) | 2.15 (1.11–4.16) | 0.024 |
| Surgical treatment (vs RT/CRT) | 0.47 (0.32–0.69) | < 0.001 |
Cox regression models above include variables listed and performed either without (top) or with (bottom)aspiration events as a covariate. All 319 patients are included in the model that does not include aspiration as a covariate. The model with aspiration excludes 135/319 patients as aspiration data was not available for these patients.
3.3. Long-term dysphagia outcomes
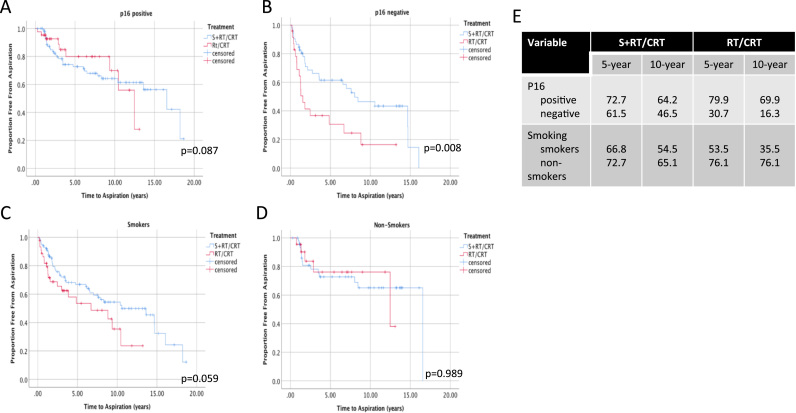
As a measure of long-term dysphagia, aspiration free survival (AFS) was estimated using Kaplan-Meier analyses. When comparing AFS between all patients treated with S+RT/CRT vs CRT, no significant differences were seen (Fig. 5 A). P16 positive and non-smoking patients had overall significantly higher AFS (Fig. 5 B, C). In p16 negative patients, S+RT/CRT was associated with higher AFS (Fig. 6, 46.5% vs 16.3% at 10 years, p = 0.008). In smokers, higher AFS was also observed with surgery (Fig. 6C), but this trend was not significant (p = 0.059).
Fig. 5.
Kaplan-Meier Estimates of Aspiration Free Survival in Patients with Oropharyngeal Cancer. The proportion of surviving patients free from aspiration post-treatment is shown stratified according to A) treatment type, B) p16 positivity and C) smoking status. P-value is shown using the Log-rank test comparing pooled differences between groups. D) 5, 10 and 15-year estimates of aspiration free survival are shown for each stratum.
Fig. 6.
Kaplan-Meier Estimates of Aspiration Free Survival in Patients with Oropharyngeal Cancer Stratified Treatment, Smoking and p16 Status. The proportion of surviving patients free from aspiration post-treatment is shown compared to surgical vs non-surgical treatments in A) p16 positive patients, B) p16 negative patients, C) smokers and D) non-smokers. P-value is shown as calculated using the Log-rank test comparing pairwise differences between strata. E) 5 and 10-year estimates of overall survival shown for each stratum.
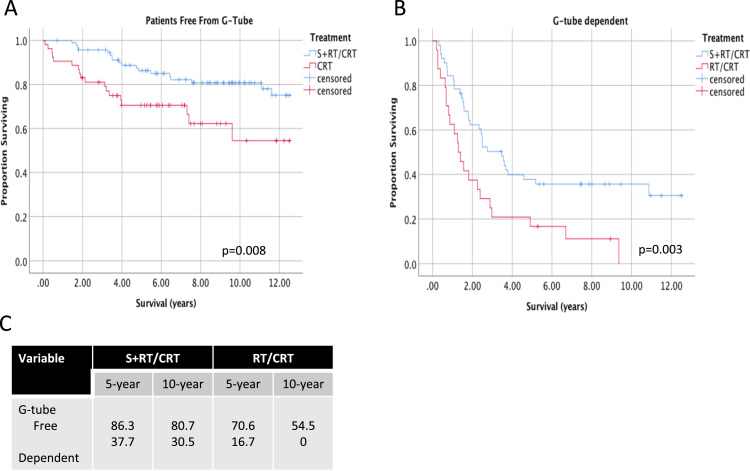
G-tube dependency was also measured as indicators of long-term dysphagia. Between treatment groups, no significant differences were seen in rates of long-term g-tube dependency (Supplementary Table 1). Significant differences in long-term OS were seen according to treatment group when stratified by g-tube dependency. All patients who were free from g-tube in the first 5 years post-treatment had improved survival. S+RT/CRT treatment was associated with significantly higher survival outcomes regardless of whether patients were g-tube dependent (Fig. 7).
Fig. 7.
Long-term survival of patients with oropharyngeal cancer stratified by G-tube dependency. Survival up to 12.5 years follow-up is shown for advanced stage OPSCC patients, stratified according to treatment type in A) patients who were free from g-tube > 1 year post-treatment and B) g-tube dependent > 1 year post-treatment. C) 5 and 10-year estimates of overall survival shown for each stratum.
4. Discussion
With the expanding population of young HPV-OPSCC survivors, understanding long-term survival and treatment sequelae in these patients is an important first step in addressing their needs. Long-term outcome studies of OPSCC survival, dysphagia and causes of death have been reported others, also with retrospective cohorts. Few studies have compared surgical and non-surgical treatments and even fewer have included HPV/p16 typing. This study is unique, as the first to report long-term outcomes on a large cohort of OPSCC patients comparing surgical and non-surgical treatments, while incorporating p16 and smoking status.
To date, long-term survival outcomes studies in OPSCC have been limited to retrospective studies and few have included HPV/p16 status [39]. Five-year OS for OPSCC have ranged from 46% to 85% including all stages [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49] and 40–85% in advanced stage cohorts [50], [51], [52], [53]. The wide range OS estimates likely reflect significant differences in clinicopathologic features and treatments between these cohorts. Studies that have reported long-term survival of OPSCC according to HPV status, with 5 and 10 year OS estimates of 77–89% [6], [54] and 50–85% [6], [39] respectively. Consistent with these studies our data estimates 5 and 10-year OS for p16 positive patients to be 71.2% and 60.2% for advanced stage OPSCC. Our study is unique not only in comparing large cohorts of surgically vs non-surgically treated OPSCC patients but also includes several important prognostic variables. In follow-up to our previous study comparing survival outcomes < 5 years post-treatment with a smaller cohort [14], this study shows smokers and p16 negative patients have improved long-term outcomes when treated with primary surgical approaches.
P16 positive versus negative OPSCCs are biologically and clinically distinct, with differences in prognostic factors [24], [25], [26], [55], [56], [57], [58], [59], [60], [61], [62]. In addition to p16 status, variables such as age, advanced stage, performance status and history of tobacco use have an increased risk of death and are important to consider when comparing survival analyses [6], [14], [27], [63], [64], [65]. Inclusion of smoking as a strong independent predictor or survival should perhaps be considered as important as p16 status. In both surgical and non-surgically treated OPCCC cohorts, several groups have shown that p16 positive smokers have worse survival than p16 positive non-smokers [1], [4], [14], [27], [66]. In our multivariate analysis of long-term OS, comparison of treatment modalities showed primary surgery was associated with a significant reduction in risk of death (HR=0.47), similar to an independent study [6].
G-tube dependence in our study was higher for CRT as compared to surgery one year post treatment and higher in the surgical cohort 5 years following treatment. This likely reflects a higher propensity for prophylactic gastronomy tube placement by the radiation oncologists to mitigate acute treatment toxicities. Similar findings were identified by Sharma et al. [67] who compared g-tube dependence in nonsurgical and transoral robotic surgery (TORS) patients. They identified increased rates of g-tube dependency among patients treated with CRT/RT at 3 and 6-months post-treatment, with no difference between groups at one year. Subjective dysphagia was not assessed in this study but previous studies have shown high rates of self-reported dysphagia [18], [68], [69], [70] following chemoradiation therapy with short and long-term follow-up after therapy.
Unique to this study is the long-term (> 10 year) follow-up for survival and dysphagia outcomes. One other study, by Kraaijenga [69] et al. identified 22 head and neck cancer survivors treated with chemoradiation therapy with a median follow-up of 11 years. They found 56% of patients had moderate to severe swallowing dysfunction with VFSS evaluations showing penetration or aspiration in 68% of patients. Our results suggest similar findings with 40–50% of survivors with evidence of some degree of aspiration on VFSS at 10-years post treatment.
A strong correlation has been identified between radiation dosing and dysphagia outcomes, with some improvement in subjective and objective swallowing function with the implementation of IMRT [27], [29], [30]. A prospective, longitudinal study by Eisbruch et al. [71] identified a TD50 (toxic dose of 50%) of 63 Gy, and a TD25 of 56 Gy for the pharyngeal constrictor muscles to be associated with a higher rate of aspiration. In the current study, patients in the RT/CRT cohort received an average of 64.5 Gy, exceeding the TD50; and patients treated with primary surgery and adjuvant therapy received on average 55 Gy. Ongoing clinical trials are examining oncologic and function outcomes in patients treated with conventional (70 Gy IMRT) and low dose (54–56 Gy) dosing in patients with HPV-related OPSCC following surgical resection [72], [73]. The outcomes of these studies will help to guide optimal radiation dosing in OPSCC.
Although this study provides insight into surgical and non-surgical treatment outcomes in OPSCC, randomized clinical trials are currently underway which may provide higher level evidence on this topic. The NRG HN002 trial is comparing survival and dysphagia outcomes of p16 positive non-smokers randomized to receive either IMRT or IMRT with cisplatin [74]. The Eastern Cooperative Oncology Group 3311 trial is addressing the potential for personalized adjuvant treatments in p16 positive OPSCC patients [75]. Based on post-operative pathologic risk stratification, patients may be observed, treated with low-dose or standard dose radiation with or without chemotherapy. The ORATOR randomized phase II trial will compare quality of life and functional outcomes of OPSCC patients treated with either TORS or RT [76], [77]. The outcomes of these trials will likely provide important guidance for the optimal treatment of HPV/p16 positive OPSCC.
The majority of post-treatment dysphagia research to date has been in the nonsurgical literature. One recent article by Dale et al. identified functional outcomes in patients treated with primary surgery [78]. They found similar functional scores on swallowing questionnaires to studies in the chemoradiation literature. Given the wide variability in chemotherapy, radiation therapy and surgical approaches (lip-split mandibulotomy versus transoral); comparisons between the cohorts, can be challenging; however, survival and functional outcomes must be assessed to optimize therapy for patients.
Results of this study should be interpreted with an acknowledgement of the following important limitations. Inherent to the retrospective study design, some data points may be inaccurate or incomplete, especially with respect to dysphagia outcomes. Our data reports outcomes from a single institution, which may not translate to other centers with different treatment approaches. The management of patients from 1998 to 2012 in this study remained largely unchanged, with no significant alterations in surgical approach or post-treatment swallowing rehabilitation. Decisions to treat patients either surgically or non-surgically was largely based on subjective patient preferences, however, bias from treating physicians, whether intentional or unintentional may have influenced treatment pathways. As such, S+RT/CRT and CRT groups are not perfectly balanced in covariates and factors which confound outcome measurements. However, a multivariate analysis including the most important known covariates supports survival outcomes demonstrated in univariate analyses. Classification of HPV positive vs negative OPSCC was done using p16 immunohistochemistry, estimated to have a 5–10% false positive and/or negative rate [54], [55], [62]. P16 staining is nevertheless widely used clinically as an excellent predictor of outcomes [39], [79], and is a recommended in the current NCCN guidelines [80].
5. Conclusions
In smokers and p16-negative OPSCC patients, primary surgery may be associated with improved long-term survival and dysphagia-related outcomes. However, given the imbalanced patient characteristics between treatment groups compared in this retrospective analysis, further prospective studies are suggested to validate these findings.
Acknowledgements
Not applicable.
Acknowledgments
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Ethics approval and consent to participate
Ethics approval for this study was obtained from the University of Alberta Health Ethics Research Board protocol (Pro00016426 HREBA.CC-16–0829).
Consent for publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from the Alberta Cancer Registry but restrictions apply to the availability of these data including health ethics approval obtained for the current study, and so are not publicly available.
Declarations of interest
None.
Funding
Funding for this study was obtained from the Alberta Head and Neck Centre for Oncology and Reconstruction Foundation.
Authors contributions
JMC was involved in all aspects of experimental design, data collection, data analysis and the primary contributor in manuscript preparation. EMH participated in data collection. DAO, JH and HS were involved in data collection and manuscript preparation. VLB is the primary investigator and was involved in experimental design, data collection, data analysis and manuscript preparation. All authors read and approved the final manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2018.09.002.
Contributor Information
Jessica M. Clark, Email: jmclark@ualberta.ca.
Emma M. Holmes, Email: emholmes@ucalgary.ca.
Jeffrey Harris, Email: Jeffrey.Harris@albertahealthservices.ca.
Hadi Seikaly, Email: Hadi.Seikaly@albertahealthservices.ca.
Vincent L. Biron, Email: vbiron@ualberta.ca.
Appendix A. Supplementary material
Supplementary material Figure 1. Comparison of survival of patients included and excluded in this study. Kaplan-Meier estimates of overall survival up to 12.5 years follow-up is shown for advanced stage OPSCC patients included in this study and those excluded due to lack of available p16 immunohistochemistry. Long-rank value is shown for comparison of groups pooled over strata.
Supplementary material
References
- 1.Chaturvedi A.K., D'Souza G., Gillison M.L., Katki H.A. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral. Oncol. 2016;60:61–67. doi: 10.1016/j.oraloncology.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison M.L., Restighini C. Anticipation of the impact of human papillomavirus on clinical decision making for the head and neck cancer patient. Hematol. Oncol. Clin. North Am. 2015;29:1045–1060. doi: 10.1016/j.hoc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Gillison M.L., Chaturvedi A.K., Anderson W.F., Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. Am. Soc. Clin. Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturgis E.M., Ang K.K. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J. Natl. Compr. Canc. Netw. 2011;9:665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 5.Xie L., Semenciw R., Mery L. Cancer incidence in Canada: trends and projections (1983–2032) Health Promot. Chronic Dis. Prev. Can. 2015;35(Suppl 1):2–186. [PubMed] [Google Scholar]
- 6.Lin B.M., Wang H., D'Souza G., Zhang Z., Fakhry C., Joseph A.W. Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer. 2013;119:3462–3471. doi: 10.1002/cncr.28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Souza G., Gross N.D., Pai S.I., Haddad R., Anderson K.S., Rajan S. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. Am. Soc. Clin. Oncol. 2014;32:2408–2415. doi: 10.1200/JCO.2014.55.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi A.K., Engels E.A., Anderson W.F., Gillison M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. 〈http://ascopubs.org/doi/10.1200/JCO.2007.14.1713〉 American Society of Clinical Oncology (Available from) [DOI] [PubMed] [Google Scholar]
- 9.Fakhry C., Andersen K.K., Eisele D.W., Gillison M.L. Oropharyngeal cancer survivorship in Denmark, 1977–2012. Oral. Oncol. 2015;51:982–984. doi: 10.1016/j.oraloncology.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Patel M.A., Blackford A.L., Rettig E.M., Richmon J.D., Eisele D.W., Fakhry C. Rising population of survivors of oral squamous cell cancer in the United States. Cancer. 2016;122:1380–1387. doi: 10.1002/cncr.29921. [DOI] [PubMed] [Google Scholar]
- 11.Bernier J., Domenge C., Ozsahin M., Matuszewska K., Lefèbvre J.-L., Greiner R.H. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004;(350):1945–1952. doi: 10.1056/NEJMoa032641. 〈http://www.nejm.org/doi/abs/10.1056/NEJMoa032641〉 Massachusetts Medical Society (Available from) [DOI] [PubMed] [Google Scholar]
- 12.Boscolo-Rizzo P., Gava A., Baggio V., Marchiori C., Stellin M., Fuson R. Matched survival analysis in patients with locoregionally advanced resectable oropharyngeal carcinoma: platinum-based induction and concurrent chemoradiotherapy versus primary surgical resection. Int. J. Radiat. Oncol.*Biol.*Phys. 2011;80:154–160. doi: 10.1016/j.ijrobp.2010.01.032. 〈http://linkinghub.elsevier.com/retrieve/pii/S036030161000132X〉 [DOI] [PubMed] [Google Scholar]
- 13.Lacau St Guily J., Rousseau A., Baujat B., Périé S., Schultz P., Barry B. Oropharyngeal cancer prognosis by tumour HPV status in France: the multicentric Papillophar study. Oral. Oncol. 2017;67:29–36. doi: 10.1016/j.oraloncology.2017.01.012. 〈http://linkinghub.elsevier.com/retrieve/pii/S1368837517300209〉 (Available from) [DOI] [PubMed] [Google Scholar]
- 14.Seikaly H., Biron V.L., Zhang H., O'Connell D.A., Côté D.W.J., Ansari K. Role of primary surgery in the treatment of advanced oropharyngeal cancer. Head Neck. 2016;38(Suppl 1):E571–E579. doi: 10.1002/hed.24042. [DOI] [PubMed] [Google Scholar]
- 15.Zenga J., Wilson M., Adkins D.R., Gay H.A., Haughey B.H., Kallogjeri D. Treatment outcomes for T4 oropharyngeal squamous cell carcinoma. JAMA Otolaryngol. Head. Neck Surg. 2015;141 doi: 10.1001/jamaoto.2015.0764. 〈http://archotol.jamanetwork.com/article.aspx?doi=10.1001/jamaoto.2015.0764〉 (Available from) (1118–10) [DOI] [PubMed] [Google Scholar]
- 16.Baxi S.S., Pinheiro L.C., Patil S.M., Pfister D.G., Oeffinger K.C., Elkin E.B. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–1513. doi: 10.1002/cncr.28588. 〈http://doi.wiley.com/10.1002/cncr.28588〉 (7 ed) (Available from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess C.B., Rash D.L., Daly M.E., Farwell D.G., Bishop J., Vaughan A.T. Competing causes of death and medical comorbidities among patients with human papillomavirus–positive vs human papillomavirus–negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol. Head Neck Surg. 2014;140 doi: 10.1001/jamaoto.2013.6732. 〈http://archotol.jamanetwork.com/article.aspx?doi=10.1001/jamaoto.2013.6732〉 (Available from) (312–11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funk G.F., Karnell L.H., Christensen A.J. Long-term health-related quality of life in survivors of head and neck cancer. Arch. Otolaryngol. Head. Neck Surg. 2012;138:123–133. doi: 10.1001/archoto.2011.234. 〈http://archotol.jamanetwork.com/article.aspx?doi=10.1001/archoto.2011.234〉 American Medical Association (Available from) [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal D.I., Lewin J.S., Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and NeckCancer. J. Clin. Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. 〈http://ascopubs.org/doi/10.1200/JCO.2006.06.0079〉 (Available from) [DOI] [PubMed] [Google Scholar]
- 20.Hutcheson K.A., Lewin J.S., Barringer D.A., Lisec A., Gunn G.B., Moore M.W.S. Vol. 118. 2012. Late dysphagia after radiotherapy-based treatment of head and neck cancer; pp. 5793–5799.〈http://doi.wiley.com/10.1002/cncr.27631〉 (Cancer). (Available from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter K.U., Lee O.E., Lyden T.H., Haxer M.J., Feng F.Y., Schipper M. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head Neck. 2013:120–125. doi: 10.1002/hed.23275. 〈http://doi.wiley.com/10.1002/hed.23275〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen N.P., Frank C., Moltz C.C., Vos P., Smith H.J., Bhamidipati P.V. Aspiration rate following chemoradiation for head and neck cancer: an underreported occurrence. Radiother. Oncol. 2006;80:302–306. doi: 10.1016/j.radonc.2006.07.031. 〈http://linkinghub.elsevier.com/retrieve/pii/S0167814006003100〉 (Available from) [DOI] [PubMed] [Google Scholar]
- 23.O'Connell D., Seikaly H., Murphy R., Fung C., Cooper T., Knox A. Primary surgery versus chemoradiotherapy for advanced oropharyngeal cancers: a longitudinal population study. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2013;42:31. doi: 10.1186/1916-0216-42-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu C.C., Biron V.L., Puttagunta L., Seikaly H. HPV status and second primary tumours in oropharyngeal squamous cell carcinoma. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2013;42:36. doi: 10.1186/1916-0216-42-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper T., Biron V.L., Adam B., Klimowicz A.C., Puttagunta L., Seikaly H. Association of keratinization with 5-year disease-specific survival in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol. Head. Neck Surg. 2015;141:250–256. doi: 10.1001/jamaoto.2014.3335. American Medical Association. [DOI] [PubMed] [Google Scholar]
- 26.Cooper T., Biron V., Adam B., Klimowicz A.C., Puttagunta L., Seikaly H. Prognostic utility of basaloid differentiation in oropharyngeal cancer. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2013;42:57. doi: 10.1186/1916-0216-42-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. Massachusetts Medical Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 7 Ed. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 29.Dziegielewski P.T., O'Connell D.A., Rieger J., Harris J.R., Seikaly H. The lip-splitting mandibulotomy: aesthetic and functional outcomes. Oral. Oncol. 2010;46:612–617. doi: 10.1016/j.oraloncology.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Dziegielewski P.T., Mlynarek A.M., Dimitry J., Harris J.R., Seikaly H. Vol. 119. Laryngoscope. Wiley Subscription Services, Inc., A Wiley Company; 2009. pp. 2369–2375. (The Mandibulotomy: Friend or Foe? Safety Outcomes and Literature Review). [DOI] [PubMed] [Google Scholar]
- 31.Biron V.L., O'Connell D.A., Barber B., Clark J.M., Andrews C., Jeffery C.C. Transoral robotic surgery with radial forearm free flap reconstruction: case control analysis. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2017;46:20. doi: 10.1186/s40463-017-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha N., Harris J., Seikaly H., Jacobs J.R., McEwan A.J.B., Robbins K.T. A phase II study of submandibular gland transfer prior to radiation for prevention of radiation-induced xerostomia in head-and-neck cancer (RTOG 0244) Int. J. Radiat. Oncol. Biol.* Phys. 2012;84:437–442. doi: 10.1016/j.ijrobp.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Qahtani K., Rieger J., Harris J.R., Mlynarek A., Williams D., Islam T. Treatment of base of tongue cancer, stage III and stage IV with primary surgery: survival and functional outcomes. Eur. Arch. Otorhinolaryngol. 2015;272:2027–2033. doi: 10.1007/s00405-014-3140-1. Springer Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
- 34.Vaz J.A., Côté D.W.J., Harris J.R., Seikaly H. Outcomes of free flap reconstruction in the elderly. Head Neck. 2013;35:884–888. doi: 10.1002/hed.23057. [DOI] [PubMed] [Google Scholar]
- 35.Seikaly H., Rieger J., O'Connell D., Ansari K., Alqahtani K., Harris J. Beavertail modification of the radial forearm free flap in base of tongue reconstruction: technique and functional outcomes. Head Neck. 2009:213–219. doi: 10.1002/hed.20953. [DOI] [PubMed] [Google Scholar]
- 36.Seikaly H., Rieger J., Zalmanowitz J., Tang J.L., Alkahtani K., Ansari K. Functional soft palate reconstruction: a comprehensive surgical approach. Head Neck. 2008:1615–1623. doi: 10.1002/hed.20919. [DOI] [PubMed] [Google Scholar]
- 37.Clark T.G., Bradburn M.J., Love S.B., Altman D.G. Survival analysis part I: basic concepts and first analyses. Br. J. Cancer. 2003:232–238. doi: 10.1038/sj.bjc.6601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradburn M.J., Clark T.G., Love S.B., Altman D.G. Survival analysis part II: multivariate data analysis--an introduction to concepts and methods. Br. J. Cancer. 2003:431–436. doi: 10.1038/sj.bjc.6601119. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dale O.T., Sood S., Shah K.A., Han C., Rapozo D., Mehanna H. Long-term survival outcomes in patients with surgically treated oropharyngeal cancer and defined human papilloma virus status. J. Laryngol. Otol. 2016:1048–1053. doi: 10.1017/S0022215116009099. [DOI] [PubMed] [Google Scholar]
- 40.Röösli C., Tschudi D.C., Studer G., Braun J., Stoeckli S.J. Outcome of patients after treatment for a squamous cell carcinoma of the oropharynx. Laryngoscope. 2009:534–540. doi: 10.1002/lary.20033. [DOI] [PubMed] [Google Scholar]
- 41.Preuss S.F., Dinh V., Klussmann J.P., Semrau R., Mueller R.-P., Guntinas-Lichius O. Outcome of multimodal treatment for oropharyngeal carcinoma: a single institution experience. Oral. Oncol. 2007;43:402–407. doi: 10.1016/j.oraloncology.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Lim Y.C., Hong H.J., Baek S.J., Park J.H., Kim G.E., Lee C.G. Combined surgery and postoperative radiotherapy for oropharyngeal squamous cell carcinoma in Korea: analysis of 110 cases. Int. J. Oral. Maxillofac. Surg. 2008;37:1099–1105. doi: 10.1016/j.ijom.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Zelefsky M.J., Harrison L.B., Armstrong J.G. Long-term treatment results of postoperative radiation therapy for advanced stage oropharyngeal carcinoma. Cancer. 1992;70:2388–2395. doi: 10.1002/1097-0142(19921115)70:10<2388::aid-cncr2820701003>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Jones A.S., Fenton J.E., Husband D.J. The treatment of squamous cell carcinoma of the tonsil with neck node metastases. Head Neck. 2003:24–31. doi: 10.1002/hed.10183. Wiley Subscription Services, Inc., A Wiley Company. [DOI] [PubMed] [Google Scholar]
- 45.Mak-Kregar S., Hilgers F.J., Levendag P.C., Manni J.J., Hart A.A., Visser O. Disease-specific survival and locoregional control in tonsillar carcinoma. Clin. Otolaryngol. Allied Sci. 1996;21:550–556. doi: 10.1111/j.1365-2273.1996.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 46.Machtay M., Perch S., Markiewicz D., Thaler E., Chalian A., Goldberg A. Combined surgery and postoperative radiotherapy for carcinoma of the base of radiotherapy for carcinoma of the base of tongue: analysis of treatment outcome and prognostic value of margin status. Head. Neck. 1997;19:494–499. doi: 10.1002/(sici)1097-0347(199709)19:6<494::aid-hed6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 47.Foote R.L., Olsen K.D., Davis D.L., Buskirk S.J., Stanley R.J., Kunselman S.J. Base of tongue carcinoma: patterns of failure and predictors of recurrence after surgery alone. Head. Neck. 1993;15:300–307. doi: 10.1002/hed.2880150406. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann M., Saleh-Ebrahimi L., Zwicker F., Haering P., Schwahofer A., Debus J. Long term results of postoperative Intensity-Modulated Radiation Therapy (IMRT) in the treatment of Squamous Cell Carcinoma (SCC) located in the oropharynx or oral cavity. Radiat. Oncol. BioMed. Cent. 2015;10:251. doi: 10.1186/s13014-015-0561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwana M.S., Wu J., Hay J., Wong F., Cheung W., Olson R.A. 25 year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral. Oncol. 2014;50:651–656. doi: 10.1016/j.oraloncology.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Bastos de Souza T.R., Pinto C.A.L., da Cunha Mercante A.M., Nishimoto I.N., Brasilino de Carvalho M., Kowalski L.P. Long-term results of surgical treatment for advanced oropharyngeal squamous cell carcinoma. Head Neck. 2014;36:1146–1154. doi: 10.1002/hed.23427. (6 ed) [DOI] [PubMed] [Google Scholar]
- 51.DeNittis A.S., Liu L., Rosenthal D.I., Machtay M. Nasopharyngeal carcinoma treated with external radiotherapy, brachytherapy, and concurrent/adjuvant chemotherapy. Am. J. Clin. Oncol. 2002;25:93–95. doi: 10.1097/00000421-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Poulsen M., Porceddu S.V., Kingsley P.A., Tripcony L., Coman W. Locally advanced tonsillar squamous cell carcinoma: treatment approach revisited. Laryngoscope. 2007:45–50. doi: 10.1097/01.mlg.0000243044.91193.32. [DOI] [PubMed] [Google Scholar]
- 53.Moore E.J., Henstrom D.K., Olsen K.D., Kasperbauer J.L., McGree M.E. Transoral resection of tonsillar squamous cell carcinoma. Laryngoscope. 2009:508–515. doi: 10.1002/lary.20124. Wiley Subscription Services, Inc., A Wiley Company. [DOI] [PubMed] [Google Scholar]
- 54.Larsen C.G., Jensen D.H., Carlander A.-L.F., Kiss K., Andersen L., Olsen C.H. Novel nomograms for survival and progression in HPV+ and HPV- oropharyngeal cancer: a population-based study of 1,542 consecutive patients. Oncotarget. Impact J. 2016;7:71761–71772. doi: 10.18632/oncotarget.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biron V.L., Kostiuk M., Isaac A., Puttagunta L., O'Connell D.A., Harris J. Detection of human papillomavirus type 16 in oropharyngeal squamous cell carcinoma using droplet digital polymerase chain reaction. Cancer. 2016;122:1544–1551. doi: 10.1002/cncr.29976. [DOI] [PubMed] [Google Scholar]
- 56.Biron V.L., Mohamed A., Hendzel M.J., Alan Underhill D., Seikaly H. Epigenetic differences between human papillomavirus-positive and -negative oropharyngeal squamous cell carcinomas. J. Otolaryngol. Head. Neck Surg. 2012;41(Suppl 1):S65–S70. [PubMed] [Google Scholar]
- 57.Biron V.L., Côté D.W.J., Seikaly H. Oropharyngeal squamous cell carcinoma and human papillomavirus-associated cancers in women: epidemiologic evaluation of association. J. Otolaryngol. Head. Neck Surg. 2011;40(Suppl 1):S65–S69. [PubMed] [Google Scholar]
- 58.Clark J., Jeffery C.C., Zhang H., Cooper T., O'Connell D.A., Harris J. Correlation of PET-CT nodal SUVmax with p16 positivity in oropharyngeal squamous cell carcinoma. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2015;44:37. doi: 10.1186/s40463-015-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barber B., Dergousoff J., Slater L., Harris J., O'Connell D., El-Hakim H. Depression and survival in patients with head and neck cancer: a systematic review. JAMA Otolaryngol. Head. Neck Surg. 2016;142:284–288. doi: 10.1001/jamaoto.2015.3171. American Medical Association. [DOI] [PubMed] [Google Scholar]
- 60.Lindsay C., Seikaly H., Biron V.L. Epigenetics of oropharyngeal squamous cell carcinoma: opportunities for novel chemotherapeutic targets. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2017;46:9. doi: 10.1186/s40463-017-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Idris S., Lindsay C., Kostiuk M., Andrews C., Côté D.W.J., O'Connell D.A. Investigation of EZH2 pathways for novel epigenetic treatment strategies in oropharyngeal cancer. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2016;45:54. doi: 10.1186/s40463-016-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isaac A., Kostiuk M., Zhang H., Lindsay C., Makki F., O'Connell D.A. Ultrasensitive detection of oncogenic human papillomavirus in oropharyngeal tissue swabs. J. Otolaryngol. Head. Neck Surg. BioMed. Cent. 2017;46:5. doi: 10.1186/s40463-016-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris B.N., Biron V.L., Donald P., Farwell D.G., Luu Q.C., Bewley A.F. Primary surgery vs chemoradiation treatment of advanced-stage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol. Head. Neck Surg. 2015;141:636–640. doi: 10.1001/jamaoto.2015.0659. American Medical Association. [DOI] [PubMed] [Google Scholar]
- 64.Hanasoge S., Magliocca K.R., Switchenko J.M., Saba N.F., Wadsworth J.T., El-Deiry M.W. Clinical outcomes in elderly patients with human papillomavirus-positive squamous cell carcinoma of the oropharynx treated with definitive chemoradiation therapy. Head Neck. 2016;38:846–851. doi: 10.1002/hed.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fakhry C., Zhang Q., Nguyen-Tân P.F., Rosenthal D., El-Naggar A., Garden A.S. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. Am. Soc. Clin. Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar B., Cordell K.G., Lee J.S., Worden F.P., Prince M.E., Tran H.H. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J.Clin. Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma A., Patel S., Baik F.M., Mathison G., Pierce B.H.G., Khariwala S.S. Survival and gastrostomy prevalence in patients with oropharyngeal cancer treated with transoral robotic surgery vs chemoradiotherapy. JAMA Otolaryngol Head Neck Surg. 2016;142:691–697. doi: 10.1001/jamaoto.2016.1106. 〈http://archotol.jamanetwork.com/article.aspx?doi=10.1001/jamaoto.2016.1106〉 (Available from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng F.Y., Kim H.M., Lyden T.H., Haxer M.J., Worden F.P., Feng M. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J. Clin. Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. 〈http://ascopubs.org/doi/10.1200/JCO.2009.24.6199〉 (Available from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kraaijenga S.A.C., Oskam I.M., van der Molen L., Hamming-Vrieze O., Hilgers F.J.M., van den Brekel M.W.M. Vol. 51. 2015. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer; pp. 787–794.〈http://linkinghub.elsevier.com/retrieve/pii/S1368837515002109〉 (Oral Oncol.). Elsevier (Available from) [DOI] [PubMed] [Google Scholar]
- 70.Vainshtein J.M., Moon D.H., Feng F.Y., Chepeha D.B., Eisbruch A., Stenmark M.H. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015;91:925–933. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisbruch A., Kim H.M., Feng F.Y., Lyden T.H., Haxer M.J., Feng M. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int. J. Radiat. Oncol.*Biol.*Phys. 2011:e93–e99. doi: 10.1016/j.ijrobp.2010.12.067. 〈http://linkinghub.elsevier.com/retrieve/pii/S0360301611000721〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goepfert R.P., Yom S.S., Ryan W.R., Cheung S.W. Development of a chemoradiation therapy toxicity staging system for oropharyngeal carcinoma. Laryngoscope. 2015;125:869–876. doi: 10.1002/lary.25023. [DOI] [PubMed] [Google Scholar]
- 73.Owadally W., Hurt C., Timmins H., Parsons E., Townsend S., Patterson J. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer. 2015;15:602. doi: 10.1186/s12885-015-1598-x. 3rd ed. (BioMed. Central) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.N.R.G. HN002. httpsclinicaltrials.govctshowNCTtermhnrank.
- 75.ECOG 3311 [Internet]. httpsclinicaltrials.govctshowNCTtermecogrank. [cited 2017 Sep 6]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT01898494?Term=ecog+3311&rank=1〉.
- 76.Nichols A.C., Yoo J., Hammond J.A., Fung K., Winquist E., Read N. Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs. Trans-Oral Robotic Surgery (ORATOR)--study protocol for a randomized phase II trial. BMC Cancer. 2013;13:133. doi: 10.1186/1471-2407-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howard J., Masterson L., Dwivedi R.C., Riffat F., Benson R., Jefferies S. Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma. In: Howard J., editor. Cochrane Database System Reviews. John Wiley & Sons, Ltd; Chichester, UK: 2016. p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dale O.T., Han C., Burgess C.A., Eves S., Harris C.E., White P.L. Long-term functional outcomes in surgically treated patients with oropharyngeal cancer. Laryngoscope. 2015;125:1637–1643. doi: 10.1002/lary.25226. [DOI] [PubMed] [Google Scholar]
- 79.Chung C.H., Zhang Q., Kong C.S., Harris J., Fertig E.J., Harari P.M. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. Am. Soc. Clin. Oncol. 2014;32:3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lydiatt W.M., Patel S.G., O'Sullivan B., Brandwein M.S., Ridge J.A., Migliacci J.C. Head and neck cancers-major changes in the American joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67 doi: 10.3322/caac.21389. (7 ed.) (122–37) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Figure 1. Comparison of survival of patients included and excluded in this study. Kaplan-Meier estimates of overall survival up to 12.5 years follow-up is shown for advanced stage OPSCC patients included in this study and those excluded due to lack of available p16 immunohistochemistry. Long-rank value is shown for comparison of groups pooled over strata.
Supplementary material
Data Availability Statement
The data that support the findings of this study are available from the Alberta Cancer Registry but restrictions apply to the availability of these data including health ethics approval obtained for the current study, and so are not publicly available.