Abstract
Trypanosoma brucei relies on two types of variant surface glycoprotein (VSG) expression sites (ESs) for RNA polymerase I (Pol I) transcription of VSG pre-mRNA. Trypanosomes developing into infectious metacyclic cells in the tsetse vector use metacyclic VSG ESs (MESs) and proliferating parasites in the mammalian host deploy bloodstream VSG ESs (BESs). Unlike the monocistronic MESs, BESs are polycistronic and their highly conserved promoters differ considerably from the MES promoters. The significance of the divergent sequences of MES and BES promoters remains to be determined. We used a reporter system to specifically test the effect of temperature on the activity of MES and BES promoters in procyclic trypanosomes and our results demonstrate that transcription from the MES promoter is largely insensitive to changes in temperature. In contrast, the BES promoter drives rapid activation of transcription upon a change of temperature from 28°C to 37°C. Additionally, endogenous BESs respond similarly to the elevation of temperature and initiate increased production of BES pre-mRNA and mRNA. Our results indicate that the sequence of the BES promoter is a specificity signal which triggers BES activation in vivo upon entry into the mammalian host.
Keywords: Trypanosoma brucei, transcription, VSG, expression site, promoter
Graphical abstract
Trypanosoma brucei rhodesiense is one of the two subspecies of the protozoan parasite T. brucei that cause human African trypanosomiasis. All members of the T. brucei group use a monolayer of variant surface glycoproteins (VSGs) on their exterior to outmaneuver the mammalian immune system by switching the expressed VSG [1,2]. Only a single VSG gene is highly active at any given time point from a large repertoire of VSG sequences in the T. brucei genome [3]. Although definitive explanation for the molecular mechanism driving monoallelic VSG expression is still elusive, the localization of the active VSG gene in an extranucleolar focus called expression site body (ESB) [4] is a defining feature of the active gene. RNA polymerase I (Pol I) transcribes the expressed VSG gene from a chromosome locus termed VSG expression site (ES), positioned adjacent to a telomere [1,2]. Two types of VSG ESs exist in the T. brucei genome. In the tsetse fly vector salivary glands trypanosomes developing to metacyclic cells use exclusively metacyclic VSG ESs (MESs) for the de novo synthesis of a VSG coat on the parasite surface, a critical aspect of their development leading to acquisition of infectivity. A ubiquitous characteristic of MESs is that they are short (3 to 6 kb) and monocistronic, i.e. there are no additional coding sequences between the MES promoter and the telomere except the VSG gene [5]. The “minimalistic” nature of MESs likely lowers the metabolic costs for synthesizing the VSG coat in trypanosomes that are in the process of acquiring transmission competence accompanied by a transition to quiescence [6]. In contrast, dividing bloodstream form trypanosomes in the mammalian host use bloodstream ESs (BESs) to transcribe the expressed VSG in any single cell. BESs are up to 60 kb long and polycistronic, containing multiple expression site associated genes (ESAGs) and a long run of 70 bp repeats in addition to the single telomeric VSG gene [1,2]. Even though recent evidence supports recombinatorial transfer of metacyclic VSGs to the active BES [7], on rare occasions, a MES can be active for VSG expression in dividing bloodstream T. brucei [8].
Although both metacyclic and bloodstream VSG ESs are transcribed by the multifunctional T. brucei RNA polymerase I (Pol I), the BES and MES promoter sequences differ considerably (Fig. 1). The highly conserved BES promoter present in all of the ~15 BESs is shorter than 70 bp [9] and binds a multi-subunit protein complex named Class I Transcription Factor A (CITFA), which also recognizes the longer procyclin and ribosomal RNA Pol I promoters [10]. The MES promoters are also short and exhibit a higher degree of heterogeneity [3,5,11], compared to the BES promoters. Despite their different sequences, MES and BES promoters are nevertheless recognized with similar high affinity by the transcription factor CITFA [12]. This factor was shown to be indispensable for transcription of rRNA, procyclin and BES VSG genes in T. brucei [10,13–15] and was demonstrated to be present not only in the nucleolus but also in the VSG ESB [14–16]. In addition, promoter occupancy of the active BES by CITFA is higher than that of silent BESs, likely reflecting a critical role for CITFA in BES activation [16].
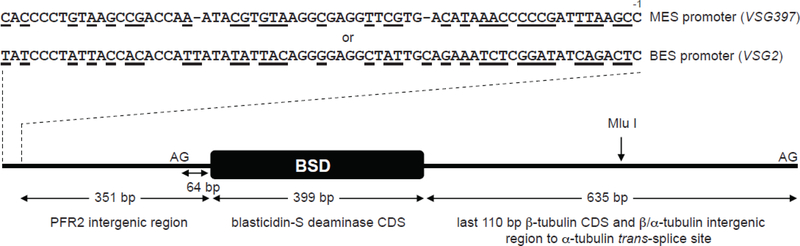
Fig. 1.
A diagram of the ES promoter reporter constructs. The difference between the two cassettes is only the identity of the promoter, MES (from the T. brucei Lister 427 VSG397 ES) or BES (from the T. brucei Lister 427 VSG2 ES). Different nucleotides between the two promoters are underlined and the position immediately upstream of the Pol I transcription start site is marked with −1. The backbone of the constructs is pBluescript II KS(+). AGs indicate the trans-splice sites upstream and downstream of the BSD coding sequence. The Mlu I restriction site present in the β/α-tubulin intergenic region was used to linearize the plasmids prior to transfection.
The reason for the divergent sequences of MES and BES promoters has so far remained unclear. Possible roles could include acting as a specificity signal for MES activation during metacyclic development, or alternatively, for MES inactivation and/or BES activation during the in situ VSG switch upon entry into the mammalian host. MES activation occurs in T. brucei cells that are attached to the tsetse salivary gland epithelium, is induced by an unknown mechanism and can be accomplished in vitro in the absence of fly tissues by forced expression of the RNA-binding protein genes RBP6 (accession number Tb927.3.2930) [6,11] or RBP10 (accession number Tb927.8.2780) [17]. Metacyclic VSGs are rapidly replaced with VSGs expressed from BESs in the initial stages of mammalian infection [18], suggesting that ES switching occurs very early in this process. The precise triggers that initiate the MES to BES VSG switching have remained unknown.
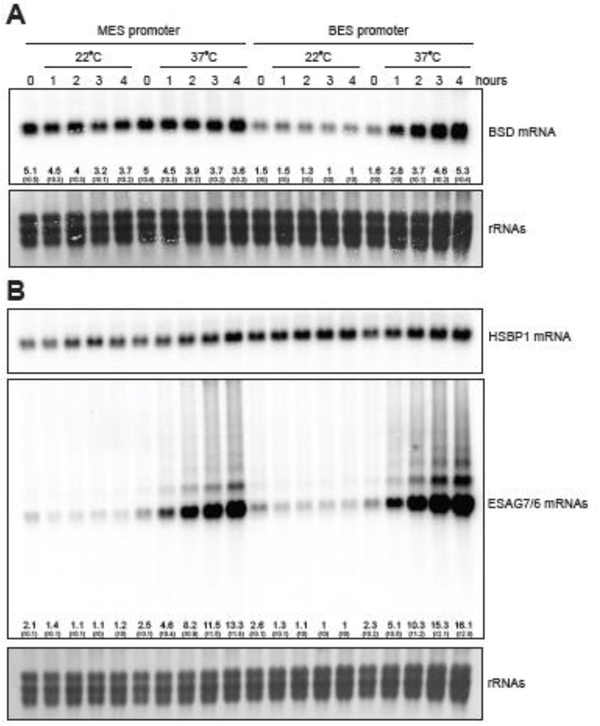
To gain insight into the process of switching from MESs to BESs upon entry into the mammalian host, we decided to study the effect of temperature on the activity of MES and BES promoters in T. b. rhodesiense parasites. We chose this particular subspecies since its procyclic form exhibits the most robust growth in vitro under our laboratory conditions when compared to other procyclic T. brucei strains, even at suboptimal temperatures. In order to minimize other variables, we chose to abstract our experiment from the influence of genome positioning and epigenetic factors that affect VSG expression at telomeres [1,2]. For this reason, we targeted our reporter constructs for integration in the actively Pol II-transcribed β/α-tubulin locus. The reporter cassettes consisted of the MES (VSG397) or the BES (VSG2) promoter, immediately followed by the paraflagellar rod protein 2 (PFR2) intergenic region containing the PFR2 trans-splice site, the blasticidin-S deaminase (BSD) coding sequence and a large portion of the β/α-tubulin intergenic segment, which serves both as a target for chromosomal integration and provides the pre-mRNA processing signals necessary for proper polyadenylation of the BSD transcript (Figs. 1 and S1). The two constructs were transfected separately into procyclic T. b. rhodesiense cells, because we wanted to avoid competition with the active BES VSG promoter in bloodstream-form cells. After selection of stable transformants with blasticidin at 28°C, equal volumes of cell cultures of the same density in the absence of blasticidin were grown overnight at 28°C in duplicate, and transferred to 22°C or 37°C. Equal volume aliquots of the cultures were used to prepare RNA samples at the zero time point (28°C) of the experiment and every hour after placing the cultures at 22°C or 37°C for 4 hours. Northern blotting was the method chosen for monitoring the levels of the BSD reporter mRNA and several other transcripts in the corresponding total RNA samples (Fig. 2). The BSD mRNA from the MES promoter construct showed limited change in steadystate levels at either 22°C or 37°C for the tested time period (Figs. 2A and S2). In contrast, the BSD mRNA had lower initial levels in the cells containing the BES promoter reporter compared to the MES construct and these levels remained low during the incubation for 4 hours at 22°C (Figs. 2A and S2). However, the temperature shift to 37°C resulted in rapid increase of BSD mRNA, noticeable even after only 1-hour incubation at the elevated temperature (Figs. 2A and S2). Because all elements (except the promoters) of our experimental design were identical between the two cell lines, including the 5ˊ- and 3ˊ-UTRs and lack of selecting drug pressure (blasticidin was removed from the cultures), we can exclude the possibility that the observed increase in BSD mRNA levels was the result of a change in pre-mRNA processing, mRNA export to the cytoplasm, mRNA translation or mRNA degradation. Our conclusion is that the observed elevation of BSD mRNA levels at 37°C in the cells containing the BES promoter reporter cassette was solely the result of increased transcription from the BES promoter.
Fig. 2.
Increase in temperature activates BES promoters. T. b. rhodesiense strain YTat 1.1, carrying either the MES or BES promoter reporter construct was grown at 28°C in Cunningham’s medium supplemented with 10% heat inactivated FBS and 2 mM L Gln. The two cultures (grown in parallel) were diluted in duplicate to 1 × 106 cells/ mL and incubated overnight at 28°C. Time point 0 hours represents the cultures at 28°C. One flask of each culture was placed at 22°C and the other at 37°C. Total RNA was prepared with Trizol reagent (Invitrogen) and Northern blotting was performed using a standard protocol. The probes used, (A) BSD, (B) HSBP1 and ESAG7/6, are indicated on the right (probe details are available upon request). The probe against ESAG7 and ESAG6 recognizes a region with high degree of identity between the two genes. Numbers in the lanes indicate the levels of the detected mRNAs from three independent experiments (± SEM) normalized relative to the HSBP1 mRNA amounts in the samples. The lowest steady-state level is set to 1. The large rRNAs were visualized by staining with methylene blue.
One of the mRNAs we chose to monitor codes for an ortholog of the mammalian heat shock factor binding protein 1 (HSBP1; accession number Tb927.9.13070) (Fig. 2B). The sequence of the protein in all available trypanosomatid species shows remarkable similarity to the human polypeptide and the structure of the T. brucei protein can be modeled on the available crystal structure of the mammalian HSBP1 [19] (data not shown). This protein has been shown to modulate the activity of heat shock factor 1 (HSF1) [20], a transcription factor that does not appear to be present in trypanosomes. Nevertheless, since this is a known transcription factor regulator and it has highly elevated levels (both protein and mRNA) in metacyclic trypanosomes in comparison to procyclics [6], we decided to test if its inducible expression in T. brucei Lister 427 (29–13) cells will trigger rapid activation of metacyclic VSG expression. Over the course of 24 hours of inducible HSBP1 expression we could not detect MES activation (data not shown). This observation does not exclude the possibility that HSBP1 in trypanosomes could be involved in regulating transcription by RNA polymerases I, II or III.
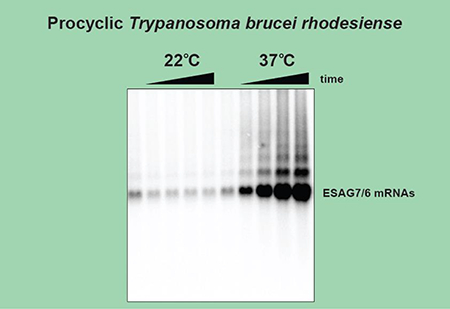
Our result demonstrating an increase in transcription from a BES promoter of the reporter mRNA at 37°C prompted us to test whether endogenous BESs responded to this temperature shift. ESAG7 and ESAG6 genes encode the two subunits of the T. brucei transferrin receptor heterodimer and are always positioned at the very beginning of the BES Pol I transcription unit that generates the VSG mRNA [21]. We used a probe that simultaneously detects both ESAG7 and ESAG6 mRNAs to visualize their abundance. There was a small decrease in the already low abundance of ESAG7/6 mRNAs in both cell lines incubated at 22°C (Figs. 2B and S2). The temperature shift to 37°C caused a rapid and very obvious increase in the steady-state levels of endogenous ESAG7/6 mRNAs (Figs. 2B and S2). We additionally observed discrete longer RNA species, likely representing partially processed pre-mRNA molecules containing 2, 3 or more protein coding sequences as well as a smear from the major mRNA band to the gel wells. The putative ESAG7-ESAG6 di-cistronic RNA has been observed previously [21], however its intensity in our northern blot combined with the presence of a smear and a higher-order polycistronic RNA species are likely indicators of inefficient pre-mRNA processing. We were able to exclude the possibility that increased stability of ESAG7/6 mRNAs is solely responsible for their rapid accumulation at 37°C, because a promoterless construct with identical UTRs (Fig. S3) integrated in endogenous BES exhibited almost identical behavior (Fig. S4), confirming that endogenous BES promoters are similarly activated at a temperature that is characteristic for a mammalian host. The increased activity of the BES promoters at higher temperature was also observed in two other T. brucei strains, TREU 927 and Lister 427 (Fig. S5).
Our results agree with previous data showing that BES promoters are generally silent in procyclic cells under normal conditions, regardless of their genome context [22]. A partial derepression in procyclics of BES promoters when placed in the rDNA spacer [22,23] or in artificial proximity to a procyclin promoter [24] is likely the result of subjecting the promoter to a high local concentration of CITFA and Pol I complexes. On plasmid episomes the BES promoter has been reported to be equally active in bloodstream and procyclic form trypanosomes, however the authors acknowledged that the transcriptional activity was low [25,26]. Additionally, numerous previous studies assessing BES promoter activity in nuclear run-on assays, while still informative, should not be regarded as an accurate readout of true levels of transcription, considering the rapid loss of activity by cooling of the cells and nuclei during the procedure for isolating trypanosome nuclei [27].
In conclusion, we demonstrate that the different sequences of MES and BES promoters determine their different behavior during the shift in temperature from the tsetse vector to the mammalian host. MES promoters are active at all temperatures tested (22°C, 28°C and 37°C) and this could explain the reason for the occasional isolation of bloodstream trypanosome clones expressing VSG from a MES [8]. In contrast, BES promoters, particularly in their natural genomic context, are highly active only at mammalian body temperature and this likely contributes to the lack of activation of BESs during the development of infectious metacyclics in the tsetse salivary glands. The specific temperature-sensitive sequence determinants of the BES promoter and the identity of the biomolecule functioning as a temperature sensor and triggering BES promoter activation are currently unknown.
Supplementary Material
Highlights.
Metacyclic VSG expression site (ES) promoters are insensitive to changes in temperature
Bloodstream VSG ES (BES) promoters are repressed at ambient temperature
BES promoters are activated at mammalian body temperature
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (http://www.nih.gov) grants AI028798 and AI110325 to C.T. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no financial conflict of interest.
Abbreviations:
- VSG
variant surface glycoprotein
- ES
expression site
- BES
bloodstream expression site
- MES
metacyclic expression site
- CITFA
class I transcription factor A
- Pol I
RNA polymerase I
- ESAG
expression site associated gene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Horn D Antigenic variation in African trypanosomes. Mol Biochem Parasitol 2014;195:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mugnier MR, Stebbins CE, Papavasiliou FN. Masters of Disguise: Antigenic Variation and the VSG Coat in Trypanosoma brucei. PLoS Pathog 2016;12:e1005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cross GA, Kim HS, Wickstead B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol Biochem Parasitol 2014;195:59–73 [DOI] [PubMed] [Google Scholar]
- [4].Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 2001;414:759–63. [DOI] [PubMed] [Google Scholar]
- [5].Ginger ML, Blundell PA, Lewis AM, Browitt A, Günzl A, Barry JD. Ex vivo and in vitro identification of a consensus promoter for VSG genes expressed by metacyclic-stage trypanosomes in the tsetse fly. Eukaryot Cell 2002;1:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christiano R, Kolev NG, Shi H, Ullu E, Walther TC, Tschudi C. The proteome and transcriptome of the infectious metacyclic form of Trypanosoma brucei define quiescent cells primed for mammalian invasion. Mol Microbiol 2017;106:74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hovel-Miner G, Mugnier MR, Goldwater B, Cross GA, Papavasiliou FN. A Conserved DNA Repeat Promotes Selection of a Diverse Repertoire of Trypanosoma brucei Surface Antigens from the Genomic Archive. PLoS Genet 2016;12:e1005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alarcon CM, Son HJ, Hall T, Donelson JE. A monocistronic transcript for a trypanosome variant surface glycoprotein. Mol Cell Biol 1994;14:5579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pham VP, Qi CC, Gottesdiener KM. A detailed mutational analysis of the VSG gene expression site promoter. Mol Biochem Parasitol 1996;75:241–54. [DOI] [PubMed] [Google Scholar]
- [10].Brandenburg J, Schimanski B, Nogoceke E, Nguyen TN, Padovan JC, Chait BT, Cross GA, Günzl A. Multifunctional class I transcription in Trypanosoma brucei depends on a novel protein complex. EMBO J 2007;26:4856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kolev NG, Ramey-Butler K, Cross GA, Ullu E, Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science 2012;338:1352–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kolev NG, Günzl A, Tschudi C. Metacyclic VSG expression site promoters are recognized by the same general transcription factor that is required for RNA polymerase I transcription of bloodstream expression sites. Mol Biochem Parasitol 2017;216:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park SH, Nguyen TN, Günzl A. Development of an efficient in vitro transcription system for bloodstream form Trypanosoma brucei reveals life cycle-independent functionality of class I transcription factor A. Mol Biochem Parasitol 2012;181:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen TN, Nguyen BN, Lee JH, Panigrahi AK, Günzl A. Characterization of a novel class I transcription factor A (CITFA) subunit that is indispensable for transcription by the multifunctional RNA polymerase I of Trypanosoma brucei. Eukaryot Cell 2012;11:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park SH, Nguyen BN, Kirkham JK, Nguyen TN, Günzl A. A new strategy of RNA interference that targets heterologous sequences reveals CITFA1 as an essential component of class I transcription factor A in Trypanosoma brucei. Eukaryot Cell 2014;13:785–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nguyen TN, Müller LS, Park SH, Siegel TN, Günzl A. Promoter occupancy of the basal class I transcription factor A differs strongly between active and silent VSG expression sites in Trypanosoma brucei. Nucleic Acids Res 2014;42:3164–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mugo E, Clayton C. Expression of the RNA-binding protein RBP10 promotes the bloodstream-form differentiation state in Trypanosoma brucei. PLoS Pathog 2017;13:e1006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hajduk S, Vickerman K. Antigenic differentiation of Trypanosoma brucei: studies on metacyclic and first parasitaemia populations. Trans R Soc Trop Med Hyg 1981;75:145–6. [DOI] [PubMed] [Google Scholar]
- [19].Liu X, Xu L, Liu Y, Tong X, Zhu G, Zhang XC, Li X, Rao Z. Crystal structure of the hexamer of human heat shock factor binding protein 1. Proteins 2009;75:1–11. [DOI] [PubMed] [Google Scholar]
- [20].Satyal SH, Chen D, Fox SG, Kramer JM, Morimoto RI. Negative regulation of the heat shock transcriptional response by HSBP1. Genes Dev 1998;12:1962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Luenen HG, Kieft R, Mussmann R, Engstler M, ter Riet B, Borst P. Trypanosomes change their transferrin receptor expression to allow effective uptake of host transferrin. Mol Microbiol 2005;58:151–65. [DOI] [PubMed] [Google Scholar]
- [22].Horn D, Cross GA. Position-dependent and promoter-specific regulation of gene expression in Trypanosoma brucei. EMBO J 1997;16:7422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rudenko G, Blundell PA, Taylor MC, Kieft R, Borst P. VSG gene expression site control in insect form Trypanosoma brucei. EMBO J 1994;13:5470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Urményi TP, Van der Ploeg LH. PARP promoter-mediated activation of a VSG expression site promoter in insect form Trypanosoma brucei. Nucleic Acids Res 1995;23:1010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zomerdijk JC, Kieft R, Shiels PG, Borst P. Alpha-amanitin-resistant transcription units in trypanosomes: a comparison of promoter sequences for a VSG gene expression site and for the ribosomal RNA genes. Nucleic Acids Res 1991;19:5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jefferies D, Tebabi P, Pays E. Transient activity assays of the Trypanosoma brucei variant surface glycoprotein gene promoter: control of gene expression at the posttranscriptional level. Mol Cell Biol 1991;11:338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kooter JM, Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res 1984;12:9457–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.