Abstract
Individuals born very preterm (<32 weeks gestation) are at increased risk for neuromotor impairments. The ability to characterize the structural and functional mechanisms underlying these impairments remains limited using existing neuroimaging techniques. Resting state-functional magnetic resonance imaging (rs-fMRI) holds promise for defining the functional network architecture of the developing brain in relation to typical and aberrant neurodevelopment. In 58 very preterm and 65 term-born children studied from birth to age 12 years, we examined relations between functional connectivity measures from low-motion rs-fMRI data and motor skills assessed using the Movement Assessment Battery for Children, 2nd edition. Across all subscales, motor performance was better in term than very preterm children. Examination of relations between functional connectivity and motor measures using enrichment analysis revealed between-group differences within cerebellar, frontoparietal, and default mode networks, and between basal ganglia-motor, thalamus-motor, basal ganglia-auditory, and dorsal attention-default mode networks. Specifically, very preterm children exhibited weaker associations between motor scores and thalamus-motor and basal ganglia-motor network connectivity. These findings highlight key functional brain systems underlying motor development. They also demonstrate persisting developmental effects of preterm birth on functional connectivity and motor performance in childhood, providing evidence for an alternative network architecture supporting motor function in preterm children.
Keywords: functional connectivity, motor development, neurodevelopmental outcome, prematurity, resting state functional MRI
1. INTRODUCTION
Preterm birth affects more than 500,000 newborns in the U.S. each year, with surviving very preterm infants (VPT; <32 weeks gestation) facing a range of life-long neurodevelopmental and neurobehavioral challenges (Beck S et al. 2010; Darlow BA et al. 2013; Anderson PJ 2014). Neuromotor abnormalities are especially prominent and life-impacting, affecting between 20-40% of all VPT survivors (Cooke RWI 2004; Larroque B et al. 2008; Williams J et al. 2010). Common conditions in this population include cerebral palsy, developmental coordination disorders, and minor motor dysfunction.
Conventional volumetric and diffusion-weighted MRI techniques have been helpful in identifying structural correlates of these commonly observed motor impairments. Cross-sectional studies using T1- and T2-weighted imaging reveal smaller regional volumes in subcortical areas that regulate motor function, including the thalamus and basal ganglia (Boardman JP et al. 2006; Boardman JP et al. 2010; Ball G et al. 2012; Loh WY et al. 2017). Longitudinal studies further demonstrate that these regional growth disturbances are evident during the neonatal period (Young JM et al. 2015) and persist into childhood (Lax ID et al. 2013) and adolescence (Nosarti C et al. 2014). Complementing these findings, diffusion tensor imaging (DTI) studies reveal microstructural alterations in key motor tracts that correlate with poorer motor performance (Thompson DK et al. 2012; Thompson DK et al. 2014; Sripada K et al. 2015; Young JM et al. 2015; Rogers CE et al. 2016; Thompson DK et al. 2016).
In contrast to these structural findings, little remains known about the effects of very preterm birth on functional brain development or how possible alterations in functional networks may relate to neuromotor outcome. Resting state-functional MRI (rs-fMRI) is a neuroimaging technique that assesses the temporal correlations in low-frequency fluctuations in the blood oxygen level dependent (BOLD) signal which occur between functionally-related brain regions independent of task (Power JD et al. 2011). This method has been used to reliably characterize the architecture of functional networks throughout the brain in both healthy and clinical populations of interest, extending and complementing information available through structural and task-based functional neuroimaging techniques (Fox MD and ME Raichle 2007). Critically, recent advances have now provided robust methods for linking functional connectivity measures to neurobehavioral functioning in order to identify the regional and brain-wide alterations that may underlie the neurodevelopmental challenges experienced by many preterm children, information currently not available from structural neuroimaging data.
Existing rs-fMRI studies demonstrate that preterm birth is associated with decreased subcortical-cortical connectivity in key motor regions, including between the thalamus and motor cortices. These prematurity-related disruptions in functional connectivity are observed in neonates (Smyser CD et al. 2010; Smyser CD et al. 2013; Toulmin H et al. 2015; Ball G et al. 2016) and persist into early childhood (Damaraju E et al. 2010; Fischi-Gomez E et al. 2015), adolescence (Myers EH et al. 2010; Constable RT et al. 2013; Wilke M et al. 2014), and adulthood (White TP et al. 2014; Scheinost D et al. 2015). However, few studies have examined the extent to which these network differences within the brain are associated with individual differences in overt behavior, including motor function. Importantly, existing studies have been largely confined to analyses of small samples of high-risk adolescents and adults with severe phenotypes, such as diagnoses of cystic periventricular leukomalacia (PVL) and spastic diplegia. These studies demonstrate alterations in individual functional networks related to impaired motor function. However, assessments of motor performance in these studies were limited to the gross motor function classification system (GMFCS) (Burton H et al. 2009; Lee JD et al. 2011). Expanded investigation including both control and preterm children with varying phenotypes, high-fidelity rs-fMRI data, and the use of standardized, comprehensive neuromotor assessments would enable improved characterization of the alterations and related compensatory changes in brain-behavior relationships attributable to VPT birth which underlie neuromotor impairments.
Enrichment analysis, a set of data-driven statistical methods derived from genetic association studies (Subramanian A et al. 2005), has recently been applied to rs-fMRI data, affording a brain-wide approach to identify functional connectivity networks with strong clusters of connections related to specific behavioral measures (Eggebrecht AT et al. 2017; Marrus N et al. 2017). Further, unlike typical brain-behavior analyses which rely on cluster correction methods and assume voxels are spatially connected (Friston KJ et al. 1994; Forman SD et al. 1995), enrichment methods explicitly incorporate a model of the network structure of the brain into analyses in order to test for relationships between behavior and spatially non-contiguous functional brain regions. Its application in both healthy and clinical populations provides a unique opportunity to delineate brain-behavior associations underlying normal and aberrant neuromotor development.
This paper employs enrichment analysis to characterize the functional brain networks associated with motor performance in a cohort of children born VPT who have been studied from birth to age 12 years. High-quality (i.e., low motion) rs-fMRI data and independently assessed, standardized measures of motor function were obtained with sufficient measurement variation to apply this dimensional analytical approach. Our specific aims were as follows:
-
1)
To compare VPT and full-term children’s performance on the Movement ABC, 2nd edition at corrected age 12 years. We hypothesized that VPT children would demonstrate poorer fine and gross motor abilities than full-term peers.
-
2)
To examine the extent to which VPT children, relative to term-born children, are characterized by altered functional connectivity within and between resting state networks that support motor performance. We hypothesized that the strength of functional connections within and between the motor cortex, subcortical regions (i.e., thalamus and basal ganglia), and the cerebellum would relate to individual variability in children’s independently assessed motor performance.
-
3)
To examine between group differences in associations between functional connectivity and motor performance at age 12 years. We hypothesized that weaker brain-behavior correlations between subcortical-cortical networks will be found in the very preterm group relative to term-born controls.
2. MATERIALS AND METHODS
2.1. Participants
As part of a prospective longitudinal study, 110 VPT-born (<32 weeks gestation) infants admitted to the regional Level III Neonatal Intensive Care Unit at Christchurch Women’s Hospital, New Zealand between 1998-2000 were recruited alongside a comparison group of 113 term-born infants (born at 37-41 weeks gestation). Term comparison children were identified from hospital birth records for the same period (n=7,200 total births) by selecting in an alternate fashion the second prior or subsequent same sex birth in the hospital delivery schedule. Exclusion criteria across both groups included congenital anomaly, family living outside the region, and non-English speaking mother. Of all eligible live births, 92% of VPT infants and 62% of full term infants were recruited.
All VPT children and a sub-sample (n=10) of full-term children underwent a neonatal term-equivalent MRI (Woodward LJ et al. 2006). These neonatal scans were independently assessed by a pediatric neurologist and a neuroradiologist for the presence and severity of cerebral white matter abnormalities (Inder TE et al. 2003). The presence of cystic PVL and intraventricular hemorrhage (IVH) on cranial ultrasound was also documented. At corrected ages 2, 4, and 9 years, children completed a pediatric neurological examination that included evaluations for cerebral palsy using the GMFCS.
2.2. MRI and Neurodevelopmental Assessments at Age 12 Years
At age 12 years, correcting for degree of prematurity, 104 VPT and 109 term-born children from this cohort returned for a half-day neurodevelopmental evaluation that included the Movement Assessment Battery for Children, 2nd edition (MABC) (Henderson SE et al. 2007) (sample retention=97%, excluding three deaths in the VPT group). Of these children, 94 (90%) VPT and 96 (88%) term children also underwent an MRI scan that included collection of rs-fMRI data. Depending on family preference, scans were done either on the same day or as close as possible to their neurodevelopmental evaluation. To minimize data loss due to strict motion criteria (see below), a small sub-sample were brought back and the rs-fMRI sequence repeated.
2.2.1. Movement ABC for Children
To assess general motor function, the MABC was administered by a senior research nurse supervised by Dr. Austin (Henderson SE et al. 2007). The MABC consists of a series of eight fine and gross motor tasks that provide measures of 1) manual dexterity, 2) aiming and catching, 3) balance, and 4) an overall measure of motor performance. The MABC is a widely used standardized measure of motor function that is reliable (Smits-Engelsman BCM et al. 2008; Brown T and A Lalor 2009) and valid across different age groups (Schulz J et al. 2011).
2.2.2. MRI Data Collection and Analysis
2.2.2.1. MRI Scanning
All MRI scans were performed on a 3T General Electric HDxt Signa System using an 8 channel head coil (GE-Medical Systems, Milwaukee, WI). Each scan consisted of a T1-weighted sequence (Sagittal BRAVO; TR/TE/TI 10.7/4.8/400 ms, flip angle 15 deg, FOV 250 mm, acquisition matrix 256 × 256 × 190, voxel size 0.98 × 0.98 × 1 mm3). The rs-fMRI data were acquired during crosshair fixation (gradient echo, echo planar imaging; TR/TE 2500/35 ms, flip angle 90 deg, FOV 240 mm, acquisition matrix 64 × 64 × 37, voxel size 3.575×3.75×4 mm3). Separate 10 minute and 5 minute rs-fMRI scans were collected on most participants, with some subjects undergoing a repeat rs-fMRI acquisition if needed to minimize motion and, in turn, optimize data quality (range 10-20 minutes, average 14 minutes/336 frames).
2.2.2.2. rs-fMRI Data Preprocessing
Functional MRI data preprocessing followed previously described methods (Power et al. 2014). Briefly, these steps included slice timing correction, intensity debanding, realignment to the first functional volume, and atlas registration to a population-specific atlas target registered to the Washington University adult atlas in Talairach space (Lancaster JL et al. 1995). Frame censoring was performed with a frame-to-frame displacement cutoff of 0.2 mm, requiring five contiguous frames per low-motion segment and at least 50 low-motion frames per run. Nuisance regression included: 1) 24 head motion parameters, 2) white matter and CSF time series, and 3) whole-brain signal (Power et al., 2014). Data were interpolated, temporally bandpass filtered (0.009-0.08 Hz), demeaned and detrended, and spatially smoothed using a 6mm FWHM Gaussian kernel. A minimum of 120 frames of low motion data after censoring (5 minutes) was required per participant to be included in subsequent analyses.
2.2.2.3. Functional Network Assignments
Three hundred regions of interest (ROIs) were utilized in the present analysis (Gratton C et al. 2018). These ROIs included 264 ROIs previously published (Power JD et al. 2011), with additional ROIs located in the subcortical gray matter and cerebellum. These ROIs were used to generate matrices consisting of the Pearson correlations between each pair of ROIs for each subject. Community detection methods were then used to assign each of the ROIs to a data-driven resting state network. Specifically, Infomap was employed to determine corresponding brain networks for each ROI (Rosvall M and C Bergstrom 2008). This approach utilizes map equation, which is a hierarchical recursive algorithm that builds upon the Louvain method for modularity. Prior work suggests Infomap-based community detection is particularly well-suited for neuroimaging data, as it more consistently retains small communities of connected ROIs across a range of edge density thresholds as compared to alternative algorithms such as Newman’s modularity (Power JD et al. 2011). Following procedures outlined previously (Eggebrecht AT et al. 2017; Marrus N et al. 2017), twelve ROIs were unable to be assigned to a resting state network and were excluded from subsequent analyses. Thus, 288 ROIs were used to investigate associations between the systems-level functional architecture of the brain and motor function as measured with MABC.
2.3. Statistical Analyses
2.3.1. Preliminary Analyses.
Independent samples t-tests were used to assess differences in gestational age and age at scan between VPT- and term-born children. A Chi-squared analysis was then used to assess between-group differences in sex. Additionally, two-tailed independent samples t-tests were used to assess differences in MABC scores between VPT and term children. Nonlinear tests of differences (i.e., Mann-Whitney U) were used for behavioral measures that were non-normally distributed based on the Shapiro-Wilk test.
2.3.2. Brain-Behavior Analyses.
Brain-behavior analyses were performed using previously published enrichment methods (Eggebrecht AT et al. 2017) (Supplementary Fig. S1). First, brain-behavior correlations were calculated separately for each group. Specifically, the correlation between each of the MABC measures and the Fisher z-transformed functional connectivity correlation measure for each pair of ROIs (n=41,328 total ROI-pairs) were calculated, generating brain-behavior correlation triangles for both groups (Supplementary Fig. S1A). Pearson r correlations between functional connectivity and behavior measures were calculated for MABC scores that were normally distributed, and Spearman rank correlations used for non-normally distributed MABC scores (Fig. 1).
Figure 1.
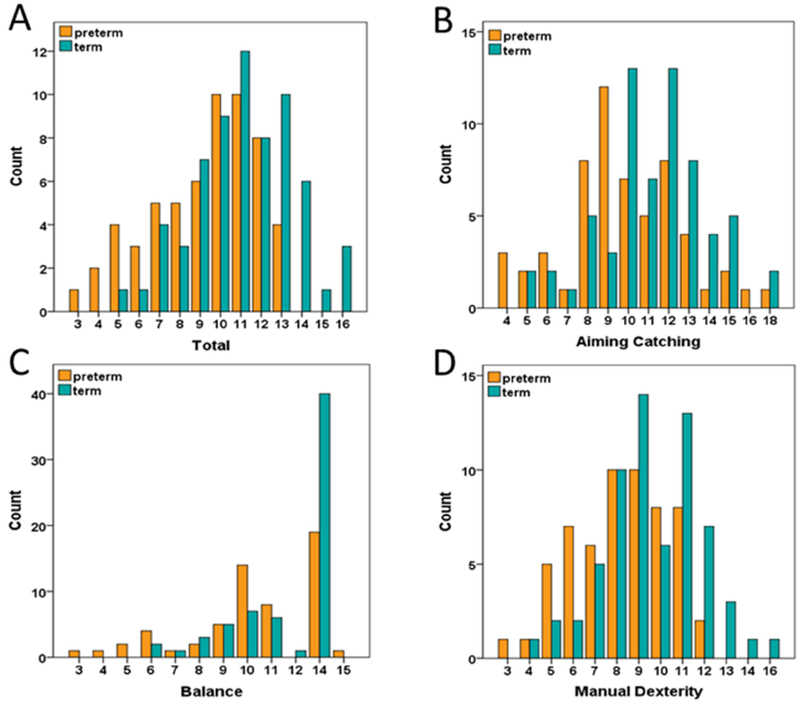
Distributions of MABC total and subscale standardized scores for very preterm (N=58) and term children (N=65).
Second, to evaluate which functional network pairs exhibited significant clustering of strong brain-behavior correlations, enrichment analysis methods were adapted from those used in large-scale genomic association studies (Rivals I et al. 2007; Khatri P et al. 2012; Backes C et al. 2014; Eggebrecht AT et al. 2017). Briefly, we tested each network pair for enrichment of strong correlation values, defined as correlations with an associated uncorrected p-value ≤0.05 (Supplementary Fig. S1B), using hypergeometric and χ2 tests (Supplementary Fig. S1C). The hypergeometric statistic, as in Fisher’s exact test, assesses the likelihood of observing a given number of strong correlations in a network pair given 1) the total number of strong correlations observed overall and 2) the total number of possible hits for that network pair (i.e., the total number or ROI-pairs within a given network pair).
Third, to test for network pair-specific differences in brain-behavior relationships between the VPT and term groups, we used a McNemar χ2 test (Supplementary Fig. S1D). Within each network pair, the McNemar statistic used the number of discordant tests (b=number of ROI pairs True for term control but False for VPT and c=the number of ROI pairs False for term control but True for VPT) between ages. The McNemar χ2 statistic is (b−c)2/(b+c), if (b+c)>25, and (|b−c|−1)2/(b+c) otherwise. Empirical significance levels were determined for the hypergeometric, χ2 enrichment test, and McNemar tests using randomization (10,000 permutations) (Backes C et al. 2014; Eggebrecht AT et al. 2017). Networks that were significantly different in their association with behavior between groups (McNemar χ2 p<0.025, accounting for comparison of two groups) and were significantly enriched for brain-behavior (hypergeometric p and χ2<0.025) were considered as networks that significantly differed between groups. All analyses conducted and visualizations generated were executed in MATLAB (Release 2015a, The Mathworks, Inc. Natick, Massachusetts, United States).
3. RESULTS
3.1. Study Cohort
Of the 190 children scanned at age 12 years, 125 participants (59 VPT and 66 term) met stringent data quality criteria for motion for rs-fMRI analysis. A further VPT child was excluded due to imaging artifacts caused by a ventriculoperitoneal shunt and a term participant because of task non-compliance at the time of their MABC assessment. Therefore, a total of 123 children (58 VPT and 65 term) were included in this analysis.
Of the VPT children who met inclusion criteria, 12 had none, 39 had mild, seven had moderate, and none had severe white matter abnormalities on neonatal MRI scans. One VPT child had a neonatal grade III IVH and another had neonatal PVL with grade I IVH. These participants were classified as having moderate white matter abnormalities. At age 9 years, two VPT children were characterized as having level II GMFCS scores, and nine VPT children were characterized as having level I GMFCS scores. No VPT subjects included in this analysis used wheelchairs, walkers, or crutches, or had high-grade cerebral palsy. In addition, no term children were characterized as having level I or greater GMFCS scores.
Table 1 describes the demographic characteristics of VPT and term children included in this analysis. As expected, birthweight and gestational age were significantly lower in the VPT group. There were no differences in gender. While all children were scanned at age 12, preterm children (mean age=12.3 years) were, on average, 2 months older chronologically at the time of their MRI scan than term-born children (mean age=12.2 years) due to prematurity-based age correction (see Methods).
Table 1.
Demographic Information
| Term (M ± SD) N=65 | Preterm (M ± SD) N=58 | df | t | p | |
|---|---|---|---|---|---|
| Gestational Age (weeks) | 39.5 ± 1.2 | 28.1 ± 2.5 | 79.97* | 32.15 | <0.001 |
| Birthweight (grams) | 3569 ± 358 | 1046 ± 288 | 121 | 42.73 | <0.001 |
| Age at Scan (years) | 12.2 ± 0.1 | 12.3 ± 0.1 | 121 | −9.18 | <0.001 |
| df | χ2 | p | |||
| Sex (M/F) | 34/31 | 26/32 | 1 | 0.686 | 0.407 |
Welch’s t used, equal variance not assumed
We also examined the extent of sample selection bias given our stringent rs-fMRI data quality control criteria that resulted in data loss (Supplementary Tables S1 and S2). With the exception of an increased proportion of small for gestational age children who were included in the study and children with more severe white matter abnormalities on term equivalent MRI who were excluded from the study, few differences between included and excluded VPT infants were evident across a wide range of infant medical and social background factors (Supplementary Table 1). Differences observed on MABC Total scores between included and excluded VPT subjects were not significant (Supplementary Table S2).
3.2. Neuromotor Performance on MABC at Age 12 Years
VPT children obtained lower scores than term children on all three MABC subscales (p<0.01), as well as the Total score (p<0.001) (Table 2). While Aiming and Catching and Manual Dexterity subscale scores were normally distributed, Balance subscale scores for both groups were positively skewed (Fig. 1). Total MABC scores were also non-normally distributed in VPT children (see Fig. 1A). Therefore, Spearman rank correlation was used in the enrichment analysis of brain-behavior relationships for Total MABC scores across both groups.
Table 2.
Movement ABC 2nd Edition Scale Scores
| Measure | Term (M ± SD) N=65 | Preterm (M ± SD) N=58 | df | t | p |
|---|---|---|---|---|---|
| Manual Dexterity | 9.6 ± 2.3 | 8.2 ± 2.2 | 120.49* | 3.382 | 0.001 |
| Aiming Catching | 11.2 ± 2.7 | 9.8 ± 2.9 | 121 | 2.758 | 0.007 |
| Median | Median | U | Z | p | |
| Balance | 14 | 10 | 1349 | 2.891 | 0.004 |
| Total Standard | 11 | 10 | 1182 | 3.589 | <0.001 |
Welch’s t used, equal variance not assumed
3.3. Resting State Networks
Based on the results of community detection analysis, a functional network model using 288 ROIs comprising 15 functional networks was used to examine brain-behavior relationships within and between the two study groups (Supplementary Table S3). Both groups demonstrated strong within and between network connectivity (Fig. 2) consistent with those reported previously in children and adults (Marek S et al. 2015).
Figure 2.
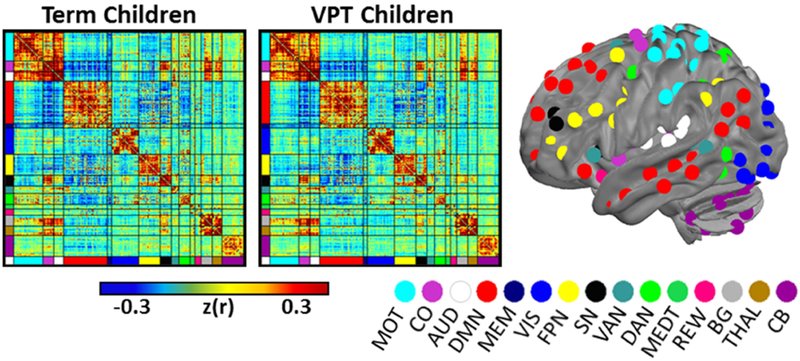
Group mean functional connectivity matrix for very preterm (center) and term (left) children. The 288 ROIs have been sorted according to the functional network model used in the study. The spatial locations of each ROI are displayed on the left lateral surface of the brain with coloring representative of the functional network assignments. Cortical network assignments as in Power et al. (2011). MOT, Motor; CO, cingulo-opercular; AUD, Auditory; DMN, Default Mode Network; MEM, Memory; VIS, Visual; FPN, frontoparietal network; SN, salience network; VAN, ventral attention network; DAN, dorsal attention network; MEDT, medial temporal; REW, reward; BG, Basal Ganglia; THAL, Thalamus; CB, cerebellum.
3.4. Brain-Behavior Results
3.4.1. Brain-behavior correlations with greater representation in term-born than VPT children
Connectivity between the thalamus and motor networks (THAL-MOT) demonstrated an enriched relationship (i.e., clustering of significant brain-behavior correlations) with MABC Total scores in term children, but not VPT children (McNemar χ2 p=2.06E-4) (Fig. 3 red square; Fig. 4A; Supplementary Fig. S4A). These results are unchanged after exclusion of VPT children with motor deficits (i.e., GMFCS scores of I/II), moderate white matter injury (Supplementary Tables S4 and S5; Supplementary Fig. S5) and use of identical statistical thresholds for each group to account for differences in group size (Supplementary Fig. S6). Greater representations of brain-behavior correlations between subcortical and cortical networks were similarly observed for each MABC subscale in term children (Figs. 5A, C, E; Supplementary Fig. S2 red squares; Supplementary Fig. S7A, C, E). Specifically, as illustrated in Figure 5, in term children, the Aiming and Catching (McNemar χ2 p=1.7E-3) and Manual Dexterity (McNemar χ2 p=1.66E-2) subscales were associated with thalamus-motor network connectivity (THAL-MOT), while enriched brain-behavior correlations were also identified between the Balance subscale and basal ganglia-motor network connectivity (McNemar χ2 p=1.37E-2) (BG-MOT; Fig. 5C). Finally, term children demonstrated greater clustering of brain-behavior correlations between Aiming and Catching scores and salience-motor network connectivity (SAL-MOT) than VPT children (McNemar χ2 p=2.41E-2) (Fig. 5A).
Figure 3.
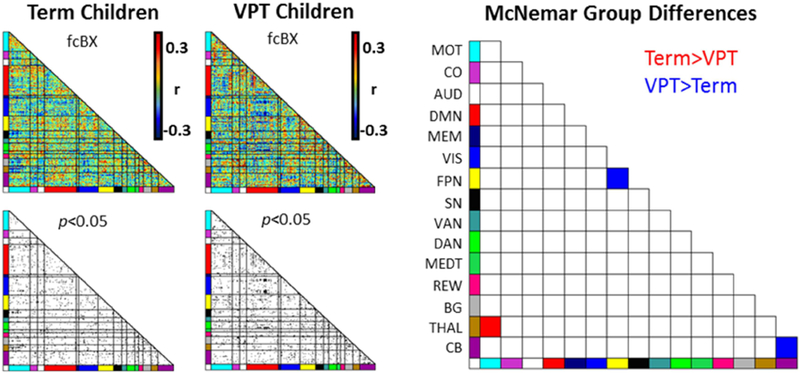
Brain-behavior differences between very preterm and term children for motor Total score. Red squares indicate network pairs that were significantly more enriched with strong rs-fMRI correlations with the MABC Total Standard Score in term than very preterm children. Blue squares indicate networks exhibiting stronger enrichment of brain-behavior correlations in very preterm than term children. MOT, Motor; CO, cingulo-opercular; AUD, Auditory; DMN, Default Mode Network; MEM, Memory; VIS, Visual; FPN, frontoparietal network; SN, salience network; VAN, ventral attention network; DAN, dorsal attention network; MEDT, medial temporal; REW, reward; BG, Basal Ganglia; THAL, Thalamus; CB, cerebellum.
Figure 4.
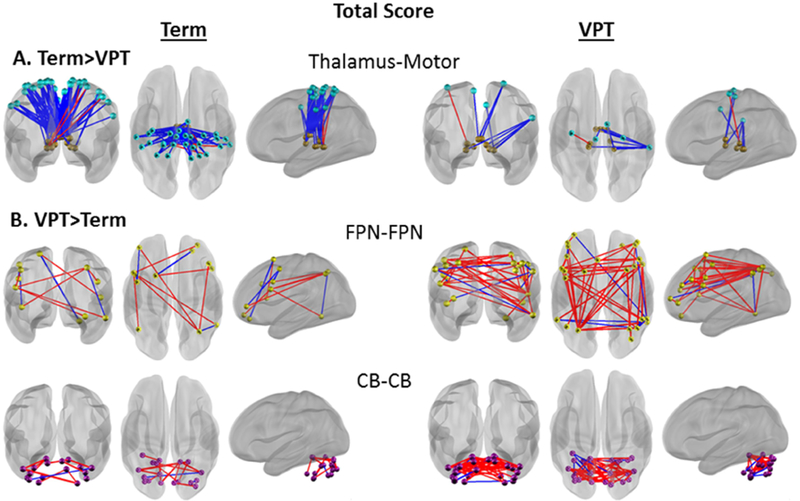
Network pair connectivity correlated with MABC Total Standard Score. Blue lines represent a negative brain-behavior correlation, red lines represent a positive brain-behavior correlation. A) Greater enrichment of strong brain-behavior correlations in term (brains on the left) than very preterm (brains on the right) children. B) Greater enrichment of strong brain-behavior correlations in very preterm than term children. All correlations shown at p<0.05. FPN, Frontoparietal Network; CB, Cerebellum.
Figure 5.
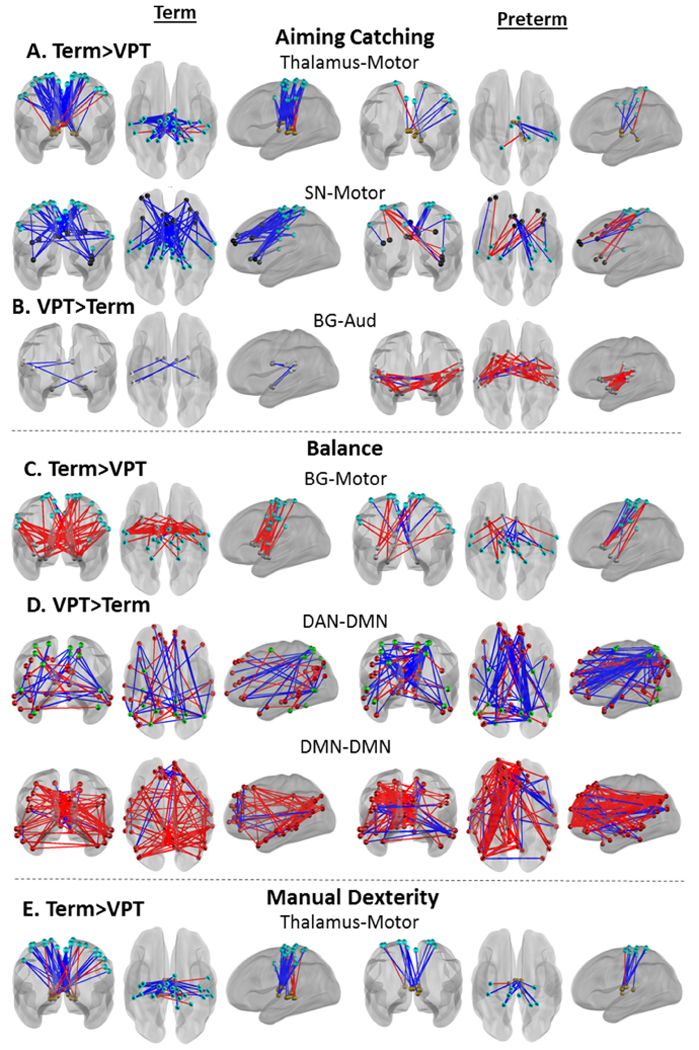
Enrichment analysis of MABC subscales suggest commonalities and differences in brain-behavior correlations supporting behavior across motor domains. Data from term children are plotted on the left, very preterm children are plotted on the right. Very preterm children exhibit a sparser pattern of brain-behavior correlations than term children (A, C, E) and a denser pattern of brain-behavior correlations in B and D. All plots represent connections with brain-behavior correlation p<0.05. Blue lines represent a negative brain-behavior correlation, red lines represent a positive brain-behavior correlation. SN, Salience Network; BG, Basal Ganglia; CB, Cerebellum; Aud, Auditory Network; DMN, Default Mode Network; DAN, Dorsal Attention Network.
3.4.2. Brain-behavior correlations with greater representation in VPT- than term-born children
In contrast, VPT-born children demonstrated a greater number of strong brain-behavior correlations with motor performance within five network pairs that were not enriched in term children (Supplementary Fig. S2). Specifically, unique to the VPT group there was evidence of enriched associations between children’s MABC Total scores and rs-fMRI correlations within the frontoparietal control (FPN-FPN) and cerebellar (CB-CB) networks (McNemar χ2 p=1.46-2 and p=2.29E-2, respectively) (Fig. 3 blue squares; Fig. 4B; Supplementary Fig. S4B). Further, stronger correlations between Aiming and Catching scores and Basal Ganglia-Auditory network connectivity (BG-AUD) were found in the VPT group (McNemar χ2 p=5.50E-3) (Fig. 5B; Supplementary Fig. S2B; Supplementary Fig. S7B). Finally, VPT children exhibited greater brain-behavior correlations between Balance subscale scores and connectivity between the dorsal attention and default mode networks (DAN-DMN) (McNemar χ2 p=2.29E-2), and within the default mode network (DMN-DMN) (McNemar χ2 p=1.67E-2) than term children (Fig. 5D; Supplementary Fig. S2C; Supplementary Fig. S7D).
3.4.3. Brain-behavior correlations shared by VPT and term-born children
Both term (Enrichment χ2 p=1.37E-2) and VPT children (Enrichment χ2 p=1.88E-2) exhibited significant clustering of strong brain-behavior correlations between Balance subscale scores and thalamus-basal ganglia network connectivity (THAL-BG; Fig. 6; Supplementary Fig. S2 green square).
Figure 6.
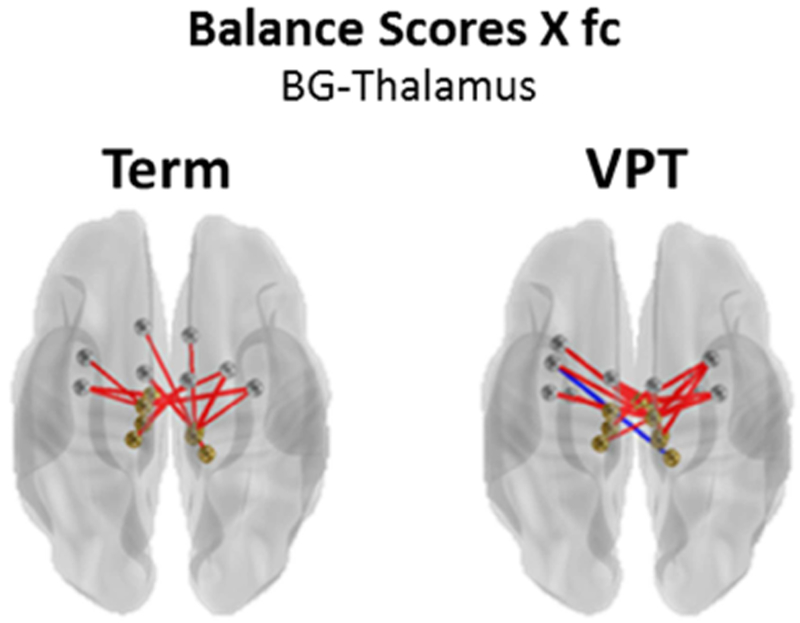
Networks with common significant enrichment of brain-behavior correlations in very preterm and term children. All correlations p<0.05. Blue lines represent a negative brain-behavior correlation, red lines represent a positive brain-behavior correlation. BG, Basal Ganglia.
4. DISCUSSION
Though recent studies have suggested that cerebral functional connectivity is disrupted in children born VPT, specific associations between these alterations in brain function and children’s motor outcomes during childhood and adolescence were unknown. This study compared brain-behavior motor performance associations between VPT and term children during this important developmental period. Standardized assessments examining multiple aspects of motor function revealed VPT children demonstrated decreased motor abilities across all areas, consistent with prior reports in this population (de Kieviet JF et al. 2009; Williams J et al. 2010). Critically, use of innovative neuroimaging analysis techniques extends earlier studies to help define the alterations in the brain’s functional network architecture that may underlie specific differences in multiple domains of childhood motor performance. Results showed that term-born children exhibited consistent associations between subcortical-motor network connectivity and motor scores, as hypothesized. In contrast, VPT children exhibited brain connectivity associated with motor behavior in a widely-distributed set of networks including the FPN, BG, DAN, DMN, CB, and auditory networks. These brain-behavior associations specific to the VPT group may be indicative of a unique alternative network architecture underlying motor function in preterm-born children. Finally, both VPT and term children exhibited enriched clustering of connections between the basal ganglia and thalamic networks associated with Balance scores, suggesting a potentially conserved functional connectivity architecture to support this ability in VPT children.
4.2. Motor functional networks are well-defined, modifiable, and measurable at rest
The brain regions critical for neuromotor performance have historically been well defined, both clinically and behaviorally, with advanced neuroimaging techniques more recently used to refine our understanding of the cerebral networks underlying motor function. While motor movements and motor learning require distinct patterns of neural activity, meta-analyses of human neuroimaging data suggest a common basis for motor behavior dependent upon a functional network centered upon a core set of regions. These include the primary motor, pre-motor, somatosensory, and supplementary motor cortices, thalamus, putamen, and cerebellum (Hardwick RM et al. 2013). Prior neuroimaging studies also demonstrate that learned automatic (e.g., habitual) motor processes specifically recruit a subset of these regions including primary motor, somatosensory, and supplementary motor cortices and striatum, with decreased activation in the cerebellum (Dayan E and LG Cohen 2011). While much of this literature has centered upon the use of task-based fMRI to study targeted individual motor behaviors, more recent rs-fMRI studies suggest that functional connections within these same regions are also readily identifiable at rest. Further, the associations between regions within the motor network are modifiable, with long-term motor learning increasing the strength of primary and supplementary motor connectivity (Albert NB et al. 2009; Xiong J et al. 2009; Ma L et al. 2011; Taubert M et al. 2011). This constellation of findings highlights the utility of rs-fMRI methods for accurate and effective investigations on the functional architecture of the brain underlying motor behavior in healthy and clinical populations of interest.
4.2. Subcortical-motor network connectivity subserves typical motor function in term children
The present findings in healthy, term-born children are consistent with this existing research, implicating subcortical-motor cortex functional connectivity as a common pathway supporting motor performance. Specifically, the MABC Total score, Aiming and Catching subscale, and Manual Dexterity subscale were all associated with thalamus-motor network connectivity, with the Balance subscale associated with BG-motor network connectivity. In addition to thalamus-motor network connectivity, other variations were observed in brain-behavior correlations among the MABC subscales. For example, term children exhibited associations between the Aiming and Catching subscale and salience-motor network connectivity. These results are consistent with a compelling study showing that salience-motor network connectivity plays a key role in attentional orienting processes when coordinating motor movements (Menon V and LQ Uddin 2010). Additionally, the need for intact executive and attention function in the salience network to perform motor tasks has also been demonstrated (Rinne P et al. 2018). In summary, our findings in term children converge with existing studies to define motor network development and demonstrate the feasibility and validity of enrichment analysis approaches to accurately understand the patterns of neural connectivity underlying motor dysfunction in high-risk children.
4.3. VPT children exhibit impaired motor performance and altered brain-behavior relationships
Deficits across a range of motor domains have been consistently reported in preterm children (de Kieviet JF et al. 2009; Williams J et al. 2010). In the present study, we utilized comprehensive assessments of motor behavior while also assessing motor sub-domains (i.e., Aiming and Catching, Balance, and Manual Dexterity), rather than simply employing a clinical outcome measure such as the GMFCS. Similar to existing research, VPT children demonstrated reduced motor abilities on the Total score and across each of the MABC subscales when compared to term children. Critically, when using these detailed motor performance data in the enrichment analysis, VPT children did not display anticipated brain-behavior relationships. Specifically, in contrast to the term children, VPT children lacked the anticipated associations between measures of motor behavior and thalamus-motor, BG-motor, and thalamus-salience network connectivity. These findings are consistent with volumetric analyses in this same cohort demonstrating decreased thalamus volumes in VPT compared to term children (Lean RE et al. 2017). These results also align with prior research that observed reduced structural connectivity within the corticospinal tract (connecting thalamus to motor cortex) relating to impaired gross motor abilities in preterm children with white matter injury (Rha DW et al. 2012; Wang S et al. 2014).
In addition to aberrant subcortical-cortical functional connectivity, VPT children exhibited related alterations in brain-behavior correlations across motor domains, a series of findings that extends and expands prior structural connectivity research. For example, VPT children exhibited enrichment of within-CB network connectivity, in which stronger CB-CB functional connectivity positively correlated with improved motor performance. While the cerebellum is believed to be involved in sensorimotor tasks (Hardwick RM et al. 2013), the CB network did not exhibit similar relationships with motor performance in term children, nor were significant associations observed between motor scores and connectivity between CB and thalamus, basal ganglia, or motor networks. These differences are of interest when considered in the context of the multiple prior investigations detailing prematurity-related reductions in cerebellar volumes and growth trajectories (Allin M et al. 2001; Volpe JJ 2009; Bouyssi-Kobar M et al. 2016). Prior work in VPT children has suggested stronger CB structural connectivity is linked with improved fine motor dexterity and visual-motor integration in adolescence (Thomas AR et al. 2017), a finding consistent with the present functional connectivity results. Similarly, preterm children with diffuse PVL with lower GMFCS scores (i.e., better motor abilities) have been shown to exhibit stronger structural connectivity within the cerebellar peduncles (Wang S et al. 2014). In addition, more recent research suggests VPT infants demonstrate altered functional connectivity patterns between the cerebellum and multiple cortical networks, suggesting the changes underlying these differences in cerebellar brain-behavior relationships may begin as early as the neonatal period (Herzmann CS et al. 2018).
More broadly, these results suggest that VPT children may rely upon an expanded, alternative set of brain-wide networks for motor function, with multiple brain-behavior associations incorporating within network connectivity (e.g., CB-CB, FPN-FPN, DMN-DMN), rather than between network connectivity (e.g., Thal-Motor). Specifically, stronger FPN-FPN and DMN-DMN network connectivity was associated with higher total motor scores. These within-network associations between motor scores and the FPN, DMN, and CB networks are convergent with structural connectivity reports suggesting that VPT children, relative to term children, have greater information flow within networks (i.e., local efficiency), but less integrated information flow between networks (Thompson DK et al. 2016). Our findings suggests that the CB, FPN, and DMN networks may provide an alternative mechanism for preserved information flow regulating VPT children’s motor abilities.
Finally, in addition to altered within-network connectivity, VPT-born children exhibited enrichment between motor performance and connectivity between the BG-AUD and DAN-DMN networks. Existing data is mixed with respect to the role of parietal and prefrontal cortices (core components of the FPN and DAN) in motor behavior (Hardwick RM et al. 2013). However, the FPN is thought to act as a hub controlling activity between other brain regions during tasks (Dosenbach NU et al. 2008; Cole MW et al. 2013), while the DAN is thought to underlie top down orienting and directing of attention (Fox MD et al. 2006; Vossel S et al. 2014). These results suggest that VPT children could be recruiting and utilizing additional functional networks, including the FPN and DAN, providing an alternative mechanism for preserved top-down control of motor movement.
4.4. Relation to previous studies of functional connectivity and motor function in VPT children
Prior rs-fMRI studies have consistently reported reduced connectivity strength in VPT children relative to term-born controls (Smyser CD et al. 2010; Smyser CD et al. 2013; Toulmin H et al. 2015; Ball G et al. 2016). These differences extend from birth (Smyser CD et al. 2010; Smyser CD et al. 2016; Rogers CE et al. 2017), through early childhood (Damaraju E et al. 2010; Fischi-Gomez E et al. 2015), adolescence (Myers EH et al. 2010; Constable RT et al. 2013; Wilke M et al. 2014), and into adulthood (White TP et al. 2014; Scheinost D et al. 2015). A limited number of functional connectivity studies have assessed the impact of preterm birth on functional connectivity and motor development. As noted previously, these studies assessed small numbers of adolescents and adults with severe phenotypes (e.g., diagnoses of cystic PVL and spastic diplegic cerebral palsy) and demonstrated alterations in motor and thalamic functional connectivity related to motor impairments in affected subjects (Burton H et al. 2009; Lee JD et al. 2011). The present study extends these reports by characterizing brain-wide differences in network connectivity patterns underlying altered motor performance. Further, by including VPT children with less severe clinical phenotypes, this study provides critical information regarding not only the deleterious alterations which underlie impaired motor function, but also the alternative mechanisms that support preserved motor performance in VPT children.
4.5. Conserved basal ganglia-thalamus network connectivity in VPT children
In the present study, differences were observed between VPT and term children in functional networks associated with a range of motor abilities. In contrast, for balance assessments, both VPT and term children demonstrated strong correlations between BG-thalamus network connectivity and balance subscale scores, despite the fact that VPT children were characterized by impaired performance in this domain. Lesion studies suggest BG-motor network connectivity is important for maintaining postural control, and aberrant BG connectivity has been implicated in a variety of motor disorders (Visser JE and BR Bloem 2005). Further, BG inhibition of the thalamus is necessary to prevent abnormal motor behaviors (e.g., dyskinesia) (Kim J et al. 2017). Our results suggest this pathway is conserved even within impaired VPT children. This may be the result of the differences in the nature of the physical requirements necessary for maintaining postural stability in comparison to the fine-tuned hand movements of manual dexterity or the coordinated multi-system movements of aiming and catching (i.e., gross versus fine motor skills) (Bos AF et al. 2013). Nonetheless, the varying effects of VPT birth on functional relationships across different aspects of motor performance highlight the need for further investigation of early brain-behavior development in VPT children.
4.6. Enrichment analysis provides an innovative network-level approach to brain-behavior analysis.
The underlying premise of enrichment analysis is that the numerous less strong associations observed within a brain-wide network architecture may provide more robust information regarding brain-behavior relationships. These patterns of associations be more readily reproducible than reporting only the strongest, sparse associations that survive traditional multiple comparisons correction (Subramanian A et al. 2005). Recently, enrichment was used to successfully investigate rs-fMRI associations with social and motor behaviors in children one and two years of age who were at low- or high-risk for developing autism (Eggebrecht AT et al. 2017; Marrus N et al. 2017). These findings, combined the present results, suggest this approach can be applied across different clinical populations and behavioral domains.
4.7. Caveats and Limitations
While these findings are compelling, some limitations need to be acknowledged. First, the network architecture used in the analysis was defined using adult neuroimaging data (Power JD et al. 2011). While research suggests that functional brain networks are highly similar between older children and adults (Marek S et al. 2015), it is possible that a data-driven network solution derived using neuroimaging data from children or adolescents may produce subtle differences in network organization that could influence measured results. In addition, due to scanning restrictions and the strict motion exclusion criteria, all of the children in the cohort with high-grade cerebral palsy or high-grade white matter injury were unfortunately excluded. Thus, although there were few significant differences between included and excluded subjects, the generalizability of these findings to children with severe motor impairments may be limited. It is possible that children with more severe neurological impairments or brain injury may display different brain-behavior relationships than the VPT children with no/mild injury in the studied sample.
5. CONCLUSIONS
This study utilized enrichment analysis to facilitate a brain-wide investigation of the relationships between motor performance and rs-fMRI functional connectivity measures in a well-characterized, longitudinal sample of VPT and term-born children at age 12 years. Anticipated relationships between motor performance and subcortical-motor network connectivity were observed in term-born children. Consistent with previous research, VPT-born children had poorer motor abilities than their same age term peers. They were also characterized by altered brain-behavior relationships, including reduced associations between motor performance and subcortical-motor network connectivity, and increased associations between motor performance and a widespread set of cortical and subcortical networks. These patterns may be indicative of an alternative network architecture underlying motor function in preterm-born children. In contrast, VPT children demonstrated similar brain-behavior relationships as term-born peers for balance measures, suggesting a preserved network architecture supporting this domain of motor performance. These findings highlight the functional brain-behavior relationships involved in motor function for both typically developing and very preterm children. They also confirm the persistence of disturbances in cerebral connectivity and demonstrate how they may relate to observed motor difficulties in school age children born very preterm.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Marie Goulden, and Carole Spencer for assistance with participant recruitment, MRI acquisition, and motor assessments. We also acknowledge Ben Seitzman, Caterina Gratton, Deanna Greene, Brad Schlaggar, and Steve Petersen for providing the 300 ROI and adult 120 Infomap network assignments.
This work was supported by the Health Research Council of New Zealand (11-283); the Neurological Foundation of New Zealand; and the National Institutes of Health (grant numbers T32 MH100019 to MDW, K01 MH103594 to ATE, and K02 NS089852 to CDS).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
REFERENCES
- Albert NB, Robertson EM, Miall RC. 2009. The resting human brain and motor learning. Curr Biol. 19:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MHS, Stewart AL, Rifkin L, Murray RM. 2001. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 124:60–66. [DOI] [PubMed] [Google Scholar]
- Anderson PJ. 2014. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 19:90–96. [DOI] [PubMed] [Google Scholar]
- Backes C, Ruhle F, Stoll M, Haas J, Frese K, Franke A, Lieb W, Wichmann H, Weis T, Kloos W, Lenhof H, Meese E, Katus H, Meder B, Keller A. 2014. Systematic permutation testing in GWAS pathway analyses: identification of genetic networks in dilated cardiomyopathy and ulcerative colitis. BMC Genomics. 15:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Arichi T, Tusor N, Cox D, Merchant N, Nongena P, Hajnal JV, Edwards AD, Counsell SJ. 2016. Machine-learning to characterise neonatal functional connectivity in the preterm brain. Neuroimage. 124:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ. 2012. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 22:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. 2010. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 88:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, Hajnal J, Allsop JM, Rutherford MA, Edwards AD. 2006. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 32:70–78. [DOI] [PubMed] [Google Scholar]
- Boardman JP, Craven C, Valappil S, Counsell SJ, Dyet LE, Rueckert D, Aljabar P, Rutherford MA, Chew AT, Allsop JM, Cowan F, Edwards AD. 2010. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage. 52:409–414. [DOI] [PubMed] [Google Scholar]
- Bos AF, Van Braeckel KN, Hitzert MM, Tanis JC, Roze E. 2013. Development of fine motor skills in preterm infants. Dev Med Child Neurol. 55 Suppl 4:1–4. [DOI] [PubMed] [Google Scholar]
- Bouyssi-Kobar M, du Plessis AJ, McCarter R, Brossard-Racine M, Murnick J, Tinkleman L, Robertson RL, Limperopoulos C. 2016. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics. 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T, Lalor A. 2009. The Movement Assessment Battery for Children--Second Edition (MABC-2): a review and critique. Phys Occup Ther Pediatr. 29:86–103. [DOI] [PubMed] [Google Scholar]
- Burton H, Dixit S, Litkowski P, Wingert JR. 2009. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res. 26:90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Vohr BR, Scheinost D, Benjamin JR, Fulbright RK, Lacadie C, Schneider KC, Katz KH, Zhang H, Papademetris X, Ment LR. 2013. A left cerebellar pathway mediates language in prematurely-born young adults. Neuroimage. 64:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RWI. 2004. Health, lifestyle, and quality of life for young adults born very preterm. Archives of Disease in Childhood. 89:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, Caprihan A. 2010. Resting-state functional connectivity differences in premature children. Front Syst Neurosci. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlow BA, Horwood LJ, Pere-Bracken HM, Woodward LJ. 2013. Psychosocial outcomes of young adults born very low birth weight. Pediatrics. 132:e1521–1528. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. 2011. Neuroplasticity subserving motor skill learning. Neuron. 72:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kieviet JF, OPiek JP, Aarnoudse-Moens CS, Oosterlaan J. 2009. Motor development in very preterm and very low-birth-weight children from birth to adolescence. JAMA. 302:2235–2242. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, Adams CM, Snyder AZ, Lewis JD, Estes AM, Zwaigenbaum L, Botteron KN, McKinstry RC, Constantino JN, Evans A, Hazlett HC, Dager S, Paterson SJ, Schultz RT, Styner MA, Gerig G, Das S, Kostopoulos P, Networkdagger I, Schlaggar BL, Petersen SE, Piven J, Pruett JR Jr. 2017. Joint Attention and Brain Functional Connectivity in Infants and Toddlers. Cereb Cortex. 27:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischi-Gomez E, Vasung L, Meskaldji DE, Lazeyras F, Borradori-Tolsa C, Hagmann P, Barisnikov K, Thiran JP, Huppi PS. 2015. Structural Brain Connectivity in School-Age Preterm Infants Provides Evidence for Impaired Networks Relevant for Higher Order Cognitive Skills and Social Cognition. Cereb Cortex. 25:2793–2805. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Imaging. 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. PNAS. 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8:700–711. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley RSJ, Frackowiak JC, Maziotta Evans AC. 1994. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1:214–220. [DOI] [PubMed] [Google Scholar]
- Gratton C, Koller JM, Shannon W, Greene DJ, Snyder AZ, Petersen SE, Perlmutter JS, Campbell MC. 2018. Emergent Functional Network Effects in Parkinson Disease. Cereb Cortex. epub 2018 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. 2013. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 67:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA, Barnett AL. 2007. Movement assessment battery for children-2. [Google Scholar]
- Herzmann CS, Snyder AZ, Kenley JK, Rogers CE, Shimony JS, Smyser CD. 2018. Cerebellar Functional Connectivity in Term- and Very Preterm-Born Infants. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. 2003. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. The Journal of Pediatrics. 143:171–179. [DOI] [PubMed] [Google Scholar]
- Khatri P, Sirota M, Butte AJ. 2012. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 8:e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim Y, Nakajima R, Shin A, Jeong M, Park AH, Jeong Y, Jo S, Yang S, Park H, Cho SH, Cho KH, Shim I, Chung JH, Paik SB, Augustine GJ, Kim D. 2017. Inhibitory Basal Ganglia Inputs Induce Excitatory Motor Signals in the Thalamus. Neuron. 95:1181–1196 e1188. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. 1995. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp. 3:209–223. [Google Scholar]
- Larroque B, Ancel P-Y, Marret S, Marchand L, André M, Arnaud C, Pierrat V, Rozé J-C, Messer J, Thiriez G, Burguet A, Picaud J-C, Bréart G, Kaminski M. 2008. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. The Lancet. 371:813–820. [DOI] [PubMed] [Google Scholar]
- Lax ID, Duerden EG, Lin SY, Mallar Chakravarty M, Donner EJ, Lerch JP, Taylor MJ. 2013. Neuroanatomical consequences of very preterm birth in middle childhood. Brain Struct Funct. 218:575–585. [DOI] [PubMed] [Google Scholar]
- Lean RE, Melzer TR, Bora S, Watts R, Woodward LJ. 2017. Attention and Regional Gray Matter Development in Very Preterm Children at Age 12 Years. J Int Neuropsychol Soc. 23:539–550. [DOI] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, Cho SR, Kim EY, Park JY, Kim CH, Kim DG, Park CI. 2011. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain. 134:1199–1210. [DOI] [PubMed] [Google Scholar]
- Loh WY, Anderson PJ, Cheong JLY, Spittle AJ, Chen J, Lee KJ, Molesworth C, Inder TE, Connelly A, Doyle LW, Thompson DK. 2017. Neonatal basal ganglia and thalamic volumes: very preterm birth and 7-year neurodevelopmental outcomes. Pediatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, Xiong J. 2011. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage. 58:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B. 2015. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol. 13:e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus N, Eggebrecht AT, Todorov A, Elison JT, Wolff JJ, Cole L, Gao W, Pandey J, Shen MD, Swanson MR, Emerson RW, Klohr CL, Adams CM, Estes AM, Zwaigenbaum L, Botteron KN, McKinstry RC, Constantino JN, Evans AC, Hazlett HC, Dager SR, Paterson SJ, Schultz RT, Styner MA, Gerig G, Network TI, Schlaggar BL, Piven J, Pruett JR Jr. 2017. Walking, gross motor development, and brain functional connectivity in infants and toddlers. Cerebral Cortex. 28:750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, Katz KH, Schneider KC, Makuch RW, Constable RT, Ment LR. 2010. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 51:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Nam KW, Walshe M, Murray RM, Cuddy M, Rifkin L, Allin MP. 2014. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin. 6:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha DW, Chang WH, Kim J, Sim EG, Park ES. 2012. Comparing quantitative tractography metrics of motor and sensory pathways in children with periventricular leukomalacia and different levels of gross motor function. Neuroradiology. 54:615–621. [DOI] [PubMed] [Google Scholar]
- Rinne P, Hassan M, Fernandes C, Han E, Hennessy E, Waldman A, Sharma P, Soto D, Leech R, Malhotra PA, Bentley P. 2018. Motor dexterity and strength depend upon integrity of the attention-control system. Proc Natl Acad Sci U S A. 115:E536–E545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivals I, Personnaz L, Taing L, Potier MC. 2007. Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics. 23:401–407. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. 2016. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatr Res. 79:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, Smyser CD. 2017. Neonatal Amygdala Functional Connectivity at Rest in Healthy and Preterm Infants and Early Internalizing Symptoms. J Am Acad Child Adolesc Psychiatry. 56:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom C. 2008. Maps of random walks on complex networks reaveal community structure. PNAS. 105:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR. 2015. Cerebral Lateralization is Protective in the Very Prematurely Born. Cereb Cortex. 25:1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Henderson SE, Sugden DA, Barnett AL. 2011. Structural validity of the Movement ABC-2 test: factor structure comparisons across three age groups. Res Dev Disabil. 32:1361–1369. [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman BCM, Fiers MJ, Henderson SE, Henderson L. 2008. Interrater reliability of the movement assessment battery for children. Physical Therapy. 88:286–294. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, Neil JJ. 2013. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS One. 8:e68098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. 2016. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex. 26:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada K, Lohaugen GC, Eikenes L, Bjorlykke KM, Haberg AK, Skranes J, Rimol LM. 2015. Visual-motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. Neuroimage. 109:493–504. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide epxression profiles. PNAS. 102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P. 2011. Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage. 57:1492–1498. [DOI] [PubMed] [Google Scholar]
- Thomas AR, Lacadie C, Vohr B, Ment LR, Scheinost D. 2017. Fine Motor Skill Mediates Visual Memory Ability with Microstructural Neuro-correlates in Cerebellar Peduncles in Prematurely Born Adolescents. Cereb Cortex. 27:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Chen J, Beare R, Adamson CL, Ellis R, Ahmadzai ZM, Kelly CE, Lee KJ, Zalesky A, Yang JYM, Hunt RW, Cheong JLY, Inder TE, Doyle LW, Seal ML, Anderson PJ. 2016. Structural connectivity relates to perinatal factors and functional impairment at 7years in children born very preterm. Neuroimage. 134:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, Egan GF. 2012. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 59:3571–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Lee KJ, Egan GF, Warfield SK, Doyle LW, Anderson PJ, Inder TE. 2014. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex. 52:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmin H, Beckmann CF, O’Muircheartaigh J, Ball G, Nongena P, Makropoulos A, Ederies A, Counsell SJ, Kennea N, Arichi T, Tusor N, Rutherford MA, Azzopardi D, Gonzalez-Cinca N, Hajnal JV, Edwards AD. 2015. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci U S A. 112:6485–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JE, Bloem BR. 2005. Role of the Basal Ganglia in Balance Control. Neural Plasticity. 12:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2009. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. 2014. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 20:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Fan GG, Xu K, Wang C. 2014. Altered microstructural connectivity of the superior and middle cerebellar peduncles are related to motor dysfunction in children with diffuse periventricular leucomalacia born preterm: a DTI tractography study. Eur J Radiol. 83:997–1004. [DOI] [PubMed] [Google Scholar]
- White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam KW, Sato JR, Allin MP, Shergill SS, Murray RM, Williams SC, Nosarti C. 2014. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 4:352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Hauser TK, Krageloh-Mann I, Lidzba K. 2014. Specific impairment of functional connectivity between language regions in former early preterms. Hum Brain Mapp. 35:3372–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Lee KJ, Anderson PJ. 2010. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Developmental Medicine & Child Neurology. 52:232–237. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. 2006. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine. 355:685–694. [DOI] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT. 2009. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 45:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Powell TL, Morgan BR, Card D, Lee W, Smith ML, Sled JG, Taylor MJ. 2015. Deep grey matter growth predicts neurodevelopmental outcomes in very preterm children. Neuroimage. 111:360–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


