Abstract
Classical theories suggest placebo analgesia and nocebo hyperalgesia are based on expectation and conditioned experience. Whereas the neural mechanism of how expectation modulates placebo and nocebo effects during pain anticipation have been extensively studied, little is known about how experience may change brain networks to produce placebo and nocebo responses. We investigated the neural pathways of direct and observational conditioning for conscious and nonconscious conditioned placebo/nocebo effects using magnetoencephalography and a face visual cue conditioning model. We found that both direct and observational conditioning produced conscious conditioned placebo and nocebo effects and a nonconscious conditioned nocebo effect. Alpha band brain connectivity changes before and after conditioning could predict the magnitude of conditioned placebo and nocebo effects. Particularly, the connectivity between the rostral anterior cingulate cortex and middle temporal gyrus was an important indicator for the manipulation of placebo and nocebo effects. Our study suggests that conditioning can mediate our pain experience by encoding experience and modulating brain networks.
Keywords: placebo, conditioning, consciousness, learning, alpha band connectivity, rostral anterior cingulate cortex
1. Introduction
Placebo analgesia and its opposite nocebo hyperalgesia (negative placebo effect) are robust phenomena observed in clinical practice and, as such, have significant and widespread applications (Scott et al., 2008; Tracey, 2010). Although placebo and nocebo effects are not direct pharmacological or physical interventions, they modulate brain circuits that can confer therapeutic effects (Colloca et al., 2008; Lu et al., 2010; Wager et al., 2004; Watson et al., 2009), both consciously and unconsciously (Jensen et al., 2015, 2012).
Conditioning is known to play an important role in placebo and nocebo effects by establishing a link between a context (cues) and the following pain stimulus, thus creating expectancies for future pain responses to the same circumstance. In addition, the mechanisms leading to the release of endogenous opioids in placebo analgesia are believed to involve conditioning factors (Amanzio and Benedetti, 1999; Enck et al., 2008; Yu et al., 2014). Classic theories of placebo and nocebo had a strong focus on direct conditioning, suggesting that higher-order areas of the brain process the expectancies for forthcoming pain based on personal exposure (Freeman et al., 2015; Wager et al., 2004). Recent studies have extended the theories to indirect conditioning where subjects observe others and learn from their experiences, and demonstrated that social observation can elicit placebo analgesia (Colloca and Benedetti, 2009; Egorova et al., 2015; Hunter et al., 2014) or nocebo hyperalgesia (Egorova et al., 2015; Świder and Ba̧bel, 2013; Vögtle et al., 2013).
Although placebo and nocebo effects are robust in general, they are largely variable across individuals. A number of psychological factors influencing the magnitude of placebo and nocebo responses have been studied, including suggestibility (Morton et al., 2010), expectation (Atlas et al., 2010; Morton et al., 2010), desire for relief (Vase et al., 2003) and belief (Zubieta et al., 2006). Neuroimaging metrics of brain function and structure provide important insights into individual variability of placebo effects. For instance, studies found that brain activities in opioid system (Petrovic et al., 2002; Scott et al., 2008; Zubieta et al., 2005) and reward system (Enck et al., 2008; Scott et al., 2007; Yu et al., 2014), brain responses for anticipation to pain stimuli (Kong et al., 2013; Wager et al., 2011, 2004) and gray matter density (Schweinhardt et al., 2009) were associated with the magnitude of individuals’ placebo responses.
Studies have suggested that learning may play an important role in placebo/nocebo effects, and a conditioning paradigm may provide a good model for us to explore how previous experience (learning) can shape our current experience (Bingel et al., 2006; Colloca et al., 2008; Kong and Benedetti, 2014; Price et al., 1999). However, the underlying neural mechanism of how conditioning mediates individual differences in placebo and nocebo response remains unknown.
In this study, we collected resting-state magnetoencephalography (MEG) data before and after direct and observational conditioning and investigated the relationships between brain connectivity changes and conscious and nonconscious conditioned placebo and nocebo effects (Egorova et al., 2015). We hypothesized that the connectivity of brain regions associated with opioid release and anticipatory anxiety, particularly the rostral anterior cingulate cortex (rACC) which is connected to the descending pain modulation system and widely reported as the key region for opioid placebo effect (Bingel et al., 2006; Enck et al., 2008; Kong and Benedetti, 2014; Wager et al., 2011, 2004; Zubieta et al., 2005), would be associated with the conditioning effect and may be used to predict the strength of conditioned placebo and nocebo responses. A unique characteristic of MEG is that it allows us to explore the functional connectivity at different frequency bands. Four frequency bands (delta, theta, alpha and beta) have been studied in this study. We are particularly interested in the alpha band (8 – 12 Hz) brain connectivity since the alpha band oscillations are capable of mediating the processing of both conscious and nonconscious stimuli, as well as modulating sensory perception (Forschack et al., 2017; Tu et al., 2016b).
2. Materials and Methods
2.1. Subjects
Thirty-seven healthy participants without any psychiatric or neurologic disorders were enrolled in the study. Participants were dropped from the study due to (1) excessive interference noise of the MEG scanner (n = 7); (2) inability to recognize facial cues (n = 2); (3) inconsistent rating during the experiment (n = 4); (4) reported back pain (n = 1); (5) inability to finish the experiment (n = 2). The final sample consisted of 21 participants (12 females; aged 25.0 ± 3.9). All protocols were approved by the Massachusetts General Hospital Institutional Review Board, and informed consent was obtained from all participants.
2.2. Pain administration
Noxious heat stimuli were delivered using a PATHWAY contact heat evoked potential stimulator system (Medoc Advanced Medical Systems, Israel). Each stimulus was delivered for 2 seconds on the medial side of the lower right leg, changing positions between runs to reduce sensitization. Heat pain temperatures were calibrated for every subject to match each individual’s tolerance levels. For calibration, an ascending heat pain sequence starting at 35 °C and slowly increasing by 1 °C up to 50 °C or the subject’s maximum tolerance was used. Gracely Sensory Scales (0–20) (Chapman et al., 1985; Gracely et al., 1978) were used to measure subjective pain ratings. Temperatures that elicited subjective intensity ratings of around 5 for low pain, 10 for moderate pain, and 15 for high pain on a scale from 0 to 20 were selected. The selected temperatures were presented several more times in a random order to ensure rating consistency, then three temperatures were selected for the experiment.
2.3. Experiment design
The experiment consisted of two phases: a conditioning and test phase, both conducted during the same day (Figure 1). The experiment and all data have never been reported before.
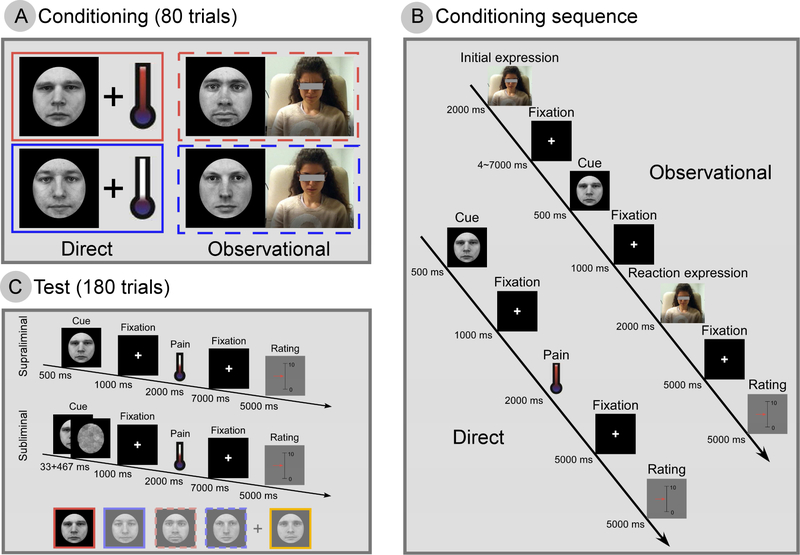
Figure 1. Experimental design.
(A) In the conditioning phase, direct cues (faces with neutral expressions) were accompanied by heat pain stimulation of high or low intensity; observational cues (different faces with neutral expressions) were accompanied by observing a model experiencing high (top row, red) or low (bottom row, blue) pain, showing both the physical reaction and subjective pain ratings of the model. Each subject participated in both direct and observational conditioning. The sequences for direct and observational conditioning are shown in (B). We have de-identified the observational model in the paper. (C) Stimuli presentation during test phase. One of five cues (four learned from conditioning and one novel, control cue) would appear either supraliminally (500 ms) or subliminally (33 ms + 467 ms mask). Identical moderate pain (~2 s) followed all cues. Subjects were instructed to rate each stimulus on a 0–10 VAS.
2.3.1. Conditioning
During the conditioning phase, subjects experienced two runs of direct and two runs of observational conditioning. Each run contained 20 trials, and in total we had 40 trials for direct and 40 trials for observational conditioning. For the direct conditioning runs, subjects saw a cue on the screen and received heat pain on the leg after 1 s. Two cues were presented, 10 times each, in a random order. One cue was presented with high pain and the other with low pain. After each pain stimulus, the subject rated the pain felt on a 0–10 visual analogue scale (VAS; 0: no pain; 10: worst pain that subjects can tolerate) using a button box and a scale displayed on the screen. It is worth mentioning that we used different pain rating scales (0–20 Gracely scale for the pain calibration phase; 0–10 VAS for the conditioning and test phases) to make subjects focus on pain experience rather than the memory of pain rating. Subjects were instructed to learn the association between the cues and pain levels.
In the observational conditioning runs, subjects watched a video of someone else (a model participant) undergoing direct conditioning without receiving pain themselves. They saw the model sitting in front of a computer with the heat probe on their leg, the cue that was presented to the model, the model’s facial reaction (painful or not painful) as he or she (one session showed a female model and the other a male) received pain stimulation, and the model’s pain rating. Two cues that were different from the direct conditioning cues were presented, 10 times each, one with high pain and the other with low pain. The model’s ratings that were shown in the video after each pain presentation averaged 2.0 ± 0.4 for low pain and 8.1 ± 0.4 for high pain. Again, subjects were instructed to learn the association between the cues and pain levels.
The timings of a typical trial for direct and observational conditioning are detailed in Figure 1B. In direct conditioning, subjects saw a cue (500 ms) and received heat pain 1000 ms after the cue. The target temperature for the pain stimulus (high or low) was maintained for 2000 ms. Subjects were instructed to rate their pain intensity 5000 ms after the pain stimulus, and the rating procedure lasted 5000 ms. In observational conditioning, subjects saw the model’s initial expression for 2000 ms. After 4000–7000 ms (pseudorandomized), they saw a cue for 500 ms, and after 1000 ms, they saw the model’s reaction (painful or not painful) to the pain stimulus for 2000 ms. Subjects observed how the model rated their pain intensity 5000 ms after the pain stimulus, and the rating procedure lasted 5000 ms. It is worth mentioning that we kept the timings from cue onset to pain rating for direct and observational conditioning the same to control for the duration of learning the association between cue and pain.
Following the conditioning phase, subjects were asked to identify the 4 learned cues from an array of 9 faces and whether the cue was direct or observational and high pain or low pain. Subjects were required to identify all 4 cues correctly before being allowed to continue.
2.3.2. Test
For the test phase, we informed subjects that they would see the 4 cues again along with an additional 5th cue that they had not seen before. Subjects were also told that some cues would appear fully visible (supraliminal) as before for 500 ms while other cues would appear very briefly (subliminal), immediately followed by a masking image (33 ms cue + 467 ms mask) so that the subject would not be able to recognize the cue. All cues were followed by the same moderately painful heat stimulus in order to test the conditioning effect. There were 3 sessions of 60 trials (in total 180 trials) for 10 different cues (5 supraliminal and 5 subliminal). For each type of cue, subjects were prompted to rate their pain sensation in 1/3 of the trials (6 out of 18 trials). In total, we collected six ratings for each of the 10 conditions (supraliminal and subliminal presentations of direct high and low, observational high and low, and neutral cues) for every participant. The timings for a typical trial in the test phase are detailed in Figure 1C.
After the test phase, subjects were shown some of the cues from the experiment, masked and unmasked, along with novel cues, masked and unmasked, and asked to identify whether the cues had been presented before. This was to confirm that recognition of the subliminal stimuli was at chance level.
2.3.3. Cues
Five forward-facing and emotionally neutral male face images taken from the Karolinska Directed Emotional Faces set (KDEF) were used as visual cues (Goeleven et al., 2008). Henceforth we refer to cues associated with a high or low level of heat pain presented in the direct conditioning sessions as ‘direct high cue’ and ‘direct low cue’, respectively. An ‘observational high cue’ and an ‘observational low cue’ associated with high observed pain or low observed pain, respectively, were presented in the observational conditioning sessions. We randomized the order of direct and observational conditioning across participants. A cue not associated with any pain level that did not appear during conditioning sessions was introduced as a control stimulus during the test phase. The assignment of facial images to a given condition was counterbalanced across participants, and all stimuli were presented using Presentation software (Version 16.3, www.neurobs.com).
2.4. MEG resting state data and structural MRI acquisition
MEG resting-state data were recorded twice for each subject, before and after the conditioning phase. The MEG data were acquired inside a magnetically shielded room using a whole-head Elekta Neuromag VectorView system (Helsinki, Finland) composed of 306 sensors arranged in 102 triplets of two orthogonal planar gradiometers and one magnetometer. The data were sampled at 1000 Hz after 300 Hz anti-aliasing low-pass filtering. Subjects were instructed to stay awake and look at a black fixation cross on a gray background at the center of the screen continuously for 6 minutes. T1-weighted, high-resolution magnetization prepared rapid acquisition gradient-echo (MPRAGE) structural images were collected on a 3T Siemens Trio whole-body MRI scanner (Siemens Medical Systems, Erlangen, Germany) using a 32-channel head coil.
2.5. MEG data preprocessing
The data were spatially filtered using the signal space separation method (Maxfilter software, Elekta Neuromag) to reduce noise generated by sources outside the brain. The signal space separation procedure also compensated for the participant’s head motion, which was acquired at 200 ms intervals using four head position indicator coils. We also used signal space projection to remove artifacts from heartbeats and eye blinks. Sensors with signal spikes where the amplitude was higher than 5SD over the whole time course were identified and dropped. The average number of sensors dropped was well below 2% for all participants (Mean ± SD: 3.5 ± 6.0).
2.6. MEG source estimation
We used the minimum-norm estimate (MNE) (Hämäläinen and Ilmoniemi, 1994) to map the MEG sensor data onto each subject’s high-resolution cortical surface generated by FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). The forward solution was computed using a boundary-element model with a single compartment bounded by the inner surface of the skull. We used the watershed algorithm in Freesurfer to generate the inner skull surface triangulations from the MRI of each participant. The source space for estimating the source current distribution consisted about 10,000 current dipoles on the cortical surface in each hemisphere. The source orientations were assumed to be perpendicular to the cortex. We estimated the sensor noise covariance matrix from the data acquired in the absence of a subject before each session.
The Freesurfer software identified 32 gyri and sulci per hemisphere based on the Desikan-Killiany Atlas, and a total of 64 cortical regions were used for further processing. We categorized these cortical regions into six groups based on their spatial and functional characteristics (Table S1) (Klein and Tourville, 2012). For the analysis, estimated source time series were extracted for each dipole within a region (Kitzbichler et al., 2015). Due to the ambiguity of individual dipole orientations, these timeseries were not averaged directly but first aligned with the dominant component of the multivariate set of timeseries before calculating the regional mean while preserving the temporal dimension. Specifically, we made use of the SVD of the data matrix. If the sign of the dot product between the first left singular vector and all other time-series in a region was negative, we inverted the time-series before averaging.
2.7. Connectivity matrix construction
The time courses were downsampled (sampling rate = 100 Hz) and band-pass filtered to delta (1 – 2Hz), theta (3 – 7 Hz), alpha (8 – 12 Hz), and beta (13 – 30 Hz). Continuous data were epoched with a length of 30 s and an overlap of 8 s. We calculated orthogonal correlations (z-transformed) between cortical regions (Hipp et al., 2012), averaged across epochs, and generated a connectivity matrix (64×64) for each frequency band and each subject. The method proposed in Hipp et al. estimates the amplitude-envelope correlation (AEC) between two time series. In the present study, we used an amplitude-based connectivity measure because in a recent MEG resting-state study (Colclough et al., 2016), the authors showed that AEC had better test-retest reliability and consistency in MEG resting-state connectivity.
2.8. Predicting conditioned placebo and nocebo effects
We hypothesized that inter-subject variation for conditioned placebo and nocebo effects might arise from the effectiveness of conditioning. Therefore, in the first step, we extracted significantly changed patterns between pre-conditioning and post-conditioning connectivity matrices for different frequency bands as features to predict the strength of placebo and nocebo effects. Specifically, resting-state connectivity matrices between pre- and postconditioning were compared across 21 subjects using paired t-test. We kept altered brain connectivity (both increased and decreased after conditioning) based on the results of t-test with an uncorrected significance level of p < 0.05. Here, we used a less strict p value because in this step (1) t-test was used as a feature screening process and we made a tradeoff between computational complexity and keeping sufficient information for the regression analyses in the next step; (2) we performed stringent nonparametric statistical tests (details in Statistical analyses) for the following analyses to keep our major results sound.
In the second step, we built multivariate linear regression (MVLR) models to predict magnitudes of placebo and nocebo effects. Due to the important role of alpha band oscillations in mediating the processing of both conscious and nonconscious stimuli as well as modulating sensory perception (Forschack et al., 2017; Tu et al., 2016b), we used significantly changed patterns between pre-conditioning and post-conditioning alpha band AEC matrices as independent variables for MVLR models. Five significantly conditioned placebo and nocebo responses, specifically consciously direct placebo/nocebo responses, consciously observational placebo/nocebo responses, and non-consciously direct nocebo responses, were used as dependent variables separately for each model. To explore potential contributions from other frequency bands, we also performed exploratory analyses by using AEC in other frequency bands as features (independent variables) to predict placebo/nocebo responses. For each model, we used recursive feature elimination (RFE) combined with support vector regression (SVR) to select the features with the most discriminative power (De Martino et al., 2008; Dosenbach et al., 2010). SVR-RFE was used to train the regression model and obtain weights for each feature. The features were ranked according to the absolute values of weights and the lowest ranking feature was discarded. Then the regression model was trained using the new feature set (without the discarded features). This procedure was repeatedly performed until the feature set was empty. To select the features with minimal prediction error, we conducted a full backward elimination procedure for each model. To reduce the risking of overfitting, all analyses were based on leave-one-out cross validation (LOOCV), and therefore all training features were selected independently of each test case by 21 iterations (Tu et al., 2016a, 2018).
Since we used a LOOCV strategy and each iteration was based on a slightly different dataset, the selected feature sets differed slightly from iteration to iteration. A consensus map which aggregated features selected in all LOOCV iterations was used and only features retained from all iterations were survived in the consensus map (Dosenbach et al., 2010; Zeng et al., 2012). The prediction power of each feature was denoted by the averaged weights across all iterations.
The performance of the regression model was evaluated by the prediction-outcome correlation, which is defined as the correlation between the actual and predicted treatment responses, as well as the mean absolute error (MAE), which is defined as mean discrepancy between real and predicted values. We used permutation tests to obtain an unbiased estimation of prediction-outcome correlation and MAE. Based on our hypothesis for alpha band connectivity, we set the alpha band results as the primary outcome and applied Bonferroni correction across five alpha band models, and the corrected significance level was 0.01. Details of the analyses are described in Statistical analyses.
Since direct and observational conditioning produced similar and correlated placebo and nocebo responses (See Results for details), we also explored the feasibility to develop a single model to predict placebo responses (direct and observational conditioned conscious placebo) as well as a single model to predict nocebo responses (direct and observational conditioned conscious nocebo, and direct conditioned nonconscious nocebo).
2.9. Statistical analyses
All statistical analyses for behavioral scores were performed using R incorporated in JASP software (Version 0.8.1, http://www.jasp-stats.org). First, we compared the subjects’ ratings after moderate heat pain stimuli following different conditioned cues, both conscious and non-conscious, using a 3-way repeated measures analysis of variance (ANOVA) between cue (high vs. neutral vs. low), conditioning type (direct vs. observational) and awareness (conscious vs. nonconscious). When observing significant effects, a post-hoc pairwise comparison (paired t-test) was conducted.
Second, we investigated placebo and nocebo responses by comparing the neutral cue and the high/low cue pain ratings (placebo: neutral – low; nocebo: high – neutral) under each type of conditioning and awareness. A 3-way repeated measures ANOVA between modulation type (placebo vs. nocebo), conditioning type (direct vs. observational) and awareness (conscious vs. nonconscious) was performed. When observing significant effects, a post-hoc pairwise comparison (paired t-test, Bonferroni corrected) was conducted.
Third, to study the relationship of placebo/nocebo effects obtained from direct and observational conditioning, we calculated the correlations for pairs: (1) direct or observational (2) conscious or nonconscious (3) placebo or nocebo effects.
The performance measures (prediction-outcome correlation and MAE) obtained from regression analyses were assessed using permutation testing. In each testing, we randomly permuted the labels (direct placebo, observational placebo, direct nocebo, or observational placebo) of the data prior to training. LOOCV was then performed on the permuted dataset and the procedure was repeated 1000 times. If the performance measures obtained from real labels exceeded the 95% confidence interval generated from the values obtained from randomly relabeled data, the regression model was considered to be performing well.
3. Results
3.1. Pain ratings during conditioning phase
The pain ratings between the low and high pain stimuli were significantly different (p < 0.001); low pain elicited an average rating of 1.6 ± 0.5 (mean ± SE) and high pain elicited an average rating of 7.4 ± 0.7, indicating the different levels of pain perception associated with high and low cues during conditioning.
3.2. Pain ratings during test phase
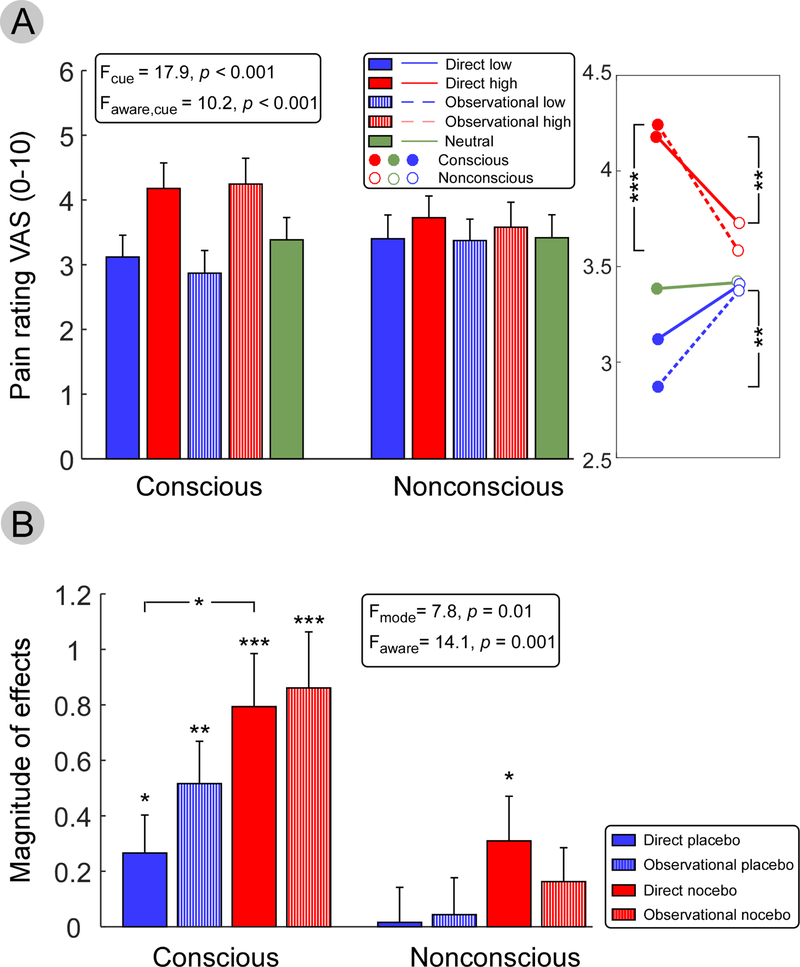
We first compared the subjective pain ratings to identical moderate heat pain stimuli, which were preceded by conscious and nonconscious cues, using repeated measures ANOVA with factors cue (high vs. neutral vs. low), conditioning type (direct vs. observational) and awareness (conscious vs. nonconscious) (Figure 2A). Results showed a main effect of cue (F2,40 = 17.89, p < 0.001); pain ratings for high pain cues (3.93 ± 0.38) were significantly higher than ratings for neutral (3.40 ± 0.35) and low cues (3.19 ± 0.34). Pairwise comparisons between low and high cues were significant in direct conscious (p < 0.001), observational conscious (p < 0.001), and direct nonconscious (p = 0.031), but not in observational nonconscious (p = 0.14). We also observed a significant effect of interaction between cue and awareness (F2,40 = 10.18, p < 0.001). Pairwise comparisons revealed pain ratings after direct and observational high cues were lower in nonconscious than conscious (p = 0.004 and p < 0.001 respectively), and pain ratings after observational low cues were higher in nonconscious than conscious (p = 0.007).
Figure 2. Behavioral scores for the test phase.
(A) Pain ratings after direct and observational conditioned cues, separated by awareness (conscious vs. nonconscious). Three-way repeated measures ANOVA (cue, awareness, and conditioning type) showed a significant main effect of cue (high, neutral, and low) and in the interaction between awareness and cue. (B) Magnitudes of direct and observational conditioned placebo and nocebo responses. Significant directly and observationally conditioned conscious placebo and nocebo effects were found, as well as directly conditioned nonconscious nocebo effects. Three-way repeated measures ANOVA (modulation mode, awareness, and conditioning type) revealed a significant main effect in modulation mode and awareness. * p < 0.05, ** p < 0.01 and *** p < 0.001.
3.3. Conditioned placebo and nocebo effects
To determine whether conditioning produced significant conditioned placebo and nocebo effects, we compared conditioned low and high cues to neutral cues under conscious and nonconscious awareness (Figure 2B). Significant conditioned placebo and nocebo effects were observed with both directly and observationally conditioned conscious cues (direct placebo: p = 0.033; observational placebo: p = 0.001; direct nocebo: p < 0.001; observational nocebo: p < 0.001). However, with nonconscious cues, only the directly conditioned nocebo effect was significant (p = 0.034).
We compared the magnitude of conditioned placebo and nocebo effects using a repeated measures ANOVA with factors modulation type (placebo vs. nocebo), conditioning type (direct vs. observational) and awareness (conscious vs. nonconscious). Results showed a main effect of modulation type (F1,20 = 7.79, p = 0.011). Nocebo effects (0.53 ± 0.18) were significantly higher than placebo effects (0.21 ± 0.14). Pairwise comparison revealed a stronger conditioned nocebo effect than conditioned placebo effect for directly conditioned conscious cues (p = 0.005). We also observed a main effect of awareness (F1,20 = 14.13, p = 0.001), indicating conditioned placebo and nocebo effects were stronger with conscious cues. Pairwise comparisons revealed a higher magnitude of directly conditioned nocebo effect (p = 0.020), observationally conditioned placebo effect (p = 0.012) and observationally conditioned nocebo effect (p = 0.003) with conscious cues than with nonconscious cues, but this effect was not found for directly conditioned placebo effect (p = 0.13).
We calculated the correlations between the effects obtained from direct and observational learning and found significant correlations between directly and observationally conditioned conscious placebo (r = 0.61, p = 0.003), conscious nocebo (r = 0.95, p < 0.001), and nonconscious placebo (r = 0.42, p = 0.05). These results indicated the experience through direct and observational learning might lead to strongly correlated placebo or nocebo effect, thus provided insights that learning from others could introduce similar effect for an individual.
3.4. Conditioning modulated brain connectivity
We first examined the grand-average spectra in sensor space (Figure S1) and source space (Figure S2) for pre- and post-conditioning data, respectively, and did not observe any significant difference of power for the whole frequency range (0–30 Hz).
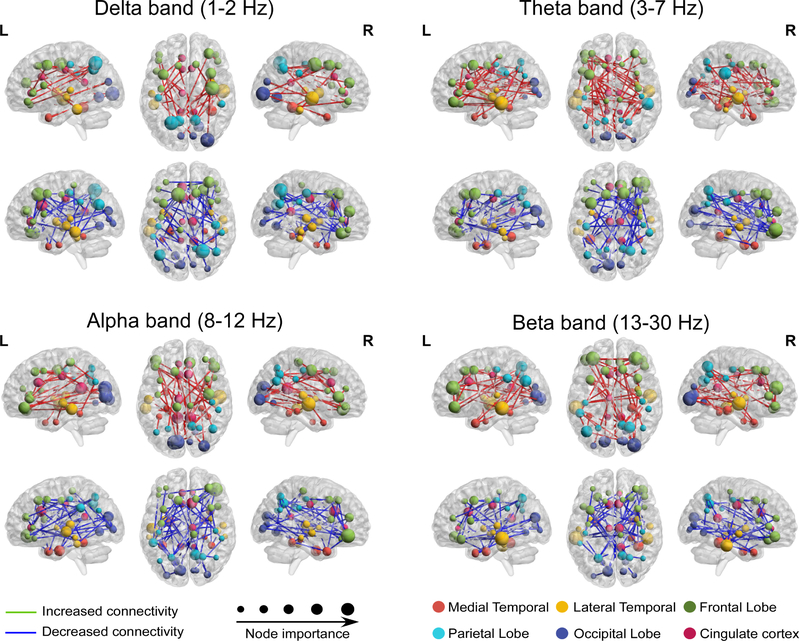
Then we calculated brain connectivity of the four frequency bands between 64 ROIs. Consistent with previous findings showing that MEG resting-state brain connectivity is band-specific (Hipp et al., 2012), we found that important nodes resided in the parietal and occipital lobes in the alpha to beta frequency ranges, in the medial temporal lobe in the theta band, and in the temporal lobe in the delta band (Figure S3).
3.5. Predict conditioned placebo and nocebo effects
Group differences in brain connectivity between pre-conditioning and post-conditioning were also band-specific (Figure 3). For different frequency bands, we built multivariate regression models with changes in brain connectivity as independent variables (features), and conditioned placebo/nocebo effects as measured by averaged pain rating change across different trials of same type as dependent variables.
Figure 3. Brain connectivity significantly changed after conditioning.
Brain activity in cortical space was bandpass filtered into four different frequency bands and brain connectivity was constructed between each pair of sources. Significantly increased (red lines) and decreased (blue lines) brain connectivities after conditioning were retained as features for regression analyses. Node importance denotes the number of connections from the node to other nodes.
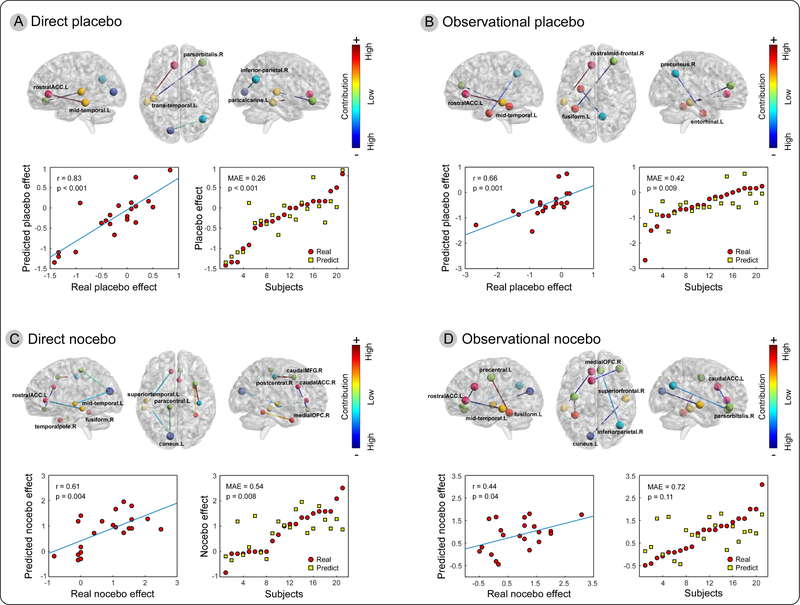
With consciously presented cues, changes in alpha band brain connectivity showed significant predictive information for both directly and observationally conditioned placebo effects. For the conditioned direct placebo effect, the changes in alpha band connectivity between the left rACC and left middle temporal gyrus (MTG), left transverse temporal gyrus and right pars orbitalis of inferior frontal gyrus (IFG), and left pericalcarine cortex and right inferior parietal lobe (IPL) significantly predicted direct placebo effects (Figure 4A). The correlation between real and predicted direct placebo effects was 0.83 (p < 0.001), and the error was 0.26 (p < 0.001). In contrast, the changes in alpha band connectivity between the left rACC and left MTG, left fusiform gyrus and right rostral middle frontal gyrus (MFG), and right precuneus and left entorhinal cortex predicted observational placebo effects with a prediction-outcome correlation of 0.66 (p = 0.001) and MAE of 0.42 (p = 0.009) (Figure 4B).
Figure 4. Changes in alpha band connectivity predicted conscious conditioned placebo and nocebo effects.
The upper panel of A-D shows the identified changes of brain connectivity that were predictive for direct and observational placebo/nocebo effects. The lower panel of each sub-figure summarizes prediction performance using prediction outcome correlation (left) and mean absolute error (right).
For the directly conditioned conscious nocebo effect, the changes in alpha brain connectivity between the left rACC and left MTG, right temporal pole and right fusiform, left superior temporal gyrus and left cuneus, left paracentral lobe and left cuneus, right caudal MFG and right postcentral gyrus, and right caudal ACC and right medial orbitofrontal cortex (OFC) predicted direct nocebo effects (Figure 4C). The correlation between real and predicted direct nocebo effects was 0.61 (p = 0.004) and the error was 0.54 (p = 0.008). For the observationally conditioned conscious nocebo effect, the changes in alpha band connectivity between the left rACC and left MTG, left precentral lobe and left fusiform, right medial OFC and right IPL, right superior frontal lobe and left cuneus, and left caudal ACC and right pars orbitalis of the IFG predicted the effect with a prediction-outcome correlation of 0.44 (p = 0.04) and MAE of 0.72 (p = 0.11) (Figure 4D).
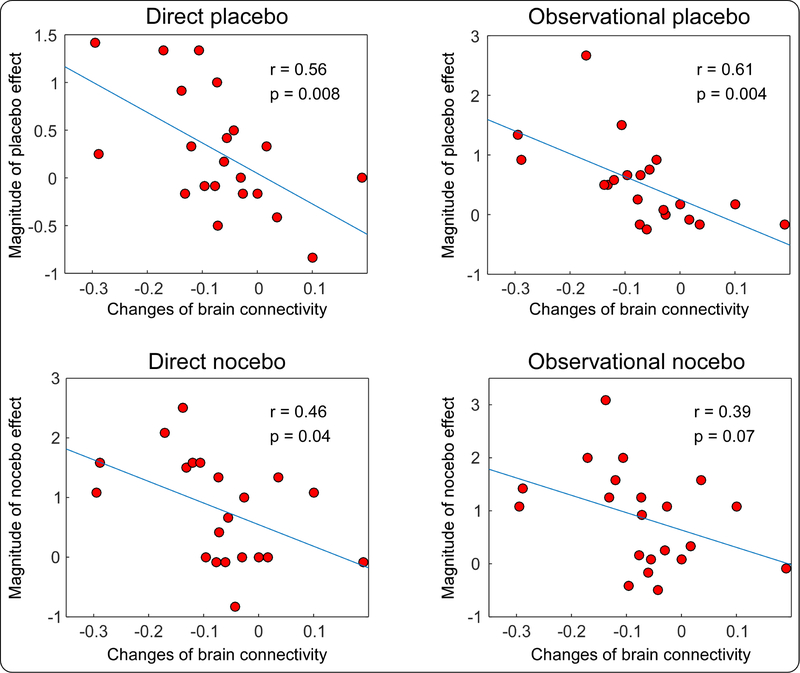
Figure 5 demonstrates that the changes of left rACC and left MTG alpha band connectivity predicted both directly and observationally conditioned placebo/nocebo responses. We directly compared the changes in brain connectivity and magnitudes of placebo/nocebo responses and found that the decrease in alpha band connectivity between the left rACC and left MTG was strongly correlated with the magnitudes of the directly conditioned placebo effect (r = 0.56, p = 0.008), observationally conditioned placebo effect (r = 0.61, p = 0.004), and directly conditioned nocebo effect (r = 0.46, p = 0.04), and was marginally correlated with the magnitude of the observationally conditioned nocebo effect (r = 0.39, p = 0.07).
Figure 5. Relationships between changes in left rACC-MTG connectivity and magnitudes of placebo/nocebo effects.
Stronger direct and observational placebo/nocebo effects were associated with decreased rACC-MTG connectivity.
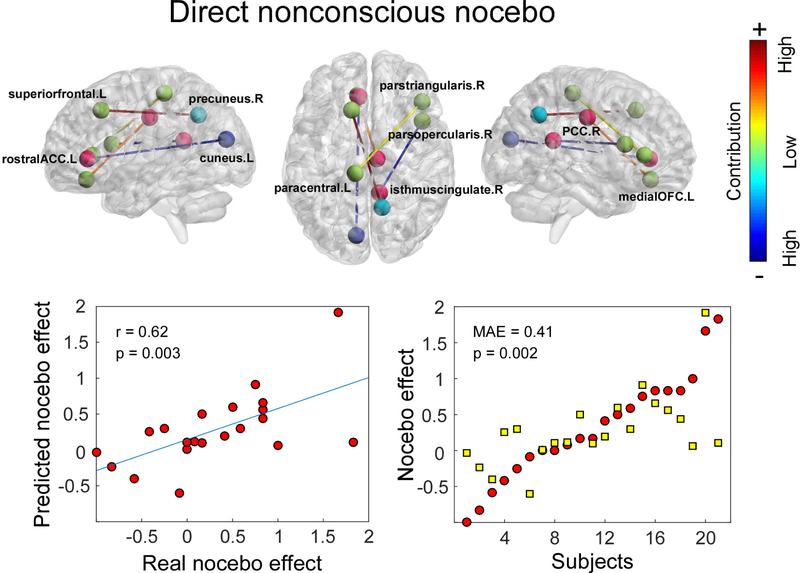
For nonconscious cues, we only observed a significant directly conditioned nocebo effect. The regression model was built for predicting such an effect. Changes in alpha band brain connectivity between the left rACC and left cuneus, left superior frontal lobe and right precuneus, left paracentral lobe and right pars triangularis gyrus of IFG, right pars opercularis gyrus of IFG and right isthmus cingulate cortex, and left medial OFC and right posterior cingulate cortex (PCC) predicted the nonconscious nocebo effect with a prediction-outcome correlation of 0.62 (p = 0.003) and MAE of 0.41 (p = 0.002) (Figure 6).
Figure 6. Alpha band connectivity predicted magnitudes of directly conditioned nonconscious nocebo effect.
Changes in alpha band brain connectivity between left rACC and left cuneus, left superior frontal lobe and right precuneus, left paracentral lobe and right pars triangularis of IFG, right pars opercularis of IFG and right isthmus of cingulate cortex, and left medial OFC and right posterior cingulate cortex (PCC) predicted nonconscious nocebo effects.
The single model for placebo effects was significantly predictive to direct conditioned conscious placebo response with a prediction-outcome correlation of 0.52 (p = 0.02) and MAE of 0.42 (p = 0.01), and observational conditioned conscious placebo response (r = 0.55, p = 0.01; MAE = 0.48, p = 0.02). The model for nocebo effects was significantly predictive to direct conditioned conscious nocebo response (r = 0.57, p = 0.01; MAE = 0.53, p = 0.01) and observational conditioned conscious nocebo response (r = 0.47, p = 0.04; MAE = 0.55, p = 0.03), but not predictive to direct conditioned nonconscious nocebo response (r = 0.16, p = 0.50; MAE = 0.80, p = 0.48).
Connectivity from other frequency bands did not show results with consistent predictive information for placebo and nocebo responses.
4. Discussion
In this study, we applied a visual cue conditioning paradigm to investigate the neural mechanism of conditioned placebo and nocebo effects using MEG. We found that after conditioning, resting-state brain connectivity showed significant changes in multiple brain regions. The changes in alpha band brain connectivity were capable of predicting the magnitude of direct and observational conditioned placebo and nocebo responses. In particular, alpha band connectivity between the left rACC and left MTG consistently predicted four responses (direct placebo, observational placebo, direct nocebo, and observational nocebo). Correlation analyses suggested that the decrease in rACC and MTG connectivity was linked with a stronger magnitude of placebo and nocebo responses.
We used a model applied in our previous studies (Egorova et al., 2015) to investigate how visual cues can modulate our pain perception. Our results showed both direct and observational conditioning produced significant conditioned placebo and nocebo effects when conscious cues were used. Interestingly, the magnitude of placebo and nocebo effects during the test phase was similar and significantly correlated for both directly and observationally conditioned cues. This result is consistent with a study that used social observation to establish conditioned placebo and nocebo learning and found no difference in the amplitude of the placebo effect between direct and observational conditioning (Colloca and Benedetti, 2009) and with our previous study (Egorova et al., 2015). Taken together, these findings suggest that classical conditioning can be extended to include learning from others’ experiences.
We only obtained a significant directly conditioned nocebo effect for nonconscious cues. For other nonconscious cues, however, we saw trends of nonconscious low pain cues reducing pain perception and high pain cues increasing pain perception. This result suggests that even for conscious physiological processes, such as pain processing, placebo and nocebo effects can operate without conscious awareness of the triggering cues, which is partly consistent with our previous studies (Egorova et al., 2015; Jensen et al., 2012).
Placebo and nocebo effects are based on expectation and experience, where experience is a form of learning that is implemented by conditioning (Büchel et al., 2014). How the conditioning stage, when associations between cue and pain are learned, modulates brain activity and consequently mediates placebo/nocebo responses remains unknown. The conditioning stage in previous studies using facial cues (Jensen et al., 2015, 2012) encoded different faces with experiences of pain perception, and facial cues have been shown to elicit stronger effects than other types of stimuli (Egorova et al., 2017). Since we used visual cues to test the conditioning effect, we would expect the learning to start from within the visual processing networks. Our results showed that brain connectivity between the rACC and MTG was significantly modulated during conditioning. The MTG is a region known to play a role in the recognition of faces. In an early imaging study, perception of faces was associated with activity in the MTG and temporal pole (Sergent et al., 1992). Subsequent positron emission tomography (PET) and fMRI studies further confirmed the role of the MTG in face recognition (Gorno-Tempini et al., 1998; Leveroni et al., 2000). Although this process is complex and needs to be accomplished by distributed brain systems, our results consistently revealed that activation of the rACC-MTG pathway in both direct and observational conditioning, as well as shed light on their underlying neural mechanism.
Studies suggest that the endogenous opioid system is essential for both placebo and nocebo effects (Amanzio and Benedetti, 1999; Benedetti et al., 1999; Fields, 2004; Petrovic et al., 2002). They are associated with opposite responses of endogenous opioid neurotransmission in a distributed network of regions (Scott et al., 2008). Functional imaging studies provide evidence that the rACC serves a critical role in placebo analgesia and nocebo hyperalgesia. Using PET, Petrovic and colleagues demonstrated increased rACC activity during both placebo and opioid analgesia (Petrovic et al., 2002). Other studies using fMRI confirmed the involvement of the rACC in placebo analgesia (Kong et al., 2009, 2006; Wager et al., 2004). Studies using fMRI functional connectivity showed that rACC brain connectivity was associated with placebo response (Kong et al., 2013). Although reports of nocebo hyperalgesia are fewer than reports of placebo analgesia, a very recent study revealed that the rACC mediates the nocebo effect (Tinnermann et al., 2017). These studies provide direct support for the role of the rACC in linking pain perception and the pain modulation pathway (Bingel et al., 2006).
In contrast to PET or fMRI studies of the brain mechanisms of placebo and nocebo effects, MEG allows the investigation of brain activity in different frequency bands. In the present study, we identified the role of alpha band connectivity in modulating subsequent conditioned pain perception. This finding is consistent with previous studies showing a clear dependence of sensory perception on alpha oscillations. A long tradition of studies has investigated the functional role of alpha rhythms by electroencephalography (EEG) or MEG, suggesting that they have an inhibitory function and may reflect top-down control processes. Alpha band oscillations also play an active role in information processing (Klimesch, 2012). Evidence from alpha amplitude-envelope correlation or coherence presents a mechanism for short and long range communication in the brain (Engel et al., 2001; Fries, 2005; Hipp et al., 2012). In this framework, it is not surprising that we found that alpha band connectivity between the rACC and MTG was associated with the magnitude of placebo/nocebo response. Indeed, expectation and experience might be encoded in the dynamics of the thalamo-cortical and cortico-cortical networks (Fries, 2005; Keil et al., 2012; Lange et al., 2012). The levels of activity in these cortical networks can be correlated or anti-correlated and determine the responsiveness of the brain to subsequent conditioned cues.
The present study has several limitations. First, we did not have repeated recordings without interventions as a control condition to rule out the effects of potential changes in spontaneous connectivity between pre- and post-conditioning resting-state MEG. Second, due to the limited sample of the MEG study, we validated the results using leave-one-out cross validation, which may not stable (Varoquaux et al., 2017). We also provided results using the approach Varoquaux et al. suggested: random splits leaving out 20% of the subjects with 50 repetitions, in Table S2 and Figure S4. Both approaches yielded similar findings and results, but future confirmatory studies would be needed to further validate our current imaging findings.
In conclusion, we investigated the neural mechanism underlying both direct and observational conditioning. Our results suggest both direct and observational conditioning can trigger conditioned placebo and nocebo effects. The learning and encoding performance during conditioning might be reflected by the alpha band brain connectivity, especially between the rACC and MTG, and may consequently mediate the magnitude of placebo and nocebo responses.
Supplementary Material
Acknowledgement
Jian Kong is supported by R21AT008707, R61 / R33 AT009310, R01AT008563 from NIH/NCCIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Jian Kong has a disclosure to report (holding equity in a startup company (MNT) and pending patents to develop new neuromodulation devices), all other authors declare no conflict of interest.
References
- Amanzio M, Benedetti F, 1999. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci 19, 484–494. doi:10.1523/JNEUROSCI.19-01-00484.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD, 2010. Brain Mediators of Predictive Cue Effects on Perceived Pain. J. Neurosci 30, 12964–12977. doi:10.1523/JNEUROSCI.0057-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Arduino C, Amanzio M, 1999. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J. Neurosci 19, 3639–3648. doi:10.1523/JNEUROSCI.19-09-03639.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Bu C, 2006. Mechanisms of placebo analgesia : rACC recruitment of a subcortical antinociceptive network. Pain 120, 8–15. doi:10.1016/j.pain.2005.08.027 [DOI] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, Eippert F, 2014. Placebo Analgesia: A Predictive Coding Perspective. Neuron 81, 1223–1239. doi:10.1016/j.neuron.2014.02.042 [DOI] [PubMed] [Google Scholar]
- Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE, 1985. Pain measurement: an overview. Pain 22, 1–31. doi:10.1016/0304-3959(85)90145-9 [DOI] [PubMed] [Google Scholar]
- Colclough GL, Woolrich MW, Tewarie PK, Brookes MJ, Quinn AJ, Smith SM, 2016. How reliable are MEG resting-state connectivity metrics? Neuroimage 138, 284–293. doi:10.1016/J.NEUROIMAGE.2016.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Benedetti F, 2009. Placebo analgesia induced by social observational learning. Pain 144, 28–34. doi:10.1016/j.pain.2009.01.033 [DOI] [PubMed] [Google Scholar]
- Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M, 2008. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain 139, 306–314. doi:10.1016/j.pain.2008.04.021 [DOI] [PubMed] [Google Scholar]
- De Martino F, Valente G, Staeren N, Ashburner J, Goebel R, Formisano E, 2008. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage 43, 44–58. doi:10.1016/j.neuroimage.2008.06.037 [DOI] [PubMed] [Google Scholar]
- Dosenbach N, Nardos B, Cohen A, Fair D, 2010. Prediction of individual brain maturity using fMRI. Science (80-. ). 329, 1358–61. doi:10.1126/science.1194144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N, Park J, Kong J, 2017. In the face of pain: the choice of visual cues in pain conditioning matters. Eur. J. Pain 21, 1243–1251. doi:doi: 10.1002/ejp.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N, Park J, Orr SP, Kirsch I, Gollub RL, Kong J, 2015. Not seeing or feeling is still believing: Conscious and non-conscious pain modulation after direct and observational learning. Sci. Rep 5, 1–9. doi:10.1038/srep16809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enck P, Benedetti F, Schedlowski M, 2008. New Insights into the Placebo and Nocebo Responses. Neuron 59, 195–206. doi:10.1016/j.neuron.2008.06.030 [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W, 2001. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci 2, 704–16. doi:10.1038/35094565 [DOI] [PubMed] [Google Scholar]
- Fields H, 2004. State-dependent opioid control of pain. Nat. Rev. Neurosci 5, 565–575. doi:10.1038/nrn1431 [DOI] [PubMed] [Google Scholar]
- Forschack N, Nierhaus T, Müller MM, Villringer A, 2017. Alpha-Band Brain Oscillations Shape the Processing of Perceptible as well as Imperceptible Somatosensory Stimuli during Selective Attention. J. Neurosci 37, 6983–6994. doi:10.1523/JNEUROSCI.2582-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S, Yu R, Egorova N, Chen X, Kirsch I, Claggett B, Kaptchuk TJ, Gollub RL, Kong J, 2015. Distinct neural representations of placebo and nocebo effect. Neuroimage 112, 197–207. doi:10.1016/j.neuroimage.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, 2005. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci 9, 474–480. doi:10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B, 2008. The Karolinska Directed Emotional Faces: A validation study. Cogn. Emot 22, 1094–1118. doi:10.1080/02699930701626582 [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS, 1998. The neural systems sustaining face and proper- name processing. Brain 121, 2103–2118. doi:10.1093/brain/121.11.2103 [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath F, Dubner R, 1978. Ratio scales of sensory and affective verbal pain descriptors. Pain 5, 5–18. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ, 1994. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput 32, 35–42. doi:10.1007/BF02512476 [DOI] [PubMed] [Google Scholar]
- Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK, 2012. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci 15, 884–890. doi:10.1038/nn.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Siess F, Colloca L, 2014. Socially induced placebo analgesia: A comparison of a pre-recorded versus live face-to-face observation. Eur. J. Pain 18, 914–922. doi:10.1002/j.1532-2149.2013.00436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J, 2015. A neural mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cereb. Cortex 25, 3903–3910. doi:10.1093/cercor/bhu275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J, 2012. Nonconscious activation of placebo and nocebo pain responses. Proc. Natl. Acad. Sci 109, 15959–15964. doi:10.1073/pnas.1202056109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil J, Muller N, Ihssen N, Weisz N, 2012. On the variability of the McGurk effect: Audiovisual integration depends on prestimulus brain states. Cereb. Cortex 22, 221–231. doi:10.1093/cercor/bhr125 [DOI] [PubMed] [Google Scholar]
- Kitzbichler MG, Khan S, Ganesan S, Vangel MG, Herbert MR, Hämäläinen MS, Kenet T, 2015. Altered development and multifaceted band-specific abnormalities of resting state networks in autism. Biol. Psychiatry 77, 794–804. doi:10.1016/j.biopsych.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Tourville J, 2012. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci 6, 171. doi:10.3389/fnins.2012.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, 2012. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci 16, 606–617. doi:10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Benedetti F, 2014. Placebo and Nocebo Effects: An Introduction to Psychological and Biological Mechanisms, in: Handbook of Experimental Pharmacology. pp. 3–15. doi:10.1007/978-3-662-44519-8_1 [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ, 2006. Brain Activity Associated with Expectancy-Enhanced Placebo Analgesia as Measured by Functional Magnetic Resonance Imaging. J. Neurosci 26, 381–388. doi:10.1523/JNEUROSCI.3556-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL, 2013. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 154, 459–467. doi:10.1016/j.pain.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub RL, 2009. An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. Neuroimage 47, 1066–1076. doi:10.1016/j.neuroimage.2009.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J, Halacz J, Van Dijk H, Kahlbrock N, Schnitzler A, 2012. Fluctuations of prestimulus oscillatory power predict subjective perception of tactile simultaneity. Cereb. Cortex 22, 2564–2574. doi:10.1093/cercor/bhr329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead L. a, Binder JR, Rao SM, 2000. Neural Systems Underlying the Recognition of Familiar and Newly Learned Faces. J. Neurosci 20, 878–886. doi:10.1523/JNEUROSCI.20-02-00878.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H-C, Hsieh J-C, Lu C-L, Niddam DM, Wu Y-T, Yeh T-C, Cheng C-M, Chang F-Y, Lee S-D, 2010. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: A 3T-fMRI study. Pain 148, 75–83. doi:10.1016/j.pain.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Morton DL, El-Deredy W, Watson A, Jones AKP, 2010. Placebo analgesia as a case of a cognitive style driven by prior expectation. Brain Res. 1359, 137–141. doi:10.1016/j.brainres.2010.08.046 [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M, 2002. Placebo and opioid analgesia--imaging a shared neuronal network. Science 295, 1737–40. doi:10.1126/science.1067176 [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS, 1999. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain 83, 147–156. doi:10.1016/S0304-3959(99)00081-0 [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC, 2009. The Anatomy of the Mesolimbic Reward System: A Link between Personality and the Placebo Analgesic Response. J. Neurosci 29, 4882–4887. doi:10.1523/JNEUROSCI.5634-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK, 2008. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65, 220–231. doi:10.1001/archgenpsychiatry.2007.34 [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK, 2007. Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects. Neuron 55, 325–336. doi:10.1016/j.neuron.2007.06.028 [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, Macdonald B, 1992. Functional Neuroanatomy of Face and Object Processing. Brain 115, 15–36. doi:10.1093/brain/115.1.15 [DOI] [PubMed] [Google Scholar]
- Świder K, Ba̧bel P, 2013. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. Pain 154, 1312–1317. doi:10.1016/j.pain.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Büchel C, 2017. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science (80-. ). 358, 105–108. doi:10.1126/science.aan1221 [DOI] [PubMed] [Google Scholar]
- Tracey I, 2010. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med 16, 1277–1283. doi:10.1038/nm.2229 [DOI] [PubMed] [Google Scholar]
- Tu Y, Tan A, Bai Y, Hung YS, Zhang Z, 2016a. Decoding Subjective Intensity of Nociceptive Pain from Pre-stimulus and Post-stimulus Brain Activities. Front. Comput. Neurosci 10, 32. doi:10.3389/fncom.2016.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Zhang Z, Tan A, Peng W, Hung YS, Moayedi M, Iannetti GD, Hu L, 2016b. Alpha and gamma oscillation amplitudes synergistically predict the perception of forthcoming nociceptive stimuli. Hum. Brain Mapp 37, 501–514. doi:10.1002/hbm.23048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YH, Fu ZN, Tan A, Huang G, Hu L, Hung YS, Zhang ZG, 2018. A novel and effective fMRI decoding approach based on sliced inverse regression and its application to pain prediction. Neurocomputing 273, 373–384. doi:10.1016/J.NEUCOM.2017.07.045 [Google Scholar]
- Varoquaux G, Raamana PR, Engemann DA, Hoyos-Idrobo A, Schwartz Y, Thirion B, 2017. Assessing and tuning brain decoders: Cross-validation, caveats, and guidelines. Neuroimage 145, 166–179. doi:10.1016/J.NEUROIMAGE.2016.10.038 [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD, 2003. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain 105, 17–25. doi:10.1016/S0304-3959(03)00073-3 [DOI] [PubMed] [Google Scholar]
- Vögtle E, Barke A, Kröner-Herwig B, 2013. Nocebo hyperalgesia induced by social observational learning. Pain 154, 1427–1433. doi:10.1016/j.pain.2013.04.041 [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK, 2011. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J. Neurosci 31, 439–452. doi:10.1523/JNEUROSCI.3420-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD, 2004. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303, 1162–1167. doi:10.1126/science.1093065 [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AKP, 2009. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145, 24–30. doi:10.1016/j.pain.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J, 2014. Placebo analgesia and reward processing: Integrating genetics, personality, and intrinsic brain activity. Hum. Brain Mapp 35, 4583–4593. doi:10.1002/hbm.22496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, Zhou Z, Li Y, Hu D, 2012. Identifying major depression using whole-brain functional connectivity: A multivariate pattern analysis. Brain 135, 1498–1507. doi:10.1093/brain/aws059 [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS, 2005. Placebo Effects Mediated by Endogenous Opioid Activity on -Opioid Receptors. J. Neurosci 25, 7754–7762. doi:10.1523/JNEUROSCI.0439-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J-K, Yau W-Y, Scott DJ, Stohler CS, 2006. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain. Behav. Immun 20, 15–26. doi:10.1016/J.BBI.2005.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.