Abstract
Introduction
Alzheimer's and Parkinson's disease (AD and PD) are distinct disorders but share similar biomarker profiles. The regions of the default mode network are implicated in these diseases and are associated with amnestic symptoms. The role of apolipoprotein-ε4 (APOE-ε4), which is associated with cognitive function, is unclear in PD.
Methods
In this work, we evaluated cortical thickness of default mode network regions that are likely affected in both early AD and PD individuals, that is, with amnestic mild cognitive impairment. We identified the prevalence of APOE-ε4 and evaluated its association with cortical atrophy.
Results
We observed significant parahippocampal atrophy and hippocampal atrophy rates in amnestic mild cognitive impairment subjects, regardless of disease origins (AD or PD). Similarly, mild cognitive impairment ε4 carriers showed significant precuneal atrophy compared with noncarriers.
Discussion
This work supports that converging changes to default mode network regions, especially the temporal lobe and precuneus, are shared in AD and PD.
Keywords: Default mode network, MCI, Alzheimer's disease, Parkinson's disease, Cortical thickness, FSL, FreeSurfer
Highlights
-
•
Alzheimer's and Parkinson's disease are distinct disorders but both show default mode network dysfunction.
-
•
We identified default mode network regions that are atrophied in both diseases, especially, in individuals with amnestic symptoms.
-
•
We identified common associations in both diseases between cortical thickness and APOE-ε4 status.
1. Introduction
Despite the clinical distinctions between Alzheimer's disease (AD) and Parkinson's disease (PD), these disorders share similar clinicopathologic features [1], [2], [3], [4]. In both, cognitive symptoms worsen over time, where early symptoms of mild cognitive impairment (MCI) can progress to dementia. Amnestic mild cognitive impairment (aMCI) is considered an early clinical symptom in AD pathology, and in PD, early executive dysfunction is followed by deficits to visual spatial and memory domains [5]. However, there exists substantial individual variation in the type of cognitive presentation and rate and extent of progression.
The apolipoprotein-ε4 (APOE-ε4) allele is a genetic risk factor that may contribute to the manifestation of amnestic symptoms in both disorders. Individuals with a heterozygous-ε4 allele have a 47% increased risk of developing AD, whereas those with a homozygous-ε4 allele have a 91% increased risk [6], [7]. Healthy older adults with an APOE-ε4 allele require an increased area of cortical activity during cognitive tasks, suggesting a genetic link to neuropathological changes that precede cognitive symptoms [8]. More so, APOE-ε4 appears to account for memory-related cognitive dysfunction in PD [9], [10], similar to that seen in aMCI attributed to AD pathology. Patients with PD with an ε4 allele have higher odds of developing dementia, lower scores on the Clinical Dementia Rating scale, and reduced episodic memory performance [6], [11], [12]. However, the causative role of APOE-ε4 on the development of PD-related dementia is still unknown, as some studies report a lack of association between the APOE-ε4 allele and PD-related dementia [13], [14].
Advancing AD is accompanied by progressive neuropathological accumulation of β-amyloid and tau protein aggregates, whereas increased α-synuclein deposition, or Lewy bodies, accumulates in PD. In vivo cerebral spinal fluid (CSF) measures of β-amyloid (Aβ42), tau, and α-synuclein can inform concomitant neuropathologic accumulation in brain parenchyma, where reduced CSF levels of β-amyloid (Aβ42) appear quite reliable in assessing concomitant brain amyloid aggregation [15]. Indeed, β-amyloid (Aβ42) is a well-described contributor to disease pathologic progression of AD and reduced levels inform the risk of progression from aMCI to AD [16], [17].
Brain regions of the default mode network appear vulnerable to β-amyloid accumulation, especially in APOE-ε4 carriers [18], [19], and symptoms of aMCI localize to this network. These regions include the posterior cingulate, medial frontal, and lateral parietal cortices, and disease-relevant changes to this network are evident in both AD and PD [20], [21], [22]. In AD, reduced connectivity between the hippocampus and the posterior cingulate accounts for aMCI presentation [21], [23], [24]. Likewise, patients with PD have reduced connectivity between the posterior cingulate and medial prefrontal regions [22], and this appears responsive to dopamine therapy [25], [26]. A limited number of studies have investigated the association between APOE-ε4 and the default mode network region in PD either with respective to functional or structural integrity.
In this study, we compare morphometric measurements in the default mode network regions (no cerebellar regions were included) between cognitively normal older adults and aMCI subjects at risk of AD, as well as aMCI subjects at risk of PD. Our objective was to identify default mode network regions that show significant atrophy in the aMCI subjects (irrespective of disease origins, i.e., AD or PD) compared with cognitively normal older adults. This goal will identify common mechanisms that can be targeted for relief of similar amnestic symptoms in the future. A supplementary objective was to evaluate neuropathologic vulnerabilities associated with APOE-ε4 carrier status in individuals with aMCI.
Recently, we investigated the differences in morphometric measurements due to variations in T1 imaging protocols in Alzheimer's Disease Neuroimaging Initiative (ADNI) and Progressive Parkinson's Markers Initiative (PPMI) [27], [28], [29], [30]. These are public databases with imaging data for AD and PD. They provide an excellent opportunity to test hypotheses of pathological and systemic overlap in the two diseases. One challenge is the different imaging parameters in the two databases that may introduce a systematic bias and increased variability, thus negatively impacting the comparisons. We have identified data from the same scanner manufacturer with identical imaging parameters in the two databases that will be leveraged for this study.
2. Methods
2.1. ADNI and PPMI databases
The ADNI (adni.loni.usc.edu) database was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD (www.adni-info.org).
The PPMI database (www.ppmi-info.org) was used to assess patients with PD. Only those subjects who were acquired with the following identical parameters in both ADNI and PPMI were selected: Siemens (Erlangen, Germany) scanner, 3T, TR/TE = 2300/2.98 ms, TI = 900 ms, resolution = 1 × 1 mm2, and slice thickness = 1–1.2 mm. Morphometric measurements especially with FreeSurfer [31] are known to be unaffected when slice thickness = 1–1.3 mm.
2.2. Subject selection and clinical phenotyping
All scans were baseline scans. ADNI comprises older adults at risk of AD, who are further subdivided into early MCI (EMCI), MCI, and late MCI (LMCI). Although the MCI classification is an older classification scheme, LMCI subjects have more severe cognitive deficits, less likely evident in early PD subjects from PPMI. Therefore, we restricted our sample to EMCI participants from ADNI but included all PD subjects from PPMI.
Despite the large number of neuropsychological assessments in ADNI and PPMI, there is very little overlap [32], [33], [34], [35], [36], [37], [38], [39]. Memory proficiency is assessed using delayed recall verbal learning tests: the Auditory Verbal Learning Test in ADNI and the Hopkins Verbal Learning Test in PPMI [40], [41]. Both tests provide similar outcomes [42], [43]. We classified patients as aMCI based on delayed recall scores of the Auditory Verbal Learning Test in ADNI and of the Hopkins Verbal Learning Test in PPMI. To accomplish this, the scores from the two assessments were z-transformed to account for differences in the scoring systems. Within ADNI, the mean result was subtracted from the individual score and then divided by the standard deviation of the scores in the cognitively normal controls. Identical transformation was performed in PPMI. Subjects with z < −0.5, that is, half a standard deviation below normative sample, were considered to be aMCI. ADNI uses a similar definition based on delayed recall to define EMCI subjects.
A total of 231 subjects were identified that completed Hopkins Verbal Learning Test/Auditory Verbal Learning Test assessments, had documented APOE-ε4 gene status, and completed a similar imaging protocol. All patients had CSF biomarkers analyzed in the same run (to minimize batch effects). We repeated the above comparisons in amyloid-negative controls (n = 49, 9 APOE-ε4), amyloid-positive MCI (n = 25, 14 APOE-ε4 carriers), and PD subjects (n = 28, 4 APOE-ε4 carriers), to obtain phenotypically similar but pathologically distinct groups. The prevalence of APOE-ε4 in PD is much lower than in AD in this study.
2.3. Image analysis and statistical considerations
For the default mode network, we included the middle temporal cortex, parahippocampal gyrus, precuneus, posterior cingulate, inferior parietal regions, medial orbitofrontal regions, and hippocampus (Fig. 1A). We applied the standard FreeSurfer (v5.1.0) pipeline comprising intensity normalization, skull tissue removal, tissue segmentation, resampling to a 1 × 1 × 1 mm3 resolution, and registration to the standard FreeSurfer brain (fsaverage) [31]. Briefly, this pipeline includes removal of nonbrain tissue using a hybrid watershed/surface deformation procedure, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures, intensity normalization [44], [45], tessellation of the gray/white matter boundary, automated topology correction, and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [46], [47]. No manual editing was performed. However, 10% of the data were randomly sampled and visually inspected to ensure accurate segmentation.
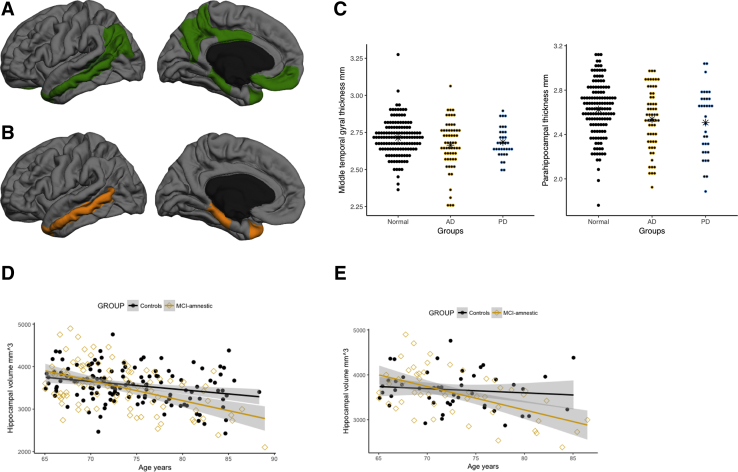
Fig. 1.
Cortical atrophy in aMCI. (A) Regions of the default mode network tested in this study. Right and left side thickness and volumes were combined. The green area near the temporal lobe is representative of the hippocampus. (B) The middle temporal lobe and the parahippocampal gyrus showed significant thinning in the aMCI phenotype compared with the cognitively normal individuals. (C) The thickness reductions in the aMCI subjects were observed regardless of disease origins. Amnestic MCI subjects from ADNI and PPMI both show decreased cortical thickness in the middle temporal gyrus and parahippocampal gyrus. (D) Although hippocampal atrophy was not significant, the slope of the relationship between hippocampal volume and age was significantly steeper in the aMCI individuals (golden ⋄) compared with the cognitively normal group (black •). (E) This relationship in (D) was maintained even when the cohort was refined to include only amyloid-negative controls, and amyloid-positive AD-MCI subject and amyloid-negative PD-MCI subjects. ∗ Denotes mean values for each group, Normal, AD, and PD. Abbreviations: AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; aMCI, amnestic mild cognitive impairment; MCI, mild cognitive impairment; PD, Parkinson's disease; PPMI, Progressive Parkinson's Markers Initiative.
We applied two linear regression models. The first evaluated association of regional atrophy in the default mode network regions and aMCI status. This association was adjusted for age and gender. Comparisons were made between cognitively normal controls and the aMCI group comprising both ADNI and PPMI together, irrespective of disease pathology. Post hoc, two separate comparisons between cognitively normal controls and the aMCI group with reduced CSF amyloid, and PD, were performed. The second regression model was used to assess regional atrophy as function of APOE-ε4 allele status while adjusting for age and gender within each group.
For the above seven regions and two evaluations (group difference and interaction with age while adjusting for age and gender), a correction for multiple comparisons was applied separately for each regression model at a false discovery rate (FDR) of 0.1. Significance was evaluated using the Benjamini Hochberg procedure. All P values reported in the text are original uncorrected P values for regions that satisfied the FDR criteria for significance. FDR-based significance is indicated for these region by an “*” in the tables with statistical results. Effect sizes are also reported (0.2 = small, 0.5 = medium, 0.8 = large [48]). Hippocampal volume comparisons were adjusted for brain size using intracranial volume (eTIV in FreeSurfer). To confirm that the differences were not only due to a large AD-aMCI sample size, we show the morphometric measurements with the aMCI groups separated by disease designation.
3. Results
3.1. Demographics
We identified 109 controls (76 men, age = 75 ± 5 years) from ADNI and 28 controls from PPMI (18 men, age = 70 ± 4 years). Also, 61 AD-MCI (47 men, age = 75 ± 6 years) and 33 PD-MCI (22 men, age = 70 ± 4 years) subjects were identified. AD-MCI PPMI subjects were significantly younger (P < .01). All outcomes were adjusted for age and gender. The incidence of APOE-ε4 in the cognitively normal older adults is similar in ADNI (25%, n = 27) and PPMI (28%, n = 8) but higher in the AD-MCI group (26%,n = 24) compared with the PD-MCI group (12%, n = 4).
3.2. Anatomical differences in cognitively normal older adults and aMCI
Individuals with aMCI from both ADNI and PPMI had reduced cortical thickness in default mode network regions, with steeper negative correlation of age and hippocampal volume. Fig. 1B illustrates the regions of the default mode network that were significantly different between controls and aMCI groups. Table 1 outlines statistical differences, where reductions in the parahippocampal gyrus and the middle temporal region are greatest in the aMCI phenotype (P = .007, P = .005, and P = .009, respectively). Amnestic MCI individuals with presumed AD and PD showed a decrease in cortical thickness (Fig. 1C) in the middle temporal and parahippocampal gyrus. Middle temporal gyral thickness values in the cognitively normal, aMCI-ADNI, and aMCI-PPMI participants were 2.71 ± 0.12 mm, 2.66 ± 0.16 mm, 2.68 ± 0.10 mm, respectively. Values for the parahippocampal gyrus were 2.62 ± 0.24 mm, 2.53 ± 0.27 mm, and 2.50 ± 0.29 mm, respectively, consistent with the reduced cortical thickness in aMCI not being driven simply by disease classification (e.g., AD or PD). No significant relationship was observed between gender and cortical thickness.
Table 1.
Temporal regions are significantly smaller in the amnestic phenotype compared with cognitively normal controls
| Regions | Effect size | P value | FDR significance | |
| Cognitively normal older adults versus mildly amnestic phenotype individuals | ||||
| Medial orbitofrontal | 0.23 | .13 | ||
| Middle temporal | 0.31 | .009 | * | |
| Parahippocampal | 0.38 | .005 | * | |
| Hippocampus | 0.08 | .05 | ||
| Inferior parietal | 0.16 | .21 | ||
| Posterior cingulate | 0.16 | .42 | ||
| Precuneus | 0.27 | .01 | ||
| r (cognitively normal) |
r (amnestic MCI phenotype) |
P value |
FDR significance |
|
| Age interactions | ||||
| Medial orbitofrontal | 0.07 | 0.07 | .99 | |
| Middle temporal | −0.19 | −0.16 | .99 | |
| Parahippocampal | −0.09 | −0.16 | .52 | |
| Hippocampus | −0.27 | −0.49 | .007 | * |
| Inferior parietal | −0.3 | −0.3 | .75 | |
| Posterior cingulate | −0.01 | −0.13 | .37 | |
| Precuneus | −0.37 | −0.5 | .13 | |
Abbreviations: FDR, false discovery rate; MCI, mild cognitive impairment.
NOTE. Bold values denote significance at P = .05 value that is uncorrected using FDR. ∗ Indicates values that are significant after correcting for multiple comparisons using FDR.
Compared with the cognitively normal older adults, the trajectory of hippocampal volume decline with age was significantly faster (P = .007) in the aMCI group (Fig. 1D). Pearson's correlation value, r, for hippocampal volume and age was −0.49 in the aMCI group compared with −0.27 in the cognitively normal individuals.
When amyloid-positive control subjects and amyloid-negative aMCI subjects were excluded, no group differences in subcortical volumes were observed. However, the estimated rate of hippocampus atrophy was significantly steeper (P = .007) in the amyloid-positive aMCI group (Fig. 1E). This finding is similar to Fig. 1D, where amyloid positivity was not considered. Pearson's correlation value, r, for hippocampal volume and age was −0.53 in the aMCI group compared with −0.11 in the cognitively normal individuals.
3.3. Anatomical differences in APOE-ε4 carriers
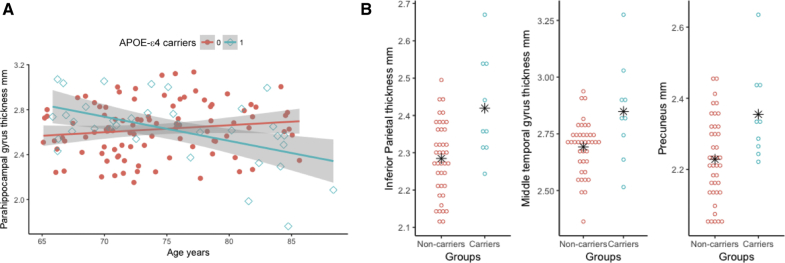
Cognitively normal APOE-ε4 carriers (n = 35) showed significantly reduced thickness in the parahippocampal gyrus with increasing age, and this survived FDR correction (Table 2 and depicted in Fig. 2A; r = −0.51, P = .0004). No relationship between parahippocampal gyrus thickness and age of noncarriers of APOE-ε4 was evident.
Table 2.
No significant difference between APOE-ε4 carriers and noncarriers within the cognitively normal individuals, and APOE-ε4 carriers within the aMCI phenotype showed cortical thinning in multiple default mode regions compared with noncarriers
| Regions | Effect size | P value | FDR significance | |
|---|---|---|---|---|
| Controls, APOE-ε4 carriers versus noncarriers | ||||
| Medial orbitofrontal | 0.08 | .71 | ||
| Middle temporal | 0.20 | .16 | ||
| Parahippocampal | 0.00 | .83 | ||
| Hippocampus | 0.11 | .69 | ||
| Inferior parietal | 0.50 | .01 | ||
| Posterior cingulate | 0.23 | .26 | ||
| Precuneus |
0.23 |
.08 |
||
| r (noncarriers) |
r (carriers) |
P value |
FDR significance |
|
| Age interactions | ||||
| Medial orbitofrontal | 0.13 | −0.02 | .35 | |
| Middle temporal | −0.06 | −0.38 | .03 | |
| Parahippocampal | 0.14 | −0.51 | .0004 | * |
| Hippocampus | −0.18 | −0.48 | .18 | |
| Inferior parietal | −0.21 | −0.46 | .75 | |
| Posterior cingulate | 0.05 | −0.16 | .28 | |
| Precuneus |
−0.29 |
−0.53 |
.17 |
|
| Effect size |
P value |
FDR significance |
||
| aMCI, APOE-ε4 carriers versus noncarriers | ||||
| Medial orbitofrontal | 0.28 | .3 | ||
| Middle temporal | 0.57 | .009 | * | |
| Parahippocampal | 0.00 | .87 | ||
| Hippocampus | 0.03 | .79 | ||
| Inferior parietal | 1.00 | .0001 | * | |
| Posterior cingulate | 0.60 | .03 | ||
| Precuneus |
0.84 |
.0002 |
* |
|
| r (noncarriers) |
r (carriers) |
P value |
FDR significance |
|
| Age interactions | ||||
| Medial orbitofrontal | −0.02 | 0.31 | .16 | |
| Middle temporal | −0.09 | −0.23 | .33 | |
| Parahippocampal | −0.09 | −0.34 | .34 | |
| Hippocampus | −0.47 | −0.56 | .53 | |
| Inferior parietal | −0.26 | −0.3 | .54 | |
| Posterior cingulate | −0.06 | −0.25 | .54 | |
| Precuneus | −0.45 | −0.59 | .32 | |
Abbreviations: aMCI, amnestic mild cognitive impairment; APOE, apolipoprotein; FDR, false discovery rate.
NOTE. Bold values denote significance at P = .05 value that is uncorrected using FDR. ∗ Indicates values that are significant after correcting for multiple comparisons using FDR.
Fig. 2.
Effect of APOE allele status in cognitively normal controls. (A) Within the cognitively normal individuals, carriers of APOE-ε4 had a faster rate of decline (Blue ⋄, high slope = −0.53) in the parahippocampal regions compared with the noncarriers (Pink •, slope = 0.14). (B) Amyloid-negative ε4 carrier controls had higher cortical thickness in the inferior parietal, middle temporal, and precuneal regions compared with ε4 noncarriers. ∗ Denotes mean values for each group. Abbreviation: APOE, apolipoprotein E.
In the amyloid-negative controls (CSF-Aβ42 >192 pg/mL), the APOE-ε4 carriers had significantly greater cortical thickness in the inferior parietal, middle temporal gyrus, and precuneus regions (Fig. 2B, inferior parietal, P = .002, effect size 1.18; middle temporal gyrus, P = .005, effect size 0.88; and precuneus, P = .01, effect size 1.08).
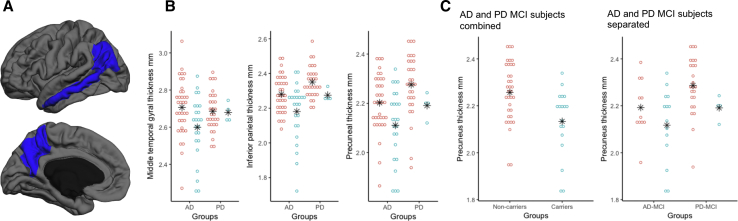
In the aMCI subjects, the middle temporal gyrus, inferior parietal region, and the precuneus had significantly reduced thickness in the 28 APOE-ε4 carriers compared with the noncarriers (Fig. 3A). Thickness values in the middle temporal, inferior parietal, and the precuneal regions in the APOE-ε4 carriers were 2.19 ± 0.12 mm, 2.61 ± 0.14, and 2.12 ± 0.13 mm, and 2.31 ± 0.10 mm, 2.69 ± 0.13 mm and 2.23 ± 0.12 mm in noncarriers (Table 2), respectively. A post hoc analysis (Fig. 3B) found that cortical thickness differences in the aMCI APOE-ε4 carriers were apparent in both ADNI and PPMI cohorts. ADNI aMCI participants with APOE-ε4 had reduced cortical thickness values in the middle temporal gyrus, inferior parietal region, and the precuneus: 2.59 ± 0.16 mm, 2.18 ± 0.16 mm, and 2.11 ± 0.14 mm, versus 2.70 ± 0.15 mm, 2.28 ± 0.10 mm, and 2.20 ± 0.11 mm in APOE-ε4 noncarriers, respectively. PPMI aMCI participants with APOE-ε4 also had reduced cortical thickness values in these same regions: 2.68 ± 0.10 mm, 2.35 ± 0.09 mm, and 2.27 ± 0.12 mm, versus 2.68 ± 0.15 mm, 2.29 ± 0.05 mm, and 2.21 ± 0.07 mm in noncarriers. Note that there were only four APOE-ε4–positive patients with PD, but they had markedly lower cortical thickness in the inferior parietal regions. In the amyloid-positive MCI subjects in ADNI and PD subjects from PPMI, precuneus region was significantly thinner (P = .001, effect size = 0.92) compared with healthy amyloid-negative control subjects. Again, this difference was observed in both AD-MCI and PD-MCI subjects as shown in Fig. 3C.
Fig. 3.
Effect of APOE allele status in aMCI. (A) Cortical regions showing significant thinning in the APOE-ε4 carriers vs. noncarriers within the aMCI phenotype. (B) Thinning cortex in the APOE-ε4 carriers (blue) compared with the noncarriers (pink) was consistent across disease pathologies. Note that there were only four APOE-ε4 carriers in the PD amnestic phenotypes. The differences in cortical thickness in the middle temporal gyrus are minimal in the PD group. (C) In the refined cohort with amyloid-negative controls, amyloid-positive AD-aMCI individuals, and amyloid-negative PD-aMCI individuals, the ε4 carriers show lower cortical thickness in the precuneal region compared with the noncarriers. This effect is observed in both AD and PD-MCI groups, that is, precuneal thinning in ε4 carriers is independent of pathologic disease origins. ∗ Denotes mean values for each group. Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; aMCI, amnestic mild cognitive impairment; MCI, mild cognitive impairment; PD, Parkinson's disease.
4. Discussion
We show that across two distinct neurodegenerative disorders, patients with clinical symptoms of aMCI localize to common regions involved in the default mode network. Furthermore, the APOE-ε4 carriers from both AD and PD populations showed similar atrophy patterns when compared with cognitively normal controls. These findings are consistent, even when accounting for amyloid status and lend support to the hypothesis that clinical symptoms of memory dysfunction converge on default mode network–related regions. That is, significantly reduced thickness of the middle temporal and parahippocampal cortices are evident in aMCI and aMCI-PD patients with lower verbal memory scores. Together, the estimated rate of hippocampal volume decline is faster than that of cognitively normal controls. Although these findings are in agreement with multiple studies [49], [50], [51] linking cortical atrophy to MCI status, especially the middle temporal gyrus, we extend these findings beyond studies of patients with AD and emphasize that these findings are consistent in patients with PD. Patients with a similar aMCI phenotype have reductions in cortical thickness in the same regions. These results emphasize the role of network-based changes and memory-related symptoms that span clinicopathologic classifications.
Using CSF-Aβ42 as a marker of aMCI patients with concomitant AD pathology and comparing this cohort to PD patients with a motor-confirmed diagnosis, we are able to emphasize the role of hippocampal volume as a marker of aMCI status between groups. Very few studies have shown hippocampal atrophy in PD, but hippocampal atrophy has been reported in advanced PD patients with dementia [52], [53]. In this analysis, we show that middle temporal and parahippocampal regions are most different as compared with healthy controls, where aMCI participants with presumed AD pathology had an estimated 1.7% smaller mesial temporal cortical thickness with an effect size of 3.59, and patients with PD had 2.8% lower thickness, with effect size = 0.48. In the remaining default motor network regions, the percent reductions ranged from 0.8% to 1.6% but were not significant. Taken together, these results emphasize that faster rates of hippocampal atrophy and thinning in the mesial temporal lobe regions in PD may be a biomarker of an aMCI subgroup and should be further validated.
Our results emphasize that the effect of APOE-ε4 on thickness of the precuneus is consistent across AD and PD groups. The precise role of APOE-ε4 in precuneal pathology is still not known, but it is clear that functional connectivity in APOE-ε4–positive patients is reduced and may emphasize the larger role of APOE-ε4 on memory-based networks, with convergence on the precuneus. Functional magnetic resonance imaging studies emphasize that default mode network deactivation, during memory tasks, does not occur in APOE-ε4–positive patients with AD. Similarly, blood flow changes in aMCI patients with AD localize to this region, the posterior cingulate, and the default mode network. What predisposes APOE-ε4–positive individuals to AD-related pathology is not known, but our results emphasize that there are differences between individuals with low CSF-Aβ42 versus normal levels. Those with normal CSF-Aβ42 levels actually have greater cortical thickness, a finding that replicates previous studies of higher IQ and educational achievement in APOE-ε4–positive patients [54], [55].
Of course, despite our best attempts at comparing patients with similar pathologic process, that is, accounting for CSF-Aβ42 levels, it is clear that systematic changes to CSF amyloid differ between PD and AD. Although patients with PD may have amyloid plaques at autopsy, the frequency of these is low, and higher CSF amyloid levels reflect this difference. Previous studies emphasize that using conventional cutoff criteria for amyloid positivity does not provide informative assessments of amyloid status in PD. It is likely that the neuropathological cascade that results in amyloid accumulation differs between PD and AD. Our study used the delayed recall scores from verbal learning tests to define aMCI individuals. This definition may not adequately capture the aMCI phenotype and is limited by the lack of common tests between the two databases and likely influenced by the varying degree of vascular pathology as well as medication history of the individuals. One approach to circumvent the differences in cognitive batteries would be to select similar tests and z-scoring them using the control individuals within each database. In summary, this work supports the hypothesis that converging changes to default mode network regions, especially the mesial temporal lobe and precuneus, are shared in patients with AD and PD. While the pathologic basis for changes to this network may differ, amnestic symptoms not only localize here but also are impacted by APOE-ε4 and CSF-amyloid levels in patients with AD. Patients with aMCI have greater rates of hippocampal atrophy. Future studies on mechanisms of network-level susceptibility despite diverse disease states may inform common pathways of degeneration.
Research in Context.
-
1.
Systematic review: This work was performed for the Biomarkers Across Neurodegenerative Disease initiative from the Alzheimer's Association, Fox Foundation, and Weston Brain Institute to identify overlapping mechanisms in distinct neurodegenerative diseases. Search terms include “default mode network,” “Alzheimer's disease,” “Parkinson's disease,” “cortical thickness,” “functional MRI,” “aMCI,” etc.
-
2.
Interpretation: We observed common regional vulnerability in both amnestic Alzheimer's disease (AD) and Parkinson's disease (PD) individuals. The genetic risk factor for AD (APOE-ε4), although rare in PD, was associated with similar cortical thinning in both diseases. This is the first study to assess AD and PD individuals together.
-
3.
Future directions: Functional magnetic resonance imaging of the common regions will determine common and unique connectivity patterns in amnestic AD and PD individuals. Further investigation is warranted in PD ε4 carriers to confirm our results due to their low prevalence. This work could lay the groundwork to identify and manage converging pathological mechanisms in disparate disorders.
Acknowledgments
Data used in preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI: www.ppmi-info.org) and Alzheimer's Disease Neuroimaging Initiative (ADNI) databases (adni.loni.usc.edu). As such, the investigators within PPMI and ADNI contributed to the design and implementation of both databases, and/or provided data, but did not participate in analysis or writing of this report. A complete list of PPMI study investigators can be found at www.ppmi-info.org/authorslist/. A complete listing of ADNI investigators can be found at www.adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
PPMI—a public-private partnership—is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GSK GlaxoSmithKline, Lilly, Lundbeck, Merck, MSD Meso Scale Discovery, Pfizer Inc., Piramal, Roche, Servier, and UCB.
Grant support: S.R. received grant support from Biomarkers Across Neurodegenerative Disease from Alzheimer's Association, Michael J Fox Foundation, and W. Garfield Weston Foundation, DC: R01NS097783-01.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.James B.D., Boyle P.A., Buchman A.S., Barnes L.L., Bennett D.A. Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry. 2011;19:961–969. doi: 10.1097/JGP.0b013e318211c219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch C.E., Cohen D.M. Aging, metabolism, and Alzheimer disease: Review and hypotheses. Exp Neurol. 1997;143:82–102. doi: 10.1006/exnr.1996.6339. [DOI] [PubMed] [Google Scholar]
- 3.Fearnley J.M., Lees A.J. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 4.Zipp F., Aktas O. The brain as a target of inflammation: Common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Zecca L., Youdim M.B., Riederer P., Connor J.R., Crichton R.R. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 6.Harhangi B.S., De Rijk M.C., Van Duijn C.M., Van Broeckhoven C., Hofman A., Breteler M.M. APOE and the risk of PD with or without dementia in a population-based study. Neurology. 2000;54:1272–1276. doi: 10.1212/wnl.54.6.1272. [DOI] [PubMed] [Google Scholar]
- 7.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bookheimer S.Y., Strojwas M.H., Cohen M.S., Saunders A.M., Pericak-Vance M.A., Mazziotta J.C., Small G.W. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks D.J. Imaging amyloid in Parkinson's disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord. 2009;24:S742–S747. doi: 10.1002/mds.22581. [DOI] [PubMed] [Google Scholar]
- 10.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 11.Monsell S.E., Besser L.M., Heller K.B., Checkoway H., Litvan I., Kukull W.A. Clinical and pathologic presentation in Parkinson's disease by apolipoprotein e4 allele status. Parkinsonism Relat Disord. 2014;20:503–507. doi: 10.1016/j.parkreldis.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsian A., Racette B., Goldsmith L.J., Perlmutter J.S. Parkinson's disease and apolipoprotein E: possible association with dementia but not age at onset. Genomics. 2002;79:458–461. doi: 10.1006/geno.2002.6707. [DOI] [PubMed] [Google Scholar]
- 13.Koller W.C., Glatt S.L., Hubble J.P., Paolo A., Tröster A.I., Schmidt K. Apolipoprotein E genotypes in Parkinson's disease with and without dementia. Ann Neurol. 1995;37:242–245. doi: 10.1002/ana.410370215. [DOI] [PubMed] [Google Scholar]
- 14.Camicioli R., Rajput A., Rajput M., Reece C., Payami H., Hao C. Apolipoprotein E ε4 and catechol-O-methyltransferase alleles in autopsy-proven Parkinson's disease: Relationship to dementia and hallucinations. Mov Disord. 2005;20:989–994. doi: 10.1002/mds.20481. [DOI] [PubMed] [Google Scholar]
- 15.Fagan A.M., Mintun M.A., Mach R.H., Lee S.Y., Dence C.S., Shah A.R. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 16.Tapiola T., Pirttilä T., Mikkonen M., Mehta P.D., Alafuzoff I., Koivisto K. Three-year follow-up of cerebrospinal fluid tau, β-amyloid 42 and 40 concentrations in Alzheimer's disease. Neurosci Lett. 2000;280:119–122. doi: 10.1016/s0304-3940(00)00767-9. [DOI] [PubMed] [Google Scholar]
- 17.Jensen M., Schröder J., Blomberg M., Engvall B., Pantel J., Ida N. Cerebrospinal fluid Aβ42 is increased early in sporadic Alzheimer's disease and declines with disease progression. Ann Neurol. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Sheline Y.I., Morris J.C., Snyder A.Z., Price J.L., Yan Z., D'Angelo G. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones D.T., Machulda M.M., Vemuri P., McDade E.M., Zeng G., Senjem M.L. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransson P., Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 22.van Eimeren T., Monchi O., Ballanger B., Strafella A.P. Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Arch Neurol. 2009;66:877–883. doi: 10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckner R.L., Snyder A.Z., Shannon B.J., LaRossa G., Sachs R., Fotenos A.F. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delaveau P., Salgado-Pineda P., Fossati P., Witjas T., Azulay J.P., Blin O. Dopaminergic modulation of the default mode network in Parkinson's disease. Eur Neuropsychopharmacol. 2010;20:784–792. doi: 10.1016/j.euroneuro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Krajcovicova L., Mikl M., Marecek R., Rektorova I. The default mode network integrity in patients with Parkinson's disease is levodopa equivalent dose-dependent. J Neural Transm. 2012;119:443–454. doi: 10.1007/s00702-011-0723-5. [DOI] [PubMed] [Google Scholar]
- 27.Rane S., Plassard A., Landman B.A., Claassen D.O., Donahue M.J. Comparison of Cortical and Subcortical Measurements in Normal Older Adults across Databases and Software Packages. J Alzheimer's Dis Rep. 2017;1:59–70. doi: 10.3233/ADR-170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller S.G., Weiner M.W., Thal L.J., Petersen R.C., Jack C.R., Jagust W. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimer's Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack C.R., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marek K., Jennings D., Lasch S., Siderowf A., Tanner C., Simuni T. The parkinson progression marker initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosser A., Hodges J.R. “Initial letter and semantic category fluency in Alzheimer's disease, Huntington's disease, and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1994;57:1389–1394. doi: 10.1136/jnnp.57.11.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abikoff H., Alvir J., Hong G., Sukoff R., Orazio J., Solomon S. Logical memory subtest of the Wechsler Memory Scale: Age and education norms and alternate-form reliability of two scoring systems. J Clin Exp Neuropsychol. 1987;9:435–448. doi: 10.1080/01688638708405063. [DOI] [PubMed] [Google Scholar]
- 35.Sunderland T., Hill J.L., Mellow A.M., Lawlor B.A., Gundersheimer J., Newhouse P.A. Clock drawing in Alzheimer's disease. J Am Geriatr Soc. 1989;37:725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn H.L., Benton A.L. Revised administration and scoring of the digit span test. J Consult Psychol. 1957;21:139. doi: 10.1037/h0047235. [DOI] [PubMed] [Google Scholar]
- 37.Test S, COWAT R, Tests TC. Judgment of Line Orientation. Clin Neuropsychol
- 38.Henderson J.M., Lu Y., Wang S., Cartwright H., Halliday G.M. Olfactory deficits and sleep disturbances in Parkinson's disease: a case–control survey. J Neurol Neurosurg Psychiatry. 2003;74:956–958. doi: 10.1136/jnnp.74.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowe S.F. Does the letter number sequencing task measure anything more than digit span? Assessment. 2000;7:113–117. doi: 10.1177/107319110000700202. [DOI] [PubMed] [Google Scholar]
- 40.Benedict H.B., Schretlen D., Groninger L., Brandt J. Hopkins Verbal Learning Test – Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 41.Schmidt M. Western Psychological Services; Los Angeles, CA: 1996. Rey Auditory Verbal Learning Test: A Handbook. [Google Scholar]
- 42.Lacritz L.H., Cullum C.M., Weiner M.F., Rosenberg R.N. Comparison of the hopkins verbal learning test-revised to the California verbal learning test in Alzheimer's disease. Appl Neuropsychol. 2001;8:180–184. doi: 10.1207/S15324826AN0803_8. [DOI] [PubMed] [Google Scholar]
- 43.Stallings G., Boake C., Sherer M. Comparison of the California verbal learning test and the Rey auditory verbal learning test in head-injured patients. J Clin Exp Neuropsychol. 1995;17:706–712. doi: 10.1080/01688639508405160. [DOI] [PubMed] [Google Scholar]
- 44.Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 46.Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. Medical Imaging. IEEE Trans. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 47.Ségonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. Medical Imaging. IEEE Trans. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101. [Google Scholar]
- 49.Bell-McGinty S., Lopez O.L., Meltzer C.C., Scanlon J.M., Whyte E.M., DeKosky S.T. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 50.Whitwell J.L., Shiung M.M., Przybelski S.A., Weigand S.D., Knopman D.S., Boeve B.F. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karas G., Sluimer J., Goekoop R., Van Der Flier W., Rombouts S.A., Vrenken H. Amnestic mild cognitive impairment: structural MR imaging findings predictive of conversion to Alzheimer disease. Am J Neuroradiol. 2008;29:944–949. doi: 10.3174/ajnr.A0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camicioli R., Moore M.M., Kinney A., Corbridge E., Glassberg K., Kaye J.A. Parkinson's disease is associated with hippocampal atrophy. Mov Disord. 2003;18:784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 53.Brück A., Kurki T., Kaasinen V., Vahlberg T., Rinne J.O. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is rel8ated to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75:1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Y.W., Lin C.H., Chen S.P., Hong C.J., Tsai S.J. Intelligence and event-related potentials for young female human volunteer apolipoprotein E ε4 and non-ε4 carriers. Neurosci Lett. 2000;294:179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]
- 55.Hubacek J.A., Pitha J., Škodová Z., Adámková V., Lánská V., Poledne R. A possible role of apolipoprotein E polymorphism in predisposition to higher education. Neuropsychobiology. 2001;43:200–203. doi: 10.1159/000054890. [DOI] [PubMed] [Google Scholar]