Abstract
Introduction
Metabolic alterations to the superior frontal gyrus (SFG) have been linked to cognitive decline. Whether these indicate structural atrophy, which could be screened for at a larger scale using noninvasive structural imaging, is unknown.
Methods
We assessed annual structural magnetic resonance imaging scans and cognitive data from 3 consecutive years from 204 participants from the AD Neuroimaging Initiative database (mean age 72.24 [8.175] years). We evaluated associations between brain structural changes and performance in the Montreal Cognitive Assessment, Everyday Cognition Visuospatial subtest (ECog Visuospatial), and Functional Assessment Questionnaire.
Results
Changes in the surface area of the SFG were associated with changes in the outcome of the ECog Visuospatial test (P < .05), but an inconsistent pattern of association was found between the 2-year global brain atrophy progression and changes in the outcome from the three cognitive tests selected.
Discussion
The extent into which (and if) changes in the SFG influence cognition warrant further evaluation in a larger period in more heterogeneous population.
Keywords: Brain, MRI, Superior frontal gyrus, Brain atrophy, Cognitive decline
Highlights
-
•
Two-year superior frontal gyrus surface area changes associated with Everyday Cognition Visuospatial.
-
•
Neurosynth meta-analysis on 224 functional magnetic resonance imaging studies in-line with our results.
-
•
Changes in global atrophy inconsistently associated with Montreal Cognitive Assessment, Functional Activities Questionnaire, and Everyday Cognition visuospatial.
1. Introduction
Disorders of cognition continue to provide profound social and economic challenges to society with our ever-increasing elderly population. Cognitive decline in adulthood represents a continuum ranging `from normal healthy aging to mild cognitive impairment (MCI) to dementia with growing severity in each cognitive grouping. Cognitive decline has been associated with a global decrease in brain volume proportionate to the degree of decline seen [1]. One hallmark of cognitive decline is deficits in executive functioning affecting skills such as working memory, multitasking, and attention [2].The prefrontal cortex has been widely associated with these functions and therefore linked to progression of overt manifestation of cognitive dysfunction.
A study using positron emission tomography scanning found that metabolic changes in the prefrontal cortex, including the superior frontal gyrus (SFG), were present in patients with MCI that progressed to Alzheimer's disease (AD) and absent in nonprogressors [3]. Given the invasiveness and expense of positron emission tomography scanning, this study has limited clinical applicability but indicated that there are specific areas of the brain that may be of interest in evaluating markers that could be better indicators of the changes across the continuum of cognitive decline, and that the SFG could be one of these areas. Structural imaging modalities such as magnetic resonance imaging (MRI) would overcome these practical barriers with their ease of use, noninvasiveness, and relatively low cost [4].
The SFG is known to be heavily involved in a variety of cognitive and motor control tasks. The lateral part is thought to have strong associations with working memory and attention, with the medial being more influential in other cognitive-related processing [5], [6]. In addition to the study from Drzezga et al., biochemical and structural changes on this brain region have been seen in other diseases that affect cognitive performance such as Parkinson's disease and schizophrenia [6]. Cortical thickness is known to be a very stable parameter [7], which illustrates cellular characteristics such as myelination, cell size, and the number of cortical neurons [8]. Previous studies have used changes in cortical thickness to separate patients of different cognitive capacity and to examine the differences between MCI “progressors” and “nonprogressors” [7]. Decrease in the SFG cortical thickness has been associated with declining cognition [7]. Furthermore, cortical surface area has been well established as a sensitive marker to brain structure changes [9]. In normal aging, it is known that the SFG has one of the greatest age-related surface area reductions in the brain [10]. Reductions in the surface area have been shown to correlate with low working memory performance in healthy populations; however, this has currently not been established in the context of cognitively impaired demographics [9] and to the best of our knowledge, it has not been analyzed in the context of progression to any form of dementia.
Therefore, the goal of this study was to investigate whether changes in global brain atrophy and SFG structural measurements were associated with performance on cognitive tests sensitive to cognitive group differentiation and indicative of cognitive decline. The ultimate aim of the study was to add to the search for structural brain imaging markers sensitive to cognitive performance that could be used for early AD diagnosis.
2. Materials and methods
2.1. Subjects
Data were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.uk), a large scale, longitudinal, ongoing collaborative study running since 2004 focused on finding biomarkers to assist in the diagnosis and prognosis of cognitive decline (ADNI, 2017). Subjects, aged 55–90 years, were selected from 55 sites in the United States and Canada including cognitively normal (CN) subjects, patients with early MCI (EMCI), others with late MCI (LMCI) and AD patients. Further information about the ADNI study and detail about selection criteria and protocols can be found at http://adni.loni.usc.edu/methods/documents/. The database was accessed in January 2017.
At the time of accessing the database, 204 subjects (IDs given in Annex 1 for reproducibility purposes) with the following selection criteria were identified:
-
•
Three consecutive scans acquired exactly 12 months apart,
-
•
Complete structural MRI data sets with T1-weighted, T2-weighted, T2*-weighted and Fluid Attenuated Inversion Recovery MRI sequences at each visit, and
-
•
Cognitive assessments done at the time of each MRI scan.
The selection and image processing of the MRI scans were done blind to any clinical, demographic, or cognitive status/data.
2.2. Cognitive assessments
All participants undertook a wide range of cognitive tests as described in http://adni.loni.usc.edu/data-samples/clinical-data/ and http://www.adni-info.org/Scientists/CognitiveTesting.html. For this study, we used the Functional Activities Questionnaire, Montreal Cognitive Assessment, and Everyday Cognition Visuospatial tests, all which were available for all selected participants at the imaged time points.
Each participant's cognitive status (i.e., CN, EMCI, LMCI, and AD) was determined from the results from the Mini-Mental State Exam (MMSE), Clinical Dementia Rating score, and the Logical Memory (II) component of the Wechsler Memory Scale [11], as described in the study by Aisen et al. [12].
2.3. MRI acquisition
All images were acquired at 3T scanners. Turbo Spin Echo T2-weighted images were acquired axially, with echo time (TE)/repetition time (TR) = 80/3000 ms, flip angle 90o, matrix 256 × 256 × 44, voxel size 0.9375 mm × 0.9375 mm × 4 mm. 2D FLAIR images were also acquired in axial orientation, with TE/TR/inversion time = 90/9000/2500 ms, matrix 256 × 256 × 35, voxel size 0.8594 mm × 0.8594 mm × 5 mm. 2D axial T2*-weighted images were acquired with TE/TR = 20/650.001 ms, matrix 256 × 256 × 44, voxel size 0.7812 mm × 0.7812 mm × 4 mm, and 3D T1-weighted MPRAGE sequences were acquired sagittally with TE/TR = 3.162/6.818, flip angle 9o, matrix 256 × 256 × 170, voxel size 1 mm × 1 mm × 1.2 mm. These sequence parameters were consistent with little variations across the sites. More information about the (ADNI-2 and ADNI-go) MRI protocols can be found in: http://adni.loni.usc.edu/methods/documents/mri-protocols/.
2.4. Image analysis
T1-weighted, FLAIR, and T2*-weighted images were spatially, rigidly, and automatically aligned to the T2-weighted using FMRIB's Linear Image Registration Tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT). The intracranial contents were extracted also fully automatically using BRICbet, a MATLAB function publicly available, which is part of the library BRIClib (https://sourceforge.net/projects/bric1936/files/MATLAB_R2015a_to_R2017b/BRIClib/). It uses either the susceptibility-weighted image (i.e., T2*-weighted) or a combination of it with a T2-weighted–based image (i.e., T2-weighted and/or FLAIR), on a pipeline that involves bias field correction and brain extraction using BET2 (http://poc.vl-e.nl/distribution/manual/fsl-3.2/bet2/index.html), followed by first-order statistical manipulations of the intensity of the brain extracted volume, combined with morphological operations. Total cerebrospinal fluid (CSF) volume and pial structures (e.g., veins and meninges) were obtained fully automatically from the MRI scans using multispectral Gaussian Clustering (i.e., clustering based on Gaussian Mixture Models using the Expectation-Maximization algorithm). Brain ventricles were extracted using nonlinear registration of the regional brain atlas for assessment of white matter hyperintensities (http://datashare.is.ed.ac.uk/handle/10283/2217) combined with the total CSF mask (i.e., which also included pial structures), also fully automatically as per [13]. All resultant binary masks were visually inspected and manually corrected only if and where required using Mango (http://ric.uthscsa.edu/mango/index.html).
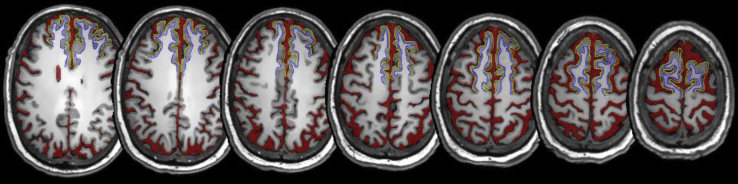
Preliminary analyses of automatic segmentations of the superior frontal cortex yielded inconsistencies in its boundaries for different subjects. Therefore, ground truth segmentations of the superior frontal cortex were generated on a randomly selected subsample of data sets from 34 subjects following a detailed protocol based on the Ono [14] and Duvernoy [15] brain atlases and considering the recommendations from Mikhael et al. (2017) [16]. Every SFG was delineated by drawing two open curves; an outer curve for the gray matter boundary with the subarachnoid space (red) and another demarcating the inner white matter boundary of the gyrus as Fig. 1 shows. The cortices were segmented on axial slices of each scan manually using mricron (https://www.nitrc.org/projects/mricron/).
Fig. 1.
Axial T1-weighted slices showing the results of the CSF and SFG cortex segmentations. Red represents ventricular and total CSF. Yellow represents gray matter outer boundary of the SFG while blue represents white matter outer boundary of the SFG. Abbreviations: SFG, superior frontal gyrus; CSF, cerebrospinal fluid.
All measurements were done independently at the three time points: year 1 (Y1), year 2 (Y2), and year 3 (Y3). The SFG outer boundary was used to calculate the cortical surface area. The cortical thickness was calculated using the distance transform of the cortex in the region delineated, which calculates the Euclidean distance between the inner and outer boundaries of the SFG at each point. Average values of the manual segmentations were used in the analyses. SFG cortical volumes were calculated by multiplying the SFG surface area by the SFG cortical thickness.
2.5. Statistical analyses
CSF was used as a proxy for brain atrophy as any increase in atrophy would equally give concomitant increases in CSF volume. We calculated the change in cognitive performance and brain atrophy measurements (i.e., ventricular CSF volume, Total CSF volume, and SFG volume all adjusted for head size, average surface area of the SFG, and mean cortical thickness of the SFG), as the difference between the measurements acquired at 2 different years, for example, (Y2 − Y1), (Y3 − Y2), and (Y3 − Y1). Any positive results in the global imaging variables and any negative results in the local SFG measurements indicate an increase in global/local atrophy, respectively. For the analyses, all brain volumetric measurements were adjusted for head size by calculating the percentage they represent in intracranial volume (ICV). We performed linear regression analyses evaluating fitness to a general linear model using MATLAB R2014b (stepwiseglm function) to assess the association between the yearly changes of the atrophy variables obtained, and the changes in the performance in the selected cognitive tests, controlling for the confounding effects of age. If an association was found, other covariates were subsequently added to the initial model to establish the extent and conditions of the associations. These were gender, baseline cognitive performance in the MMSE test, years of education, family history of dementia/AD, and cardiovascular and endocrino-metabolic risk factors. We used backward stepwise general linear models to evaluate the results of our linear model and appraised the model fitness while adding or removing variables using both the Akaike Information Criterion and the P value for the chi-squared test of the change in the deviance by adding or removing the term. The model with all possible interactions was the largest model to consider in the evaluation. Paired samples t-test and correlations were calculated using IBM SPSS Statistics ver. 21 (release 21.0.0.0, 64 bit ed.) to evaluate differences between variables and changes between years (nonstandardized correlation coefficients B and P values given). The results were re-evaluated doing a percentile bootstrap on the pair difference and adjusted for multiple comparisons using the Robust Statistical MATLAB Toolbox ([17]—MATLAB implementation, in//github.com/Cpernet/Robust_Statistical_Toolbox). Finally, Neurosynth (http://www.neurosynth.org/) was used to investigate on the positive associations found. Neurosynth is a platform for automatically synthesizing the results of meta-analyzing thousands of neuroimaging studies [18]. We queried the term-based meta-analyses database for the cognitive function(s) that resulted associated with the brain imaging variable(s) analyzed. Such query triggers a giant meta-analysis comparing the coordinates reported for studies with and without the term entered, producing statistical inference maps and posterior probability maps that display the likelihood of a given term being used in a study if activation is observed at a particular voxel.
3. Results
The sample included 71 CN, 65 EMCI, 61 LMCI, and 7 AD ADNI participants with mean age of 72.24 (8.175) years. Age, gender, and years of education were balanced among cognitive groups. Detailed demographics for each cognitive group can be seen in Supplementary Table S1 (Annex 2). The median number of cardiovascular risk factors (0: none, 1: cardiovascular disease or smoking, 2: both) was one across the four cognitive groups. None of the individuals who provided data for this analysis changed the cognitive group within the period analyzed.
The descriptive statistics of the imaging and cognitive parameters explored are shown in Table 1 and illustrated in Supplementary Figs. S2–S10 of Annex 2. The average surface area in the SFG decreased in LMCI and AD patients over the 2 consecutive years analyzed but the pattern was inconsistent in CN and EMCI groups. SFG surface area measurements at each year were highly correlated (0.85 < B < 0.89, P < .0001) and yearly changes were not significant (Y1→Y2 confidence interval (CI) [−400 + 1016] P = .4, Y2→Y3 CI [−877 + 381] P = .4, and Y1→Y3 CI [−625 + 754] P = .8). SFG cortical thickness measurements at each year were less correlated (0.52 < B < 0.75, P < .005), with a positive change between years 1 and 2 (CI [0.01, 0.23] P = .03) that is, however, not significant when accounting for multiple comparison correction (Y2→Y3 CI [−0.25 0.15] P = .6 Y1→Y3 CI [−0.06 0.21] P = .3). SFG volumes expressed as % in ICV were all highly correlated (0.57 < B < 0.74, P < .001). Yearly volumetric changes were highly correlated with 2-year volumetric changes (0.62 < B < 0.81, P < .001). However, no correlation was observed between SFG yearly volumetric changes (i.e., Y1→Y2 and Y2→Y3). Paired t-tests revealed only borderline significant differences between SFG volumes as % in ICV in years 1 and 2 (P = .041).
Table 1.
Descriptive statistics of the imaging and cognitive variables used
| Cognitive group | Parameter | n | Mean (SD) |
||
|---|---|---|---|---|---|
| Y1 → Y2 (i.e., Y2 − Y1) | Y2 → Y3 (i.e., Y3 − Y2) | Y1 → Y3 (i.e., Y3 − Y1) | |||
| CN | Total CSF (% in ICV) | 67 | −1.74 (13.78) | 1.62 (12.67) | −0.12 (13.53) |
| Ventricular CSF (% in ICV) | −0.05 (1.20) | 0.22 (1.21) | 0.17 (1.35) | ||
| Average SFG surface area (mm2) | 12 | −529.92 (1389.19) | 695.97 (1412.53) | 135.33 (1405.31) | |
| Mean SFG cortical thickness (mm) | −0.11 (0.21) | −0.10 (0.44) | −0.23 (0.37) | ||
| SFG volume (% in ICV) | −0.26 (0.51) | −0.16 (0.69) | −0.47 (0.84) | ||
| FAQ | 65 | 0.34 (2.57) | −0.11 (0.61) | 0.23 (2.68) | |
| MoCA | 0.32 (5.65) | −0.26 (4.76) | 0.06 (5.57) | ||
| ECog visuospatial | 0.11 (2.89) | 0.20 (2.88) | 0.31 (3.23) | ||
| EMCI | Total CSF (% in ICV) | 60 | −1.08 (10.52) | 1.73 (10.48) | 0.65 (12.49) |
| Ventricular CSF (% in ICV) | 0.09 (0.94) | 0.23 (1.05) | 0.32 (1.16) | ||
| Average SFG surface area (mm2) | 10 | 308.07 (1573.98) | 609.77 (1092.89) | 768.59 (1864.95) | |
| Mean SFG cortical thickness (mm) | −0.08 (0.26) | 0.22 (0.57) | 0.13 (0.49) | ||
| SFG volume (% in ICV) | −0.0046 (0.44) | 0.35 (0.29) | 0.30 (0.74) | ||
| FAQ | 57 | 1.03 (3.19) | 0.67 (3.05) | 1.70 (4.32) | |
| MoCA | −0.19 (6.56) | 0.58 (4.77) | 0.39 (7.03) | ||
| ECog visuospatial | 1.79 (4.36) | −1.10 (4.36) | 0.68 (4.24) | ||
| LMCI | Total CSF (% in ICV) | 55 | 1.26 (10.38) | 0.90 (9.51) | 2.16 (10.76) |
| Ventricular CSF (% in ICV) | 0.11 (0.81) | 0.22 (0.65) | 0.33 (1.04) | ||
| Average SFG surface area (mm2) | 10 | −264.88 (2571.63) | −116.05 (1964.07) | −857.12 (1868.00) | |
| Mean SFG cortical thickness (mm) | −0.14 (0.39) | 0.12 (0.53) | −0.03 (0.25) | ||
| SFG volume (% in ICV) | −0.22 (0.50) | 0.11 (0.45) | −0.23 (0.68) | ||
| FAQ | 54 | 0.68 (3.16) | 1.96 (4.08) | 2.65 (5.21) | |
| MoCA | 0.04 (6.67) | −1.81 (7.27) | −1.78 (8.27) | ||
| ECog visuospatial | −1.28 (6.54) | 1.59 (5.43) | 0.31 (7.15) | ||
| AD | Total CSF (% in ICV) | 7 | −5.62 (17.68) | 1.96 (1.30) | −3.66 (17.67) |
| Ventricular CSF (% in ICV) | −0.30 (1.34) | 0.28 (0.27) | −0.02 (1.39) | ||
| Average SFG surface area (mm2) | 2 | −557.58 (3.67) | −1900.96 (1624.75) | −2458.54 (1621.08) | |
| Mean SFG cortical thickness (mm) | −0.10 (0.21) | −0.21 (0.39) | −0.31 (0.17) | ||
| SFG volume (% in ICV) | −0.19 (−0.17) | −0.60 (0.69) | −0.79 (0.52) | ||
| FAQ | 3 | 4.33 (2.89) | 4.33 (1.15) | 8.67 (4.04) | |
| MoCA | −2.67 (7.23) | −10.00 (14.42) | −12.67 (10.02) | ||
| ECog visuospatial | −2.33 (6.11) | 9.33 (10.60) | 7.00 (5.20) | ||
Abbreviations: FAQ, Functional Activities Questionnaire; SFG, superior frontal gyrus; AD, Alzheimer's disease; CN, cognitively normal; ICV, intracranial volume; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; ECog, Everyday Cognition; MoCA, Montreal Cognitive Assessment.
NOTE. The “−” sign represents average decrease of the parameter in a year (Y)/2-year time.
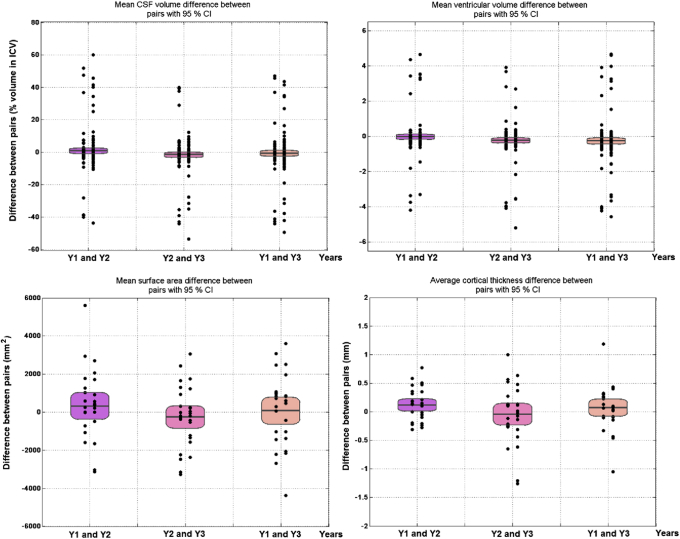
The total volume of CSF (used as proxy for global atrophy) showed a consistent pattern of increase in the LMCI group only, and therefore, the magnitude of the changes in global atrophy between years were not significantly different (Y1→Y2 CI [−0.9 + 2.6] P = .3, Y2→Y3 CI [−3.1 + 0.13] P = .07, Y1→Y3 CI [−2.6 + 1.17] P = .4). In contrast to total CSF volume, brain ventricular size experienced either no change at year 1 (i.e., decrease of ∼1% considered to be within the error margins) or a slight increase at year 2, in all cognitive groups (Y1→Y2 CI [−0.18 0.11 P = .7, Y2→Y3 CI [−0.39, −0.08], P = .004; Y1→Y3 CI [−0.44, −0.07], P = .01, Fig. 2). Paired samples correlation tests showed that only the global CSF measurements from year 2 and year 3 were modestly correlated (B = 0.18, P = .010), whereas all CSF ventricular measurements were highly correlated (0.6 < B < 0.7, P < .0001).
Fig. 2.
Paired differences of the brain MRI measurements took on 2 consecutive years (first two columns from left to right) and 2 years apart (third column from left to right) after being adjusted for head size (i.e., percent in ICV) show significance in the transitions from years 1 and 2 to 3 even after correcting for multiple comparisons. Abbreviation: MRI, magnetic resonance imaging.
All cognitive variables evaluated were highly correlated (FAQ 0.80 < B < 0.92, MoCA 0.52 < B < 0.72, ECog visuospatial 0.62 < B < 0.65, P < .0001), but only the measurements of the FAQ were significantly different between years (P < .0001). The changes between the yearly FAQ results (i.e., Y2 − Y1 and Y3 − Y2) and the changes in the results of the FAQ across 2 years (i.e., Y3 − Y1) were also significantly different (P < .001) even after correcting for multiple comparisons. The changes in the results from the other two cognitive tests evaluated did not differ significantly.
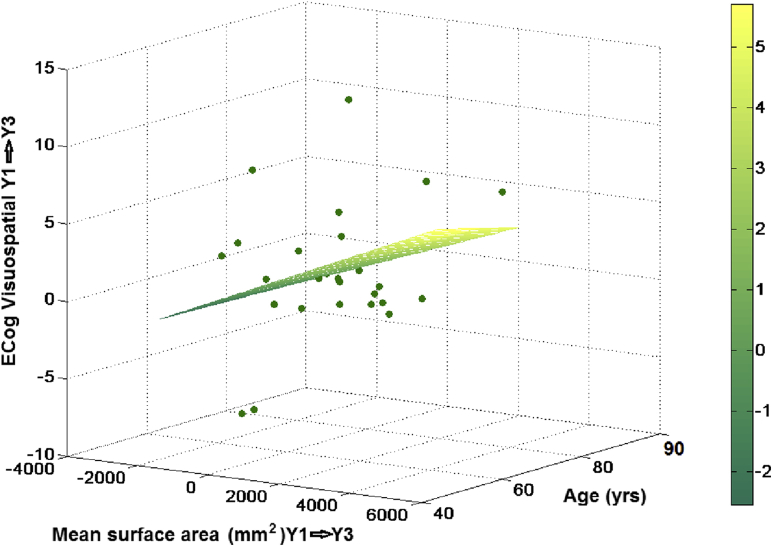
From the 204 individuals pseudorandomly selected, only 177 had all variables to explore associations between changes in the imaging (i.e., global brain atrophy) parameters and changes in the outcome of the cognitive tests selected. Regression analyses showed that changes in the surface area of the SFG were associated with changes in the outcome of the ECog visuospatial test (P = .046–0.047) when the model was adjusted for age only (Fig. 3). Adjusting the model also for baseline cognitive performance (MMSE test results) did not change the significance in the results. Including gender in the model increased the significance in the associations (.01 < P < .02 between years 1 and 2 and .02 < P < .04 between years 1 and 3). Changes in the SFG volume between years 1 and 3 were also associated with changes in the results of the same test (i.e., ECog visuospatial) (B = 3.36, P = .009 in the model only adjusted for age, and B = 3.32, P = .012 in the model adjusted for age and MMSE). Adjusting for the rest of the covariates added in stepwise manner did not change the significance of the association. It only disappeared when interaction factors between covariates were additionally considered in the models (Supplementary Table S2 Annex 2). The stepwise general linear models also confirmed significance in the association between changes in the surface area and the outcome of the ECog visuospatial test between years 1 and 3 (B = 0.001, P < .001, model fitness P < .001). The best model fit had gender, baseline cognitive performance in the MMSE test, years of education, family history of dementia/AD, cardiovascular and endocrino-metabolic risk factors as covariates and interaction factors between the cognitive variables and the risk factors used as covariates (Supplementary Table S2 Annex 2). No associations of the changes in SFG surface area were found with changes in the other two cognitive tests (i.e. FAQ and MoCA) and neither between changes in the cortical thickness of the SFG and changes in the outcome from any of the three cognitive tests, as the SFG measurements were excluded as predictors in these models (Supplementary Table S2 Annex 2). However, interestingly, a general linear model considering all covariates, and interactions between SFG volume and age, SFG volume and family history of dementia, and cognitive variables used as covariates and risk factors, showed association between 2-year changes in the SFG volume and the outcome from MoCA (Supplementary Table S2 Annex 2).
Fig. 3.
Plot of the results of the regression model that had the 2-year change in mean surface area and age as predictors and the change in the results of the ECog Visuospatial test in the same period as outcome variable.
The results of the exploratory analysis to evaluate the associations between changes in the general atrophy measurements (total CSV and ventricular size) and the changes in the outcome of the cognitive tests are shown in Table 2. Best model fitness was achieved when the FAQ test results were the outcome variable, and for changes between years 1 and 2 to 3 when the MoCA results were the outcome variable. Among these models, the change in both general brain atrophy indicators appeared to be associated with the change in the FAQ test from years 2 to 3 and MoCA from years 1 to 3, although at significance .001 < P < .05.
Table 2.
Results from the exploratory analysis (i.e., using stepwise general linear modeling) of the associations between changes in the general atrophy measurements and the changes in the outcome of the cognitive tests
| General linear model |
Estimate (P value) for change in total CSF volume (% in ICV) | Estimate (P value) for change in ventricular volume (% in ICV) | ||
|---|---|---|---|---|
| Outcome variable | Predictors | Model fitness (P value) | ||
| FAQ(Y1→Y2) | CSF(Y1→Y2), age, MMSE, years education, FH dementia, gender, EMRF, CVRF | 6.43e–05** | −0.0406 (0.124) | n/a |
| FAQ(Y1→Y3) | CSF(Y1→Y3), BV(Y1→Y3), age, MMSE, years education, gender, EMRF, CVRF | 2.88e–05** | −1.30 (0.0049)* | 6.90 (0.104) |
| FAQ(Y2→Y3) | CSF(Y2→Y3), BV(Y2→Y3), age, MMSE, years education, gender, EMRF, CVRF | 0.000431** | 0.930 (0.020)* | −10.979 (0.0022)* |
| MoCA(Y1→Y2) | CSF(Y1→Y2), BV(Y1→Y2), MMSE, years education, FH dementia, gender, EMRF, CVRF | 0.00126* | 3.177 (0.009)* | −41.028 (0.019)* |
| MoCA(Y1→Y3) | age, MMSE, years education, FH dementia, EMRF, CVRF | 6.78e–05** | n/a | n/a |
| MoCA(Y2→Y3) | CSF(Y2→Y3), BV(Y2→Y3), age, MMSE, gender, EMRF, CVRF | 8.37e–05** | −1.707 (0.0188)* | −1.871 (0.0748) |
| ECog Visuospatial (Y1→Y2) | age, MMSE, years education, FH dementia, gender | 0.00225* | n/a | n/a |
| ECog Visuospatial (Y1→Y3) | CSF(Y1→Y3), age, MMSE, FH dementia, gender, EMRF | 0.00187* | −1.131 (0.0054)* | n/a |
| ECog Visuospatial (Y2→Y3) | CSF(Y2→Y3), BV(Y2→Y3), age, MMSE, years education, CVRF | 0.0554 | 1.358 (0.0333)* | −13.127 (0.0221)* |
Abbreviations: FAQ, Functional Activities Questionnaire; CSF, % cerebrospinal fluid volume in ICV; BV, % brain ventricular volume in ICV; MMSE, Mini-Mental State Examination results at baseline; FH dementia, family history of dementia; EMRF, endocrino-metabolic risk factors; CVRF, cardiovascular risk factors; Y1, year 1; Y2, year 2; Y3, year 3; n/a, not applicable due to term not included as predictor in the model.
NOTE. *P < .05; **P < .001.
NOTE. Past medical history of cardiovascular risk factors refers to smoking, other risk factors mentioned in the participant's medical history, and previous medical reports of having (or not) any cardiovascular disease. The latter referred to/included the presence of coronary or peripheral artery disease, mild stroke, hypertensive or rheumatic heart disease, cardiomyopathy, carditis, heart arrhythmia, or thromboembolic disease. The most common risk factors described in the participant's medical history are hypertension and hypercholesterolemia.
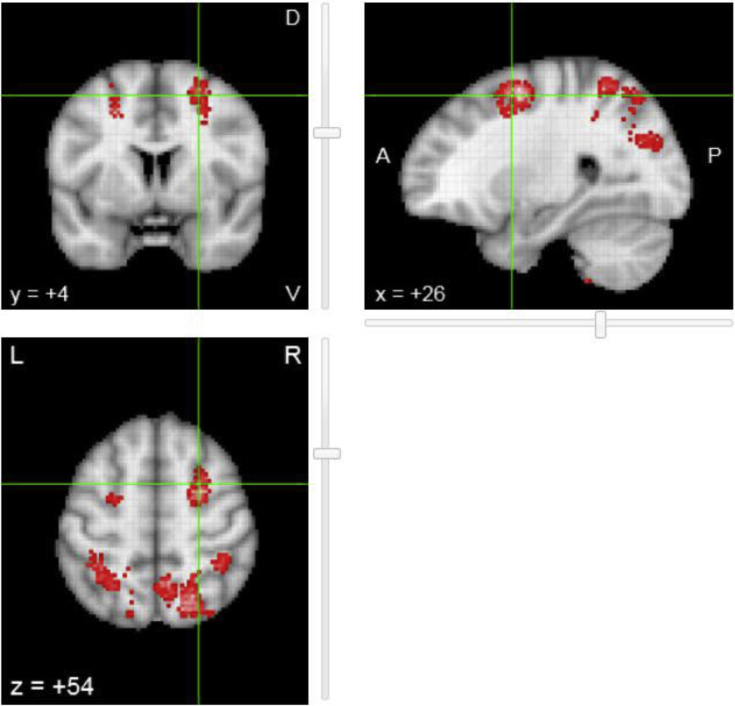
As our analysis showed association between the volume and surface area of the SFG and the ECog visuospatial test, we investigated whether our finding was in-line with the published literature searching in Neurosynth for neural correlates of visuospatial cognition. The search resulted in 224/11,406 studies in the database. The reverse inference map resulting from the meta-analysis of the selected publications, after false discovery rate correction (expected false discovery rate 0.01 as per website documentation) is shown in Fig. 4. As can be appreciated, the SFG was one of the regions that appear to be preferentially related with the term visuospatial. The csv file with the results of the search is provided as Supplementary Material (Annex 3).
Fig. 4.
Reverse inference map of the brain regions that were preferentially related to the term visuospatial in the 224 studies selected. The locations that show the red-to-white foci appear reported more often in articles that include the term visuospatial in their abstracts than articles that do not.
4. Discussion
4.1. Main findings
Two-year changes in the volume and surface area of the SFG were associated with changes in the outcome of the ECog visuospatial test. No differences across years and no association were found between changes in the cortical thickness of the SFG and changes in the outcome from any of the three cognitive tests evaluated. The change in global atrophy indicators showed an inconsistent pattern of associations (i.e., better model fitness corresponded with .001 < P < .05) with the change in all the three cognitive tests evaluated.
Cortical surface area is known to be a sensitive marker of brain structure changes [9]. The SFG has been reported having one of the greatest age-related surface area reductions in normal brains [10]. However, in our CN group, this was not observed, but rather in our LMCI and AD groups. In general, despite the yearly changes in this parameter not being significant, the changes in a 2-year period were associated with the changes in the ECog visuospatial test in the same period. Studies evaluating visuospatial working memory tasks have inconsistently detected activation in the prefrontal cortical areas depending presumably on the type of memory and control tasks used. An early functional magnetic resonance imaging study showed that visuospatial working memory tasks engage a network of distributed brain areas and areas in the dorsal visual pathway are engaged in mnemonic processing of visuospatial information [19]. Furthermore, the meta-analysis including other 223 functional magnetic resonance imaging studies showed the SFG being a brain region preferentially related to the term visuospatial. Our finding, not previously reported, is in-line with these results.
Some factors may confound the fact that total brain atrophy, as measured by alterations in total and ventricular CSF volume, did not have a consistent pattern of association with change in the performance of the cognitive tests evaluated. The automated software used to extract the CSF volumes have previously been found to have varying degrees of accuracy with measurement errors in up to 12% of the MRI scans [20], [21]. These errors can also be accounted for by the effects of pulsatile CSF flow and nonuniformities in clinical head coils [20] despite the latter being corrected. The veins present in the subdural space have also been shown to stiffen and thicken with age, increasing their area of distribution, which reduces levels of CSF and confounding its use as a proxy for atrophy [22]. It must also be acknowledged that we were assessing very subtle changes and, given our very small AD population and the fact that no subjects changed cognitive status during the analysis, these atrophy rates were expected to be minimal [23], [24]. These errors in acquisition and confounding factors may explain the negative % changes seen in the descriptive statistical results (Table 1).
4.2. Strengths and limitations
Some strengths of our study include the use of the ADNI database, which encompasses a large variety of centers from across the United States and Canada making site-specific and general errors less likely. When correcting the semiautomatic ICVs and segmenting the SFG cortices, the investigator was blind to the clinical data. Thus, there was no risk of bias before image analysis. Using both an automated segmentation and thorough manual correction process when mapping the ICVs also decreased the risk of error from the brain extraction that was used to generate the automatic volumes of CSF used in the analyses of global atrophy. The sample was of a moderately large longitudinal sample size increasing the validity of the results.
However, we also acknowledge that the study has limitations. Primarily, all the data acquired from the analysis are progressive and relative to the measurements at the first year that had data available. Thus, someone with AD may have already declined structurally to a level where structural changes are minimal and no longer perceivable. The 2-year duration of the changes was a very small period to assess alterations, given the variability in annual rates of atrophy and the subtlety of changes expected. Although the segmentation adhered to a strict protocol, there was no interobserver reliability testing. Furthermore, the cohort was a population of convenience; the individuals participating in the ADNI database all agreed to have intensive serial procedures over a long period of time and do not have lacunas, or mass lesions at the time of recruitment, therefore not being representative of the general population. In addition, the sample was highly skewed away from patients with AD due to the blind selection process.
4.3. Implications for future research
Future studies should reaffirm or refute findings from this study to further define the role of the SFG in cognitive decline. Using a larger, more evenly distributed sample should be a priority in the future to establish whether the relationships found indeed exist or not. The better distribution may be achieved from increasing the sample size; our low AD cohort may have just been down to chance. Having more longitudinal data than 3 years of brain scans would also increase the time for structural changes to manifest. Furthermore, having intraobserver reliability testing of the SFG segmentations would be required.
The cognitive tests chosen should be expanded in the future to incorporate a wide battery of cognitive tests. We suggest that future analyses focus on specific aspects of cognition such as organization or verbal learning, avoiding general screening tools that incorporate several cognitive processes as these may be less sensitive to subtle changes. It would be interesting to use this same method to evaluate other areas in the prefrontal cortex found to have changes in relative cerebral glucose metabolic rate in the article by Drzezga et al. [3].
Research in Context.
-
1.
Systematic review: Current literature on the role of age-related changes in the superior frontal gyrus (SFG) identified using conventional structural magnetic resonance imaging, in cognitive decline, was systematically reviewed in Web of Science. One study that used positron emission tomography linked metabolic alterations to the SFG to cognitive decline. Whether these metabolic changes are indicative of structural atrophy in a period of 2 consecutive years, which could be screened for at a larger scale using noninvasive, less expensive structural imaging is unknown and was therefore the aim of our investigation.
-
2.
Interpretations: Our findings suggest that subtle changes in the average measurement of surface area—but not cortical thickness—of the SFG influence the outcome of the visuospatial cognitive function, whereas a 2-year period yields inconclusive results on the global brain atrophy changes and their association with cognitive changes in such period. Indeed, the SFG resulted as one of the more prominent regions preferentially related to the term visuospatial as per 224/11406 functional magnetic resonance imaging studies.
-
3.
Future directions: We propose to repeat the analysis in a wider sample more representative of the general population, focus on specific aspects of cognition such as organization or verbal learning, avoiding general screening tools that incorporate several cognitive processes as these may be less sensitive to subtle changes and study whether differences between observers/segmentation methods affect the results.
Acknowledgments
This work was funded by the Row Fogo Charitable Trust (M.V.H.) grant no. BRO-D.FID3668413 and the College of Medicine and Veterinary Medicine of The University of Edinburgh through the BMedSci Honours Programme (S.R.). Funding from SINAPSE-SPIRIT (a Scottish Funding Council HR09021 grant), the Tony Watson Scholarship, and Toshiba Medical Visualization Systems Edinburgh (TMVSE) are also acknowledged. The study was conducted independently of the funders who do not hold the data and did not participate in the study design or analyses.
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC.; Johnson and Johnson Pharmaceutical Research and Development LLC.; Lumosity; Lundbeck; Merck and Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.07.010. All of the data and analysis code that relate to this article can be freely accessible from https://datashare.is.ed.ac.uk/handle/10283/3156 or http://dx.doi.org/10.7488/ds/2414.
Supplementary data
References
- 1.Ritchie S.J., Dickie D.A., Cox S.R., Valdés Hernández M.C., Corley J., Royle N.A. Brain volumetric changes and cognitive ageing during the eighth decade of life. Hum Brain Mapp. 2015;36:4910–4925. doi: 10.1002/hbm.22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan R., Shum D., Toulopoulou T., Chen E. Assessment of executive functions: Review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Drzezga A., Lautenschlager N., Siebner H., Riemenschneider M., Willoch F., Minoshima S. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 4.Braskie M.N., Thompson P.M. A focus on structural brain imaging in the Alzheimer's Disease Neuroimaging Initiative. Biol Psychiatry. 2014;75:527–533. doi: 10.1016/j.biopsych.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisgueheneuc F., Du Levy R., Volle E., Seassau M., Duffau H., Kinkingnehun S. Functions of the left superior frontal gyrus in humans: A lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 6.Li W., Qin W., Liu H., Fan L., Wang J., Jiang T. Subregions of the human superior frontal gyrus and their connections. Neuroimage. 2013;78:46–58. doi: 10.1016/j.neuroimage.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Julkunen V., Niskanen E., Muehlboeck S., Pihlajamaki M., Kononen M., Hallikainen M. Cortical thickness analysis to detect progressive mild cognitive impairment: A reference to alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28:404–412. doi: 10.1159/000256274. [DOI] [PubMed] [Google Scholar]
- 8.Li M., Chen H., Wang J., Liu F., Wang Y., Lu F. Increased cortical thickness and altered functional connectivity of the right superior temporal gyrus in left-handers. Neuropsychologia. 2015;67:27–34. doi: 10.1016/j.neuropsychologia.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Nissim N.R., O'Shea A.M., Bryant V., Porges E.C., Cohen R., Woods A.J. Frontal structural neural correlates of working memory performance in older adults. Front Aging Neurosci. 2017;8:1–9. doi: 10.3389/fnagi.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemaitre H., Goldman A.L., Sambataro F., verchinski B.A., Meyer-Lindenberg A., Weinberger D.R. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33:617.e1–617.e9. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler D. Psychological Corporation; London: 1997. WMS-III: Wechsler Memory Scale Administration and Scoring Manual; pp. 1–212. [Google Scholar]
- 12.Aisen P.S., Petersen R.C., Donohue M., Gamst A., Raman R., Thomas R.G. Clinical core of the Alzheimer's Disease Neuroimaging Initiative: Progress and Plans. Alzheimers Dement. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdes Hernandez M.C., Armitage P.A., Thrippleton M.J., Chappell F., Sandeman E., Munoz Maniega S. Rationale, design and methodology of the image analysis protocol for studies of patients with cerebral small vessel disease and mild stroke. Brain Behav. 2015;5:e00415. doi: 10.1002/brb3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono M., Kubuk S., Abernathy C.D. 1st. Thieme Medical Publishers; New York: 1990. Atlas of the Cerebral Sulci. [Google Scholar]
- 15.Duvernoy H.M. Springer-Verlag; New York, NY: 1999. The Human Brain: Surface, Blood Supply, and Three-dimensional Anatomy; pp. 1–491. [Google Scholar]
- 16.Mikhael S., Hoogendoorn C., Valdés Hernández M.C., Pernet C. A critical analysis of neuroanatomical software protocols reveals clinically relevant differences in parcellation schemes. Neuroimage. 2018;170:348–364. doi: 10.1016/j.neuroimage.2017.02.082. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox R. 4th ed. Academic Press; 2016. Introduction to Robust Estimation and Hypothesis Testing. [Google Scholar]
- 18.Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson S., Martinkauppi S., Rama P., Salli E., Korvenoja A., Aronen H.J. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- 20.Malko J.A., Hoffman J.C., Green R.C. MR measurement of intracranial CSF volume in 41 elderly normal volunteers. AJNR Am J Neuroradiol. 1991;12:371–374. [PMC free article] [PubMed] [Google Scholar]
- 21.de Bresser J., Portegies M.P., Leemans A., Biessels G.J., Kappelle L.J., Viergever M.A. A comparison of MR based segmentation methods for measuring brain atrophy progression. Neuroimage. 2011;54:760–768. doi: 10.1016/j.neuroimage.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 22.Aribisala B.S., Valdes Hernandez M.C., Royle N.A., Morris Z., Munoz Maniega S., Bastin M.E. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur Radiol. 2013;23:1084–1092. doi: 10.1007/s00330-012-2677-x. [DOI] [PubMed] [Google Scholar]
- 23.Fox N.C., Schott J.M. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet. 2004;363:392–394. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 24.Chan D., Janssen J.C., Whitwell J.L., Watt H.C., Jenkins R., Frost C. Change in rates of cerebral atrophy over time in early-onset Alzheimer's disease: longitudinal MRI study. Lancet. 2003;362:1121–1122. doi: 10.1016/S0140-6736(03)14469-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.