Abstract
Although anti-retroviral therapies have greatly extended the lives of HIV infected individuals, current treatments are unable to completely eliminate virally infected cells. A number of latency reversing agents have been proposed for use in a “shock and kill” strategy to reactivate latent HIV, thus making it vulnerable to killing mechanisms. Procyanidin trimer C1 (PC1) is a flavonoid found in multiple plant sources including grape, apple, and cacao, which has antioxidant and anti-inflammatory properties. We determined that PC1 reactivates latent HIV in cell line and primary cell models of HIV, through activation of the MAPK pathway. Notably, PC1 reactivates latent HIV without increasing surface markers of T cell activation. Combining several therapeutics, which activate HIV transcription through different mechanisms, is the most efficient approach to clinically reactivate latent reservoirs. We utilized PC1 (MAPK agonist), kansui (PKC agonist), and JQ1 (BET bromodomain inhibitor) in a triple combination approach to reactivate latent HIV in cell line and primary cell models of HIV latency. When used in combination, low concentrations which fail to reactivate HIV as single treatments, are effective. Thus, several mechanisms, using distinct activation pathways, act together to reactivate latent HIV.
Introduction
Latency is the principal obstacle to complete eradication of HIV [1]. Transcriptionally silent virus evades anti-retroviral therapies (ART), which target viral proteins expressed during the replication cycle [2, 3]. In spite of undetectable plasma viremia while on ART, suppressed patients have rapid viral rebound following treatment interruption [4, 5]. As a consequence, HIV+ individuals must remain on life-long ART. Although HIV infection in the era of ART has become a more manageable chronic infection, problems with adherence to drug regimens, co-morbidities, and the emergence of drug resistance emphasize the need for continued research into HIV cure [6–8]. Since the barrier to cure is the persistence of latent HIV, targeting this persistent and transcriptionally silent virus is critical.
A “shock and kill” strategy has been proposed to target latently infected cells, in which virus is shocked out of latency by compounds which activate HIV transcription, making virally infected cells available to cellular and immune killing mechanisms [9]. HIV is dependent on cellular transcriptional machinery, including nuclear factor kappa B (NF-κB), activator protein 1 (AP1), and positive transcription elongation factor b (P-TEFb) [10]. P-TEFb is a critical transcription factor (TF) required for HIV gene expression. Cellular P-TEFb exists in equilibrium between an inactive state, bound to Hexim1 and 7SK snRNA, and a free active state that is recruited to the HIV long terminal repeat (LTR) by NF-κB and the HIV transactivator of transcription (Tat) [11, 12]. Once at the LTR, P-TEFb phosphorylates negative elongation factors and RNA polymerase II (RNAPII), thus permitting productive elongation [13]. NF-κB is regulated by its inhibitor, IκBα [14]. Upon activation, IκBα is phophorylated, degraded, and released; freeing NF-κB for nuclear transclocation. The mitogen activated protein kinases (MAPK) signaling cascade phosphorylates JNK, p38, and extracellular signal-related kinase (ERK), which regulate AP1 expression [15]. Proposed latency reversing agents (LRAs) target these TF to reactivate latent HIV and purge hidden HIV reservoirs.
A number of LRAs are currently being investigated to determine which reactivate latent HIV through separate but complementary cellular pathways; and each group of LRA has its own advantages and caveats [16]. Histone deacetylase and BET bromodomain inhibitors (HDACi and BETi), such as JQ1, robustly activate HIV through the release of P-TEFb from 7SK snRNP [17, 18]. However, these compounds do not work in human primary T cells [19, 20]. Resting CD4+ T cells have low expression of P-TEFb and other cyclin dependent kinases (CDKs) which are abundant in T cell lines and necessary for efficient transcriptional elongation and co-transcriptional processing of nascent transcripts [10, 21]. Therefore, it is crucial to include a compound that increases P-TEFb levels for JQ1 to function properly. Protein kinase C (PKC) agonists, including ingenol, induce a robust T cell response including activation of inflammatory response genes and cell proliferation [22–25]. A crude extract from Euphorbia kansui (kansui) contains 12 ingenols and other bioactive compounds, which may contribute to its overall ability to activate T cells without much toxicity [26–28]. Kansui reactivates latent HIV in cell lines and primary T cell models of latency, increases cellular levels of the P-TEFb components cyclin T1 (CycT1) and CDK9, and reactivates latent virus from cells isolated from ART suppressed patients [21]. In spite of the appeal of PKC agonists, there has been caution using these agents as a single treatment since concentrations which reactivate latent HIV could induce global T cell activation and cytokine release if delivered clinically [29, 30]. Ideally, a lower dose of PKC agonist could be used in combination with other compounds which reactivate HIV, but do not activate T cells. MAPK signaling activates HIV through induction of AP1 expression [31, 32]. Procyanidin C1 trimer (PC1), a flavonoid isolated from Theobroma cacao (cacao), reactivates cell line models of HIV through the MAPK pathway and act synergistically with the potent PKC agonist PMA [33]. PC1 is also commercially available as a general wellness supplement, derived from a variety of plant sources including grape seed. The use of MAPK agonists to reactivate latent HIV has not been investigated as rigorously as PKC, HDACi, or BETi, but has great potential for use in combinatorial therapeutic approaches. The most effective proposed latency reversal strategies include two or more compounds which reactivate latent HIV by different mechanisms [21, 29, 30]. Thus, several mechanisms, using distinct activation pathways, will act together to reactivate latent HIV.
In this study, we determined the potential of PC1 isolated from grape seed as an LRA. Using cell line and primary CD4+ T cell models of HIV latency, we found that PC1 reactivates latent HIV without concurrently activating these lymphocytes. Indeed, the combination of PC1, kansui, and JQ1 activated cellular TFs via separate pathways and reactivated HIV synergistically from latency.
Materials and methods
Cell lines, PBMC, and primary CD4+ T cells
2D10 cells (obtained from Dr. Jonathan Karn at Case Western Reserve University) are a Jurkat based HIV latency cell line model that contains attenuated Tat and d2EGFP in the place of Nef. Reactivation of latent HIV in 2D10 cells was measured by d2EGFP expression by flow cytometry. Trima residuals from healthy donors, from Trima aphoresis collection and enriched for PBMC, were obtained from Blood Center of the Pacific (San Francisco, CA). Bulk peripheral blood mononuclear cells (PBMCs) were cultured 3 days on tissue cultured plastic to allow for macrophages to adhere. Non-adherent PBMCs were negatively selected for purified CD4+ T cells, or used directly in PMBC experiments. CD4+ T cells were selected from bulk PBMC using negative bead selection (Dynal CD4+ untouched beads, Invitrogen). Primary CD4+ T cells were activated and expanded using CD3/CD28 beads (Invitrogen) and 30 U/ml interleukin 2 (IL-2) for 5 days.
Procyandin Trimer C1
Procyanidin Trimer C1 (PC1) was obtained from ChemFaces Biochemical (CFN99560, Wuhan, China) and reconstituted in DMSO. Working stocks were stored at -20°C.
Cell culture and reactivation conditions
Cells were maintained in RPMI 1640 supplemented with 10% FBS and Penicillin/Streptomycin at 37°C with 5% CO2. Cells were stimulated at a concentration of 1 x 106 cells/ml with PC1 at indicated concentrations, DMSO (1 μl/ml), kansui extract (Baoji F.S. Biological Development Co. Ltd. (Shanxi, China)), or JQ1 (Martin Delaney Collaboratory of AIDS Researchers for Eradication (CARE)). Cells were seeded in triplicate wells of a 24 well plate, and stimulated for 24 hours. Approximately 5–10 x 106 cells/5-10 ml on 10 cm plates were stimulated to obtain lysates for protein expression analysis.
Generation of HIV-1 infectious titers and infections
Infectious stocks of HIV-1 were generated by transfecting 293T cells with 15 μg of pNL4.3-Nef(+)-HSA (NL4.3 HSA) (National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pNL4-3.HSA.R+.E- from Dr. Nathaniel Landau at the NYU School of Medicine [34, 35]) and 3 μg of VSV-G using calcium phosphate. Viral supernatants were harvested and filtered through a 0.45 μm disc at 48 hours post transfection. Approximately 0.5 x 106 pg p24 of infectious virus were added per 1 x 106 activated CD4+ T cells. Cells were spinoculated for 90 min at 2000 rpm with polybrene (2 μg/ml). 24 hours post infection, cells were washed twice to thoroughly remove initial infectious virus.
Human CD4+ T cells model of HIV latency
Primary CD4+ T cells were activated, infected with HIV-1 NL4.3 HSA, and given decreasing concentrations of IL-2 over 12 days in culture to induce quiescence and HIV latency, as previously described [21]. Latently infected and uninfected control cells were stimulated for 24 hours and samples were collected for flow cytometry analysis.
Flow cytometry analysis
Cells were harvested 24 hours post reactivation and washed in cold PBS and 0.5 x 106 cells were allotted to each tube. Cells were stained with PE mouse anti-human CD69 (555531, BD Biosciences), PE anti-human CD25 (555432, BD Biosciences), FITC rat anti-mouse HSA (553261, BD Biosciences), or fixed immediately to analyze GFP expression. Cells were fixed in 2% paraformaldehyde and analyzed using the BD Biosciences FACScaliber and CellQuest Pro software at the UCSF Parnassus Flow Cytometry Core. Cells were gated on the live lymphocyte gate using the forward and side scatter plot, and the percentage of live lymphocytes in 10,000 collected total cells was used as an estimate for cell viability.
Western blotting analysis of protein expression
Whole cell lysates were generated using lamemmli buffer (Bio-rad) in the presence of proteinase inhibitor cocktail. Lysates were run on 10% SDS-PAGE and transferred onto a nitrocellulose membrane. Membranes were cut at approximately 60 kDa; the top portion of the membrane was used to probe for CycT1 (75 kDa) and the bottom portion was used to probe for phospho-(p-) (42 kDa) and total IKBα (40 kDa), p- and total ERK (42 kDa), and β-actin (55 kDa). Membranes were blocked in 5% non-fat milk (NFM) for at least 1 hour and blotted overnight with mouse anti-human cyclin T1 antibody (sc-271348, Santa Cruz Biotechnology), mouse anti-human p-ERK (P-p44/42 MAPK) (9106S, Cell Signaling), rabbit anti-human ERK (p44/42 MAPK) (9102S, Cell Signaling), mouse anti-human p-IKBα (9246S, Cell Signaling), mouse anti-human IKBα (48145S, Cell Signaling) and rabbit anti-human β-actin (ab8227, abcam) antibodies in 5% NFM. Membranes were washed 3 x with PBS with 0.05% Tween 20, and then blotted for 1 hour with HRP anti-rabbit IgG, in 5% NFM. After washing 3 x with PBS with 0.05% Tween 20; membranes were treated with ECL Plus chemiluminescence reagent (Promega) for 5 minutes and imaged using Odyssey Fc imaging system and Image Studio software (LI-COR). Reprobed membranes were stripped with NewBlot Stripping Buffer (LI-COR) then washed 3 x with PBS.
Calculation of drug synergy
To determine whether combination treatment resulted in synergistic activation of HIV we used the Bliss independence model previously described [29]. The equation faxy,p = fax + fay- (fax)(fay), wherein fax,p is the prediction based on the individual effects of PC1 (fax) and kansui + JQ1 (fay). The individual effects were calculated by the equation fax/y/xy = (% GFP positive combination treatment—% GFP positive DMSO)/ (% GFP positive kansui 500 μg/ml—% GFP positive DMSO). The difference between the observed and predicted values (Δfaxy = faxy,o—faxy,p) was calculated. A value greater than 0 signified synergy, a value equal to 0 indicated Bliss independence, and a value less than 0 indicated antagonism.
Statistical analysis
Statistical analysis was performed using a Student t test, two-tailed distribution, and assuming equal variances. Further regression analysis was performed to confirm the significance of the triple combination treatments in relation to the effects of each single and double combination treatment, wherein an F statistic of less than 0.05 was considered significant.
Results
PC1 reactivates latent HIV in a cell line model of HIV latency
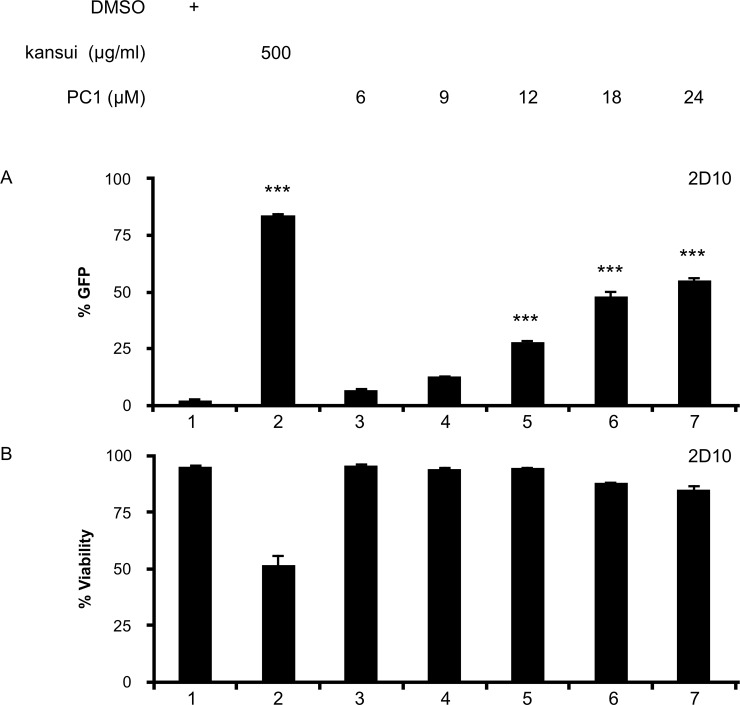
For this study, we obtained PC1 isolated from grape seed, which is the source of many commercially available PC1 wellness supplements. Preliminary experiments were performed in 2D10 cells, which are Jurkat cells that stably express GFP driven by the HIV LTR [36]. GFP was measured by flow cytometry to determine HIV reactivation. A crude extract of kansui, containing the PKC agonist ingenol, was used as a positive control [21]. Kansui treatment resulted in a 42-fold increase in GFP expression over the DMSO control (Fig 1A, lane 2). A dose dependent increase in GFP was observed with increasing concentrations of PC1 (Fig 1A, lanes 3–7). GFP expression was significantly increased in cells treated with 12, 18, and 24 μM PC1, ranging from 12 to 24-fold over the DMSO control (Fig 1A, lanes 5–7). Importantly, none of the concentrations of PC1 tested were toxic to 2D10 cells (Fig 1B, lane 3–7) compared to kansui, which decreased their viability by 45% (Fig 1B, lane 2). These preliminary studies provided the proof of concept that PC1 from grape seed reactivates latent HIV, and effective concentrations were similar to those observed with PC1 from cacao [33].
Fig 1. PC1 reactivates latent HIV in a cell line model of HIV latency.
2D10 cells were stimulated for 24 hours with DMSO, kansui, or PC1 at the indicated concentrations. (A) GFP expression was measured by flow cytometry. (B) Percent viability was estimated using forward/side scatter and the percentage of live lymphocytes in 10,000 total cells analyzed. Triplicate stimulations were performed. Error bars represent standard error of the mean. (***p<0.001).
PC1 activates the MAPK pathway
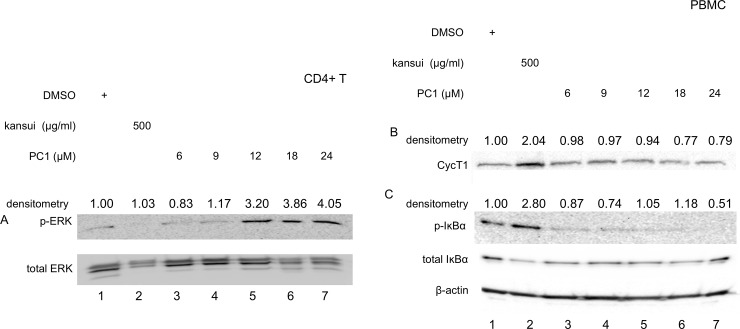
PC1 from cacao activates the MAPK pathway, but not the PKC pathway, in a Jurkat model of HIV latency [33]. To determine if PC1 from grape seed activates the same cellular pathway, human primary CD4+ T cells were stimulated with increasing concentrations of PC1 for 24 hours. PC1 activated the MAPK signaling cascade through phosphorylation of ERK (p-ERK), where levels of total ERK protein remained constant (Fig 2A). A threshold of p-ERK was achieved in cells treated with 12 μM PC1 (Fig 2A, lane 5), and levels of phosphorylation did not increase with 18 and 24 μM PC1 (Fig 2A, lanes 6 and 7). In contrast, kansui did not induce p-ERK (Fig 2A, lane 2). These results agree with previously published reports that PC1 from cacao activates the MAPK signaling cascade [33].
Fig 2. PC1 activates ERK.
Human CD4+ T cell or PBMC were stimulated for 24 hours with DMSO, kansui, or PC1 at indicated concentrations. Whole cell lystates were separated on 10% SDS PAGE. Membranes were probed with specific antibodies for (A) p-ERK and total ERK (B) CycT1, (C) and p-IκBα and total IκBα, and β-actin. Densitometric data were collected, normalized to appropriate loading controls with the DMSO negative control set to one. Results are representative of western blots from three healthy donors.
PKC signaling activates NF-κB and increases cellular expression of P-TEFb [37]. Resting primary PBMC express low levels of CycT1, with some variability in expression between donors (Fig 2B, lane 1). Treatment with kansui induced a robust increase in CycT1 expression (Fig 2B, lane 2) and phophorylation of IκBα, as a measure of NF-κB activation (Fig 2C, lane 2). However, no concentration of PC1 tested increased levels of CycT1 or p-IκBα (Fig 2B and 2C, lanes 3–7).
PC1 does not increase markers of T cell activation
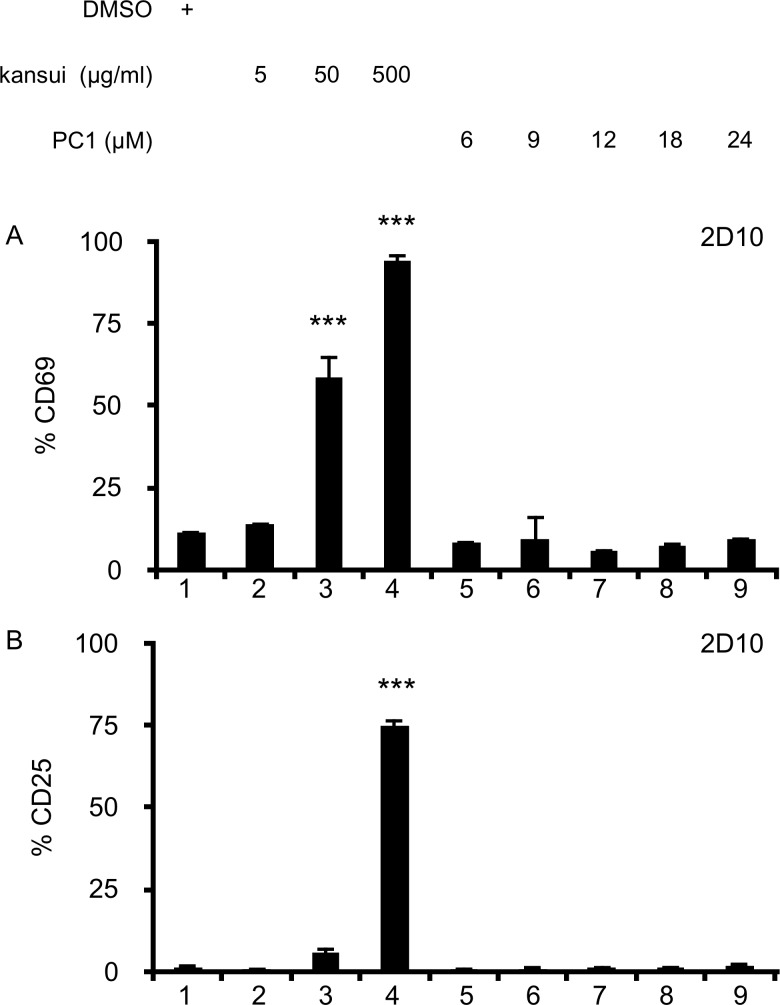
Treatment of 2D10 cells with kansui resulted in a dose dependent increase in the early T cell activation marker, CD69 (Fig 3A, lane 2–4). Kansui also induced expression of the high affinity IL-2 receptor, CD25. However, CD25 induction was more all or none, with only the highest dose of kansui inducing CD25 (Fig 3B, lanes 2–4). No concentration of PC1 tested increased CD69 or CD25 expression on 2D10 cells (Fig 3A and 3B, lanes 5–9). However, this finding is not surprising given the lack of NF-κB activation (Fig 2B, lanes 3–7).
Fig 3. PC1 does not increase markers of T cell activation.
2D10 cells were stimulated for 24 hours with DMSO, kansui, or PC1 at the indicated concentrations. (A) CD69 and (B) CD25 expressions were measured by flow cytometric analyses. Triplicate stimulations were performed. Error bars represent standard error of the mean. (***p<0.001).
PC1 reactivates latent HIV in a primary CD4+ T cell model of HIV latency
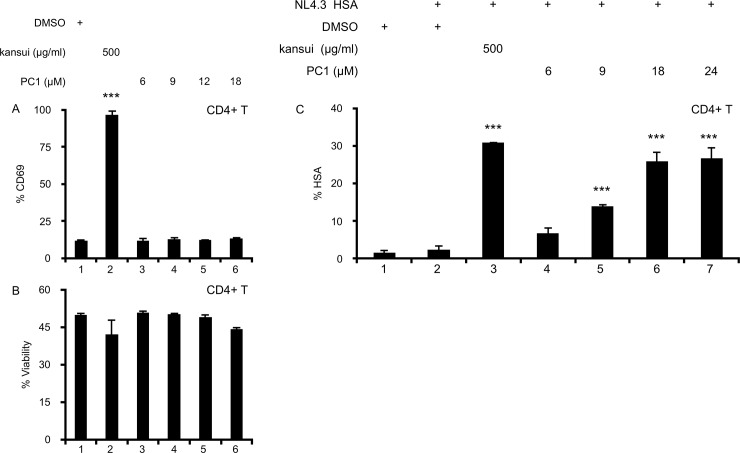
While PC1 activated HIV transcription in 2D10 cells (Fig 1A), it was important to validate effects of proposed therapeutic reagents on primary cells. Immortalized cell lines express high levels of P-TEFb, CDK11, and NF-κB, which are lacking in resting CD4+ T cells [18, 38]. Therefore, it is important to verify that LRAs are not just effective in cell lines. In these cells, kansui stimulation resulted in robust CD69 expression (Fig 4A, lane 2), PC1 did not induce the expression of CD69 at any concentration tested (Fig 4A, lanes 3–6). Moreover, no concentration of PC1 reduced the viability of primary CD4+ T cells (Fig 4B, lanes 3–6).
Fig 4. PC1 reactivates latent HIV in a primary CD4+ T cell model of HIV latency.
(A) Uninfected human primary CD4+ T cells were stimulated for 24 hours with DMSO, kansui, or PC1 at the indicated concentrations. Cells were stained with anti-CD69 antibody and measured by flow cytometric analyses. (B) Percent viability was estimated using forward/side scatter and the percentage of live lymphocytes was determined in 10,000 total cells analyzed. (C) Human primary CD4+ T cells were infected with VSV-G-pseudotyped NL4.3 HSA and maintained over 12 days with decreasing concentrations of IL-2 to establish latency as previously described [21]. Uninfected cells were maintained in the same conditions. At 12 days post infection, cells were stimulated for 24 hours with DMSO, kansui, and PC1. Cells were stained with anti-HSA antibody. Data are presented as a fold change in HSA expression over the uninfected control cells. Experiments were repeated with three independent donors, each with three technical repeats. A representative experiment, from a single donor is presented. Error bars represent standard error of the mean. (***p<0.001).
Using our in vitro HIV latency model [21], we tested the ability of PC1 to reactivate latent HIV in primary human CD4+ T cells. Activated T cells were infected with VSV-G-pseudotyped HIV-1 NL4.3 HSA R+E- (NL4.3 HSA) that expresses the murine heat shock antigen (HSA), which allows reactivated cells to be quantified using flow cytometry. Following infection, cells were driven into quiescence through gradually decreasing concentrations of IL-2, and at day 12 post infection, HSA was no longer detectable on the surface of infected cells (Fig 4C, lane 2). Uninfected cells were maintained in the same conditions (Fig 4C, lane 1). Cells were stimulated for 24 hours with kansui and indicated concentrations of PC1. Kansui induced a 15-fold increase in HSA expression (Fig 4C, lane 3). Reactivation in primary lymphocytes was approximately 2.5 fold less than observed in 2D10 (Fig 1 compared to Fig 5). This result was expected due to the abundant expression of key cellular transcription factors, such as P-TEFb, CDK11, and NF-κB in immortalized cell lines [18, 38], which make them easier to reactivate. This difference highlights the importance of verifying cell line responses in primary cell models. While 6 μM PC1 had no effect (Fig 4C, lane 4), 9 μM PC1 increased HSA expression 6-fold (Fig 4C, lane 5). At higher concentrations of PC1, 18 and 24 μM, a 12.5-fold increase was observed, nearly equivalent to that of the kansui positive control (Fig 4C, lane 6 and 7). Thus PC1 alone also reactivates latent HIV in primary CD4+ T cells, without global lymphocyte activation.
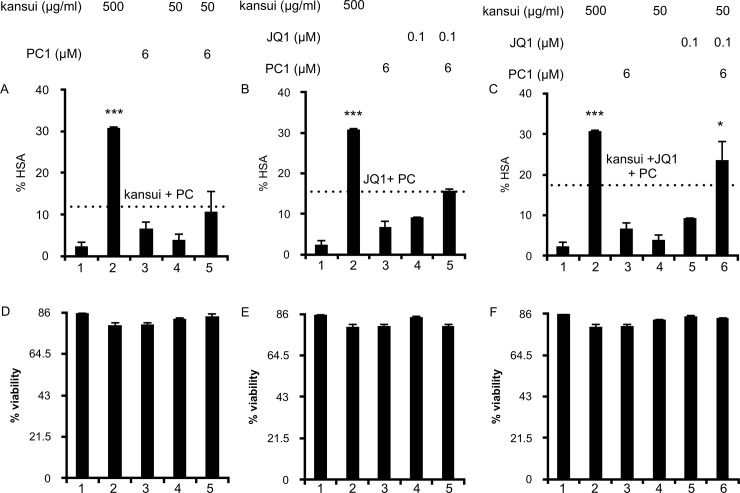
Fig 5. Combination therapy of low dose PC1, kansui, and JQ1 reactivates latent HIV in a primary CD4+ T cell model of HIV latency.
Human primary CD4+ T cells were infected with VSV-G-pseudotyped HIV-1 NL4.3 HSA and maintained over 12 days with decreasing concentrations of IL-2 to establish latency as previously described. Uninfected cells were maintained in the same conditions. At 12 days post infection, cells were stimulated for 24 hours with: (A) single and double PC1 (6 μM) and kansui (50 μg/ml) (B) single and double PC1 (6 μM) and JQ1 (0.1 μM) (C) single and triple PC1 (6 μM), kansui (50 μg/ml), and JQ1 (0.1 μM). Cells were stained with anti-HSA antibody and measured by flow cytometric analyses. Data are presented as a fold change in HSA expression over uninfected control cells. Dashed line is equal to the additive effects (sum % HSA of the single treatments), and a synergistic effect was observed if double and triple treatments surpassed this line. Synergy was validated using Bliss Independence calculation of drug synergy. Experiments were repeated with three independent donors, each with three technical repeats. A representative experiment, from a single donor is presented. (D-F) Uninfected CD4 + T cells were treated as above. Percent viability was estimated using forward/side scatter and the percentage of live lymphocytes in 10,000 total cells analyzed. Error bars represent standard error of the mean. (*p<0.05, **p<0.01, ***p<0.001).
Combination therapy of PC1, kansui, and JQ1 reactivates latent HIV in a primary CD4+ T cell model of HIV latency
The most effective proposed latency reversal strategies include two or more compounds which reactivate latent HIV by different mechanisms [29, 30]. When used in combination, effective concentrations can be reduced to avoid potentially toxic side effects. Because PC1 reactivates HIV through the MAPK pathway, it is ideal to use with a PKC agonist (kansui) and a compound that activates P-TEFb (JQ1).
Latently infected CD4+ T cells were treated with the indicated concentrations of PC1, kansui, and JQ1 either as single, double, or triple combinations. Unstimulated infected cells did not express HSA (Fig 5A–5C, lane 1). Cells were treated with the high concentration of kansui (500 μg/ml) as a positive control, which induced a 15-fold increase in HSA expression (Fig 5A–5C, lane 2). Cells treated with 6 μM PC1 (Fig 5A and 5B, lane 3), kansui (Fig 5A, lane 4), or JQ1 (Fig 5B, lane 4) alone resulted less than 2.5-fold increases in HSA. When 6 μM PC1 was used in combination with kansui or JQ1, HSA expression increased 2-fold over kansui or JQ1 alone (Fig 5A and 5B, lane 5). The additive effect of PC1 and kansui or JQ1 is indicated by the dashed line on each graph, and neither double treatment surpassed this additive effect (Fig 5A and 5B, lane 5) [39]. However, when used as a triple combination, 6 μM PC1 increased expression of HSA 3.5-fold over kansui and 2.5-fold over JQ1 (Fig 5C, lanes 4–6). More importantly, the triple combination of 6 μM PC1, 50 μg/ml kansui, and 0.1 μM JQ1 resulted in a synergistic response, which surpassed the sum of all three single treatments (Fig 5C, lanes 3–6), and was nearly as effective as the high dose of kansui (Fig 5C, lanes 2 and 6). Synergy was confirmed by the Bliss Independence for HIV drug combinations model [29], in which the difference between predicted and observed effects was greater than 1.
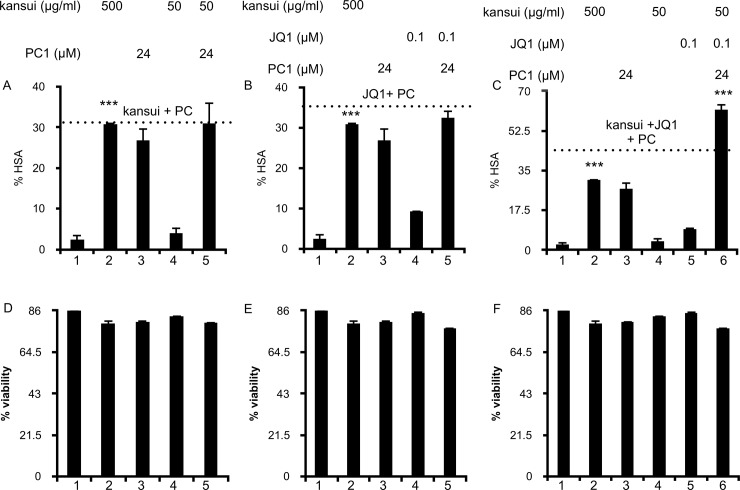
The triple combination of PC1, kansui, and JQ1 had even greater efficacy with the higher dose of PC1. 24 μM PC1 did not have a synergistic effect in double combinations with kansui and JQ1 (Fig 6A and 6B, lanes 5); the 12.5-fold induction of HSA in each of these treatments was most likely only the effect of PC1 (Fig 6A and 6B, lanes 3 and 5). However, a triple combination with 24 μM PC1 resulted in a 30-fold induction of HSA (Fig 6C, lane 6), and was greater than the additive effects of all three single treatments, as indicated by the dashed line (Fig 6C, lane 6). Synergy was confirmed by the Bliss Independence for HIV drug combinations model [29], in which the difference between predicted and observed effects was greater than 1. Furthermore, the triple combination of 24 μM PC1, 50 μg/ml kansui, and 0.1 μM JQ1 resulted in 2-fold higher induction than the high concentration of kansui alone (Fig 6C, lanes 2 and 6).
Fig 6. Combination therapy of high dose PC1, kansui, and JQ1 reactivates latent HIV in a primary CD4+ T cell model of HIV latency.
Human primary CD4+ T cells were infected with VSV-G-pseudotyped HIV-1 NL4.3 HSA and maintained over 12 days with decreasing concentrations of IL-2 to establish latency as previously described. Uninfected cells were maintained in the same conditions. At 12 days post infection, cells were stimulated for 24 hours with: (A) PC1 (24 μM) and kansui (50 μg/ml) (B) PC1 (24 μM) and JQ1 (0.1 μM) (C) e PC1 (24 μM), kansui (50 μg/ml), and JQ1 (0.1 μM). Cells were stained with anti-HSA antibody and measured by flow cytometry analysis. Data are presented as a fold change in HSA expression over the uninfected control cells. Dashed line is equal to the additive effects (sum % HSA of the single treatments), and a synergistic effect was observed if double and triple treatments surpassed this line. Synergy was validated using Bliss Independence calculation of drug synergy. Experiments were repeated with three independent donors, each with three technical repeats. A representative experiment, from a single donor is presented.(D-F) Uninfected cells were treated as above. Percent viability was estimated using forward/side scatter and the percentage of live lymphocytes in 10,000 total cells was analyzed. Error bars represent standard error of the mean. (*p<0.05, **p<0.01, ***p<0.001).
Importantly, no loss in cell viability was observed with double or triple combination treatments including 6 μM (Fig 5D–5F) or 24 μM PC1 (Fig 6D–6F). Our previous data indicate that a combination of 50 μg/ml kansui and 0.1 μM JQ1 induces expression of the early T cell activation marker, CD69 without inducing cellular toxicity [21]. PC1 treatment does not induce T cell activation as measured by expression of CD69 (Fig 3A), nor does it activate NF-κB (Fig 2B), which indicates it should not further impact T cell activation by the low dose combination of kansui and JQ1.
Taken together the triple combination of PC1, kansui, and JQ1 effectively reactivated latent HIV by activating different but complimentary signaling pathways. When used as a combination, lower concentrations of all 3 compounds were effective at reactivating latent HIV. However, as PC1 reactivated latent HIV without T cell activation, the higher dose of PC1 may also represent a safer strategy in our triple combination therapy.
Discussion
In this study we determined that PC1 reactivates latent HIV and works best in a triple combination therapy with kansui and JQ1. PC1 reactivated 2D10 in a dose dependent manner without toxicity. PC1 activated the MAPK signaling pathway, through phosphorylation of ERK. Furthermore, PC1 did not induce CD69 or CD25 expression, indicating that it reactivates HIV without T cell activation. PC1 effectively reactivated HIV in an immortalized cell line and primary CD4+ T cell models of HIV latency. Finally, suboptimal doses of PC1, kansui, and JQ1 reactivated latent HIV more effectively than single doses of any compound, and the highest dose of PC1, when used in triple combination, reactivated latent HIV more effectively than even the highest concentration of kansui.
When considering compounds for therapeutic use, high effective concentrations in vitro could translate to even larger clinical doses. In our study, we determined that 18–24 μM PC1 had the highest efficacy, which is a relatively large amount. Clinical dosing strategies could be adjusted to accommodate these effective doses, but this finding is not ideal. However, PC1 treatment does not increase the expression of markers of T cell activation, as measured by CD69 and CD25, and likely would not induce global T cell activation, which indicates that higher therapeutic concentrations should be tolerated without toxic side effects. Additionally, we determined that a triple combinatorial approach, using a lower dose of PC1, reactivated latent HIV as well as a high dose of kansui. Thus, the effective dose of PC1 is significantly reduced when used in combination with kansui and JQ1, at a concentration more ideal for its therapeutic use.
Our study examined three LRAs, which reactivate latent HIV through three different pathways, but when combined reactivate more potently than any single treatment. However, we did not exhaust the complete list of available LRAs to test in combination with PC1. The PKC agonists prostratin and bryostatin activate resting CD4+T cells and latent HIV in vitro [40, 41]. However both compounds are too toxic and cost prohibitive to manufacture for widespread clinical applications [42, 43]. Our previous studies indicate that a crude extract of kansui reactivates latent HIV to the same degree as the purified PKC agonist ingenol [21]. Kansui has been used in traditional Chinese medicine for thousands of years with minimal toxicity and is orally bio-available [44]. Other LRAs currently under investigation include: HDACi, such as Panobinostat, Romidepsin, SAHA and valproic acid [45–49]. These all robustly activate HIV in cell line models of latency, by inducing chromatin stress and subsequently releasing of P-TEFb from its inactive complex. All of these compounds reactivate latent HIV through similar mechanisms as JQ1 [17, 18]. Thus, although we did not test an extensive panel of LRAs for our studies, we chose appropriate and representative compounds for an effective combinatorial approach.
Our study utilized immortalized and primary T cell models of HIV latency, but we did not test HIV+ ART suppressed patients. In our previous study we determined that our in vitro primary cell latency model provided a good predictor for responses in patient samples [21]. Thus, concentrations of kansui and JQ1 that effectively reactivated latent HIV in this in vitro latency model were equally effective in PBMC isolated from HIV+ ART suppressed patients. This in vitro model uses gradual reductions in IL-2 concentrations and the removal of T cell receptor engagement over several weeks to bring activated HIV-infected primary CD4+ T cells into a quiescent state. In addition to our model, a number of other primary CD4+ T cells have been designed as a surrogates for studies of proviral latency in vivo. A comprehensive review and comparison of several of these models was performed by Spina et al. [19]. In that study, reactivation varied among these models, particularly in the ability to respond to HDACi without additional stimuli, which could indicate that cells in some of these models are not fully resting [18]. In spite of these differences, every model tested reactivated latent HIV following treatment with PKC agonists, albeit at different magnitudes [19]. Our latency model is resistant to reactivation by HDACi and BETi alonge and requires a strong PKC agonist to first increase expression of P-TEFb [21, 22], thus it is appropriate to test the efficacy of the triple combination of PC1, kansui, and JQ1.
Combinatorial therapies have the greatest potential to effectively purge the HIV latent reservoir [29, 30]. PC1, JQ1, and kansui activate cellular transcription factors through distinct pathways, which combined lead to additive effects on HIV reactivation as well as to reduced effective concentrations of individual compounds. By combining different compounds, lower doses of a kansui can be administered, reducing global T cell activation. Furthermore, treatment with JQ1 activates P-TEFb transiently, which induces a feedback loop that is characterized by increased expression of Hexim1 and the subsequent inactivation of P-TEFb in the 7SK snRNP [50]. In the meantime, Tat is synthesized, which can utilize P-TEFb even from the inactive complex [10]. Whereas HIV transcription is maintained, cells return to their resting state. Additionally, PC1 activates through MAPK signaling without T cell activation. Therefore together this combination may prevent inflammatory gene expression and the resulting cytokine storm, while still potently reactivating latent HIV. Double combinations of LRAs have been extensively researched, including combinations of PKC agonists and HDACi/BETi, which resulted in synergistic reactivation [23, 29, 30, 33]. Our triple combinatorial approach expands on this research and includes compounds which reactivate latent HIV at higher concentrations. When used together at lower concentrations, all three potently reactivate latent HIV at more manageable therapeutic concentrations.
Kansui and PC1 are both compounds derived from natural plant sources, which represent affordable, readily available raw materials which can easily be made into therapeutic agents [51]. Kansui has been used for thousands of years in traditional Chinese medicine for treatment of fluid retention, ascites, and cancer; consumed as a tea in its raw form [52–54]. Kansui is a member of the Euphorbia family of plants, which are found in abundance world–wide. Euphorbia, are the main plant source of ingenol, which in addition to its ability to reactivate latent HIV, has been developed into a topical treatment of actinic keratosis world-wide and used as the cancer therapeutic Aveloz in Brazil [53, 55]. PC1 is found in a variety of plant sources including grape, apple, cinnamon, and cacao, which are all ubiquitous sources of raw material [56]. The plant sources of PC1 have natural metabolic and anti-inflammatory properties as well as reported cardiovascular and anti-cancer benefits [57–60]. While natural product based therapeutics are often viewed as alternative medical treatments, scientific discovery of therapeutic compounds has a rich history of plant derived drugs, including aspririn, taxol, and quinine [51]. Taking advantage of these natural products has the potential to yield affordable and more easily scalable production of LRAs.
Acknowledgments
We thank the Peterlin lab, in particular Zeping Luo, Koh Fujinaga, Fang Huang, and Marie Leoz for technical expertise, discussions, and careful reading of the manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by National Institutes of Health grants (https://www.nih.gov/): R01 AI049104 (Peterlin, PI) and P50 GM082250 (HARC grant). DCC was supported by a grant from the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research, P30-AI027763. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–51. Epub 2013/11/19. 10.1016/j.cell.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taube R, Peterlin M. Lost in transcription: molecular mechanisms that control HIV latency. Viruses. 2013;5(3):902–27. Epub 2013/03/23. 10.3390/v5030902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiralli Lester GM, Henderson AJ. Mechanisms of HIV Transcriptional Regulation and Their Contribution to Latency. Molecular biology international. 2012;2012:614120 Epub 2012/06/16. 10.1155/2012/614120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science (New York, NY). 1997;278(5341):1295–300. Epub 1997/11/21. [DOI] [PubMed] [Google Scholar]
- 5.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science (New York, NY). 1997;278(5341):1291–5. Epub 1997/11/21. [DOI] [PubMed] [Google Scholar]
- 6.Marin B, Thiebaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23(13):1743–53. Epub 2009/07/03. 10.1097/QAD.0b013e32832e9b78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis. PLoS medicine. 2016;13(11):e1002183 Epub 2016/11/30. 10.1371/journal.pmed.1002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyidogan P, Anderson KS. Current perspectives on HIV-1 antiretroviral drug resistance. Viruses. 2014;6(10):4095–139. Epub 2014/10/25. 10.3390/v6104095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG. HIV: Shock and kill. Nature. 2012;487(7408):439–40. Epub 2012/07/28. 10.1038/487439a [DOI] [PubMed] [Google Scholar]
- 10.Cary DC, Fujinaga K, Peterlin BM. Molecular mechanisms of HIV latency. The Journal of clinical investigation. 2016;126(2):448–54. Epub 2016/01/06. 10.1172/JCI80565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedore SC, Byers SA, Biglione S, Price JP, Maury WJ, Price DH. Manipulation of P-TEFb control machinery by HIV: recruitment of P-TEFb from the large form by Tat and binding of HEXIM1 to TAR. Nucleic acids research. 2007;35(13):4347–58. Epub 2007/06/20. 10.1093/nar/gkm443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He N, Pezda AC, Zhou Q. Modulation of a P-TEFb functional equilibrium for the global control of cell growth and differentiation. Molecular and cellular biology. 2006;26(19):7068–76. Epub 2006/09/19. 10.1128/MCB.00778-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujinaga K, Irwin D, Huang Y, Taube R, Kurosu T, Peterlin BM. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Molecular and cellular biology. 2004;24(2):787–95. Epub 2004/01/01. 10.1128/MCB.24.2.787-795.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annual review of biophysics. 2013;42:443–68. Epub 2013/03/19. 10.1146/annurev-biophys-083012-130338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. The Journal of biological chemistry. 1995;270(28):16483–6. Epub 1995/07/14. [DOI] [PubMed] [Google Scholar]
- 16.Cary DC, Peterlin BM. Targeting the latent reservoir to achieve functional HIV cure. F1000Research. 2016;5. Epub 2016/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. The Journal of biological chemistry. 2012;287(43):36609–16. Epub 2012/09/07. 10.1074/jbc.M112.410746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartholomeeusen K, Fujinaga K, Xiang Y, Peterlin BM. Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. The Journal of biological chemistry. 2013;288(20):14400–7. Epub 2013/03/30. 10.1074/jbc.M113.464834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS pathogens. 2013;9(12):e1003834 Epub 2014/01/05. 10.1371/journal.ppat.1003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blazkova J, Chun TW, Belay BW, Murray D, Justement JS, Funk EK, et al. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. The Journal of infectious diseases. 2012;206(5):765–9. Epub 2012/06/27. 10.1093/infdis/jis412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cary DC, Fujinaga K, Peterlin BM. Euphorbia Kansui Reactivates Latent HIV. PloS one. 2016;11(12):e0168027 Epub 2016/12/16. 10.1371/journal.pone.0168027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandelo Jose D, Bartholomeeusen K, da Cunha RD, Abreu CM, Glinski J, da Costa TB, et al. Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology. 2014;462–463:328–39. Epub 2014/07/12. 10.1016/j.virol.2014.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, Cai I, et al. Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation. PLoS pathogens. 2015;11(7):e1005066 Epub 2015/08/01. 10.1371/journal.ppat.1005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. Journal of neurovirology. 2008;14(4):309–17. Epub 2008/09/10. 10.1080/13550280802132832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, Clements JE, et al. Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PloS one. 2014;9(5):e97257 Epub 2014/05/16. 10.1371/journal.pone.0097257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LY, Wang NL, Yao XS, Miyata S, Kitanaka S. Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. Journal of natural products. 2002;65(9):1246–51. Epub 2002/09/28. [DOI] [PubMed] [Google Scholar]
- 27.Hou JJ, Wu WY, Liang J, Yang Z, Long HL, Cai LY, et al. A single, multi-faceted, enhanced strategy to quantify the chromatographically diverse constituents in the roots of Euphorbia kansui. Journal of pharmaceutical and biomedical analysis. 2014;88:321–30. Epub 2013/10/12. 10.1016/j.jpba.2013.08.049 [DOI] [PubMed] [Google Scholar]
- 28.Shi QW, Su XH, Kiyota H. Chemical and pharmacological research of the plants in genus Euphorbia. Chemical reviews. 2008;108(10):4295–327. Epub 2008/09/27. 10.1021/cr078350s [DOI] [PubMed] [Google Scholar]
- 29.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. The Journal of clinical investigation. 2015;125(5):1901–12. Epub 2015/03/31. 10.1172/JCI80142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, Ait-Ammar A, et al. An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS pathogens. 2015;11(7):e1005063 Epub 2015/08/01. 10.1371/journal.ppat.1005063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. The Journal of biological chemistry. 1999;274(39):27981–8. Epub 1999/09/17. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. Journal of virology. 1999;73(4):3460–6. Epub 1999/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hori T, Barnor J, Huu TN, Morinaga O, Hamano A, Ndzinu J, et al. Procyanidin trimer C1 derived from Theobroma cacao reactivates latent human immunodeficiency virus type 1 provirus. Biochemical and biophysical research communications. 2015;459(2):288–93. Epub 2015/03/03. 10.1016/j.bbrc.2015.02.102 [DOI] [PubMed] [Google Scholar]
- 34.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. Journal of virology. 1995;69(11):6705–11. Epub 1995/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–44. Epub 1995/02/01. 10.1006/viro.1995.1016 [DOI] [PubMed] [Google Scholar]
- 36.Kim YK, Mbonye U, Hokello J, Karn J. T-cell receptor signaling enhances transcriptional elongation from latent HIV proviruses by activating P-TEFb through an ERK-dependent pathway. Journal of molecular biology. 2011;410(5):896–916. Epub 2011/07/19. 10.1016/j.jmb.2011.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun MK, Alkon DL. The "memory kinases": roles of PKC isoforms in signal processing and memory formation. Progress in molecular biology and translational science. 2014;122:31–59. Epub 2014/02/04. 10.1016/B978-0-12-420170-5.00002-7 [DOI] [PubMed] [Google Scholar]
- 38.Yu W, Ramakrishnan R, Wang Y, Chiang K, Sung TL, Rice AP. Cyclin T1-dependent genes in activated CD4 T and macrophage cell lines appear enriched in HIV-1 co-factors. PloS one. 2008;3(9):e3146 Epub 2008/09/06. 10.1371/journal.pone.0003146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsden MD, Wu X, Navab SM, Loy BA, Schrier AJ, DeChristopher BA, et al. Characterization of designed, synthetically accessible bryostatin analog HIV latency reversing agents. Virology. 2018;520:83–93. Epub 2018/05/26. 10.1016/j.virol.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez M, de Vinuesa AG, Sanchez-Duffhues G, Marquez N, Bellido ML, Munoz-Fernandez MA, et al. Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Current HIV research. 2010;8(6):418–29. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 41.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. Journal of virology. 2002;76(16):8118–23. Epub 2002/07/23. 10.1128/JVI.76.16.8118-8123.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaufelberger DE, Koleck MP, Beutler JA, Vatakis AM, Alvarado AB, Andrews P, et al. The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. Journal of natural products. 1991;54(5):1265–70. Epub 1991/09/01. [DOI] [PubMed] [Google Scholar]
- 43.Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Current opinion in infectious diseases. 2014;27(1):29–35. Epub 2013/12/04. 10.1097/QCO.0000000000000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bensky D, Clavey S, Stoger E. Chinese Herbal Medicine: Materia Medica (ed Third). Vista, CA: Eastland Press; 2004. [Google Scholar]
- 45.Rasmussen TA, Schmeltz Sogaard O, Brinkmann C, Wightman F, Lewin SR, Melchjorsen J, et al. Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Human vaccines & immunotherapeutics. 2013;9(5):993–1001. Epub 2013/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei DG, Chiang V, Fyne E, Balakrishnan M, Barnes T, Graupe M, et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS pathogens. 2014;10(4):e1004071 Epub 2014/04/12. 10.1371/journal.ppat.1004071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. The Journal of biological chemistry. 2009;284(11):6782–9. Epub 2009/01/13. 10.1074/jbc.M807898200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Routy JP, Tremblay CL, Angel JB, Trottier B, Rouleau D, Baril JG, et al. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV medicine. 2012;13(5):291–6. Epub 2012/01/27. 10.1111/j.1468-1293.2011.00975.x [DOI] [PubMed] [Google Scholar]
- 49.Boehm D, Calvanese V, Dar RD, Xing S, Schroeder S, Martins L, et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell cycle (Georgetown, Tex). 2013;12(3):452–62. Epub 2012/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu P, Xiang Y, Fujinaga K, Bartholomeeusen K, Nilson KA, Price DH, et al. Release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein (snRNP) activates hexamethylene bisacetamide-inducible protein (HEXIM1) transcription. The Journal of biological chemistry. 2014;289(14):9918–25. Epub 2014/02/12. 10.1074/jbc.M113.539015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cary DC, Peterlin BM. Natural Products and HIV/AIDS. AIDS research and human retroviruses. 2018;34(1):31–8. Epub 2017/12/12. 10.1089/AID.2017.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu XD, Zhang Q. Clinical progress in the treatment of severe acute pancreatitis with integrative Chinese and Western medicine. Chinese journal of integrative medicine. 2007;13(3):235–40. Epub 2007/09/28. 10.1007/s11655-007-0235-1 [DOI] [PubMed] [Google Scholar]
- 53.Kupchan SM, Uchida I, Branfman AR, Dailey RG Jr., Fei BY. Antileukemic principles isolated from euphorbiaceae plants. Science (New York, NY). 1976;191(4227):571–2. Epub 1976/02/23. [DOI] [PubMed] [Google Scholar]
- 54.Xing F, Tan Y, Yan GJ, Zhang JJ, Shi ZH, Tan SZ, et al. Effects of Chinese herbal cataplasm Xiaozhang Tie on cirrhotic ascites. Journal of ethnopharmacology. 2012;139(2):343–9. Epub 2011/11/24. 10.1016/j.jep.2011.10.040 [DOI] [PubMed] [Google Scholar]
- 55.Anderson L, Schmieder GJ, Werschler WP, Tschen EH, Ling MR, Stough DB, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. Journal of the American Academy of Dermatology. 2009;60(6):934–43. Epub 2009/05/27. 10.1016/j.jaad.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 56.De Rosso M, Panighel A, Vedova AD, Gardiman M, Flamini R. Characterization of Non-Anthocyanic Flavonoids in Some Hybrid Red Grape Extracts Potentially Interesting for Industrial Uses. Molecules (Basel, Switzerland). 2015;20(10):18095–106. Epub 2015/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sung NY, Yang MS, Song DS, Byun EB, Kim JK, Park JH, et al. The procyanidin trimer C1 induces macrophage activation via NF-kappaB and MAPK pathways, leading to Th1 polarization in murine splenocytes. European journal of pharmacology. 2013;714(1–3):218–28. Epub 2013/06/19. 10.1016/j.ejphar.2013.02.059 [DOI] [PubMed] [Google Scholar]
- 58.Byun EB, Sung NY, Byun EH, Song DS, Kim JK, Park JH, et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-kappaB signaling through TLR4 in macrophages. International immunopharmacology. 2013;15(2):450–6. Epub 2012/12/25. 10.1016/j.intimp.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 59.Khan N, Khymenets O, Urpi-Sarda M, Tulipani S, Garcia-Aloy M, Monagas M, et al. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients. 2014;6(2):844–80. Epub 2014/02/26. 10.3390/nu6020844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andujar I, Recio MC, Giner RM, Rios JL. Cocoa polyphenols and their potential benefits for human health. Oxidative medicine and cellular longevity. 2012;2012:906252 Epub 2012/11/15. 10.1155/2012/906252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.