Abstract
When elevated serum hCG is discovered during the work up of a gynecologic tumor, it is paramount to identify the source of hCG prior to initiation of treatment. Potential sources of hCG include viable intrauterine pregnancy, ectopic pregnancy, miscarriage, gestational trophoblastic disease, pituitary hCG production, phantom hCG (heterophilic antibody interference), and tumor production. Here, we present a case of elevated hCG in a young women with a large complex pelvic mass. Prior to treatment initiation, the patient underwent work up for hCG elevation, which was ultimately found to be from tumor production. Pathologic examination revealed the mass to be a mucinous adenocarcinoma of the ovary with aberrant expression of hCG, rather than the more typical hCG producing germ cell tumor. We detail the preoperative evaluation process of hCG elevation. Additionally, we discuss the role of hCG in ovarian cancer and influence on tumorigenesis and management.
Keywords: hCG, Epithelial ovarian cancer, Mucinous ovarian adenocarcinoma
Highlights
-
•
Evaluation of elevated hCG in a premenopausal patient is complex but necessary prior to surgical intervention.
-
•
Abberant hCG production resulting in elevated serum hCG can be seen in mucinous adenocarcinomas of the ovary.
-
•
hCG production in ovarian cancer may be a useful marker for surveillance.
1. Case report
Our patient is a 19 year-old G0 female who presented with abdominal bloating, weight gain, and discomfort with exercise. Physical exam was significant for a palpable abdominopelvic mass. CT imaging of the abdomen and pelvis revealed a large, complex right adnexal mass measuring 27 × 20 × 16 cm with multiple solid nodules, the largest approximately 4 cm, causing hydronephrosis. CT also demonstrated a thickened, heterogeneous endometrium. Serum tumor markers were obtained, including Ca-125, CEA, Ca 19-9, LDH, hCG, and AFP. Ca-125 was 46. Serum quantitative hCG was elevated at 94. Point of care urine hCG was also positive. The patient reported continuous oral contraceptive use for management of dysmenorrhea and denied recent sexual activity. Pelvic ultrasound did not identify an intra- or extra-uterine pregnancy. hCG was repeated 48 h later and demonstrated an inappropriate rise of 68%. A serum progesterone was checked and resulted at 0.9, a level with a sensitivity of 75% and a specificity of 98% for nonviable pregnancy. (Verhaegen et al., 2012)
One week later, the patient's hCG was rechecked and again noted to be slowly rising at 227. Despite the positive urine hCG, assessment was undertaken to rule out heterophilic antibody interference. The patient underwent a serum hCG serial dilution assay. Results are shown in Table 1. The linearity of the results indicated a true serum hCG rather than heterophilic antibody interference. As point of care urine hCG testing was persistently positive, a quantitative analysis of the urine sample using the serum analysis machine was run. Quantitative urine hCG testing revealed an undetectable pregnancy component but elevated tumor marker component at 702. The conclusion was the hCG was secondary to tumor production of altered hCG compounds with no production of intact hCG.
Table 1.
Heterophilic antibody interference assay results.
| Concentration | hCG (mIU/mL) |
|---|---|
| Initial result | 227 |
| 1:2 Dilution | 105 |
| 1:6 Dilution | 35.3 |
| 1:11 Dilution | 18.45 |
Serial serum hCG dilutions were performed as evaluation for heterophilic antibody interference. The linearity of the hCG during serial dilutions is most consistent with true hCG production rather than heterophilic antibody interference.
The patient ultimately underwent an exploratory laparotomy and right salpingo-oophorectomy for fertility sparing treatment of a suspected germ cell neoplasm. Frozen pathology demonstrated at least mucinous low malignant potential tumor on limited sampling. However, a usual amount of atypia was seen along with a villoglandular pattern that raised concern for an invasive carcinoma versus metastatic mucinous tumor from GI origin. Given uncertainty of frozen section, further staging surgery was deferred at that time. Final pathology, though negative for a germ cell neoplasm, demonstrated a mucinous adenocarcinoma with several foci of undifferentiated carcinoma. Given elevated serum hCG, hCG staining on the tumor tissue was performed which demonstrated aberrant hCG expression. The tumor was negative for markers of choriocarcinoma (SALL4 and GATA3). PAX8 was found to be positive throughout, consistent with adenocarcinoma of mullerian origin.
Since the patient was incompletely staged at the time of primary surgery, decision was made to return to the operating room for pelvic and paraaortic lymph node dissection, omentectomy, peritoneal biopsies, and appendectomy with platinum-based chemotherapy thereafter. Prior to second surgery she underwent oocyte retrieval and cryopreservation. hCG at that time had decreased to 69. Final pathology revealed a stage IIIa1 mucinous adenocarcinoma of the ovary, with one right paraaortic lymph node positive for metastasis (4 mm). Nodal tissue was positive for hCG expression. Eleven days after staging, the patient received her first cycle of chemotherapy. Before adjuvant chemotherapy, hCG was 36. hCG became undetectable after the 1st cycle of chemotherapy and remained undetectable throughout the completion of her chemotherapy. Four months after completion of chemotherapy, hCG was found to be slightly elevated at 9. Ca-125 was normal at 16. CT imaging was performed that unfortunately demonstrated multiple boney metastases. Pathology from bone biopsy confirmed recurrent mucinous adenocarcinoma.
2. Discussion
There are numerous causes of elevated hCG in reproductive aged women, the most common of which is pregnancy (Table 2). When discovered during the work up of a gynecologic tumor, it is important to identify the source of hCG before initiating treatment. In order to narrow the differential it is beneficial to understand hCG from a molecular basis. Three distinct forms of hCG are recognized and vary both in form and function; regular hCG, hyperglycosylated hCG (hCG-H), and the free beta subunit of hCG. Regular hCG and hCG-H are dimers consisting of an alpha and beta subunit. While the beta subunit is unique to the hCG molecule, the alpha subunit is identical to that of luteinizing hormone, follicle-stimulating hormone, and thyroid-stimulating hormone. (Elliott et al., 1997)
Table 2.
Causes of elevated hCG in a reproductive aged woman.
| Sources of hCG |
|---|
| Pregnancy |
| Phantom hCG |
| Pituitary hCG |
| Solid malignancy |
| Gestational trophoblastic disease |
Regular hCG is produced by syncytiotrophoblasts in normal pregnancy in order to maintain the corpus luteum prior to adequate placental progesterone production. (Herr et al., 2007) Additionally, regular hCG is found in noninvasive trophoblastic disease and can also be elevated due to physiologic pituitary secretion around the time of menopause. Perimenopausal secretion of hCG is stimulated by GnRH in response to high levels of circulating LH. (Cole et al., 2009) hCG-H is produced by extravillous cytotrophoblastic cells and promotes invasion. (Handschuh et al., 2007) Unlike complete and partial moles, which are primarily made up of syncytiotrophoblasts, invasive trophoblastic disease and choriocarcinoma are composed mainly of cytotrophoblastic cells and therefore produce predominantly hCG-H. (Cole et al., 2006) Finally, the free beta subunit of hCG (free βhCG) or its degradation product, β-core fragment, can be elevated both gynecologic and non-gynecologic malignancies. (Marcillac et al., 1992) Furthermore, elevated free βhCG is thought to facilitate cellular proliferation and impair apoptosis to promote tumorigenesis within ovarian cancers and is felt to be an overall poor prognostic sign. (Guo et al., 2011; Iles et al., 1996; Iles, 2007)
In addition to understanding the distinct molecular types of hCG, it is equally important to understand the different hCG assays commercially available. At our institution, 2 different serum hCG assays are in use. The first is a pregnancy specific test which recognizes regular hCG but may not recognize hCG-H or free βhCG. The second available hCG assay has less molecular specificity and, as such, recognizes regular hCG as well as the other forms of hCG.
Another important limitation of hCG testing is the phenomenon of heterophilic antibody interference, or “phantom hCG.” This can occur due to the two-site nature of hCG immunoassays. If a patient has preexisting heterophilic antibodies against animal antigens, these antibodies can interfere with the assay and cause cross linking complexes between two anti-hCG antibodies without the hCG molecule being present, resulting in a false positive test. (Boscato and Stuart, 1986; Vladutiu et al., 1982) Reported causes of heterophilic antibody interference include frequent animal or animal byproduct exposure, recent mononucleosis, IgA deficiency, and E.coli septicemia. (Knight et al., 2005; Covinsky et al., 2000) In order to reduce this phenomenon, manufactures have included nonspecific animal antibodies in the assays to saturate and neutralize possible heterophilic antibodies. Additionally, heterophilic antibodies are not usually detected in urine samples, as the molecular complex is too large to cross the glomerular basement membrane (Vladutiu et al., 1982), and therefore, a negative urine hCG assays can be used as a confirmatory test if phantom hCG is expected. To further evaluate for phantom hCG, serial serum dilution can be performed by the laboratory. There will not be linear dilution of serum hCG levels with heterophilic antibodies, as would be seen with the intact hCG molecule. Results from serial dilution of hCG in our patient suggested that heterophilic antibody interference was not the cause of elevated hCG.
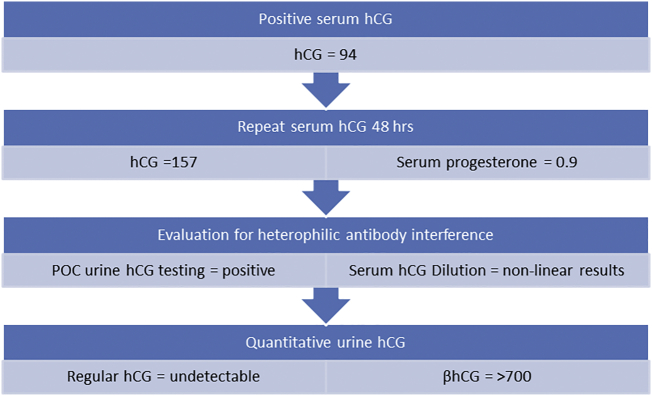
The work up of elevated hCG in our patient led us to preoperative conclusion that the elevated hCG identified was ultimately due to tumor production (Fig. 1). The final pathologic evaluation of our patient's tumor confirmed aberrant hCG expression of tumor cells and corroborated our preoperative suspicion. Interestingly, pathology ruled out suspected germ cell tumor, as this tumor type is more classically thought of as an hCG producing entity. Pathology instead showed a mucinous epithelial adenocarcinoma. Our patient's serum hCG normalized throughout her treatment but unfortunately began to rise 4 months after completion of primary treatment. Work up at that time confirmed recurrent disease, thus demonstrating the utility of serum hCG as a tumor marker for treatment response and surveillance in this particular patient.
Fig. 1.
hcG testing algorithm. Below is the algorithm used in our patient for work up of elevated serum hCG. The results are consistent with hCG due to tumor production. Final pathology of the patient's tumor revealed a mucinous adenocarcinoma of the ovary with aberrant hCG expression.
With epithelial ovarian cancers there is new literature to suggest overexpression of hCG, specifically free βhCG, may play an active role in tumorigenesis. (Guo et al., 2011) Gue et al. were able to show that overexpression of free βhCG in a cellular model increased cell proliferation and anchorage-independent growth, induced cell cycle progression, and downregulated apoptosis. Additionally, they showed that injecting cells which overexpressed hCG into mice actually induced tumor development. Furthermore, there is some published data suggesting that free βhCG correlates with severity of disease and prognosis with epithelial ovarian cancer. In a study with 111 patients with epithelial ovarian cancer, elevated hCG correlated with poor overall survival (RR 2.31, p = 0.006); however, this correlation was not found to be independent in multivariate analysis. (Ind et al., 1997) A similar study with 173 ovarian cancer patients did show free βhCG to correlate with poor prognosis in both a univariate and in multivariate analysis (HR 2.2, p = 0.0003). In their model, hCG was used to stratify patients in two risk groups independent of grade and stage. Overall, 5 year survival was 65% when hCG normal but only 19% with elevated serum hCG (p < 0.0001). (Vartiainen et al., 2008)
To date, there has been one previously reported case of paraneoplastic hCG production in a mucinous ovarian adenocarcinoma. (Goldstein et al., 2016) The elevated hCG in that case wasn't identified until the patient was enrolling onto a clinical trial for second line therapy following disease progression after surgery and chemotherapy. Whether discovered prior to surgery or prior to other cytotoxic treatment regimens, it is important to quickly be able to establish the source of hCG both to ensure the patient is not pregnant, as well as to prevent delay of treatment or unnecessarily begin treatment for ectopic pregnancy or trophoblastic disease. In the case of our patient, the hCG was identified as paraneoplastic without a delay in treatment and was confirmed by pathologic examination of her tumor. Furthermore, in our patient, hCG became a useful marker for treatment response and surveillance.
Authors' contributions
VW, HW, AN reviewed and interpreted the available literature. VW wrote the manuscript with input from all authors; all authors read and approved the final version of the manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
References
- Boscato L.M., Stuart M.C. Incidence and specificity of interference in two-site immunoassays. Clin. Chem. 1986;32(8):1491–1495. [PubMed] [Google Scholar]
- Cole L.A. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol. Oncol. 2006;102(2):145–150. doi: 10.1016/j.ygyno.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Cole L.A., Khanlian S.A., Muller C.Y. Normal production of human chorionic gonadotropin in perimenopausal and menopausal women and after oophorectomy. Int. J. Gynecol. Cancer. 2009;19(9):1556–1559. doi: 10.1111/igc.0b013e3181a40cf2. [DOI] [PubMed] [Google Scholar]
- Covinsky M. An IgM lambda antibody to Escherichia coli produces false-positive results in multiple immunometric assays. Clin. Chem. 2000;46(8 Pt 1):1157–1161. [PubMed] [Google Scholar]
- Elliott M.M. Carbohydrate and peptide structure of the alpha- and beta-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997;7(1):15–32. doi: 10.1007/BF02778058. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A non-pregnant woman with elevated beta-HCG: a case of para-neoplastic syndrome in ovarian cancer. Gynecol. Oncol. Rep. 2016;17:49–52. doi: 10.1016/j.gore.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. Overexpression of the beta subunit of human chorionic gonadotropin promotes the transformation of human ovarian epithelial cells and ovarian tumorigenesis. Am. J. Pathol. 2011;179(3):1385–1393. doi: 10.1016/j.ajpath.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschuh K. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. Endocrinology. 2007;148(10):5011–5019. doi: 10.1210/en.2007-0286. [DOI] [PubMed] [Google Scholar]
- Herr F. HCG in the regulation of placental angiogenesis. Results of an in vitro study. Placenta. 2007;28(Suppl A):S85–S93. doi: 10.1016/j.placenta.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Iles R.K. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol. Cell. Endocrinol. 2007;260-262:264–270. doi: 10.1016/j.mce.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Iles R.K. Urinary concentration of human chorionic gonadotrophin and its fragments as a prognostic marker in bladder cancer. Br. J. Urol. 1996;77(1):61–69. doi: 10.1046/j.1464-410x.1996.82910.x. [DOI] [PubMed] [Google Scholar]
- Ind T. Serum concentrations of cancer antigen 125, placental alkaline phosphatase, cancer-associated serum antigen and free beta human chorionic gonadotrophin as prognostic markers for epithelial ovarian cancer. Br. J. Obstet. Gynaecol. 1997;104(9):1024–1029. doi: 10.1111/j.1471-0528.1997.tb12061.x. [DOI] [PubMed] [Google Scholar]
- Knight A.K. Frequent false positive beta human chorionic gonadotropin tests in immunoglobulin A deficiency. Clin. Exp. Immunol. 2005;141(2):333–337. doi: 10.1111/j.1365-2249.2005.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillac I. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52(14):3901–3907. [PubMed] [Google Scholar]
- Vartiainen J. Combination of serum hCG beta and p53 tissue expression defines distinct subgroups of serous ovarian carcinoma. Int. J. Cancer. 2008;122(9):2125–2129. doi: 10.1002/ijc.23322. [DOI] [PubMed] [Google Scholar]
- Verhaegen J. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345 doi: 10.1136/bmj.e6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladutiu A.O. Heterophilic antibodies interfering with radioimmunoassay. A false-positive pregnancy test. JAMA. 1982;248(19):2489–2490. [PubMed] [Google Scholar]