Abstract
BACKGROUND
Pharyngeal contractility is critical for safe bolus propulsion. Pharyngeal contractile vigor can be measured by Pharyngeal Contractile Integral (PhCI): product of mean pharyngeal contractile amplitude, length, and duration. We characterized PhCI in neonates and examined the hypothesis that PhCI differs with mode of stimulation.
METHODS
Nineteen neonates born at 38.6(34-41) weeks gestation were evaluated at 42.9 (40.4-44.0) weeks postmenstrual age using high resolution manometry (HRM). PhCI was calculated using: a) Conventional, and b) Automated Swallow Detection algorithm (ASDA) methods. Contractility metrics of all pharyngeal regions were examined using mixed statistical models during spontaneous and adaptive state (pharyngeal and oral stimulus) swallowing.
RESULTS
PhCI of oral stimuli swallows were distinct from pharyngeal stimuli and spontaneous swallows (P<0.05). Correlation between conventional and ASDA methods was high (P < 0.001). PhCI increased with swallows for pharyngeal stimulation (P <0.05) but remained stable for swallows with oral stimulation. PhCI differed between proximal and distal pharynx (P < 0.001).
CONCLUSIONS
PhCI is a novel reliable metric capable of distinguishing 1) proximal and distal pharyngeal activity, 2) effects of oral and pharyngeal stimulation, and 3) effects of prolonged stimulation. Changes in pharyngeal contractility with maturation, disease, and therapies can be examined with PhCI.
INTRODUCTION
Swallowing function is facilitated by the coordination of lingual, oral, pharyngeal, upper esophageal sphincter (UES), esophageal body, and lower esophageal sphincter (LES) systems (1, 2). Coordinated pharyngeal and laryngeal mechanics are critical for bolus propulsion to maintain aerodigestive health (3-6). Proper aerodigestive safety involves airway protection via pharyngo-glottal closure reflex and deglutition apnea, as well as bolus propulsion through pharyngo-UES-contractile reflex and pharyngeal reflexive swallowing (3, 7-11). Pharyngeal swallowing functions include pharyngeal rhythm kinetics, contractile force dynamics, regulatory modulation, and cross-systems interactions across the neuro-aero-digestive systems. Robust pharyngeal contractility is essential to trigger pharyngo-esophageal peristalsis when pharyngeal provocation occurs through oral feeding or during gastroesophageal reflux (GER) events (12-14).
Human neonates have dynamic maturational physiology and diverse pathophysiology influenced by birth gestation, pulmonary function and/or neurological development. Their pharyngeal and airway interactions are effective in health (3, 9, 11, 15), and impaired in disease (16, 17). Pharyngeal dysfunctions manifest as chronic dysphagia, oral feeding difficulties, inability to handle supra-esophageal reflux events, and/or airway compromise. These dysfunctions may belong to any of three anatomical regions in adults, i.e. velopharynx, oropharynx, and hypopharynx. However, in neonates the oropharynx is underdeveloped (18). Current knowledge of segmental pharyngeal motility functions in neonates is not well understood. Identifying these segmental motility functions and their coordination can shed light on pharyngeal impairments in neonates.
Video-fluoroscopy studies (VFSS) are generally used to track pharyngeal bolus movement in healthy infants who could swallow oral barium. However, high risk infants who have aerodigestive concerns and are on respiratory support have difficulties with swallowing barium and therefore VFSS studies are difficult to conduct and interpret. In contrast, crib-side manometry studies permit measurement of luminal pressure patterns generated by contractions of the pharyngeal musculature. In previous studies using water-perfusion manometry methods, we have measured spontaneous and adaptive responses of pharyngo-esophageal motility reflexes in neonates (3, 9-11). The advent of high resolution manometry (HRM) advanced our understanding of pressure topography. Closely spaced sensors (1 cm apart) in HRM catheters improve regional measurements of the contiguous and non-contiguous reflexes. Using HRM, we have measured pharyngeal rhythm responses and the effects of oral and pharyngeal provocation on pharyngo-esophageal propulsive and protective reflexes in infants (16, 19).
Recent studies in adults using HRM methods revealed that pharyngeal peristaltic function can be quantifiable across its whole length and also recognized age-related changes (20-24). Additionally, important pharyngeal function metrics such as pharyngeal contractile integral (PhCI = mean contractile amplitude >20 mmHg × contractile length × contractile duration) (21, 22, 24, 25), maximal pharyngeal contraction (P-Max), and regional differences have been described (20, 21, 26). However, systematic examination of these pharyngeal peristaltic motor functions has not been characterized in human neonates. HRM has also enabled identification of segmental regions in adults (27). In neonates, since the oropharyngeal region is underdeveloped, it is possible to identify the base of tongue manometrically but it is necessary to use simultaneous videomanometry to identify the segments accurately.
We have undertaken this study to characterize the functions of pharynx in oral-fed thriving human neonates. Our aims were to: 1) characterize PhCI in oral fed neonates, and test the hypothesis that pharyngeal vigor (measured by PhCI) differs between spontaneous and adaptive (pharyngeal and oral stimuli) state swallows, 2) compare proximal and distal pharyngeal (separated at base of tongue) motility functions in neonates, and 3) develop an automated swallow detection algorithm to derive PhCI and correlate with conventional methods. We hypothesized that the proximal and distal PhCI are distinct in neonates and are modified by different loci of stimulation.
MATERIALS AND METHODS
Subjects
The study included 19 neonates (12 males, born at median 38.6 weeks, ranging from 34 – 41 weeks) admitted to Nationwide Children’s Hospital neonatal nurseries for transitional problems. Neonates were exclusively orally fed at evaluation, and were not dependent on any respiratory support. These infants were enrolled as part of our ongoing pathophysiology of aerodigestive reflexes research study protocol. Exclusion criteria included gastrointestinal or congenital anomalies. Institutional Review Board approval and written informed parental consent were obtained in compliance with the Health Insurance Portability and Accountability Act. Patient safety was continuously monitored by the physician and a registered nurse at the crib-side during the study procedures.
Manometry Methods and Experimental Protocol
Solid state HRM [Solar GI, HRM Laborie Medical Technologies, Mississauga, ON, Canada] concurrent with provocative pharyngeal manometric and oral feeding methods were adapted (3, 4, 9, 12, 16, 28). Catheters included solid state HRM catheter (6 French) with 25 uni-directional pressure sensors spaced 1 cm apart (Unitip High Resolution Catheter, Unisensor, Portsmouth, NH) for pharyngo-esophageal motility, and a custom designed 5.25 French silastic catheter (Dentsleeve International, Mui Scientific, Mississauga, ON, Canada) for pharyngeal infusions. After HRM catheter placement, the infusion catheter was placed into the opposing nares positioned at 1 cm above UES border so that the infusion port was at the level of the pharynx. The infusion catheter was connected to the HRM system via a pressure transducer (TNF-R disposable, Laborie Medical Technologies, Mississauga, ON, Canada). During spontaneous (resting) state, 10 spontaneous swallows in each subject were identified that propagated as primary peristalsis sequences occurring in the absence of any provocation. Adaptive stimuli were administered via pharyngeal or oral routes during periods of pharyngo-esophageal quiescence. Pharyngeal stimuli consisted of 0.3 mL of saline (given in triplicate per subject) infused into the pharynx through the infusion catheter (3, 5, 11). Oral stimuli consisted of a single nutritive oral bottle feeding session administered over a 3-minute period (28).
Data Analysis and Data Validation
Pharyngeal contractile characteristics were analyzed by stimulus state (spontaneous, pharyngeal stimulation and oral stimulation) and region (proximal and distal) as described in Figure 1. The pharynx was divided into proximal and distal regions at the tongue base which was identified as the swallow related high pressure zone (27). Pharyngeal contractile activity was defined as >20 mmHg (waveform amplitude) in the pharyngeal region to reduce intra-bolus pressure (IBP) effects. Pharyngeal contraction measures included length (cm), amplitude (mmHg), and duration (sec) of contraction, and PhCI (mmHg.cm.s). PhCI was defined as the product of mean contractile amplitude (a), pharyngeal length of contraction (l) and contractile duration (τ) of the pharyngeal segment in the HRM contour plot (21, 22, 24, 25).
Figure 1. Study Design.

The figure shows contour plots for: A) Spontaneous swallow, B) Pharyngeal stimulus induced swallows, and C) Oral stimulus induced swallows at the 20 mmHg iso-contour line, the white arrows indicate the presentation of stimulus. D) The pharyngeal region of interest (black box) was identified from the upper border of the proximal pharynx to the upper esophageal sphincter upper border. The pharyngeal region can be distinguished manometrically into proximal and distal regions at the tongue base as shown by the redboxes. PhCI values were calculated if the 20mmHg isocontour threshold was achieved.
Where: a is the mean pharyngeal pressure of contraction, τ is the duration between the start and stop of the 20 mmHg isocontour, and l is the length of contraction of the pharyngeal region of interest.
Additionally, for adaptive state (pharyngeal or oral stimulation) swallows, pharyngeal response frequency (Hz) was defined as the number of pharyngeal swallows divided by duration between the first and last pharyngeal swallow (10). The PhCI slope, which is the change in PhCI during progressive swallows, was compared between oral and pharyngeal stimulations.
Algorithms Employed to measure Pharyngeal Contractile Integral
Traditionally in adult studies, contractile integral values are determined manually using built-in esophageal pressure topography tools from the pharyngo-esophageal manometry analysis software (MMS, v 9.5, Laborie Medical Technologies) (21, 22, 25, 29). This metric was initially developed for the distal contractile integral (DCI) in the Esophageal Motility Disorder Chicago Classifications, and calculated by drawing a box around the region of interest (distal esophagus) (29), which has also been established in children (30). A threshold of 20 mmHg is set to reduce the effects of intra bolus pressure (IBP). Similar methods for calculating the DCI have also been applied to the pharyngeal region in adults (21, 22, 24, 25). This method can be referred to as the “Conventional Method.” However, there is a limitation to this method among neonates. Adult swallows are voluntary, instructional and sporadic, unlike neonates whose swallows are clustered, involuntary, and are highly variable within and between clusters. Manually drawing a region of interest box to calculate PhCI can be labor intensive, tedious and subject to human error, thus requiring multiple verifications to be accurate and consistent.
To mitigate the above limitation, an “automated swallow detection algorithm” was developed using Python (v. 3.6.2) to identify pharyngeal contractile activity with minimal user inputs (time frame epochs of spontaneous and adaptive states, proximal pharyngeal channel, proximal junction, and upper border of the UES during pharyngeal contraction). HRM data file in .csv format was imported to Python. Scipy (v 0.19.1) was used to linearly interpolate this data and Matplotlib (v 2.0.2) to generate contour plots. Pharyngeal swallows in the contour plots were identified in each epoch and individual swallows were isolated using the 20mmHg contour lines around them to calculate PhCI instead of using a region of interest box. This prevents inflation of PhCI from the region of interest box overlapping on IBP and also reduces the manual effort required to analyze swallows.
Time frame epochs for the automated analysis selected during the spontaneous state included a duration of quiescence for a 10 second period followed by subsequent spontaneous pharyngeal swallow culminating in quiescence (6, 13). Epochs for analysis of pharyngeal stimulus induced swallows were selected to include a quiescent state prior to the onset of pharyngeal infusion stimulus until the restoration of aerodigestive quiescence after a reflex response (3, 10, 11, 16). Epochs for analysis during the 3-min oral stimulation challenge were selected from before the onset of oral stimulus to the stimulus offset and until restoration of quiescence (28).
An example of the conventional and automated swallow detection algorithms can be observed in Figure 2, where the fixed rectangles or regions of interest for PhCI in the conventional algorithm can be noted. Any values over the 20 mmHg isocontour may contribute to the PhCI in this region, thus potentially inflating the PhCI values. In contrast, the automated detection algorithm isolates the contractile data associated with the pharyngeal swallow above the 20 mmHg isocontour, thus alleviating the potential for inflation.
Figure 2. Analytical Methods.

The left panel shows the raw HRM data. The right panels show the conventional method and the automated detection algorithm for calculating PhCI. These regions of interest calculate any values within this window above the 20 mmHg isocontour threshold, thus having the potential of artificially elevated values. In contrast, the automated detection algorithm identifies and isolates the pharyngeal contractions first and then calculates only those values associated with the identified pharyngeal contraction, thus alleviating the potential for inflation while defining accuracy.
Statistical Analysis
Parametric tests, nonparametric tests, mixed models, and violin plots were used to compare pharyngeal contractile characteristics (PhCI, duration, length, amplitude, and frequency) during spontaneous (no stimulation) and adaptive (pharyngeal or oral stimulation) states and further subdivided into proximal and distal regions. Due to the presence of repeated measures, mixed statistical models were used to test the effect of elapsed time on PhCI between spontaneous and adaptive states. Correlation and mixed regression models were used to test the association between pharyngeal stimuli induced PhCIs and the oral stimulus within the neonate. To evaluate the agreement of the PhCI values generated by the algorithm with those from the MMS software, two-way intra-class correlation for agreement (ICC) and Pearson correlation were calculated. Data are presented as mean ± SEM, unless otherwise noted. P-values < 0.05 were considered significantly different. Analyses were performed in R (v. 3.3.3) and SAS (v. 9.3).
RESULTS
Demographic characteristics
Nineteen subjects were evaluated at 42.9 (40.4 – 44.0) weeks PMA. Their weights 3.8 (2.4 – 6.1) kg, lengths 52.3(48 – 59.5) cm and head circumferences 35.1(32.5 – 41) cm were appropriate for growth centiles. All infants were physiologically stable and orally feeding at evaluation and at discharge.
Determination of PhCI
A total of 1608 pharyngeal swallows were examined. The mean overall pharyngeal length of contraction was 3.36 ± 0.01 cm (range 2.50 – 4.50 cm). During pharyngeal contractions, the mean contractile amplitude was 69 ± 0.5 (range 20 to 169) mmHg, and mean contractile duration was 0.52 ± 0.01 (range 0.22 to 3.98) sec. Overall PhCI calculated was 85 ± 1 (range 6 to 393) mmHg.sec.cm.
We then compared the pharyngeal contractile characteristics (amplitude, length, duration, and PhCI) during spontaneous state swallows and adaptive state swallows (Table 1 and Figure 3). Differences were evident between proximal and distal pharyngeal regions (Table 2 and Figure 4). The frequency of pharyngeal contractions from pharyngeal stimulation (0.68 ± 0.05 Hz), and from oral stimulation (0.64 ± 0.04 Hz) revealed no significant difference (P = 0.2).
Table 1.
Characteristics of Pharyngeal contractions compared during spontaneous and adaptive states.
| Pharyngeal contraction Characteristics | Spontaneous swallows (N=178) | Pharyngeal Stimulus Induced swallows (N=160) | Oral Stimulus Induced swallows (N=1270) |
|---|---|---|---|
| Amplitude, mmHg | 72 ± 5 | 72 ± 5 | 71 ± 5 |
| Duration, sec | 0.65 ± 0.03 | 0.68 ± 0.03 | 0.50 ± 0.02*† |
| PhCI, mmHg.s.cm | 106 ± 11 | 114 ± 11 | 87 ± 11*† |
Data represents mean ± SE.
P<0.05 vs. spontaneous swallow.
P<0.05 vs pharyngeal stimulus swallow
Figure 3. Overall PhCI between spontaneous and adaptive stimuli.
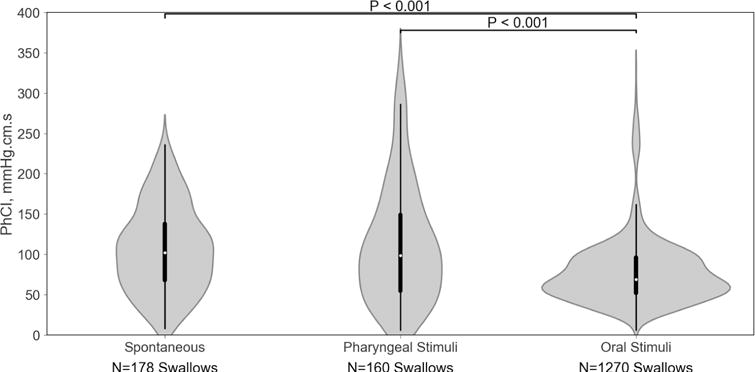
Depicted are violin plots for the pharyngeal PhCI values for the entire pharyngeal region between states. The white dot represents the median, the thick black bar represents the interquartile range, and the thin black line represents the 95% confidence interval. The gray areas around the black lines represent the data distribution using kernel density estimation, with wider areas indicating a higher probability of PhCI in that region and narrower areas indicating a lower probability of PhCI. Note the oral stimuli (nutritive swallowing) resulted in a wider base and significantly lower PhCI.
Table 2.
Comparison of Proximal and Distal pharyngeal characteristics averaged over spontaneous and adaptive state swallows.
| Characteristic | Proximal | Distal | P-value |
|---|---|---|---|
| Length, cm | 2.36 ± 0.06 | 1.00 ± 0.06 | <0.0001 |
| Amplitude, mmHg | 74 ± 5 | 77 ± 5 | <0.0001 |
| Duration, sec | 0.52 ± 0.02 | 0.51 ± 0.02 | <0.0001 |
| Contractile Integral mmHg.s.cm | 63 ± 5 | 30 ± 5 | <0.0001 |
Data presented as Mean ± SE
Figure 4. Characterization of regional PhCI during (a) spontaneous swallowing, (b) pharyngeal stimulation, and (c) oral stimulus.
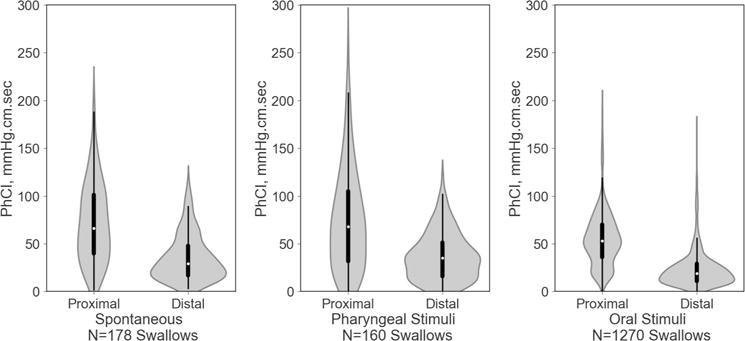
Note the wider bases and significantly lower PhCI in the distal regions.
Slopes of overall PhCI were examined to assess the rate of change in contractility; PhCI increased more with pharyngeal stimulation (ß ± SE, 1.26 ± 0.64 mmHg.s.cm, P<0.05) than with oral stimulation (ß ± SE, 0.02 ± 0.01 mmHg.s.cm, P<0.05), and comparison of the slopes showed they were different (P<0.001). PhCI and frequency for oral stimulation were correlated with pharyngeal PhCI (r=0.50, P=0.03) and frequency (r=0.40, P=0.04) respectively. Agreement between conventional and automated algorithm methods for determining the PhCI values were examined. An ICC of 0.88 was observed (P<0.0001). The Pearson correlation of the PhCI values generated by the automated swallow detection algorithm and those from conventional methods was 0.92 (P<0.001) as shown in Figure 5.
Figure 5. Correlation of PhCI between Conventional and Automated Algorithm methods.
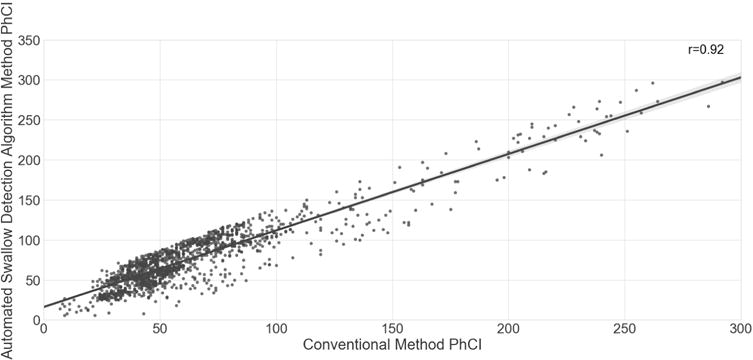
Correlation of PhCI values was high (r = 0.92, P<0.001) between the conventional method and the automated swallow detection algorithm method for the 1608 swallows.
DISCUSSION
In this study, we validated the methods and characterized the pharyngeal contractile integral (PhCI) in oral-fed neonates and found that: 1) pharyngeal vigor is distinct during oral feeding (vs spontaneous and pharyngeal stimulation), 2) PhCI increased with sequential swallows for pharyngeal stimuli, while remaining stable during oral feeding, 3) PhCI is distinct in proximal and distal pharyngeal regions, and 4) automated algorithms had high correlation with conventional methods.
We have previously reported that basal (spontaneous) swallowing briefly activates swallowing-respiratory pause interactions (9, 15) and electro-cortical arousal response (31). Thus, these sequences have the capability of activating multiple neural networks at brainstem and supranuclear cortical levels. This is further demonstrated by the distinct oral stimuli PhCI (compared to spontaneous or pharyngeal stimulus). This is likely due to the facial, palatal and lingual muscles (regulated by V, VII, IX, and XII cranial nerves) that facilitate lingual peristalsis (32) and palatal coordination in moving oral bolus into pharynx, thus resulting in the lower PhCI. This latter scenario is dependent on oral sucking skills and the duration of infant feeding. It is also likely that the oral bolus presentation may be preparatory to the succeeding pharyngeal swallowing phase characteristics (33). We speculate that graded pharyngeal stimulation can be helpful in evoking the downstream reflexes which can help with peristalsis and bolus propulsion, and also with respiratory-swallowing cross-systems interactions during maturation, health and disease.
The slope of the oral stimulus induced PhCI remained stable, in contrast to the increasing slope of pharyngeal stimulus induced PhCI. In adults, external resistive load has been used for pharyngeal rehabilitation, in which PhCI decreases over elapsed time thus indicating fatigue (22). However, determinants of fatigue are unclear in neonates, and may underlie in the PhCI, frequency and duration of pauses between the bursts. Further studies are needed to understand the basis for symptoms of dysphagia in the context of pharyngeal functions, which may provide clues to potential steps to avoid troublesome symptoms. These manifestations may include decreased endurance and fatigue (22), and cardiorespiratory symptoms (5, 6, 34). From our studies, we believe that the pharyngeal stimuli may strengthen pharyngeal vigor by encouraging higher pharyngeal contractile vigor, but this is dependent on appropriate sensory-motor components of pharyngeal stimulus-induced airway-digestive protective reflexes (3, 5, 9-11).
Distinction between proximal and distal pharyngeal regions is important to develop better understanding of swallowing physiology. Exploring these regional differences may lead to better diagnostic precision. Significant differences were noted with proximal and distal regions, with median length, duration, and PhCI significantly elevated in the proximal region. If distal function is challenged, alternative therapies (such as spoon-feeding) can help stimulate the velopharynx and facilitate bolus propulsion (35). If distal pressures are higher over prolonged periods or if the aboral gradient does not exist, such scenarios may suggest UES dysfunction.
Clinical Implications
Pharyngeal contractility is the driving force for initiating primary peristalsis, triggering bolus clearance, and ensuring airway protection when bolus is presented to pharynx via mouth (as in oral feeding) or from esophagus (as in reflux or regurgitation). In adults, PhCI has been used to detect swallowing impairment (36) and studies are underway to assess dysphagia using similar HRM metrics (37). In contrast to the limited time periods with video-fluoroscopy swallow studies, the current study demonstrates that our methods are applicable at the crib-side in the evaluation of neonatal swallowing pathophysiology during different activity states safely and over prolonged testing periods. In addition, the effects of therapeutic interventions such as feeding rehabilitation and swallowing therapies, medical, or surgical therapies on pharyngeal function can be safely evaluated at the crib-side before and after the procedures. To understand additional functions, these methods require further refinement to account for bolus clearance, circumferential pressure, and varying pressure dynamics, and can be built upon the critical steps established in this study. Further studies are needed: 1) to establish normative values and identify respiratory and pharyngeal-motility interaction parameters for the mechanistic diagnosis of dysphagia, life-threatening events and related swallowing impairments, 2) to investigate the role of the pharynx with aerodigestive malfunctions, maturational and neurogenic dysphagia, as well as syndromic neonates who have feeding concerns (38), 3) longitudinally to determine the differential growth of the proximal and distal segments and their functions, and to clarify when adult-type functions are attainable (24, 26), and 4) to develop precision therapies for oral feeding under controlled conditions.
In conclusion, PhCI is a marker for proximal and distal pharyngeal contractile vigor, and is distinct between different modes stimulation. Automated rapid and reproducible analysis of PhCI is possible for precise diagnosis of pharyngeal function in neonates. These methods can be safely applied at crib side over prolonged periods to examine changes in pharyngeal contractile activity in health and disease, across maturation, and for evaluation of therapies designed to improve pharyngeal function.
Acknowledgments
We are grateful to Carissa Collins, BS, BME, for technical support with manometric waveforms and contour plots; Brittany Durr, RN, MHA, for clinical coordination efforts and data extraction; and Lai Wei, PhD for statistical consultations.
STATEMENT OF FINANCIAL SUPPORT: This study was supported in part by National Institutes of Health Grants RO1 DK-068158 (Jadcherla), and PO1 DK-068051 (Shaker, Lang, Jadcherla)
Footnotes
AUTHOR CONTRIBUTIONS
SRJ developed the concepts, study design, obtained grant funding and ethical approvals from IRB. MK, GB, and RS were also associated in concepts. SRJ and KAH performed high resolution pharyngeal manometry studies for data acquisition. SRJ, VP, KAH, and SN performed data analysis. VP developed automated analytical algorithms. SN performed statistical analysis and interpretation. JD provided guidance with mathematical concepts and analytical algorithms. SRJ, VP, KAH, SN, JD, MK, GB and RS performed data verification and interpretation. VP, KAH, and SN created figures and tables. SRJ, VP, KAH, SN, JD, MK, GB and RS were involved with manuscript writing, editing, and approval of final version.
DISCLOSURE STATEMENT: The authors declare no conflicts of interest.
CATEGORY OF STUDY: Translational
References
- 1.Arvedson JC, Lefton-Greif MA. Anatomy, physiology, and development of feeding. Semin Speech Lang. 1996;17:261–268. doi: 10.1055/s-2008-1064103. [DOI] [PubMed] [Google Scholar]
- 2.Miller L, Clave P, Farre R, et al. Physiology of the upper segment, body, and lower segment of the esophagus. Ann N Y Acad Sci. 2013;1300:261–277. doi: 10.1111/nyas.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and Lower Esophageal Sphincter Kinetics are Modified During Maturation: Effect of Pharyngeal Stimulus in Premature Infants. Pediatr Res. 2014;77:99–106. doi: 10.1038/pr.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasenstab KA, Sitaram S, Lang IM, Shaker R, Jadcherla SR. Maturation Modulates Pharyngeal-Stimulus Provoked Pharyngeal and Respiratory Rhythms in Human Infants. Dysphagia. 2017;32:509–519. doi: 10.1007/s00455-017-9833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasenstab KA, Jadcherla SR. Respiratory Events in Infants Presenting with Apparent Life Threatening Events: Is There an Explanation from Esophageal Motility? J Pediatr. 2014;165:250–255 e251. doi: 10.1016/j.jpeds.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dua K, Surapaneni SN, Kuribayashi S, Hafeezullah M, Shaker R. Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. Am J Physiol Gastrointest Liver Physiol. 2011;301:G197–202. doi: 10.1152/ajpgi.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dua K, Surapaneni SN, Kuribayashi S, Hafeezullah M, Shaker R. Protective role of aerodigestive reflexes against aspiration: study on subjects with impaired and preserved reflexes. Gastroenterology. 2011;140:1927–1933. doi: 10.1053/j.gastro.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol. 2009;104:2572–2582. doi: 10.1038/ajg.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jadcherla SR, Hasenstab KA, Sitaram S, Clouse BJ, Slaughter JL, Shaker R. Effect of Nasal Non-Invasive Respiratory Support Methods on Pharyngeal Provocation Induced Aero-digestive Reflexes in Infants. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1006–1014. doi: 10.1152/ajpgi.00307.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and lower esophageal sphincter kinetics are modified during maturation: effect of pharyngeal stimulus in premature infants. Pediatr Res. 2015;77:99–106. doi: 10.1038/pr.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrattan KE, Sivalingam M, Hasenstab KA, Wei L, Jadcherla SR. The physiologic coupling of sucking and swallowing coordination provides a unique process for neonatal survival. Acta Paediatr. 2016;105:790–797. doi: 10.1111/apa.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–38. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 14.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr. 2006;149:77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol. 2007;102:2286–2293. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen PS, Gulati IK, Shubert TR, et al. Pharyngeal stimulus-induced reflexes are impaired in infants with perinatal asphyxia: Does maturation modify? Neurogastroenterol Motil. 2017;27 doi: 10.1111/nmo.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun R, Sitton M, Tipnis NA, et al. Endoscopic cricopharyngeal myotomy for management of cricopharyngeal achalasia (CA) in an 18-month-old child. Laryngoscope. 2013;123:797–800. doi: 10.1002/lary.23545. [DOI] [PubMed] [Google Scholar]
- 18.Arvedson JC, Brodsky L. Pediatric Swallowing and Feeding: Assessment and Management. Singular Publishing Group; San Diego, California: 1992. [Google Scholar]
- 19.Shubert TR, Sitaram S, Jadcherla SR. Effects of pacifier and taste on swallowing, esophageal motility, transit, and respiratory rhythm in human neonates. Neurogastroenterol Motil. 2016;309 doi: 10.1111/nmo.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nativ-Zeltzer N, Logemann JA, Zecker SG, Kahrilas PJ. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography-a normative study of younger and older adults. Neurogastroenterol Motil. 2016;28:721–731. doi: 10.1111/nmo.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao H, Mei L, Sharma T, Kern M, Sanvanson P, Shaker R. A human model of restricted upper esophageal sphincter opening and its pharyngeal and UES deglutitive pressure phenomena. Am J Physiol Gastrointest Liver Physiol. 2016;311:G84–90. doi: 10.1152/ajpgi.00145.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaker R, Sanvanson P, Balasubramanian G, Kern M, Wuerl A, Hyngstrom A. Effects of laryngeal restriction on pharyngeal peristalsis and biomechanics: Clinical implications. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1036–1043. doi: 10.1152/ajpgi.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon KJ, Park JH, Jung IS. Videofluoroscopic and manometric evaluation of pharyngeal and upper esophageal sphincter function during swallowing. J Neurogastroenterol Motil. 2014;20:352–361. doi: 10.5056/jnm14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balasubramanian G, Sharma T, Kern M, Mei L, Sanvanson P, Shaker R. Characterization of pharyngeal peristaltic pressure variability during volitional swallowing in healthy individuals. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern MK, Balasubramanian G, Sanvanson P, Agrawal D, Wuerl A, Shaker R. Pharyngeal Peristaltic Pressure Variability, Operational Range and functional Reserve. Am J Physiol Gastrointest Liver Physiol. 2017;312:G516–G525. doi: 10.1152/ajpgi.00382.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen SP, Jones CA, McCulloch TM. Pharyngeal swallowing pressures in the base-of-tongue and hypopharynx regions identified with three-dimensional manometry. Laryngoscope. 2017;127:1989–1995. doi: 10.1002/lary.26483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119:369–376. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jadcherla SR, Stoner E, Gupta A, et al. Evaluation and management of neonatal dysphagia: impact of pharyngoesophageal motility studies and multidisciplinary feeding strategy. J Pediatr Gastroenterol Nutr. 2009;48:186–192. doi: 10.1097/MPG.0b013e3181752ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldani HA, Staiano A, Borrelli O, Thapar N, Lindley KJ. Pediatric esophageal high-resolution manometry: utility of a standardized protocol and size-adjusted pressure topography parameters. Am J Gastroenterol. 2010;105:460–467. doi: 10.1038/ajg.2009.656. [DOI] [PubMed] [Google Scholar]
- 31.Jadcherla SR, Parks VN, Peng J, et al. Esophageal sensation in premature human neonates: temporal relationships and implications of aerodigestive reflexes and electrocortical arousals. Am J Physiol Gastrointest Liver Physiol. 2012;302:G134–144. doi: 10.1152/ajpgi.00067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow SM. Central pattern generation involved in oral and respiratory control for feeding in the term infant. Curr Opin Otolaryngol Head Neck Surg. 2009;17:187–193. doi: 10.1097/MOO.0b013e32832b312a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logemann JA. Swallowing disorders. Best Pract Res Clin Gastroenterol. 2007;21:563–573. doi: 10.1016/j.bpg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Jadcherla SR, Hasenstab KA, Shaker R, Castile RG. Mechanisms of cough provocation and cough resolution in neonates with bronchopulmonary dysplasia. Pediatr Res. 2015;78:462–469. doi: 10.1038/pr.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Engel-Hoek L, van Hulst KC, van Gerven MH, van Haaften L, de Groot SA. Development of oral motor behavior related to the skill assisted spoon feeding. Infant Behav Dev. 2014;37:187–191. doi: 10.1016/j.infbeh.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 36.O’Rourke A, Humphries K, Lazar A, Martin-Harris B. The pharyngeal contractile integral is a useful indicator of pharyngeal swallowing impairment. Neurogastroenterol Motil. 2017;29:1–7. doi: 10.1111/nmo.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryu JS, Park DH, Kang JY. Application and Interpretation of High-resolution Manometry for Pharyngeal Dysphagia. J Neurogastroenterol Motil. 2015;21:283–287. doi: 10.5056/15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia. 2014;29:2–16. doi: 10.1007/s00455-013-9494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


