Abstract
Purpose:
To identify factors related to graft rejection following Descemet stripping automated endothelial keratoplasty (DSAEK) in the Cornea Preservation Time Study (CPTS).
Design:
Cohort study within a multicenter randomized clinical trial.
Methods:
1330 eyes of 1090 subjects undergoing DSAEK were randomized to receive a donor cornea with preservation time (PT) of 0–7 days (N=675) or 8–14 days (N=655) and followed for three years. Central endothelial cell density (ECD) was determined by a central image analysis reading center. Multivariable Cox models adjusted for PT, recipient diagnosis, and surgeon effect were used to identify factors associated with rejection.
Results:
Cumulative probability of definite graft rejection was 3.6% (99% CI 2.5% to 5.3%). Younger recipient age was associated with graft rejection [p<0.001; HR: 0.53 (0.33, 0.83) per decade]. PT, donor-recipient gender mismatch, recipient diagnosis, recipient race, graft size, discontinuation of topical corticosteroids, and immune-modulators, prior immunizations within three months, and prior glaucoma surgery were not associated with rejection (p>0.01). Among clear grafts with an ECD measurement at baseline and 3 years (N=913), endothelial cell loss (ECL) was greater in eyes that experienced a rejection episode (N=27) than in those that did not (N=886) (48% vs 38%, p=0.03). Twelve of 44 eyes (27%) with definite graft rejection subsequently failed, comprising 15% of the 79 failures in the CPTS.
Conclusions:
Graft rejection is uncommon after DSAEK and more likely with younger age, in a study cohort mostly > 50 years old. Rejection increases ECL, but it is not a leading cause of DSAEK failure.
Introduction
Over the last decade, Descemet stripping automated endothelial keratoplasty (DSAEK) has become the most common surgical procedure for the management of corneal endothelial cell failure in the United States.1 Its acceptance has been driven by excellent single-site studies that established the efficacy and safety of DSAEK, advanced and simplified surgical techniques2,3 and provided useful outcome data.4–9 However, these studies provided limited information about the association of donor, recipient, operative, and postoperative factors with the success of the procedure. There also have been conflicting results on the rejection rate of this procedure (ranging from 0–45%10–17) and the factors influencing this rate.
The Cornea Preservation Time Study (CPTS) was a large, prospective, randomized, double-masked clinical trial designed to examine the relationship between donor preservation time (PT), graft success, and endothelial cell loss.18 The CPTS established that there was no difference in graft success or endothelial cell loss for donor tissue preserved up to 11 days.19,20 Secondary analyses demonstrated that donor diabetes, recipient diagnosis of pseudophakic/aphakic corneal edema (PACE), and operative complications were associated with lower graft success and greater endothelial cell loss.21,22
In addition, because of the varying reported rejection rates with DSAEK,10–17 another pre-planned secondary outcome of the CPTS was the occurrence of rejection episodes. These data were collected with the a priori objective of identifying donor, recipient, and operative factors that might be associated with graft rejection, as well as the consequences of these rejection episodes on subsequent graft failure and endothelial cell loss from the largest prospective study of DSAEK to date.
Methods
The protocol was approved by institutional review boards for participating clinical sites and eye banks (e-Table1; supplemental material at AJO.com), and each participant provided written informed consent. Enrollment occurred between April, 2012, and February, 2014, and follow-up ended in June, 2017 with 1,330 cases (1,090 subjects) entered into the CPTS, performed across 40 sites involving 70 surgeons. Study oversight was provided by an independent data and safety monitoring committee. Details of the study methods have been reported previously,18–20 and methods relevant to this paper are summarized below. Briefly, eyes with primary endothelial dysfunction, Fuchs endothelial corneal dystrophy (FECD) or PACE, were randomized to receive a donor cornea from either the “0–7d PT group” (PT ≤7 days) or the “8–14d PT group” (PT 8–14 days) which was stratified by surgeon. Surgeons used multiple surgical techniques and corticosteroid regimens. The full protocol is available at https://clinicaltrials.gov/ct2/show/NCT01537393.
Donor corneas met current Eye Bank Association of America (EBAA) standards for DSAEK with a minimum endothelial cell density (ECD) ≥2,300 cells/mm2.23 Eligibility criteria for participants have also been previously published.18 Recipients could be 30 to 91 years old with eyes undergoing DSAEK for FECD or PACE, and eyes were excluded if they had complicated histories including previous penetrating keratoplasty (PKP) or DSAEK, tube shunts, uncontrolled glaucoma, anterior chamber intraocular lenses or significant anterior synechiae. Participants could enroll both eyes, if eligible. Preoperatively, a history of ocular surgery (particularly glaucoma surgery), diabetes, smoking, and FECD, other corneal dystrophies, and current medications (including systemic and topical corticosteroids, anti-glaucoma medications and systemic or topical immune-modulators) was obtained. Eye banks or surgeons prepared the donor corneas according to their customary technique. Preoperative care, surgical technique, and postoperative care were provided according to each investigator’s routine.
The Corneal Image Analysis Reading Center at Case Western Reserve University and University Hospitals Eye Institute (CIARC, Cleveland, OH), served as the reading center for endothelial image analysis to determine ECD and also was responsible for quality control measures at the eye banks and clinical sites. Details of the image quality, certification procedures, and endothelial imaging devices for each of the study eye banks and clinical sites have been previously published.18,20,24 Details of image capture by the eye banks and clinical sites, and determination of the ECD by the CIARC are also detailed in these publications.18,20,24 The CIARC-determined ECD using a variable frame method conducted by two independent masked readers, along with adjudication if the two readings differed by ≥ 5%.18,20,25
Follow-up examinations were performed at 1 day, 1 week, and 1, 6, 12, 24, and 36 months postoperatively. For participants who consented to extended follow-up, visits were also completed at 48 months and if possible within the study period, a 60-month visit was completed. Central recipient stroma clarity was assessed at every visit and graded on a validated three-point scale18: clear, equivocal, and cloudy. Each study visit also included documentation of topical medications, including systemic and topical corticosteroids, and systemic or topical immune-modulators. Whether the participant received any immunizations in the last 3 months prior to the appointment and, if yes, the type of immunization (e.g. influenza, tetanus, herpes zoster, pneumococcus, unknown vaccines, or other) was also recorded. Data from visits between scheduled study examinations were captured if a corneal abnormality was noted, including a suspected rejection episode. If active rejection was diagnosed, data from subsequent visits tracking resolution or worsening of the rejection episode were also captured.
Graft rejection was assessed using a modification of the Collaborative Corneal Transplantation Studies (CCTS) classification.26–28 The rejection types are summarized in Table 1. All principal investigators at each clinical site were trained on assessing both definite and probable/possible rejection signs at an investigator meeting prior to commencement of study. Graft rejection episodes were managed at the discretion of each investigator. Criteria for determining the principal cause of graft failure have been previously published.18,19
Table 1.
CPTS Graft Rejection Classification
|
Statistical Analysis
All eyes that underwent DSAEK were included in the analysis (N=1330). Results were analyzed by eyes, rather than patients. The outcome was time to the first definite graft rejection episode occurring up to three years after DSAEK. In all models, time was categorized based on intervals corresponding to the study examinations required by the protocol. Mild and severe definite graft rejection episodes were combined in the analysis. Data were censored at the time of non-rejection failure or the time of the last visit. No adjustments for multiple comparisons were made. Cumulative incidence plots based on Kaplan-Meier estimates29 of the distribution of time to the first graft rejection episode were constructed for each PT group.
The association of factors with graft rejection was evaluated using Cox proportional hazard models with the discrete logistic model option applied. A base model for evaluating candidate predictive factors included PT and the recipient diagnosis. All models included surgeon as a random effect to accommodate the potential correlation in graft rejection among DSAEKs performed by the same surgeon (“surgeon effect”). All candidate factors that were considered are listed in Table 2 and e-Table 2 (supplemental material at AJO.com). Each factor was first evaluated by adding the factor to the base model. Candidate factors were selected for inclusion in a final multivariable model in two stages. In stage 1, baseline recipient and donor factors associated with p < 0.10 were included in a multivariable backward model selection procedure.30 In stage 2, all factors selected in stage 1 were retained and operative and postoperative factors associated with p < 0.10 were included in another multivariable backward model selection procedure to yield a final model.
Table 2.
Association between donor, recipient, and operative risk factors and definite graft rejection episode through 3 years in the Cornea Preservation Time Study
| 3-yr Graft Rejection (99% CI) | Base Modela | Multivariable Modelb | ||||
|---|---|---|---|---|---|---|
| N=1330 | Hazard Ratio (99% CI) | p-value | Hazard Ratio (99% CI) | p-value | ||
| Significant Don or/Recipien t Factors | ||||||
| Recipient Age | <0.001 | <0.001 | ||||
| <51 years | 15 | 16.7% (2.9%, 67.7%) | ||||
| 51–60 years | 178 | 7.2% (3.5%, 14.5%) | ||||
| 61–70 years | 459 | 3.8% (2.0%, 7.1%) | 0.53 (0.33, 0.83) per decade | 0.53 (0.33, 0.83) per decade | ||
| 71–80 years | 502 | 2.4% (1.1%, 5.2%) | ||||
| 81–90 years | 173 | 1.9% (0.4%, 8.3% | ||||
| >90 years | 3 | 0.0% (0.0%, 0.0%) | ||||
| Other Don or/Recipient Factors | ||||||
| Donor Gender | 0.03 | 0.02 | ||||
| Female | 486 | 5.2% (3.1%, 8.8%) | 1.93 (0.88, 4.24) | 2.02 (0.92, 4.45) | ||
| Male | 844 | 2.7% (1.5%, 4.7%) | 1 [Ref] | 1 [Ref] | ||
| Recipient Diagnosis | 0.84 | 0.68 | ||||
| FECD | 1255 | 3.6% (2.5%, 5.4%) | 1 [Ref] | 1 [Ref] | ||
| PACE | 75 | 3.1% (0.5%, 17.9%) | 0.87 (0.13, 5.78) | 1.35 (0.20, 9.38) | ||
| Preservation Time Group | 0.85 | 0.91 | ||||
| 0–7 days | 675 | 3.5% (2.0%, 6.0%) | 1 [Ref] | 1 [Ref] | ||
| 8–14 days | 655 | 3.7% (2.2%, 6.4%) | 1.06 (0.49, 2.31) | 1.04 (0.47, 2.27) | ||
| Recipient Race/Ethnicity | 0.08 | 0.11 | ||||
| Black/African American | 47 | 2.6% (0.2%, 29.6%) | 0.77 (0.06, 10.78) | 0.55 (0.04, 7.83) | ||
| White | 1207 | 3.3% (2.2%, 5.0%) | 1 [Ref] | 1 [Ref] | ||
| Other | 76 | 9.0% (3.2%, 23.8%) | 2.90 (0.84, 9.96) | 2.60 (0.75, 9.05) | ||
Base models adjusted for PT, recipient diagnosis, random surgeon effect
Multivariable model adjusted for preservation time, recipient diagnosis, random surgeon effect. Recipient age was retained in the final model via backward selection. For factors not retained in the final model, the hazard ratio and p-value were obtained from the final model with the factor of interest included.
CI = confidence interval.
Time dependent Cox models were also built for discontinuation of topical corticosteroid use, discontinuation of topical immune-modulator use, whether any prior immunizations were received over the past three months, whether any influenza immunizations were received over the past three months, and whether any pneumococcus immunizations were received over the past three months. All analyses above were repeated with the inclusion of possible rejection episodes.
Descriptive summary statistics were calculated for the subset of eyes experiencing a definite rejection episode. A Cox model adjusting for recipient diagnosis and a random surgeon effect was constructed to compare failure rates between PT groups among eyes that ultimately failed due to any cause after experiencing a rejection episode. Student’s two sample t-test was used to compare pre-operative ECD between eyes that failed and those that survived.
The subset of eyes that did not experience graft failure were evaluated comparing ECD at three years and percent change in ECD from baseline to 3 years between eyes with and eyes without definite rejection episodes using two sample Wilcoxon tests. Two sample Wilcoxon tests were used to compare ECD at three years and percent change in ECD from baseline to three years between eyes with severe and mild rejection episodes. All statistical analyses were conducted using SAS, version 9.4 (SAS Inc).31 All reported p-values are two-sided.
Results
The donor and recipient cohort have previously been described.18 The mean recipient age of the 1090 DSAEK recipients at the time of enrollment was 70 ± 9 years (mean ± SD). 663 (61%) were female, and 983 (90%) were white. 1015 (93%) of the subjects had FECD, and the remaining 75 (7%) had PACE.
Definite Rejection
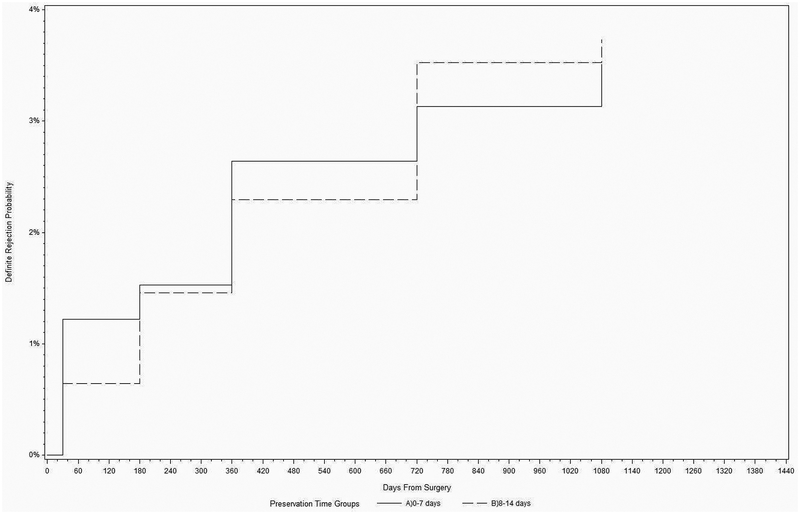
The three-year cumulative probability of a definite graft rejection episode was 3.6% (99% C.I.; 2.5% - 5.3%). During the follow-up period, 44 of the 1330 eyes (3.3%) in 44 of the 1090 subjects (4.0%) experienced one or more definite graft rejection episodes. Of the 44 definite graft rejection episodes, 22 (50%) were mild and 20 (45%) were severe. Severity was not determined for the remaining 2 episodes (5%), but these two grafts subsequently failed due to rejection. 22 (50%) of the 44 definite graft rejection episodes occurred prior to the one-year visit, whereas 16 (36%) occurred in between the one and two year visits, and 6 (14%) occurred in between the two and three year visits. The risk of definite rejection did not differ between the two PT groups (log rank p=0.85; Figure 1).
Figure 1.
Cumulative incidence for the first definite graft rejection episode in the Cornea Preservation Time Study
In a univariate analysis, there was a higher risk of definite rejection with female donors, non-white race, and younger recipient age. Those three factors were selected as candidate variables to be included in the final multivariable model (Table 2); however, donor gender and recipient race were removed from the final model during the backwards selection process. For every decade increase in recipient age, the hazard ratio (99% CI) was 0.53 (0.33, 0.83), indicating a decreased risk for rejection for older recipients compared to younger ones. In similar analyses, longer PT, gender mismatch between recipient and donor, recipient diagnosis, graft size, and recipient race all were found not to be associated with rejection (e-Table 2; supplemental material at AJO.com). No significant associations were detected for the immunization and topical corticosteroid or immune-modulator variables (Table 3). Among the 445 eyes with follow-up beyond 3 years that had not experienced a rejection episode, no eyes had a definite rejection episode in the 4th year.
Table 3.
Hazard ratios for Immunizations and steroid discontinuations of as time-dependent covariates for definite graft rejection episode through 3 years in the Cornea Preservation Time Study
| Na | Hazard Ratio (99% CI) | p-value |
|---|---|---|
| 571 | 0.82 (0.24, 2.85) | 0.67 |
| 509 | 0.94 (0.27, 3.29) | 0.90 |
| 120 | 1.51 (0.11, 21.48) | 0.69 |
| 86 | 1.16 (0.08, 16.86) | 0.88 |
| 22 | 7.12 (0.46, 111.35) | 0.06 |
CI = confidence interval.
Number of eyes with the one or more occurrences during follow-up
Combined Definite, Probable/Possible Rejection
The three-year cumulative probability of a definite or probable/possible rejection episode was 6.0% (99% CI; 4.5% - 8.0%). Seventy-four of the 1330 eyes (5.6%) met the definition of definite or probable/possible rejection episode. Results of a multivariable analysis evaluating factors associated with definite, probable, or possible graft rejection episodes were similar to those of definite graft rejection episodes only (e-Table 3 through e-Table 5; supplemental material at AJO.com), with younger recipient age being the only significant factor ([HR: 0.63 (0.44, 0.89) per decade); p<0.001). Among the 445 eyes with follow-up beyond 3 years that had not experienced a graft rejection episode, 2 had a possible rejection episode in the 4th year.
Subset of Eyes with Definite Rejections
Twelve of 44 eyes (27%) that experienced definite rejection episodes subsequently failed, while the remainder cleared with treatment. These 12 failures comprised 15% of the 79 failures for all causes (Table 4) in 1% (12 of 1330 eyes) of all the eyes in the study. Four of 22 (18%) grafts with rejection episodes in the 0–7 day PT group failed, while 8 of 22 (36%) of grafts with rejection episodes in the 8–14 day PT group failed (p=0.20). Of the 42 eyes that had active definite rejection episodes, 28 (67%) were on topical corticosteroids at the time of rejection, 2 (5%) were on topical immune-modulators at the time of rejection, 1 (2%) was on both topical corticosteroids and immune-modulators, and the remainder 11 (26%) were not on either topical corticosteroids or immune-modulators.
Table 4.
Characteristics of 44 eyes with a definite graft rejection episode by graft failure status at 3 years in the Cornea Preservation Time Study
| Yes | 5 (31%) | 11 (69%) |
| mean ± SD | ||
| Recipient Age (years) | 67 ± 8 | 65 ± 10 |
| Screening ECD (EB determined - cells/mm2) | 2834 ± 380 | 2713±291 |
| Pre-Operative ECD (EB determined - cells/mm2) | 2799 ± 325 | 2709 ± 260 |
| Pre-Operative ECD (CIARC determined - cells/mm2)a | 2769 ± 423 | 2667 ± 324 |
| median (IQR) | ||
| ECD before rejection (cells/mm2)a,b | 2000 (962, 2726) | 2443 (1830, 2765) |
| ECD after rejection (cells/mm2)c | 665 (481, 772) | 1453 (984, 2303) |
| mean ± SD | ||
| Difference in ECD after and before rejection (cells/mm2)a,b,c | −1356 ± 984 | −658 ± 632 |
| median (IQR) | ||
| Days between ECD before and after rejectiona,b,c | 175 (173, 716) | 321 (196, 378) |
| Days from last ECD before rejection to rejectiona,b | 147 (111, 267) | 138 (82, 309) |
Excludes one eye that did not fail. This eye did not have a CIARC determined pre-operative ECD.
For eyes with no ECD measurements at follow-up prior to rejection, the baseline value was used as ECD prior to rejection, and the first visit time was used as the time of measurement. One eye did not have a baseline ECD value or any ECD measurements before rejection. This eye was excluded from tabulations involving ECD before rejection.
Nine eyes that failed and two eyes that did not fail did not have any ECD screenings after rejection. One eye that did not fail had an ECD screening after rejection but no screening before rejection. Only eyes with an ECD screening available before and after rejection were included in the tabulations of differences between before and after rejection. The difference in the number of days from lasted screening before rejection to the date of rejection could not be calculated for the eye with no measurement prior to rejection.
PACE = pseudophakic/aphakic corneal edema; FECD = Fuchs endothelial corneal dystrophy; ECD = endothelial cell density; EB = eye bank; CIARC = Cornea Image Analysis Reading Center
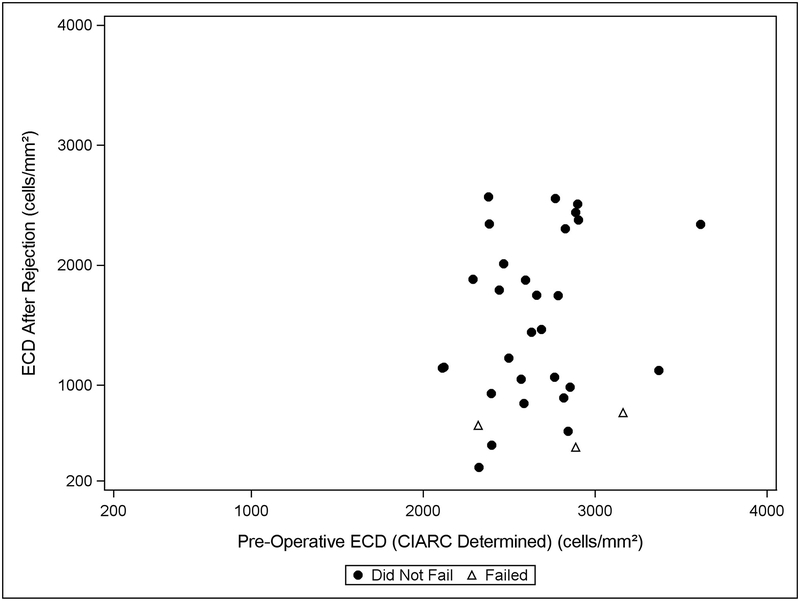
Among 3 eyes with rejection episodes that ultimately failed, ECD (when available) was reduced by 1356 ± 984 cells/mm2 (mean ±SD). For 29 eyes with rejection episodes that ultimately remained clear and had an ECD determination available after rejection, the mean (±SD) was a loss of 658 cells/mm2 (± 632) (Table 4 and Figure 2). Similarly, the median (IQR) number of days between ECD determination occurring before and after rejection was 175 days (173, 716) for grafts that went on to failure. For grafts that did not fail, it was 321 days (196, 378). The median (IQR) ECL from the measurement before rejection to after rejection among all eyes experiencing rejections with an ECD determination available after rejection was 26% (13%, 60%). The mean (±SD) eye bank-determined preoperative ECD for grafts that had rejection and failed was 2799 cells/mm2 (± 325), while the mean (±SD) eye bank-determined preoperative ECD for those that had rejection and survived was 2709 cells/mm2 (± 260) (t-test p=0.35).
Figure 2.
Endothelial cell density after the first definite graft rejection episode vs. pre-operative endothelial cell density
For those eyes (N = 28) that had at least one definite rejection episode but remained clear with 3-years follow-up and had an analyzable image at the exam compared to those eyes (N = 917) that did not experience any rejection episode or other postoperative complications and had clear grafts with an analyzable image, the central ECD at 3 years was 1349 ± 752 cells/mm2 vs. 1693 ± 623 cells/mm2, respectively (p=0.01). Among eyes with rejection episodes that had a central ECD determination available at 3 years, the mean (±SD) central ECD at 3 years was 1268 cells/mm2 (±814) for eyes with severe rejections vs. 1419 cells/mm2 (±714) for eyes with mild ones (p=0.45). Among eyes that had an ECD measurement at baseline and 3 years (N=913), ECL for the eyes that had a rejection (N=27) but had clear grafts at 3 years was 48% compared to 38% for those eyes that had clear grafts at the same time point but no prior history of rejection (N=886) (p=0.03). ECL for eyes with severe rejections at 3 years was 48% compared to 49% for eyes with mild rejections.
Discussion
We found an overall low cumulative probability of a definite rejection episode for DSAEK of 3.6% and of a possible, probable or definite rejection of 6.0% in the CPTS with 50% of the definite rejection episodes occurring within the first postoperative year. This rate is lower than rates reported in many previous studies of DSAEK10–17 and well below the rejection rate of 17% for PKP in one large series.16 Immunologic graft rejection of corneal transplants is less likely than that of solid organs, presumably because the cornea is normally avascular, isolating it from the immune system. Theoretically, rejection of DSAEK grafts should be less likely than that of penetrating grafts because DSAEK grafts are smaller, containing less foreign antigen, and are more isolated from the vascular system, lymphatic system, and peripheral Purkinje processing cells. DSAEK grafts also present allo-antigens via the anterior chamber, which tends to induce tolerance, rather than rejection (anterior chamber-associated immune deviation, ACAID).32,33
The incidence of rejection episodes is dependent on the definition that is used for a rejection episode. In general, the definition used in this study is broader than that used for other studies, which often require the presence of keratic precipitates. For example, Allan et al10 reported 7.5% rejection in the first two years after deep lamellar endothelial keratoplasty (DLEK) and DSAEK based on defining rejection as “any anterior chamber inflammatory episode with keratic precipitates on the transplanted endothelium needing an unscheduled increase in topical steroid medication”. Hjordtal et al34, reported a rejection rate of 5% through 5 years after DSAEK (although all episodes occurred in the first two years), based on defining rejection as “precipitates on the corneal graft but not on the peripheral recipient cornea, either scattered or in the form of a Khodadoust line along with an increase in central corneal thickness”. Finally, Ezon et al35, also reported a post DSAEK rejection rate of 5% with a median follow-up of 29 months, based on defining rejection as the presence of keratic precipitates on the endothelium, a rejection line, or sub-epithelial infiltrates. Considering that our definition was broader (i.e. allowed for other signs of inflammation and corneal thickness changes as well as keratic precipitates) and we are reporting on rejection events up to 48 months after DSAEK, our lower 3.6% cumulative probability of a definite rejection episode across multiple surgeons and sites supports a much lower rate of rejection in DSAEK compared to that of PKP16.
The rejection rate in the CPTS, although nearly double the mean rejection rate of 1.9% for DMEK reported by the American Academy of Ophthalmology Ophthalmic Technology Assessment36, was comparable to the rates in several DMEK studies utilizing topical corticosteroid therapy37–40 and lower than the 6% rate when corticosteroids were discontinued.41 Meta-analyses either support no significant difference in the rejection rates between the two procedures42 or a slight advantage to DMEK.43
After a comprehensive multivariable analysis of the association of 25 donor, recipient, operative, and postoperative factors with the occurrence of a definite rejection episode, the only statistically significant factor identified was recipient age; for every decade increase in recipient age, the risk for rejection decreased (HR: 0.53; 99% CI (0.33, 0.83). However, it is important to note that there were only 15 recipients <50 years old; thus, this finding principally applies to recipients over 50 years old. A higher risk for rejection of penetrating grafts has been well recognized in the pediatric population.44 To our knowledge this is the first report that among recipients older than 50 years old, graft rejection following DSAEK is more likely in younger recipients, as has been noted with high rejection risk PKP cases.27 This may be the result of the immune system becoming less active with increasing age,45
Notably, the following factors were not associated with a higher risk for a rejection episode: donor age, recipient gender, gender mismatch between donor and recipient, recipient diagnosis, recipient race, individuals with history of use of glaucoma medications or past less complex glaucoma surgery (e.g. trabeculectomy, laser trabeculoplasty), PT, graft size, and prior immunizations within three months of the rejection episode. The CDS, another major prospective clinical trial found that PACE, prior use of glaucoma medications, and glaucoma filtering surgery were associated with higher risk for graft rejection following PKP.46,47 Female recipient gender following PKP in the CDS,47 as well as donor-recipient gender mismatch following PKP (63% of cases) and endothelial keratoplasty (37%) from United Kingdom cornea transplant registry data48 were also associated with a higher risk of graft rejection. However, both the CDS and the UK registry studies involved more complicated eyes with PACE and glaucoma, predominantly performing PKP. A recent study of DMEK also did not find donor-recipient gender mismatch to increase the risk of rejection.49 Longer PT could theoretically lower graft rejection risk because of reduced antigen load with time,50 but the CPTS did not support this theory. Graft size has been considered a risk factor for rejection, but primarily for large grafts over 9 mm for therapeutic PKP keratoplasty.51 PKP studies have not shown an effect for typical donor diameters between 7.5 and 8.5 mm.52,53 The CPTS confirms the findings of Terry et al for DSAEK who found no significant impact on rejection rate for 8.0 mm and 8.5 mm grafts.54 Race has also been considered a risk factor in keratoplasty.55 However, 90% of the subjects in the CPTS were Caucasian (primarily because 94% of our cases were for the surgical management of FECD)18 and we were not able to detect an effect of race on rejection. It has been hypothesized that recent immunizations might increase the risk of corneal graft rejection56; however, the CPTS was not able to identify immunization within 3 months as a risk factor.
Only 12 (27%) of the grafts in the CPTS that experienced a definite rejection episode went on to failure from any cause after 3 years of follow-up and only 8 of these demonstrated a clear path from rejection to failure. This compares with PKP in the CDS with 5 years of follow-up and more complicated PACE which showed a somewhat higher 37% (92 of 247 eyes) of eyes with a rejection going on to failure.47 Thus, the rate of reversal of rejection is somewhat better for DSAEK than it is for PKP, with only 18% (8 of 44 eyes) of eyes with a rejection episode going on to failure. Additionally, the overall rate of failure of DSAEK from rejection was very low in the CPTS (8 out of 1330 eyes, 1%) and in the literature.42,43
A definite rejection episode caused a significant decrease in ECD in grafts that went on to fail. Also, grafts that survived at least one definite rejection episode and were clear at 3 years had a significantly lower ECD and greater percentage cell loss (48% vs. 38%) at 3 years compared to those grafts that had had an uncomplicated postoperative course. Price et al. has also shown a significant correlation between rejection episodes and ECD at 5 years after both DSEK and DMEK.49
The strengths of this report include its design as a prospective multi-center, multi-surgeon large study with the collection of major factors that could have been associated with graft rejection by trained observers, and use of multivariable analytical modeling to identify the influential factors that might influence the development of a graft rejection episode. Follow-up was sufficient to determine the impact of these rejection episodes on subsequent graft failure and endothelial cell loss.
The limitations include the fact that rejection episodes between study visits may not have been captured, particularly if the patient was not symptomatic or was managed elsewhere, outside the practice of the investigator, although we suspect that was rare. Although corticosteroid and immunomodulatory therapy was tracked, the specific agents utilized were not; thus specific management approaches to the rejection episodes cannot be evaluated. Although the reporting of 99% confidence intervals does not fully adjust for the multiplicity effect of evaluating 25 factors, the p value for the association of graft rejection and age was <0.001, making chance an unlikely explanation for this finding. For some factors the lack of finding a significant association with graft failure may have had inadequate precision from the small number of graft rejections observed. The lack of detection of an association of the discontinuation of topical corticosteroids and immune-modulators may be an example of this with the confidence intervals for the hazard ratios wide (Table 2). Finally, exclusion criteria prevented enrollment of potentially more complicated PACE eyes with anterior chamber intraocular lenses, more extensive anterior synechiae, and eyes with more advanced glaucoma including tube shunts. The CPTS could not confirm the increased risk of rejection associated with PACE47 and previous use of glaucoma medications and filtering surgery46 as in the CDS because these eyes were excluded from the CPTS. Thus, our findings only apply to less complicated DSAEK cases performed for FECD and uncomplicated PACE.
In conclusion, we found that younger DSAEK recipients are more likely to reject their grafts than older recipients, with the caveat that few of the study participants were <50 years old. Within this age limit, the younger DSAEK patients may bear closer observation and consideration for more prolonged topical corticosteroid therapy. Studies are suggested for DMEK patients to determine if recipient age is a factor in these cases. Importantly, the CPTS found that the rejection rate was not increased for patients receiving immunizations for influenza or other infections. Therefore, physicians can feel confident in advising their DSAEK patients that they can proceed with immunizations without increasing their risk of graft rejection. Remarkably, the CPTS also found that other donor, recipient and operative factors were not associated with a higher rejection rate including donor age, PT, graft size, recipient gender, donor-recipient gender mismatch, and operative complications. Finally, the CPTS provides the best view of the rejection rate for DSAEK in a multi-center, multi-surgeon study. The rate was on the lower spectrum of reported rejection rates at approximately 3% of eyes and 4% of subjects, and only 1% of the grafts failed due to rejection. These data support the concept that DSAEK has made a major impact in lowering the failure rate of corneal transplants from immunologic rejection.
Supplementary Material
Highlights.
DSAEK rejection rates are low (3.6%) after 3 years in the CPTS.
Younger recipient age is associated with a higher rejection rate.
Acknowledgements
Funding/Support: Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MDEY20797 and EY20798. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health. Additional support provided by: Eye Bank Association of America (Washington DC), The Cornea Society (Fairfax, VA), Vision Share, Inc. (Ann Arbor, MI), Alabama Eye Bank (Birmingham, AL), Cleveland Eye Bank Foundation (Cleveland, OH), Eversight (Ann Arbor, MI), Eye Bank for Sight Restoration (New York, NY), Iowa Lions Eye Bank (Iowa City, IA), Lions Eye Bank of Albany (Albany, NY), San Diego Eye Bank (San Diego, CA), and SightLife (Seattle, WA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The comprehensive list of participating CPTS clinical sites, investigators and coordinators; eye bank investigators; members of the Operations, Executive, Eye Bank Advisory, Data and Safety Monitoring Committee; Coordinating Center, Cornea Image Analysis Reading Center (CIARC), and Data Management and Analysis Center Staff; and the National Eye Institute staff have been previously published (Cornea 2015;34:601–608; JAMA Ophthalmology 2017;135:1401–09)
all co-authors have seen and agree with each of the changes made to this revised manuscript and to the way his or her name is listed.
Supplemental Material available at AJO.com
Financial Disclosures: The following authors have financial disclosures with companies that manufacture corneal storage solutions (considered relevant to this work): Mark Terry (Bausch & Lomb), and W. Barry Lee (Bausch & Lomb) (part of CPTS Study Group but not authored on this paper).
Additionally Maureen Maquire: Genentech/Roche (payment for service on a data monitoring committee for a clinical trial); Shahzad Mian: Shire (research grant) and VisionCare (research grant); Jonathan Song: Sun Pharmaceuticals (consulting) and Kula Pharmaceutical (consulting); Loretta Szczotka-Flynn: Johnson & Johnson Vision Care, Inc. (consulting), Alcon Laboratories (consulting), Robin Chalmers (adjudication panel); R. Doyle Stulting: Abbott Medical Optics (consultant), Alcon Laboratories (consultant, lecture fees), Calhoun Vision Inc (consultant), Cambium Medical Technologies (consultant, equity owner), EyeYon (consultant, equity owner), Hydrolens (consultant, equity owner), Intelon (consultant), Ocumetrics (consultant), OPHTEC (consultant), Optovue (consultant), Promisight (consultant, equity owner), TearLab (consultant, equity owner); Pankaj Gupta: Eversight Ohio (Associate Medical Director); Mark Terry: Bausch & Lomb (royalties on instruments for DSAEK and unrestricted educational grant for EKG breakfast), Moria (unrestricted educational grant for EKG breakfast), Envisia (Safety Monitoring Board for Glaucoma Study). All other authors have no financial disclosures related to this manuscript.
References
- 1.Eye Bank of America. 2017 Eye Banking Statistical Report. 2018, http://restoresight.org/wp-content/uploads/2016/03/2015-Statistical-Report.pdf. Accesses August 2, 2017.
- 2.Terry MA, Shamie N, Chen ES, Hoar KL, Friend DJ. Endothelial keratoplasty a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology. 2008;115(7):1179–1186. [DOI] [PubMed] [Google Scholar]
- 3.Busin M, Bhatt PR, Scorcia V. A modified technique for descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell loss. Arch Ophthalmol. 2008;126(8):1133–1137. [DOI] [PubMed] [Google Scholar]
- 4.Terry MA. Endothelial keratoplasty: a comparison of complication rates and endothelial survival between precut tissue and surgeon-cut tissue by a single DSAEK surgeon. Trans Am Ophthalmol Soc. 2009;107:184–191. [PMC free article] [PubMed] [Google Scholar]
- 5.Terry MA, Shamie N, Chen ES, Hoar KL, Phillips PM, Friend DJ. Endothelial keratoplasty: the influence of preoperative donor endothelial cell densities on dislocation, primary graft failure, and 1-year cell counts. Cornea. 2008;27(10):1131–1137. [DOI] [PubMed] [Google Scholar]
- 6.Li JY, Terry MA, Goshe J, Davis-Boozer D, Shamie N. Three-year visual acuity outcomes after Descemet’s stripping automated endothelial keratoplasty. Ophthalmology. 2012;119(6):1126–1129. [DOI] [PubMed] [Google Scholar]
- 7.Terry MA, Straiko MD, Goshe JM, Li JY, Davis-Boozer D. Descemet’s stripping automated endothelial keratoplasty: the tenuous relationship between donor thickness and postoperative vision. Ophthalmology. 2012;119(10):1988–1996. [DOI] [PubMed] [Google Scholar]
- 8.Price MO, Fairchild KM, Price DA, Price FW, Jr. Descemet’s stripping endothelial keratoplasty five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118(4):725–729. [DOI] [PubMed] [Google Scholar]
- 9.Wacker K, Baratz KH, Maguire LJ, McLaren JW, Patel SV. Descemet Stripping Endothelial Keratoplasty for Fuchs’ endothelial corneal dystrophy: Five-year results of a prospective study. Ophthalmology. 2016;123(1):154–160. [DOI] [PubMed] [Google Scholar]
- 10.Allan BD, Terry MA, Price FW Jr., Price MO, Griffin NB, Claesson M. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26(9):1039–1042. [DOI] [PubMed] [Google Scholar]
- 11.Terry MA, Chen ES, Shamie N, Hoar KL, Friend DJ. Endothelial cell loss after Descemet’s stripping endothelial keratoplasty in a large prospective series. Ophthalmology. 2008;115(3):488–496. [DOI] [PubMed] [Google Scholar]
- 12.Price MO, Baig KM, Brubaker JW, Price FW Jr. Randomized, prospective comparison of precut vs surgeon-dissected grafts for descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 2008;146(1):36–41. [DOI] [PubMed] [Google Scholar]
- 13.Jordan CS, Price MO, Trespalacios R, Price FW Jr. Graft rejection episodes after Descemet stripping with endothelial keratoplasty: part one: clinical signs and symptoms. Brit J Ophthalmol. 2009;93(3):387–390. [DOI] [PubMed] [Google Scholar]
- 14.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818–1830. [DOI] [PubMed] [Google Scholar]
- 15.Price MO, Jordan CS, Moore G, Price FW Jr. Graft rejection episodes after Descemet stripping with endothelial keratoplasty: part two: the statistical analysis of probability and risk factors. Brit J Ophthalmol.2009;93(3):391–395. [DOI] [PubMed] [Google Scholar]
- 16.Anshu A, Price MO, Price FW Jr. Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119(3):536–540. [DOI] [PubMed] [Google Scholar]
- 17.Maier P, Reinhard T, Cursiefen C. Descemet stripping endothelial keratoplasty--rapid recovery of visual acuity. Deutsches Arzteblatt international. 2013;110(21):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lass JH, Szczotka-Flynn LB, Ayala AR, et al. Cornea preservation time study: methods and potential impact on the cornea donor pool in the United States. Cornea. 2015;34(6):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenwasser GO, Szczotka-Flynn LB, Ayala AR, et al. Effect of cornea preservation time on success of Descemet Stripping Automated Endothelial Keratoplasty: A randomized clinical trial. JAMA Ophthalmol. 2017;135(12):1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lass JH, Benetz BA, Verdier DD, et al. Corneal endothelial cell loss 3 Years after successful Descemet Stripping Automated Endothelial Keratoplasty in the Cornea Preservation Time Study: A randomized clinical trial. JAMA Ophthalmol. 2017;135(12):1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry MA, Aldave AJ, Szczotka LB, et al. Donor, recipient, and operative factors asssociated with graft success in the Cornea Preservation Time Study. Ophthalmology. https://doi.org/10.1016/j.ophtha.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lass JH, Benetz BA, Patel SV, et al. Donor, recipient, and operative factors Influencing endothelial cell loss in the Cornea Preservation Time Study. JAMA Ophthalmol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eye Bank Association of America. Medical Standards. 2018, June 2015. http://www.corneas.org/repository/docs/SurgeonDocs/EBAA-Medical-Standards-with-Appendices-June-2015.pdf. Accessed August 2, 2017.
- 24.Sayegh RR, Benetz BA, Lass JH. Specular microscopy In: Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis, Management. Vol 1: Elsevier; 2016:160–179. [Google Scholar]
- 25.Benetz BA, Gal RL, Ruedy KJ, et al. Specular microscopy ancillary study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res. 2006;31(4):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collaborative Corneal Transplantation Studies Research Group. The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110(10):1392–1403. [PubMed] [Google Scholar]
- 27.Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101(9):1536–1547. [DOI] [PubMed] [Google Scholar]
- 28.Collaborative Corneal Transplantation Studies Research Group. Design and methods of The Collaborative Corneal Transplantation Studies. The Collaborative Corneal Transplantation Studies Research Group. Cornea. 1993;12(2):93–103. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 30.Derksen S, Keselman HJ. Backward, forward, and stepwise automated subset selection algorithms: Frequency of obtaining authentic and noise variable. Brit J Mathematical and Statistical Psychology. 1992;45:265–282. [Google Scholar]
- 31.Team RC. R: A language and environment for statistical computing. Vienna, Austria: 2018. [Google Scholar]
- 32.Hori J Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation. J Ocular Biol, Diseases, and Informatics. 2008;1(2–4):94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. IOVS. 1996;37(13):2700–2707. [PubMed] [Google Scholar]
- 34.Hjortdal J, Pedersen IB, Bak-Nielsen S, Ivarsen A. Graft rejection and graft failure after penetrating keratoplasty or posterior lamellar keratoplasty for fuchs endothelial dystrophy. Cornea. 2013;32(5):e60–63. [DOI] [PubMed] [Google Scholar]
- 35.Ezon I, Shih CY, Rosen LM, Suthar T, Udell IJ. Immunologic graft rejection in descemet’s stripping endothelial keratoplasty and penetrating keratoplasty for endothelial disease. Ophthalmology. 2013;120(7):1360–1365. [DOI] [PubMed] [Google Scholar]
- 36.Deng SX, Lee WB, Hammersmith KM, et al. Descemet Membrane Endothelial Keratoplasty: Safety and outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295–310. [DOI] [PubMed] [Google Scholar]
- 37.Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118(12):2368–2373. [DOI] [PubMed] [Google Scholar]
- 38.Veldman PB, Dye PK, Holiman JD, et al. The S-stamp in Descemet Membrane Endothelial Keratoplasty safely eliminates upside-down graft implantation. Ophthalmology. 2016;123(1):161–164. [DOI] [PubMed] [Google Scholar]
- 39.Heinzelmann S, Bohringer D, Eberwein P, Reinhard T, Maier P. Outcomes of Descemet membrane endothelial keratoplasty, Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch Clin Exp Ophthalmol. 2016;254(3):515–522. [DOI] [PubMed] [Google Scholar]
- 40.Monnereau C, Quilendrino R, Dapena I, et al. Multicenter study of descemet membrane endothelial keratoplasty: first case series of 18 surgeons. JAMA Ophthalmol. 2014;132(10):1192–1198. [DOI] [PubMed] [Google Scholar]
- 41.Price MO, Scanameo A, Feng MT, Price FW Jr. Descemet’s Membrane Endothelial Keratoplasty: Risk of Immunologic Rejection Episodes after Discontinuing Topical Corticosteroids. Ophthalmology. 2016;123(6):1232–1236. [DOI] [PubMed] [Google Scholar]
- 42.Pavlovic I, Shajari M, Herrmann E, Schmack I, Lencova A, Kohnen T. Meta-analysis of postoperative outcome parameters comparing Descemet Membrane Endothelial Keratoplasty versus Descemet Stripping Automated Endothelial Keratoplasty. Cornea. 2017;36(12):1445–1451. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Liu L, Wang W, et al. Efficacy and safety of Descemet’s membrane endothelial keratoplasty versus Descemet’s stripping endothelial keratoplasty: A systematic review and meta-analysis. PloS one. 2017;12(12):e0182275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulias-Canizo R, Gonzalez-Salinas R, Hernandez-Zimbron LF, Hernandez-Quintela E, Sanchez-Huerta V. Indications and outcomes of pediatric keratoplasty in a tertiary eye care center: A retrospective review. Medicine (Baltimore). 2017;96(45):e8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn SP, Gal RL, Kollman C, et al. Corneal graft rejection 10 years after penetrating keratoplasty in the cornea donor study. Cornea. 2014;33(10):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stulting RD, Sugar A, Beck R, et al. Effect of donor and recipient factors on corneal graft rejection. Cornea. 2012;31(10):1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hopkinson CL, Romano V, Kaye RA, et al. The influence of donor and recipient gender Incompatibility on corneal transplant rejection and failure. Am J Transplant. 2017;17(1):210–217. [DOI] [PubMed] [Google Scholar]
- 49.Price DA, Kelley M, Price FW Jr., Price MO. Five-year graft survival of Descemet Membrane Endothelial Keratoplasty (EK) versus Descemet Stripping EK and the effect of donor sex matching. Ophthalmology. 2018. https://doi.org/10.1016/j.ophtha.2018.03.050 [DOI] [PubMed] [Google Scholar]
- 50.Kamiya K, Hori J, Kagaya F, et al. Preservation of donor cornea prevents corneal allograft rejection by inhibiting induction of alloimmunity. Exp Eye Res. 2000;70(6):737–743. [DOI] [PubMed] [Google Scholar]
- 51.Sony P, Sharma N, Vajpayee RB, Ray M. Therapeutic keratoplasty for infectious keratitis: a review of the literature. CLAO J. 2002;28(3):111–118. [PubMed] [Google Scholar]
- 52.Baradaran-Rafii A, Karimian F, Javadi M-A, et al. Corneal graft rejection: Incidence and risk factors. Iran J Ophthalmic Res. 2007;2:7–14. [Google Scholar]
- 53.Inoue K, Amano S, Oshika T, Tsuru T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scandinavica. 2001;79(3):251–255. [DOI] [PubMed] [Google Scholar]
- 54.Terry MA, Li J, Goshe J, Davis-Boozer D. Endothelial keratoplasty: the relationship between donor tissue size and donor endothelial survival. Ophthalmology. 2011;118(10):1944–1949. [DOI] [PubMed] [Google Scholar]
- 55.Ling JD, Mehta V, Fathy C, et al. Racial disparities in corneal transplantation rates, complications, and outcomes. Semin Ophthalmol. 2016;31(4):337–344. [DOI] [PubMed] [Google Scholar]
- 56.Wertheim MS, Keel M, Cook SD, Tole DM. Corneal transplant rejection following influenza vaccination. Brit J Ophthalmol. 2006;90(7):925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.