Abstract
Purpose:
To compare the performance of the pattern standard deviation (PSD) values derived from the central 12 locations of the 24–2 visual field test (C24–2) to the entire 10–2 test for detecting central visual field abnormalities in eyes with, suspected or at risk of having glaucoma.
Design:
Cross-sectional, case-control study
Methods:
Eyes with, suspected or at risk of having glaucoma, based on masked grading of optic disc stereophotographs and/or ocular hypertension (intraocular pressure ≥22 mm Hg) were included as cases (n=523). Eyes from healthy participants were included as controls (n=107) to allow the two tests to be compared at matched specificities. The sensitivity to detect cases at 95% specificity using PSD values derived from the entire 10–2 test and C24–2 were compared.
Results:
The sensitivity of the 10–2 and C24–2 PSD values was not significantly different between the 10–2 and C24–2 at matched specificities (35.9% and 35.4% respectively; P=0.900). There was also a substantial agreement between the cases detected by both methods (kappa=0.80±0.04), and a very strong association between the PSD values from the two methods (R2=0.91).
Conclusions:
10–2 and 24–2 tests identified a similar number of eyes with, suspected or at risk of having glaucoma as having central visual field abnormalities using PSD values. These findings do not mean that 10–2 tests are not useful, but highlight the need for further studies to determine the potential advantages of 10–2 tests through equivalent comparisons against 24–2 tests to ensure appropriate recommendations are made about its incorporation into the glaucoma standard-ofcare.
INTRODUCTION
Visual field testing remains one of the most important tools in glaucoma clinical management, allowing the nature of visual function loss to be characterized and monitored over time. Its results are crucial for understanding both the current state and future risk of functional disability for individuals affected by this disease.1,2 An accumulating body of evidence has recently revealed that glaucomatous damage can often affect the macula region, even in the early stages of this disease.3 This region is particularly important for daily functioning, and previous studies have reported that central visual function loss is strongly associated with self-reported quality of life.4–6 As such, evidence-based guidance on how to optimally detect and monitor central visual function loss would be tremendously beneficial for clinicians, but this remains to be established.
Recent studies have evaluated whether performing dedicated central visual field tests (such as with the 10–2 strategy on the Humphrey Field Analyzer [HFA]; Carl Zeiss Meditec, Dublin, CA)6–10 or modifying the conventional stimulus patterns (such as the 24–2 strategy on the HFA)11,12 would improve the detection of central visual dysfunction. Some studies have recommended that 10–2 visual field tests should be performed in the clinical management of patients with glaucoma, on the basis that: a) summary metrics on the 24–2 visual field test often missed topographically consistent abnormalities detected on the 10–2 test and optical coherence tomography (OCT) macular scans,7 b) glaucoma eyes with normal 24–2 results often had abnormal 10–2 results,8 and that c) the 10–2 binocular mean sensitivity was more strongly associated with vision-related quality of life than the same measure from the 24–2 test.6
However, all of these studies have compared the results of the entire 24–2 test with those from the 10–2 test, rather than evaluating the topographically equivalent central locations of the 242 test. Instead, two previous studies9,10 demonstrated that abnormalities in these central locations of the 24–2 test were highly associated with those from the 10–2 test. Thus, it remains to be determined whether, and the extent to which, central visual field abnormalities can be detected with the 24–2 central locations when compared to the 10–2 test. To examine this, we used the pattern standard deviation (PSD) measure to compare the two tests at topographically matched locations, and at matched specificities. Although PSD values are not used clinically in isolation to detect visual field abnormalities, they have been widely used for defining the presence and identifying the development of visual field abnormalities in research studies.13–16 Thus, this comparison could provide important insights into understanding the interpretation of central visual dysfunction defined by 10–2 versus the central locations of 24–2 test patterns in research studies.
This study was therefore undertaken to compare the summary statistics from 10–2 and 24–2 tests at matched specificities and topographical locations for defining central visual field abnormalities for glaucoma research.
METHODS
Participants included in this study were evaluated in two prospective observational studies of optic nerve structure and visual function longitudinally in glaucoma – the Diagnostic Innovations in Glaucoma Study (DIGS) and the African Descent and Glaucoma Evaluation Study (ADAGES).15 Institutional review board approvals were attained from each of the sites involved in the studies, and they were conducted in adherence with the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act, and all participants provided written informed consent.
The details of the testing performed by the participants in these studies have been described previously.15 In brief, all participants underwent an annual comprehensive ophthalmologic evaluation that included a medical history review, best-corrected visual acuity measurements, slit lamp biomicroscopy, dilated fundus examination and optic disc stereophotography, and intraocular pressure and pachymetry measurements. Participants included in this study also performed visual field testing with the Swedish Interactive Thresholding Algorithm Standard 24–2 and 10–2 strategy on the HFA II-i (Carl Zeiss Meditec, Dublin, CA), approximately semi-annually.
As this was a cross-sectional, case-control study, eyes with, suspected or at risk for having glaucoma were included as cases, and healthy eyes as controls. The cases included eyes considered to have glaucomatous optic neuropathy based on the masked grading of the optic nerve appearance on stereophotographs by at least 2 graders as described in detail previously.15 It also included eyes with ocular hypertension, which was defined as having an intraocular pressure of 22 mm Hg or greater, were also included. The control group included eyes from healthy participants who had an unremarkable ophthalmological examination and intraocular pressures below 22 mm Hg. In addition, all the eyes included in this study were required to have a bestcorrected visual acuity of 20/40 or better, open angles on gonioscopy and the participants were required to be older than 18 years of age. Participants that had any other ocular or systemic disease that could affect the optic nerve or the visual field were excluded from this study. Visual fields were not utilized in the classification of cases or controls.
Visual Field Testing
All eyes included were required to have a reliable 10–2 and 24–2 visual field test performed on the same day, and a visual field test was considered unreliable if there was > 33% fixation losses or > 33% false negative errors (except when the mean deviation [MD] was < −12 dB), or > 15% false positive errors. All tests were reviewed for the presence of artifacts, including inappropriate fixation, fatigue, inattention or learning effects, eyelid or rim artifacts, or any evidence that the results were affected by a condition other than glaucoma (e.g. homonymous hemianopia). This quality review was performed by experienced graders at the University of California, San Diego (UCSD) Visual Field Assessment Center,17 and any test with such artifacts was excluded from this study.
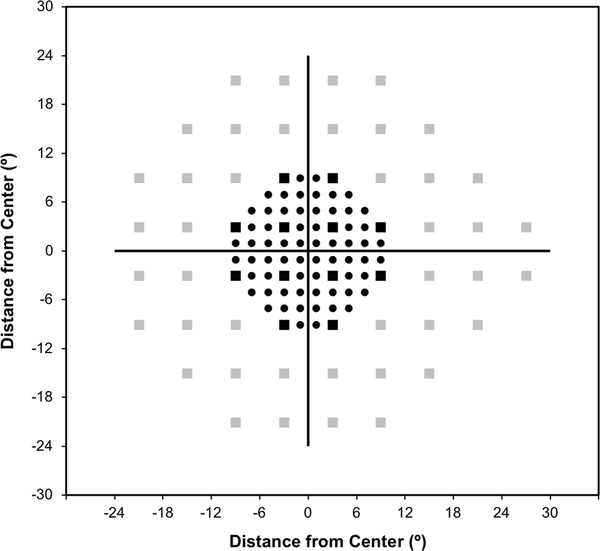
In order to compare the diagnostic performance of the 24–2 and 10–2 test, the central 12 locations of the 24–2 (or C24–2) were evaluated due to its close spatial correspondence with the region sampled by the 10–2 test; the locations sampled by both tests are shown in Figure 1.
Figure 1:
Plot of the 10-2 and 24-2 visual field test locations for a left eye, shown using circular and square markers respectively. The central 12 locations of the 24-2 test are highlighted using the black square markers.
The primary outcome measure used for detecting central visual field abnormalities was the measure of pattern standard deviation (PSD), which is conventionally calculated as follows:
Where n represents the total number of test locations, s indicates the standard deviation (SD) of the normal sensitivity at each individual location i, and TD represents the total deviation (or deviation from normal values) and where MD is the weighted average of the TD. In essence, the PSD represents a weighted SD of the point-wise difference in TD from MD from the normal shape of the hill of vision.18 However, since the weighting applied to the C24–2 and 10–2 by the device manufacturer differs, the PSD values will differ. To obtain comparable PSD estimates for the 10–2 and C24–2 for the ease of comparison, its calculations can be simplified by removing the constant used to account for the normal shape of the hill of vision as follows:
This PSD value thus simply represents the SD of the point-wise difference in TD at each location from MD relative to normal variability. The findings of this study remain unchanged when using the original method to calculate the PSD (data not shown), but this method was used to demonstrate that the 10–2 and C24–2 PSD values were highly comparable (see Results section).
Statistical Analysis
The PSD values from the 10–2 and C24–2 were compared using a Wilcoxon sign-rank test (as these values are not normally distributed), and coefficients of determination were calculated to determine the strength of the relationship between these two parameters. Their diagnostic performance for discriminating between healthy and glaucoma eyes were compared using a Wald test of the difference in sensitivity at 95% specificity, using a bootstrap resampling procedure (n = 1000 resamples).19 The upper 5th percentile of the PSD values from the healthy eyes were then used as a cut-off to classify eyes as having normal or abnormal central visual field results at 95% specificity. Cohen’s kappa was calculated to estimate the level of agreement between the 10–2 and C24–2 PSD values for identifying eyes as having central visual field abnormalities.
RESULTS:
Participant Characteristics
A total of 523 eyes with, suspected or at risk of having glaucoma from 300 participants were included as cases, and 107 eyes from 67 healthy participants were included as controls. The characteristics of these participants and eyes are presented in Table 1. The median PSD of the 10–2 and C24–2 for all eyes were 1.01 dB (IQR = 0.80 to 1.99 dB) and 1.04 dB (IQR = 0.76 to 2.06 dB) respectively, and were not significantly different from each other (P = 0.076). Of interest, the upper 5th percentile of the 10–2 and C24–2 PSD values (used as the cut-offs to determine if an eye was normal or abnormal at 95% specificity) was 1.76 dB and 1.51 dB respectively. In addition, there was a strong and statistically significant relationship between these two parameters (R2 = 0.91 for both eyes, P < 0.001), as illustrated in Figure 2. This strong relationship is observed even when considering only PSD values >2 dB (R2 = 0.94 for both eyes, P < 0.001).
Table 1:
Characteristics of the eyes and participants in this study
| Healthy (n = 107 eyes) | Glaucoma (n = 523 eyes) | |
|---|---|---|
| Age (years)* | 64 ± 11 | 70 ± 11 |
| 24-2 MD (dB) | −0.26 (0.72 to −1.44) | −1.76 (−4.98 to 0.09) |
| 24-2 PSD (dB) | 1.78 (1.52 to 2.32) | 2.33 (1.67 to 6.20) |
| Study Parameters# | ||
| 10-2 MD (dB) | 0.17 (−1.17 to 0.83) | −1.25 (−4.42 to 0.25) |
| 10-2 PSD (dB) | 0.83 (0.69 to 1.10) | 1.11 (0.79 to 3.64) |
| C24-2 MD (dB) | −0.43 (−1.54 to 0.60) | −1.19 (−4.88 to 0.03) |
| C24-2 PSD (dB) | 0.86 (0.76 to 1.05) | 1.06 (0.81 to 2.93) |
Data presented either as mean ± standard deviation or median (interquartile range); MD = mean deviation, PSD = pattern standard deviation,
= analyzed by participants,
= parameters are unweighted and does not include multiplication by a constant used to account for the normal shape of the hill of vision (see Methods section)
Figure 2:
Scatterplot of the pattern standard deviation (PSD) of the central twelve locations of the 24-2 visual field test (C24-2) against the PSD of the 10-2 visual field test, with the black line representing the line of equality between these two measures.
Diagnostic Performance of the 10–2 and C24–2 Pattern Standard Deviation
The sensitivity of detecting the cases at 95% specificity using the 10–2 and C24–2 PSD was not significantly different (35.9% and 35.4% respectively; P = 0.900). This finding remained similar when including only cases with an MD ≥ −6 dB (18.9% and 17.9% respectively; P = 0.850) and MD ≥ −12 dB (27.6% and 27.0% respectively; P = 0.910); these findings are summarized in Table 2. Note that the area under the receiver operating characteristic curve for discriminating between cases and controls using the 10–2 and C24–2 PSD was also not significantly different when evaluating all eyes (P = 0.680), or when including only cases with an MD ≥ −6 dB (P = 0.650) and ≥ −12 dB (P = 0.690). There was also a substantial level of agreement between the 10–2 and C24–2 for detecting the cases (kappa = 0.80 ± 0.04), with 26 out of 188 cases (13.8%) with abnormal 10–2 PSD values having normal C24–2 PSD values, and 23 out of 185 cases (12.4%) with abnormal C24–2 PSD values having normal 10–2 PSD values.
Table 2:
Sensitivity (%) of detecting cases (defined as eyes with, suspected or at risk of having glaucoma) using the pattern standard deviation derived from the 10-2 visual field test and central 12 locations of the 24-2 visual field test (C24-2)
| 10-2 | C24-2 | P-Value | |
|---|---|---|---|
| MD ≥ −6 dB (n = 515) | 18.9 | 17.9 | 0.850 |
| MD ≥ −12 dB (n = 570) | 27.6 | 27.0 | 0.910 |
| All Eyes (n = 630) | 35.9 | 35.4 | 0.900 |
MD = mean deviation of the 24-2 visual field test
Examples of Findings in this Study
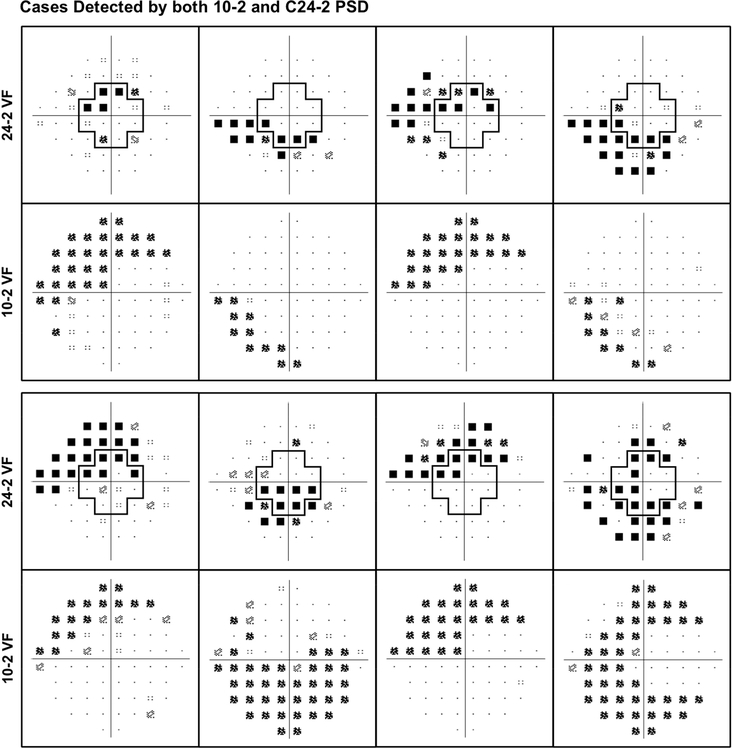
Eight examples of cases (or eyes with, suspected or at risk for having glaucoma) detected as having abnormal central visual field results using both the 10–2 and C24–2 are shown in Figure 3. These examples illustrate how the 24–2 visual field test revealed a similar pattern of central visual field abnormalities as the 10–2 test when evaluating the central 12 locations.
Figure 3:
Examples of cases (eyes with, suspected or at risk of having glaucoma) detected as having abnormal central visual field sensitivity based on the pattern standard deviation (PSD) values of the 10-2 test and central 12 locations of the 24-2 test (C24-2).
Two examples of cases detected as having abnormal C24–2 PSD values but normal 10–2 PSD values are also shown in Figure 4, demonstrating how the 10–2 test can miss central visual field abnormalities detected by the C24–2. Two further examples of the opposite scenario – where the C24–2 missed central visual field abnormalities detected by the 10–2 test – are also shown in Figure 4.
Figure 4:
Two examples of cases (eyes with, suspected or at risk of having glaucoma) detected as having abnormal central visual field sensitivity based on the pattern standard deviation (PSD) values of the central 12 locations of the 24-2 test (C24-2), but not the 10-2 test (left). Two further examples of the opposite case – namely, eyes with abnormal 10-2 but normal C24-2 PSD values – are also shown (right).
DISCUSSION
This study revealed that the PSD values from the 10–2 test and central twelve locations of the 24–2 test were not significantly different in their ability to detect central visual field abnormalities in eyes with, suspected or at risk of having glaucoma. As such, these findings suggest that when using this summary measure in a research setting, they perform equivalently. However, future studies are needed to determine the potential advantages of 10–2 testing in clinical practice setting, since summary measures are not used in isolation to detect central visual field abnormalities clinically. Future studies are also needed to determine the potential value-add the 10–2 visual field test for characterizing the nature of central visual field loss. This study also underscores the importance of its comparisons against topographically equivalent central locations of the 24–2 test at matched specificities, to better understand its potential role in the glaucoma standard of care.
This study observed a close association between central visual field abnormalities on the 10–2 and 24–2 tests in this study, as consistent with previous studies.9,10 A previous study also reported that 18% of the eyes with a repeatable 10–2 visual field defect did not have a repeatable 24–2 defect within the central twelve locations, but 15% of the eyes with a repeatable 24–2 visual field defect within the central twelve locations also did not have a repeatable 10–2 defect.9 This was a similar trend observed in this study, where the frequencies were 13.8% and 12.4% respectively with the specific PSD criterion used. Based on the very strong correlation between the 10–2 and C24–2 PSD values and the substantial agreement between the cases detected with these measures, it appears that the central 12 locations of the 24–2 test provides similar information regarding the non-uniformity of visual sensitivity (based on PSD values) as the 10–2 test.
Moreover, this study suggests that there is information in the 24–2 test that can be utilized to detect the presence or development of central visual field abnormalities in a clinical research setting, to a similar extent as 10–2 tests when using the PSD measure.
This is the first study to our knowledge that equivalently compared the 10–2 and 24–2 visual field tests in their independent ability to detect central visual field abnormalities in eyes with, suspected or at risk of having glaucoma, through evaluating only the central locations of the 24–2 test and ensuring that there were compared at matched specificities. The finding that the PSD measure from these two methods performed similarly may be attributed to a few different reasons. First, it is possible that highly narrow and localized glaucomatous defects under-sampled by the 24–2 central locations (that could be better detected by the 10–2 test) occur infrequently. Second, even if such local defects are under-sampled by the 24–2 central locations, previous studies have suggested that small fixation drifts – even those present in excellent fixators – can make it difficult accurately detect such defects,20–22 which would affect both the 10–2 and 24–2 tests. In fact, a recent study reported that visual field testing even at a sampling density twice the resolution of the 10–2 test showed no significant improvement for detecting a loss of variance (which is also captured by the PSD measure used in this study) than a the sampling density of the 24–2 test.23
However, these findings should not be taken to suggest that 10–2 visual field testing is not useful, but rather simply that the PSD values – a commonly-used measure for defining the presence of visual field abnormalities – derived from both methods identified a similar number of eyes as having PSD values outside the normal range. Instead, the additional value of the increased sampling density provided by the 10–2 test could be better realized using other measures or methods, such as an expert qualitative assessment or artificial intelligence.24–26 Future studies enlisting such alternative methods are paramount to determine whether central visual field damage is indeed missed without the spatial resolution of the 10–2 testing paradigm, and how often this occurs. Dismissing the potential value of the 10–2 test based on the findings of this study alone may be detrimental if this test does indeed substantially improve the characterization of visual function loss in this tremendously important region.4–6 However, its routine incorporation into clinical practice without evidence-based support could also have adverse consequences, such as an increased burden on public healthcare resources if these tests are routinely added, or a delay in the time to detect visual field progression if the test was performed in lieu of a 24–2 test.27
Future studies need to also determine the value-add of a 10–2 test in an era where OCT imaging – being a powerful tool for detecting glaucomatous macular damage3,28 – is becoming increasingly ubiquitous in clinical settings, to better understand how to optimize the use of healthcare resources in glaucoma clinical management in this regard. If 10–2 testing does indeed provide substantial advantages, a potential way to improve the detection of central visual field abnormalities without substantially increasing testing burden could potentially be achieved through the use of a new test pattern similar to the 24–2 test, but with an increased sampling density of the central visual field. This new test pattern could alleviate the need for clinicians to have to choose one test over another for detecting central visual field abnormalities.
A key limitation needs to be acknowledged when interpreting the findings of this study, and that is that the 10–2 and C24–2 PSD measures were compared in their ability to discriminate between a population with eyes that either had a glaucomatous optic disc appearance on masked grading of stereophotographs or eyes with OHT, and a healthy population. These two populations are not defined specifically based on whether glaucomatous macular damage or central visual field loss is present, and therefore the diagnostic performance for detection of central visual field damage was not evaluated. Nevertheless, the likelihood for the presence of such central visual field abnormalities are substantially different between these two populations. As such, the withinsubject comparison of the two measures remains robust and relevant. Furthermore, this study only compared the ability for the 10–2 and C24–2 PSD measures for detecting central visual field abnormalities, but not compare their ability to characterize the nature and extent of visual field loss. The increased sampling density of the 10–2 visual field test is likely advantageous for this task, and future studies are needed to examine this. Finally, it should be acknowledged as with most new research findings that the C24–2 PSD is not currently available on the printouts of the commercial HFA devices, and would thus need to be calculated separately.
In conclusion, this study showed that there is no significant difference in the proportion of eyes with, suspected or at risk of having glaucoma with an abnormal PSD result detected on the 10–2 visual field test and central twelve locations of the 24–2 test. These findings should not be interpreted as demonstrating an absence of clinical value for the 10–2 test, but rather the need for future studies to clearly demonstrate its potential value through equivalent comparisons with the 24–2 test to ensure appropriate recommendations are made about its incorporation into the glaucoma standard of care.
Supplementary Material
ACKNOWLEDGEMENTS
a. Funding/Support: Supported in part by National Institutes of Health/National Eye Institute Grants EY021818 (FAM) EY011008, EY14267, EY027510 and EY019869 (LMZ), and Core Grant P30EY022589; EY026574 (LMZ); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen; a National Health & Medical Research Council Early Career Fellowship (#1104985, ZW). The sponsors or funding organizations had no role in the design or conduct of this research.
b. Financial Disclosures: Zhichao Wu: None; Felipe A. Medeiros: Financial support – Alcon Laboratories (Fort Worth, TX), Bausch & Lomb (Garden City, NY), Carl Zeiss Meditec (Jena, Germany), Heidelberg Engineering (Heidelberg, Germany), Merck (White House Station, NJ), Allergan (Irvine, CA), Topcon (Livermore, CA), Reichert (Dewey, NY), National Eye Institute (Bethesda, MD); Research support – Alcon Laboratories (Fort Worth, TX), Allergan (Irvine, CA), Carl Zeiss Meditec (Jena, Germany), Reichert (Dewey, NY); Consultant – Allergan (Irvine, CA), Carl-Zeiss Meditec, (Jena, Germany), Novartis (Basel, Switzerland); Robert N. Weinreb: Financial support – Heidelberg Engineering, Carl Zeiss Meditec, Genentech, Konan, Optovue, Topcon, Optos, Centrvue; Consultant – Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Eyenovia, Novartis, Sensimed, Unity, Valeant; Linda M. Zangwill: Financial Support – National Eye Institute, Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems Inc.; Consultant – Merck; Recipient – Optovue, Topcon Medical Systems.
c. Other Acknowledgments such as Statisticians, Medical Writers, Expert contributions: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ramulu P Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol 2009;20(2):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders LJ, Medeiros FA, Weinreb RN, Zangwill LM. What rates of glaucoma progression are clinically significant? Exp Rev Ophthalmol 2016;11(3):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood DC, Raza AS, de Moraes CGV, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res 2013;32:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology 2016;123(3):552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Lin C, Waisbourd M, et al. The Impact of Visual Field Clusters on Performancebased Measures and Vision-Related Quality of Life in Patients With Glaucoma. Am J Ophthalmol 2016;163:45–52. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg DM, De Moraes CG, Prager AJ, et al. Association between undetected 10–2 visual field damage and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol 2017;125(7):742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grillo LM, Wang DL, Ramachandran R, et al. The 24–2 Visual Field Test Misses Central Macular Damage Confirmed by the 10–2 Visual Field Test and Optical Coherence Tomography. Trans Vis Sci Tech 2016;5(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Moraes CG, Hood DC, Thenappan A, et al. 24–2 Visual Fields Miss Central Defects Shown on 10–2 Tests in Glaucoma Suspects, Ocular Hypertensives, and Early Glaucoma. Ophthalmology 2017;124(10):1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan-Mee M, Karin Tran MT, Pensyl D, Tsan G, Katiyar S. Prevalence, Features, and Severity of Glaucomatous Visual Field Loss Measured With the 10–2 Achromatic Threshold Visual Field Test. Am J Ophthalmol 8// 2016;168:40–51. [DOI] [PubMed] [Google Scholar]
- 10.Park H-YL, Hwang B-E, Shin H-Y, Park CK. Clinical Clues to Predict the Presence of Parafoveal Scotoma on Humphrey 10–2 Visual Field Using a Humphrey 24–2 Visual Field. Am J Ophthalmol 2016;161:150–159. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich AC, Raza AS, Ritch R, Hood DC. Modifying the conventional visual field test pattern to improve the detection of early glaucomatous defects in the central 10. Trans Vis Sci Tech 2014;3(6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, McKendrick AM, Turpin A. Choosing two points to add to the 24–2 pattern to better describe macular visual field damage due to glaucoma. Br J Ophthalmol 2015;99(9):12361239. [DOI] [PubMed] [Google Scholar]
- 13.Thomas R, Parikh R, Muliyil J, Kumar RS. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: a population-based study. Acta Ophthalmol Scand 2003;81(5):480–485. [DOI] [PubMed] [Google Scholar]
- 14.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):701–713. [DOI] [PubMed] [Google Scholar]
- 15.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros FA, Lisboa R, Zangwill LM, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology 2014;121(1):100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol 2010;128(5):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijl A, Lindgren G, Olsson J. A package for the statistical analysis of visual fields. Paper presented at: Seventh International Visual Field Symposium, Amsterdam, September 19861987. [Google Scholar]
- 19.Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. The Stata Journal 2009;9(1):1. [PMC free article] [PubMed] [Google Scholar]
- 20.Maddess T The Influence of Sampling Errors on Test–Retest Variability in Perimetry. Invest Ophthalmol Vis Sci February 1, 2011. 2011;52(2):1014–1022. [DOI] [PubMed] [Google Scholar]
- 21.Maddess T Modeling the relative influence of fixation and sampling errors on retest variability in perimetry. Graefes Arch Clin Exp Ophthalmol 2014;252(10):1611–1619. [DOI] [PubMed] [Google Scholar]
- 22.Numata T, Maddess T, Matsumoto C, et al. Exploring Test–Retest Variability Using HighResolution Perimetry. Trans Vis Sci Tech 2017;6(5):8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Numata T, Matsumoto C, Okuyama S, et al. Detectability of Visual Field Defects in Glaucoma With High-resolution Perimetry. J Glaucoma 2016;25(10):847–853. [DOI] [PubMed] [Google Scholar]
- 24.Goldbaum MH, Sample PA, Chan K, et al. Comparing machine learning classifiers for diagnosing glaucoma from standard automated perimetry. Invest Ophthalmol Vis Sci 2002;43(1):162–169. [PubMed] [Google Scholar]
- 25.Bizios D, Heijl A, Bengtsson B. Trained artificial neural network for glaucoma diagnosis using visual field data: a comparison with conventional algorithms. J Glaucoma 2007;16(1):20–28. [DOI] [PubMed] [Google Scholar]
- 26.Ceccon S, Garway-Heath DF, Crabb DP, Tucker A. Exploring early glaucoma and the visual field test: Classification and clustering using bayesian networks. IEEE journal of biomedical and health informatics 2014;18(3):1008–1014. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Saunders LJ, Daga FB, Diniz-Filho A, Medeiros FA. Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients. Ophthalmology 2017;124(6):786–792. [DOI] [PubMed] [Google Scholar]
- 28.Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res 2017;57:46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.