Abstract
Background
Chronic kidney disease (CKD) is a risk factor for vascular disease and stroke. The spectrum of brain injury and microstructural white matter abnormalities in children with CKD is largely unknown.
Methods
Cross sectional study at two North American pediatric hospitals. A cohort of 49 children, 29 with CKD, including renal transplant (mean age 14.4 ± 2.9 years; range 9–18), and 20 healthy controls (mean age 13.7 ± 3.1 years; range 9–18) had their conventional brain magnetic resonance images (MRIs) reviewed by one neuroradiologist to determine the prevalence of brain injury. Fractional anisotropy (FA) maps calculated from diffusion tensor imaging (DTI) were generated to compare white matter microstructure in CKD compared to controls, using tract-based spatial statistics (TBSS).
Results
Focal and multifocal white matter injury was seen on brain MRI in 6 children with CKD (21%). Relative to controls, CKD subjects showed reduced white matter fractional anisotropy and increased mean diffusivity and radial diffusivity in the anterior limb of the internal capsule, suggestive of abnormal myelination.
Conclusion
Cerebral white matter abnormalities, including white matter injury, are under-recognized in pediatric CKD patients. Brain imaging studies through progression of CKD are needed to determine the timing of white matter injury and any potentially modifiable risk factors.
Introduction
Chronic kidney disease (CKD) is a risk factor for vascular disease and stroke [1]. In the 1980’s, brain CT of children with CKD showed cerebral atrophy in many of the affected children [2–4]. In the past three decades, the care of children with CKD has changed dramatically. Improved renal nutrition, especially for the infants, elimination of aluminum as a phosphate binder, use of erythropoietin for anemia, and improved dialysis treatment and timely transplantation, have all contributed to improve neurological outcomes. Despite these advances, it is largely unknown whether the occurrence of brain injury has improved. Emerging studies show neurocognitive deficits in individuals with CKD [5–8]. These deficits can lead to difficulties at school, and in turn may limit their future employment options and decrease their social independence [9, 10]. Neurocognitive deficits can also increase the likelihood of non-adherence to treatments in adolescents with CKD. The spectrum of structural abnormalities in the brain that underlie these cognitive deficits remains largely unknown to date, and need to be described.
The only contemporary pediatric neuroimaging studies in children with CKD include a relatively homogeneous Finnish cohort, affected predominantly by congenital nephrotic syndrome [11, 12]—which is not common in North America. Brain MRI of these children who had received a kidney transplant before five years of age, showed high rates of stroke. These strokes were associated with a later age at transplantation, longer time on dialysis, and a history of hemodynamic crisis [12]. These data suggest that (a) brain abnormalities are associated with CKD, and (b) potentially modifiable risk factors, such as hypertension, or timing of renal transplantation, can be identified. However, children with congenital nephrotic syndrome are not representative of most children with CKD. Therefore, additional studies in more typical pediatric CKD population are warranted.
The aim of our study was to characterize the spectrum of brain injury and white matter microstructural brain abnormalities in children with CKD studied with MRI. This included the comparison of brain white matter integrity in CKD and transplant patients to that of healthy controls. Due to the lack of prior neuroimaging data in this population, we used a whole brain voxel-wise analysis to look for white matter differences between control, CKD and transplant groups. We hypothesized that the CKD group would have impaired white matter integrity relative to controls.
Materials and Methods
Study Population
Between 2008 and 2010, we prospectively enrolled 49 children, 29 with CKD (including 2 peritoneal dialysis, and 10 renal transplant recipients), and 20 age-matched healthy controls (recruited from the community) at two academic centers in North America.
Inclusion criteria for cases: 1) school-age children over 8 years of age (to avoid need for sedation for neuroimaging), 2) CKD, including patients with a functioning renal transplant of more than one year. Exclusion criteria: 1) known neurologic comorbidity, 2) hospitalization within the previous week, or existing acute illness, 3) claustrophobia, 4) dental braces.
This study was approved by institutional review boards, BC Children’s Hospital H07-01271, and UNC IRB Study #03-0830 (previously 03-MED-33), and participants and or their parents provided informed written consent.
Magnetic Resonance Imaging
Each site used imaging parameters to obtain comparable MR signal to noise despite scanning at different field strengths (see Supplemental Methods S1). This enabled combining the Diffusion Tensor Imaging (DTI) scans for analysis with a single pipeline, as detailed below. The anatomical MRI images from both sites were reviewed by a single neuroradiologist who was blinded to the clinical history of the participants.
Diffusion Tensor Imaging (DTI) analysis
DTI provided a quantitative measure of regional brain development as reflected by water diffusion in each 3-dimensional pixel of the MR image. Water diffusion can be characterized as an ellipsoid, with the shape of the ellipsoid represented by eigenvalues (λ1, λ2, λ3) [13]. λ1 corresponds to axial diffusion (AD), and is considered to reflect axonal integrity, while λ2 and λ3 correspond to radial diffusion ((λ2+ λ3)/2 [RD]) and primarily reflect myelin integrity. Mean diffusivity (MD) reflects the average of λ1, λ2, and λ3 [14]. The degree of directionality is represented by fractional anisotropy (FA), reflecting the standard deviation of λ1, λ2, and λ3. With increasing white matter maturation and myelination MD decreases and FA increases. The DTI measures of MD, FA, AD, and RD are also sensitive to degeneration and injury in the nervous system [15–18].
Two methods of analysis were used to compare white matter maturation between children with CKD, children with renal transplantation, and controls. Images from both sites were included in both analyses. (A) Images were analyzed using a whole brain voxel-based comparison using tract based spatial statistics (TBSS). (B) To complement TBSS findings, a region of interest (ROI) based analysis was used to calculate diffusion parameters from ROIs in readily identifiable white tracts with functional significance.
(A) Tract-based Spatial Statistics (TBSS)
TBSS was done with the FMRIB Software Library (FSL) using procedures well established in our laboratory [19]. All participants’ fractional anisotropy (FA) maps were placed into a common space using a nonlinear registration [20]. Common space was determined by comparing the FA maps of all subjects to each other, and choosing the subject whose brain was most representative of the group. The mean FA map was then thinned to create a mean FA-skeleton that represented the main fiber tracts common to all subjects (threshold of FA>0.2). FA data from each participant were projected onto the mean FA-skeleton to compare FA across all voxels (voxel-wise analysis). Registration and transformation steps were also applied to MD, AD and RD. Data for each of these measures also were projected onto the mean FA-skeleton for voxel-wise analysis.
Values of FA, MD, AD, and RD were compared across CKD, transplant, and control groups. To account for the large number of comparisons in the image, threshold-free clustered results were calculated (p<0.05) to detect anatomically localized clusters with consistent group differences, using age, gender, and glomerular filtration rate (GFR) as covariates[21]. Effect sizes were calculated for any significant group differences in an effort to show the magnitude of any group differences. Control participants did not have blood taken and therefore did not have GFR data for inclusion in the analyses.
(B) ROI analysis
As the TBSS skeleton is limited to white matter with sufficiently high FA (threshold >0.2), ROIs (4×4 mm) were also placed in the anterior limb of the internal capsule (ALIC), posterior limb of the internal capsule (PLIC), and optic radiations. [22] These white matter tracts are readily identifiable and neurodevelopmentally important for child development as they contain thalamocortical projection fibers that are important for the visual system (optic radiations), the motor system (corticospinal tract in PLIC) and for behavioral and emotional regulation networks (ALIC). Within these tracts, the ROIs included areas displaying group differences in the TBSS analysis using anatomical colour map. [22] The diffusion parameters were then calculated from these ROIs. ROIs were placed twice on separate days to determine intra-rater reliability.
Statistical Analysis
Multivariable linear regression analysis was carried out using Stata 12.1 (Stata Corporation, College Station, Texas). In comparing control, CKD, and transplant groups, the white matter microstructural measures (MD, FA, AD and RD), age and scan site were included in the regression model in order to determine the independent effect of group on outcome variables. Age was included as a covariate given that FA and MD typically change with brain maturation from childhood into adolescence [23]. As each site used a different scanner, site was included in order to allow each outcome variable to vary by site, despite comparable images acquired at each site. Intra-rater reliability for ROI based FA measures was high, with an intra-correlation coefficient of 0.9488 (95% CI 0.92824 – 0.961) with a mean difference of 0.002 and Bland and Altman limits of agreement of −0.05 to 0.06 [24].
Results
Patient characteristics are summarized in Table 1. A total of 29 children with CKD (mean age of 14.4 ± 2.9 years; range 9–18), and 20 healthy age matched controls (mean age of 13.7 ± 3.1 years; range 9 – 18) were considered for the neuroimaging analysis.
Table 1.
Patient characteristics
| Control (N = 20) |
CKD (N = 19) |
Transplant (N = 10) |
|
|---|---|---|---|
|
| |||
| Age (yrs) | 13.7±3.1 | 14.4±2.9 | 14.5±2.9 |
|
| |||
| Male (%) | 12 (60) | 11 (58) | 5 (50) |
|
| |||
| Race/Ethnicity | |||
| Caucasian | 16 (80) | 13 (68.4) | 7 (70) |
| African American | 4 (20) | 1 (5.3) | 2 (20) |
| Asian | 4 (21) | 1 (10) | |
| Aboriginal | 1 (5.3) | ||
|
| |||
| Primary renal disease | |||
| Congenital/Hereditary | – | 11 (58) | 5 (50) |
| Posterior urethral valves | 4 | 1 | |
| Hypodysplasia | 3 | 2 | |
| Polycystic kidney disease | 2 | 2 | |
| Nephronophthisis | 1 | ||
| Alport | 1 | ||
| Glomerulonephritis | – | 8 (42) | 5 (50) |
| Focal segmental glomerulosclerosis | 3 | 1 | |
| ANCA vasculitis | 1 | 1 | |
| Lupus nephritis | 2 | ||
| IgA nephropathy | 1 | ||
| Immune complex glomerulonephritis | 3 | 1 | |
Anatomical MRI
The neuroradiologist identified focal and multifocal white matter injury on conventional MRI (Fig. 1) in 6 children with CKD, including transplant (21%). The white matter injury was subclinical and chronic, distributed non-uniformly in the periventricular white matter and outside of the white matter tracts of the internal capsule. The primary renal disease of the 6 patients with white matter injury were renal dysplasia, posterior urethral valves, nephronophthisis, focal segmental glomerulosclerosis, pauci-immune glomerulonephritis, and cortical necrosis. Given the cross sectional study design, it was not possible to determine the timing of brain injury in the course of CKD progression. As shown in Table 2, only 2/6 (33%) children with white matter injury had hypertension, controlled with medications, compared with 15/23 (65%) of CKD children without white matter injury who had hypertension. Hypertension was defined as having blood pressure readings ≥95th percentile for age, gender, and height, or if the patient was receiving antihypertensive medications. Blood pressure was controlled if blood pressure was below the 90th percentile for age, gender, and height on current antihypertensive medications. None of the 6 subjects with cerebral white matter injury were on dialysis, and two of them were post-first transplant. Both transplant recipients were 14 years old, and the primary renal disease for one was posterior urethral valves and for the other, it was focal segmental glomerulosclerosis, diagnosed four years prior to transplant. Of the total of 29 subjects with CKD, only two were on dialysis, and they had a normal brain MRI.
Figure 1.
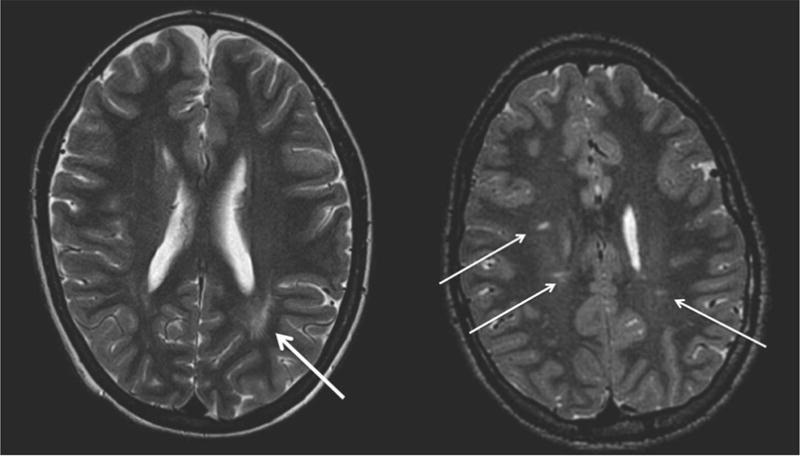
Focal and multifocal white matter injury was identified on conventional MRI in 6 children with CKD, including transplant (21%).
Table 2.
Clinical characteristics of subjects with and without brain white matter injury
| White matter injury (N = 6) |
No white matter injury (N = 23) |
|
|---|---|---|
|
| ||
| Age (Mean ±SD) | 14.8 ± 2.8 | 14.3 ± 2.9 |
|
| ||
| Male Sex (%) | 4 (66%) | 13 (57%) |
|
| ||
| CKD Stage (including transplant) | ||
| Stage 2 | 1 | 7 |
| Stage 3 | 2 | 8 |
| Stage 4 | 1 | 3 |
| Stage 5 | 2 | 5* |
|
| ||
| Duration of CKD (months), median | 62 (12, 120) | 97 (57, 158) |
| (IQR) | ||
|
| ||
| Hypertension (%) | 2 (33) | 15 (65) |
|
| ||
| Hemoglobin, g/L (±SD) | 124.7 ± 19.4 | 125.4 ± 14.0 |
Staging of CKD was based on the GFR categories outlined in the KDIGO 2012 clinical practice guideline for the evaluation and management of CKD, combining stage 3a and 3b [33].
2/5 were on peritoneal dialysis
Whole Brain Voxel-Based Group Comparisons
DTI for 40 children were included in the final TBSS analysis, 9 cases being excluded because of unacceptable image quality due to artifacts caused by excessive head motion during data acquisition: 3 controls, 3 CKD, and 3 transplants. Using threshold-free cluster analysis, a number of differences between CKD, transplant patients and controls are evident. The CKD group displayed lower FA (a measure of white matter microstructural integrity) than the control group, in the ALIC (Fig. 2) (p=0.05). We found that regions within the optic radiation in the right hemisphere had significantly lower radial diffusivity in CKD patients than that of controls (p=0.05). Finally, there was a significant decrease in axial diffusivity (CKD<Control) in the anterior aspect of the right ALIC (p=0.05).
Figure 2.
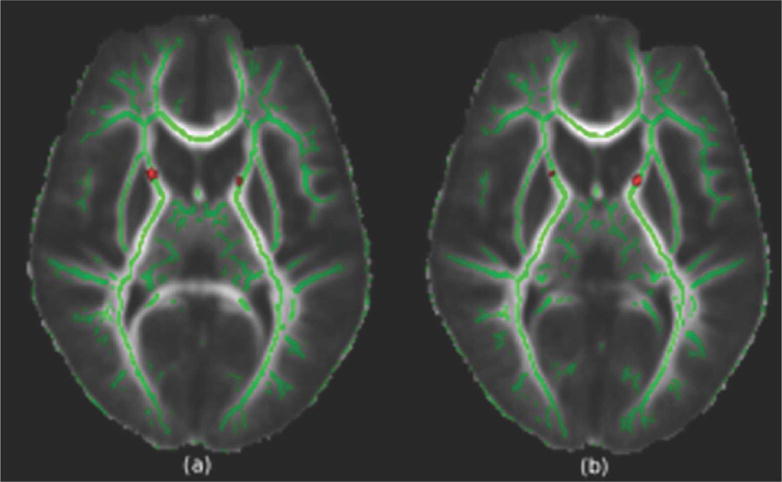
Regions displaying a significant decrease in fractional anisotropy in the tract based spatial statistics analysis (t>3, p=0.05) superimposed on the mean FA image. Green denotes the white matter FA skeleton. Compared to controls, the chronic kidney disease group showed decreases in the right and left anterior limb of the internal capsule.
Region of Interest Analysis
ROIs were placed bilaterally in each of the subjects’ native space for (i) the anterior aspect of the PLIC, (ii) the anterior aspect of the ALIC, and (iii) the optic radiations. Differences in FA, MD, AD and RD from the ROIs are summarized in Tables 3–5. Neither site nor age had a significant effect on any of the measures.
Table 3.
Group effects on fractional anisotropy (FA) and mean diffusivity (MD) for each region of interest at p < 0.05. There were significant decreases in FA in the left ALIC in chronic kidney disease (CKD) and transplant groups compared to control group. There was a significant increase in MD in the left ALIC and left optic radiation in the transplant group (ALIC = Anterior limb of the internal capsule, PLIC = Posterior limb of the internal capsule).
| Brain Region | Group | Mean difference from controls | 95% CI | p | Mean difference from controls | 95% CI | p | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fractional Anisotropy (FA) | Mean Diffusivity (MD) | ||||||||
| Left ALIC | CKD | −0.0859 | −0.127 | −0.044 | <0.001* | 0.032 | −0.001 | 0.066 | 0.06 |
| Transplant | −0.0973 | −0.157 | −0.038 | 0.002* | 0.075 | 0.027 | 0.124 | 0.003* | |
|
| |||||||||
| Right ALIC | CKD | −0.021 | −0.066 | 0.024 | 0.4 | −0.012 | −0.059 | 0.035 | 0.6 |
| Transplant | −0.055 | −0.120 | 0.010 | 0.09 | 0.006 | −0.061 | 0.074 | 0.9 | |
|
| |||||||||
| Left PLIC | CKD | 0.020 | −0.014 | 0.055 | 0.2 | −0.043 | −0.077 | −0.010 | 0.01* |
| Transplant | −0.028 | −0.077 | 0.021 | 0.3 | −0.028 | −0.065 | 0.030 | 0.5 | |
|
| |||||||||
| Right PLIC | CKD | −0.007 | −0.043 | 0.029 | 0.7 | −0.036 | −0.065 | −0.006 | 0.02* |
| Transplant | −0.048 | −0.099 | 0.003 | 0.07 | −0.003 | −0.046 | 0.039 | 0.9 | |
|
| |||||||||
| Left Optic | CKD | 0.011 | −0.028 | 0.051 | 0.6 | −0.016 | −0.058 | 0.027 | 0.5 |
| Radiation | Transplant | −0.010 | −0.067 | 0.048 | 0.7 | −0.069 | 0.007 | 0.130 | 0.03* |
|
| |||||||||
| Right Optic | CKD | −0.03 | −0.086 | 0.027 | 0.3 | −0.006 | −0.054 | 0.043 | 0.8 |
| Radiation | Transplant | 0.009 | −0.072 | 0.090 | 0.8 | −0.000 | −0.084 | 0.056 | 0.7 |
Table 5.
Significant (p<0.05) differences in the tract based statistics (TBSS) and region of interest (ROI) analysis methodologies. The only difference found by both methodologies was a decrease in FA in the chronic kidney disease (CKD) group in the left ALIC (ALIC = Anterior limb of the internal capsule, PLIC = Posterior limb of the internal capsule, FA = fractional anisotropy, MD = mean diffusivity, RD = Radial diffusivity, λ1 = axial diffusivity).
| CKD | Transplant | ||||
|---|---|---|---|---|---|
|
| |||||
| Region of interest | Diffusion Measure | TBSS Analysis | ROI Analysis | TBSS Analysis | ROI Analysis |
| Left ALIC | FA | Decrease | Decrease | – | Decrease |
| MD | – | Increase** | – | Increase | |
| RD | – | Increase | – | Increase | |
| λ1 | Decrease | – | – | – | |
|
| |||||
| Right ALIC | FA | Decrease | – | – | – |
|
| |||||
| Left PLIC | FA | – | – | Decrease | – |
| MD | – | Decrease | – | – | |
| RD | – | Decrease | – | – | |
|
| |||||
| Right PLIC | MD | – | Decrease | – | – |
| λ1 | – | Decrease | – | – | |
|
| |||||
| Left Optic Radiation | MD | – | – | – | Increase |
| RD | Decrease | – | – | Increase | |
p=0.058
(i) Anterior Limb of the Internal Capsule (ALIC)
There were significant differences found in the left ALIC. When compared to the control group, subjects with CKD had decreased FA (p<0.001), a significant increase in RD (p=0.002), and a marginal increase in MD (p=0.06). The transplant group showed a comparable decrease in FA compared to the control group (p=0.002), accompanied by increases in both RD (p=0.001) and MD (p=0.003).
(ii) Posterior Limb of the Internal Capsule (PLIC)
As shown in Tables 3–5, in the PLIC, differences were seen only in the CKD group relative to control. In the left PLIC, there were significant decreases in MD (p=0.01) and RD (p=0.02). On the right, there were significant decreases in MD (p=0.02) and axial diffusivity (p=0.02). There were no significant differences in FA in the PLIC.
(iii) Optic Radiations
There were no significant differences in FA found in the CKD or transplant groups. The transplant group demonstrated a significant increase in the left optic radiation in both MD (p=0.03) and RD (p=0.034) [Tables 3–5]. There were no differences in the right optic radiation.
Discussion
The care of children with CKD has improved tremendously over the past three decades to be more brain protective. Improved renal nutrition, avoidance of aluminum-based phosphate binders, enhanced dialysis therapies, and timely renal transplantation are each expected to improve neurologic outcome. Yet the actual impact of these interventions on brain health has not been examined with contemporary neuroimaging. In a recent comprehensive review of neuroimaging studies in patients with kidney disease spanning over 35 years, only 13 of the 43 studies involved children, and 10 of the 13 pediatric studies were from the 1980s and early 90s using CT scans [25]. The only contemporary pediatric brain MRI studies involved a cohort of renal transplant recipients, transplanted before the age of 5 years, majority of whom had congenital nephrotic syndrome of the Finnish type [11, 12], not a common cause of end-stage renal disease in North America [26]. Interestingly, the white matter lesions observed on MRI in our cohort of children with CKD share features with the small punctate foci confined to the white matter reported by Valanne et al. in one third of their pediatric cohort who had received a renal allograft before 5 years of age [12]. Our study indicates that these cerebral white matter findings are also observed in children with “typical” CKD.
In our study, white matter abnormalities were seen on conventional MRI in 21% of children with CKD, and not in any of the controls. In a recent report of incidental brain MRI findings in 1400 youths (age range 8–23 years) volunteering for research, there were 148 (10%) incidental findings, of which only 12 (0.9%) required any clinical follow-up[27]. The incidental findings included brain lesions other than white matter injury suggesting that the findings observed in our cohort of children with CKD are unexpected in the general population. Furthermore, in our study, DTI analysis revealed that relative to controls, CKD subjects (including transplant recipients) had reduced white matter fractional anisotropy (FA) and increased mean diffusivity (MD) and radial diffusivity (RD) in the anterior limb of the internal capsule, suggesting impaired white matter myelination in this region. Both methods of DTI analysis, namely ROI-based and TBSS, detected a mean decrease in FA in the left ALIC in the CKD group. The biologic significance of this finding is reinforced by the fact that three diffusion measures differed in this region.
Sensory and motor information is sent between the pyramids of the medulla and the cerebral cortex through ascending and descending fibers in the internal capsule [28]. The connections of the internal capsule have been mapped out previously using diffusion imaging [29]. A recent study by Sullivan et al [30] found correlations between diffusion measures in the internal capsule and neurocognitive scores. Specifically, they found a positive correlation between FA and scores on verbal and non-verbal fluency and problem solving. This is concordant with findings in other cohorts that CKD patients score lower in language and executive function [7, 31] and supports our results of a decreased FA in the ALIC. CKD patients also display deficits in memory [32]. In a study of schizophrenic patients, Levitt et al. found a significant positive correlation between FA in the left ALIC and performance on memory tasks [33]. Interestingly, in a prospective community-based study of adults including 40 with normal cognition, 94 with mild impairment and 11 with mild Alzheimer’s, episodic memory and executive function were associated with white matter hyperintensities in the ALIC [34]. Given the potential vascular etiology to the cognitive impairments in this population, these findings and those from our study, support the need for future studies to examine the link between white matter imaging changes and neurocognitive function in children with CKD.
A possible shortcoming of our study is the cross sectional design. Also, the scans at the two different sites were acquired at different field strengths (1.5T at BCCH and 3.0T at UNC). However, site had no significant effect on the left ALIC after including it as a variable in the regression analysis. Therefore, it is unlikely that site of MRI scan biased our results. We also recognize our relatively small sample size. Therefore, we limited the number of hypotheses tested, applied threshold-free cluster analysis in our whole brain voxel-based group comparisons, and limited our region of interest-based diffusion MRI analyses to three anatomically defined regions.
This study focuses attention on the importance of brain health in the care of children with CKD. A longitudinal study during the progression of CKD, as well as post-renal replacement therapy, looking for white matter changes over time, would allow better understanding of the timing of white matter injury and identifying potential modifiable risk factors. Advances in the care of children with CKD is anticipated to further improve the brain health of this vulnerable population.
Supplementary Material
Table 4.
Group effects on radial diffusivity (RD) and axial diffusivity (λ1) for each region of interest at p < 0.05. There were significant increases in RD in the left ALIC in the Chronic Kidney Disease (CKD) group and in the left ALIC and left optic radiation in the transplant group compared to control group. There was a significant decrease in RD the left PLIC in the CKD group compared to the control group. There was a significant decrease in (λ1) in the CKD group in the right PLIC compared to control group. (ALIC = Anterior limb of the internal capsule, PLIC = Posterior limb of the internal capsule).
| Brain Region | Group | Mean difference from controls | 95% CI | p | Mean difference from controls | 95% CI | p | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Radial diffusivity (RD) | Axial diffusivity (λ1) | ||||||||
| Left ALIC | CKD | 0.064 | 0.025 | 0.104 | 0.002* | −0.046 | −0.109 | 0.017 | 0.2 |
| Transplant | 0.099 | 0.043 | 0.155 | 0.001* | 0.013 | −0.078 | 0.103 | 0.8 | |
|
| |||||||||
| Right ALIC | CKD | −0.002 | −0.054 | 0.049 | 0.9 | −0.048 | −0.124 | 0.028 | 0.2 |
| Transplant | 0.006 | −0.037 | 0.112 | 0.3 | −0.05 | −0.159 | 0.060 | 0.4 | |
|
| |||||||||
| Left PLIC | CKD | −0.053 | −0.097 | −0.001 | 0.02* | −0.053 | −0.112 | 0.006 | 0.08 |
| Transplant | 0.005 | −0.058 | 0.067 | 0.9 | −0.067 | −0.153 | 0.018 | 0.1 | |
|
| |||||||||
| Right PLIC | CKD | −0.017 | −0.053 | 0.020 | 0.4 | −0.078 | −0.142 | −0.014 | 0.02* |
| Transplant | 0.034 | −0.019 | 0.087 | 0.2 | −0.070 | −0.162 | 0.022 | 0.1 | |
|
| |||||||||
| Left Optic | CKD | −0.028 | −0.087 | 0.032 | 0.4 | −0.012 | −0.095 | 0.072 | 0.8 |
| Radiation | Transplant | 0.093 | 0.007 | 0.178 | 0.03* | 0.071 | −0.049 | 0.190 | 0.2 |
|
| |||||||||
| Right Optic | CKD | 0.020 | −0.047 | 0.087 | 0.5 | −0.062 | −0.132 | 0.009 | 0.08 |
| Radiation | Transplant | −0.000 | −0.078 | 0.114 | 0.7 | −0.000 | −0.124 | 0.079 | 0.7 |
Acknowledgments
Statement of Financial Support:
This study was funded by the British Columbia Children’s Hospital Telethon Foundation Grant, by the Renal Research Institute at the University of North Carolina School of Medicine, and by NCTraCTS Institute at the University of North Carolina School of Medicine. Additional support for completion of the data analyses and for some of the data collected for this manuscript was provided by the Chronic Kidney Disease in children prospective cohort study (CKiD), with Principal Investigators at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, Ph.D.). The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.ihsph.edu/ckid.
The funders of this study had no role in the study design, data collection or analysis, interpretation of data, or writing the report; or the decision to submit the report for publication.
Footnotes
Disclosure: There are no conflicts of interest for any of the authors.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–23. [PubMed] [Google Scholar]
- 2.Schnaper HW, et al. Cerebral cortical atrophy in pediatric patients with end-stage renal disease. Am J Kidney Dis. 1983;2(6):645–50. doi: 10.1016/s0272-6386(83)80046-8. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg A, et al. Computerized tomography of the brain in children with chronic renal failure. Int J Pediatr Nephrol. 1985;6(2):121–6. [PubMed] [Google Scholar]
- 4.Trompeter RS, et al. Neurological complications of arterial hypertension. Arch Dis Child. 1982;57(12):913–7. doi: 10.1136/adc.57.12.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gipson DS, et al. The nervous system and chronic kidney disease in children. Pediatr Nephrol. 2004;19(8):832–9. doi: 10.1007/s00467-004-1532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerson AC, et al. Neurocognitive outcomes in children with chronic kidney disease: Current findings and contemporary endeavors. Ment Retard Dev Disabil Res Rev. 2006;12(3):208–15. doi: 10.1002/mrdd.20116. [DOI] [PubMed] [Google Scholar]
- 7.Gipson DS, et al. Memory and executive functions in pediatric chronic kidney disease. Child Neuropsychol. 2006;12(6):391–405. doi: 10.1080/09297040600876311. [DOI] [PubMed] [Google Scholar]
- 8.Gipson DS, et al. The central nervous system in childhood chronic kidney disease. Pediatr Nephrol. 2007;22(10):1703–10. doi: 10.1007/s00467-006-0269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Icard PF, et al. The transition from childhood to adulthood with ESRD: educational and social challenges. Clin Nephrol. 2008;69(1):1–7. doi: 10.5414/cnp69001. [DOI] [PubMed] [Google Scholar]
- 10.Tjaden LA, et al. Impact of Renal Replacement Therapy in Childhood on Long-Term Socioprofessional Outcomes: A 30-year Follow-Up Study. J Pediatr. 2016;171:189–95. e1–2. doi: 10.1016/j.jpeds.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Qvist E, et al. Neurodevelopmental outcome in high-risk patients after renal transplantation in early childhood. Pediatr Transplant. 2002;6(1):53–62. doi: 10.1034/j.1399-3046.2002.1o040.x. [DOI] [PubMed] [Google Scholar]
- 12.Valanne L, et al. Neuroradiologic findings in children with renal transplantation under 5 years of age. Pediatr Transplant. 2004;8(1):44–51. doi: 10.1046/j.1397-3142.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 13.Moseley ME, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176(2):439–45. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 14.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox RJ, et al. A preliminary validation study of diffusion tensor imaging as a measure of functional brain injury. Arch Neurol. 2008;65(9):1179–84. doi: 10.1001/archneur.65.9.1179. [DOI] [PubMed] [Google Scholar]
- 16.Vaillancourt DE, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–84. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGraw P, et al. Krabbe disease treated with hematopoietic stem cell transplantation: serial assessment of anisotropy measurements–initial experience. Radiology. 2005;236(1):221–30. doi: 10.1148/radiol.2353040716. [DOI] [PubMed] [Google Scholar]
- 18.Klawiter EC, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–60. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalization. FMRIB Technical report TR07JA2. 2007 from www.fmrib.ox.ac.uk/analysis/techrep.
- 21.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 22.Wakana S, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 23.Clayden JD, et al. Normative Development of White Matter Tracts: Similarities and Differences in Relation to Age, Gender, and Intelligence. Cereb Cortex. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 25.Moodalbail DG, et al. Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol. 2013;8(8):1429–48. doi: 10.2215/CJN.11601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JM, Martz K, Blydt-Hansen TD. Pediatric kidney transplant practice patterns and outcome benchmarks, 1987–2010: a report of the North American Pediatric Renal Trials and Collaborative Studies. Pediatr Transplant. 2013;17(2):149–57. doi: 10.1111/petr.12034. [DOI] [PubMed] [Google Scholar]
- 27.Gur RE, et al. Incidental findings in youths volunteering for brain MRI research. AJNR Am J Neuroradiol. 2013;34(10):2021–5. doi: 10.3174/ajnr.A3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schunke M, et al. Thieme atlas of anatomy : head and neuroanatomy. Vol. 1. Stuttgart: Thieme; 2010. p. 414. (THIEME Atlas of Anatomy Series). [Google Scholar]
- 29.Zarei M, et al. Two-dimensional population map of cortical connections in the human internal capsule. J Magn Reson Imaging. 2007;25(1):48–54. doi: 10.1002/jmri.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan EV, et al. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48(14):4155–63. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper SR, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1824–30. doi: 10.2215/CJN.09751110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fennell RS, et al. A longitudinal study of the cognitive function of children with renal failure. Pediatr Nephrol. 1990;4(1):11–5. doi: 10.1007/BF00858429. [DOI] [PubMed] [Google Scholar]
- 33.Levitt JJ, et al. Fractional anisotropy and radial diffusivity: diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr Res. 2012;136(1-3):55–62. doi: 10.1016/j.schres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Smith EE, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492–9. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


