Abstract
Purpose:
To compare race-related differences in estimated rate of change of Bruch’s membrane opening-minimum rim width (BMO-MRW) and circumpapillary retinal nerve fiber layer thickness (RNFLT) in healthy, glaucoma suspect and glaucoma eyes of individuals of European (ED) and African descent (AD).
Design:
Prospective cohort study.
Methods:
This study investigated rate of change of BMO-MRW and RNFLT in 124 healthy, 227 glaucoma suspect and 177 glaucoma eyes followed for approximately 3 years and tested with optical coherence tomography every 6 months. Suspect eyes had a history of untreated IOP ≥ 22 mmHg or suspicion of glaucoma by optic disc photograph assessment without repeatable abnormal standard automated perimetry (SAP) results. Glaucoma eyes had repeatable abnormal SAP results (GHT ONL or PSD ≤ 5%). Mixed effects models were used to estimate the rate of change after controlling for age, mean follow-up IOP, central corneal thickness, axial length, and BMO area.
Results:
A race-related difference in rate of change of global BMO-MRW but not average RNFLT in suspect eyes was observed. Rate of change of BMO-MRW was −1.82 μm/year and −2.20 μm/year in ED and AD suspect eyes, respectively (p=0.03). Rate of change of RNFLT was −0.64 μm/year and −0.75 μm/year in ED and AD suspect eyes, respectively (p=0.75). No race-related differences in change rate were found in healthy or glaucoma eyes.
Conclusion:
Race is an important consideration when assessing structural change, particularly minimum rim width, in glaucoma suspect eyes. Differences in rate of structural change may help explain racial disparities in glaucoma susceptibility.
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness in individuals of African descent (AD).1-5 African Americans are at a greater risk of developing primary open angle glaucoma (POAG) and show an up to six times higher incidence of POAG than individuals of European descent (ED), according to the Baltimore Eye Study. 6-11 In addition, POAG progresses more rapidly in AD than in ED eyes12 and AD individuals are many times more likely than ED individuals to develop POAG-associated visual impairment.2
Findings from the African Descent and Glaucoma Evaluation Study (ADAGES) indicate that AD individuals with glaucoma have worse central and peripheral visual field (VF) damage13, have more variable VF results and are more likely to be rapid progressors based on VF assessment (Medeiros FA, et al. IOVS 2011;55:ARVO E-Abstract 2117). However, the rate of neuroretinal rim loss is similar between AD and ED eyes with glaucoma.14 In addition, ADAGES results have demonstrated significant differences in optic disc morphology between healthy AD and ED groups including deeper confocal scanning laser ophthalmoscopy-measured cup depth, thinner optical coherence tomography (OCT)-measured macular thickness and thicker OCT-measured retinal nerve fiber layer (RNFL) in AD individuals, after controlling for differences in optic disc area between groups15 (see also Girkin et al., Kashani et al., and Racette et al.,16-18 for similar OCT results and Tjon-Fo-Sang et al 19 for similar differences in scanning laser polarimetry-measured RNFLT). Differences in prevalence of glaucoma have been reported among regional AD populations20 and subsequently, differences in RNFLT between races was shown to be marginally associated with biogeographic ancestry.21
Because evidence suggests that the rate of change in structural measurements is influenced by race (e.g., Wilson et al.12), the current study investigated whether ancestry-related differences exist in the estimated rate of change of Bruch’s membrane opening minimum rim width (BMO-MRW) and circumpapillary RNFL thickness (RNFLT) in healthy, glaucoma suspect and glaucoma eyes of individuals of African and European descent. The presence of such differences would suggest the need for different monitoring strategies for individuals of different biogeographic ancestry.
METHODS
The current study was a prospective longitudinal cohort study. Study participants were selected from two prospective longitudinal studies designed to evaluate optic nerve structure and visual function in glaucoma: The African Descent and Glaucoma Evaluation Study (ADAGES) and the UC San Diego Diagnostic Innovations in Glaucoma Study (DIGS). The 3-site ADAGES collaboration included the Hamilton Glaucoma Center at the Shiley Eye Institute, University of California, San Diego (La Jolla, CA; data coordinating center); the Edward S. Harkness Eye Institute, Columbia University Medical Center (New York, NY); and the Department of Ophthalmology, University of Alabama, Birmingham (Birmingham, AL). Protocols of the two studies are identical and have been described elsewhere.22 Enrollment of participants was based on the inclusion/exclusion criteria specified below. Informed consent was obtained from each participant and each institution’s Human Subjects Committee approved all methodology. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. ADAGES and DIGS are registered as cohort clinical trials [http://www.clinicaltrials.gov (identifiers NCT00221923 and NCT00221897; September 14, 2005)].
Participants
Eligible participants had best corrected visual acuity of 20/40 or better, spherical refractive error less than 5.0 diopters (D), cylinder less than 3.0 D, and open angles on gonioscopy. All participants were at least 18 years old. Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery). Eyes with coexisting retinal disease, uveitis, or non-glaucomatous optic neuropathy also were excluded. Diabetic participants with no evidence of retinal involvement were included. Healthy eyes, glaucomatous eyes and eyes suspected of having glaucoma were included in the longitudinal analyses.
Healthy eyes had healthy appearing optic discs and RNFLs based on masked assessment of digital stereoscopic photographs with no history of repeatable abnormal standard automated perimetry (SAP, HFA II, Carl Zeiss Meditec Inc., Dublin, CA, USA) visual field (VF) results and no history of elevated intraocular pressure (IOP) (all IOP ≤ 21 mm Hg). Normal VFs were defined based on mean deviation (MD) and pattern standard deviation (PSD) within 95% confidence limits (95% CI), and a Glaucoma Hemifield Test (GHT) within normal limits.
Glaucoma eyes had open angles on gonioscopy, and at least 3 consecutive and reliable (defined below) SAP VF examinations with either PSD ≥ 5% or a GHT result outside of the 99% normal limits.
Glaucoma suspect eyes had suspicious appearance of the optic disc (neuroretinal rim narrowing, excavation, or suspicious RNFL defects by masked stereophotograph assessment) or elevated IOP (≥ 22 mm Hg), with normal VF results at baseline, as defined above. In addition, suspect eyes never had two consecutive and reliable abnormal VFs during the course of the study.
For photograph assessment, two graders, masked to the participants’ race, age, and clinical diagnosis evaluated simultaneous stereophotographs presented on a 22-inch or larger computer monitor according to a standard protocol [developed by the UC San Diego Imaging Data Evaluation and Analysis (IDEA) Center] using a stereoscopic viewer. In case of discrepancies between the 2 graders, adjudication was completed by a third experienced grader.
All subjects underwent an annual comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, dilated funduscopic examination, and stereoscopic optic disc photography. Semi-annual examination included tonometry, spectral domain (SD)-OCT imaging (SD-OCT circular scan, SD-OCT ONH cube scan and SD-OCT ONH radial scan), and VF testing. In addition, central corneal thickness (CCT, measured using the Pachmate 2, DGH Technology Inc., Exton, PA, USA), axial length (measured using the IOLMaster, Carl Zeiss Meditec Inc., Dublin, CA, USA) and Bruch’s membrane opening area were measured for inclusion in mixed-effects models.
Visual Field Testing
All patients underwent SAP testing using the Swedish Interactive Thresholding Algorithm (SITA) Standard 24-2 strategy at baseline and during follow-up. All VFs were evaluated by UC San Diego Visual Field Assessment Center (VisFACT) personnel based on a standardized protocol. Visual fields with more than 33% fixation losses or false-negative errors or more than 15% false-positive errors were automatically excluded. Visual fields exhibiting a learning effect (i.e., initial tests showing consistent improvement of visual field indices) also were excluded. Visual fields were further reviewed for lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (e.g., homonymous hemianopia), and inattention. VisFACT personnel requested repeats of unreliable visual field test results, and these were obtained whenever possible.
Spectral Domain Optical Coherence Tomography
Study eyes were imaged with SDOCT (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany, software version 5.2.0.3). Spectralis OCT uses a dual-beam SDOCT, a confocal laserscanning ophthalmoscope with a wavelength of 870 nm and an infrared reference image to obtain images of ocular microstructures. The instrument has an acquisition rate of 40,000 A-scans per second.
Spectralis OCT incorporates a real-time eye-tracking system that couples confocal laser-scanning ophthalmoscope and SDOCT scanners to adjust for eye movements and to ensure that the same location of the retina is scanned over time. To estimate the BMO-MRW, we used the Enhanced Depth Imaging (EDI) scan centered on the optic disc (48 B-scans with 1024 A-scans each). RNFLT was measured using the 3.45 mm high resolution circle RNFL scan centered on the optic disc (single B-scan with 1536 A-scans). Quality assessment of OCT scans was evaluated by Imaging Data Evaluation and Analysis (IDEA) Center experienced examiners masked to the subjects’ results of other tests.
Retinal Layer Segmentation
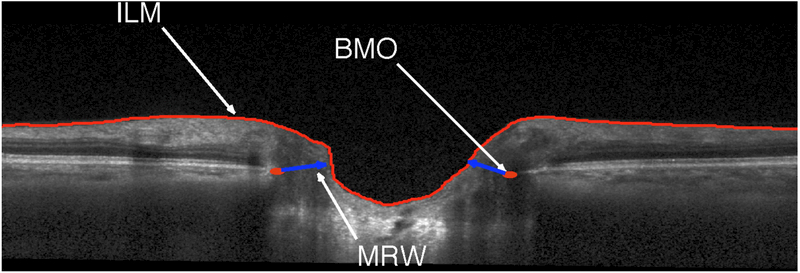
Raw 3D SD-OCT images were exported to a numerical computing language (MATLAB; MathWorks, Natick, MA). The San Diego Automated Layer Segmentation Algorithm (SALSA)23,24 was used to automatically segment the Bruch's membrane opening (BMO) and the internal limiting membrane (ILM) on each ONH radial scan to calculate the BMO-MRW defined as the shortest distance from BMO to ILM (Figure 1).25,26 Global and sectoral RNFLT measurements were provided by commercially available Spectralis software.
Figure 1.
Example of San Diego Automated Layer Segmentation Algorithm identified Bruch’s membrane opening minimum rim width (BMO-MRW) defined on a single Spectralis b-scan as the minimum distance between the BMO and internal limiting membrane (ILM).
Statistical Analyses
Descriptive statistics were used to compare demographic characteristics by group (healthy, glaucoma suspect and glaucoma study participants). Chi2 tests were used to compare categorical variables and t-tests were used to compare continuous variables.
Mixed effects models were used to calculate the estimated rates of change (slopes) for BMO-MRW and RNFLT loss from baseline. Models included group (healthy vs. glaucoma vs. suspect) and ancestry (AD vs. ED), time, and the interaction term group X time. The models were adjusted for age, mean IOP during follow-up, CCT, axial length, and BMO area.
Statistical analysis was performed using SAS, Version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
The study included 42 eyes of 27 AD healthy subjects, 82 eyes of 42 ED healthy subjects, 94 eyes of 54 AD glaucoma suspect patients, 133 eyes of 72 ED glaucoma suspect patients, 95 eyes of 47 AD glaucoma patients and 82 eyes of 43 ED glaucoma patients. A summary of the demographic variables and measurements at baseline of each group are shown in Table 1. Glaucoma patients and suspects were significantly older (p < 0.001) and had worse VF mean deviation (MD) (p < 0.001) than healthy eyes at baseline (one-way ANOVA with post-hoc Tukey test).
Table 1.
Demographics and baseline ophthalmic measurements from African descent and European descent individuals within diagnostic groups
| Healthy Eyes | Glaucoma Suspect Eyes | Glaucoma Eyes | ANOVA* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ED | All | P- value |
AD | ED | All | P- value |
AD | ED | All | P- value |
P-v alue |
|
| Number of patients | 27 | 42 | 69 | 54 | 72 | 126 | 47 | 43 | 90 | ||||
| Number of eyes | 42 | 82 | 124 | 94 | 133 | 227 | 95 | 82 | 177 | ||||
| Sex, % female | 74 | 73 | 73.5 | 0.40 | 70 | 62 | 66 | 0.03 | 55 | 51 | 53 | 0.02 | 0.03 |
|
Age at baseline (years) |
64 ± 12 | 54 ± 12 | 59 ± 12 | 0.01 | 68 ± 11 | 68 ± 10 | 68 ± 11 | 0.70 | 67 ± 9 | 73 ± 12 | 70 ± 11 | 0.01 | <0.001 |
|
Median Number of visits |
3 (3, 4) | 5 (3, 9) | 5 (3,9) | 0.007 | 6 (3, 10) | 4 (3, 9) | 5 (3,10) | 0.20 | 8 (3, 10) | 8 (4, 10) | 8 (3,10) | 0.30 | 0.01 |
| Mean Followup time (years) | 2.9 ± 1.2 | 1.9 ± 1.2 | 2.4 ± 1.22 | <0.001 | 2.8 ± 1.2 | 2.5 ± 1.2 | 2.6 ± 1.2 | 0.14 | 3.5 ± 0.8 | 3.2 ± 1.04 | 3.35 ± 1.01 | 0.01 | 0.007 |
| Axial length (mm) | 23.7 ± 0.9 | 23.7 ± 0.8 | 23.7 ± 0.85 | 0.50 | 24.04 ± 1.04 | 24.1 ± 1.0 | 24.07 ± 1.01 | 0.70 | 23.8 ± 1.3 | 23.8 ± 1.2 | 23.8 ± 1.25 | 0.04 | 0.05 |
| Disc area (mm2) | 2.08 ± 0.33 | 2.05 ± 0.41 | 2.07 ± 0.35 | 0.62 | 2.10 ± 0.41 | 2.03 ± 0.52 | 2.06 ± 0.48 | 0.39 | 2.17 ± 0.5 | 2.12 ± 0.62 | 2.15 ± 0.53 | 0.33 | 0.43 |
| VF Mean deviation (dB) | 0.22 (−1.43, 2.1) |
−0.44 (−2.36, −2.29) |
−0.11 (−2.36, 2.1) |
0.02 | −0.17 (−4.8, −2.7) |
0.1 (−3.9, 2.7) |
−0.03 (−4.8,2.7) |
0.27 | −7.95 (−33.69, −0.51) |
−8.3 (−29.17, −.67) |
−8.3 (−33.69, −0.51) |
0.40 | <0.001 |
| CCT (μm) | 528.5 ± 31.5 | 561.3 ± 38.6 | 544.9 ± 35.4 | <0.001 | 535.5 ± 42.3 | 553.4 ± 37.7 | 544.4 ± 41.2 | 0.001 | 525.0 ± 37.0 | 573.4 ± 41.0 | 549.2 ± 40.0 | 0.02 | 0.006 |
| Mean IOP during follow-up (mmHg) | 14.3 ± 2.4 | 14.5 ± 2.8 | 14.4 ± 2.6 | 0.60 | 16.0 ± 4.2 | 17.1 ± 4.2 | 16.55 ± 4.2 |
0.06 | 14.8 ± 3.5 | 13.8 ± 3.8 | 14.3 ± 3.6 | 0.07 | 0.007 |
Abbreviations: VF, visual field; CCT, central corneal thickness, IOP, intraocular pressure. Variability measurements are ± SD, ranges or 95 percent confidence intervals. Wilcoxon rank sum test P-values reported representing the differences between AD and ED eyes.
P-values represent differences across diagnostic groups.
Baseline global and sectoral BMO-MRW thickness derived from the ONH radial scans are presented in Table 2. Healthy eyes had thicker baseline BMO-MRW compared to glaucoma suspect and glaucoma eyes using one-way ANOVA with post-hoc Tukey test (p < 0.001). There was no statistically significant difference in baseline BMO-MRW measurement between AD and ED eyes in any diagnostic group (all comparisons p ≥ 0.07).
Table 2.
Baseline Bruch’s membrane opening minimum rim width (MRW) from African descent and European descent individuals within diagnostic groups
| Healthy Eyes | Glaucoma Suspect Eyes | Glaucoma Eyes | ANOVA* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ED | Total | P- value |
AD | ED | Total | P- value |
AD | ED | Total | P- value |
P- value |
|
|
Global MRW (μm) |
285 ± 44 | 306 ± 72 | 295.5 ± 65 | 0.09 | 260 ± 77 | 264 ± 73 | 262 ± 73 | 0.35 | 200 ± 81 | 208 ± 87 | 204 ± 84 | 0.27 | <0.001 |
| Nasal MRW (μm) | 302 ± 80 | 320 ± 76 | 311 ± 78 | 0.15 | 270 ± 77 | 279 ± 80 | 274.5 ± 78 | 0.19 | 239 ± 115 | 232 ± 96 | 235.5 ± 105 | 0.60 | <0.001 |
| Temporal MRW (μm) | 217 ± 58 | 200 ± 54 | 208.5 ± 56 | 0.10 | 185 ± 49 | 198 ± 60 | 191.5 ± 55 | 0.07 | 156 ± 62 | 163 ± 61 | 159.5 ± 61 | 0.31 | <0.001 |
|
Inferior MRW (μm) |
321 ± 62 | 340 ± 73 | 330.5 ± 68 | 0.12 | 280 ± 81 | 289 ± 80 | 284.5 ± 80 | 0.36 | 222 ±114 | 223 ± 92 | 222.5 ± 111 | 0.48 | <0.001 |
| Superior MRW (μm) | 301 ± 73 | 310 ± 79 | 305.5 ± 76 | 0.27 | 267 ± 81 | 273 ± 72 | 270 ± 76 | 0.26 | 222 ± 90 | 220 ± 96 | 221 ± 93 | 0.46 | <0.001 |
Abbreviations: MRW, minimum rim width. All values are mean (±SD) and Wilcoxon rank sum test P-values reported representing the differences between AD and ED eyes.
P-values represent differences across diagnostic groups.
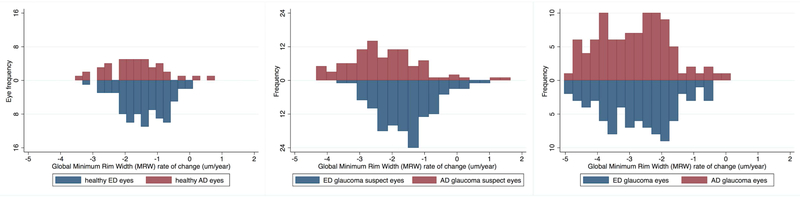
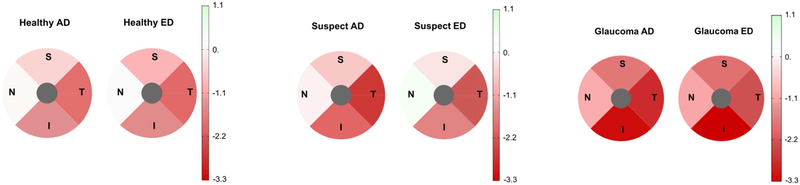
Mixed effects model-estimated rates of structural loss of global and sectoral BMO-MRW in healthy, glaucoma suspect and glaucoma eyes adjusted for age, mean IOP during follow-up, CCT, axial length, and BMO area are presented in Table 3. Global, temporal and inferior BMO-MRW showed a significant change over time (change greater than 0 μm/year evidenced by 95% confidence intervals) for all diagnostics groups. Nasal and superior BMO-MRW showed a significant change over time in glaucoma eyes only. In suspect eyes, the rate of change of global, temporal and inferior BMO-MRW was faster in AD vs ED eyes (all comparisons p ≤ 0.03). In healthy and glaucoma eyes, there was no difference in rate of change between AD and ED eyes (all comparisons p ≥ 0.06). Distributions of rates of change in BMO-MRW (μm/year) in ED and AD eyes for healthy, suspect and glaucoma groups are shown in Figure 2. Pie charts showing BMO-MRW rates of change over time by sector in ED and AD eyes for healthy, suspect and glaucoma groups are shown in Figure 3. Values shown also are adjusted for age, mean IOP during follow-up, CCT, axial length and BMO area.
Table 3.
Estimated rate of change (and 95 percent confidence intervals) in Bruch’s membrane opening minimum rim width (MRW) for African descent and European descent individuals within diagnostic groups. Values shown are adjusted for age, mean IOP during follow-up, CCT, axial length, and Bruch’s membrane opening area.
| Healthy eyes | Glaucoma suspect eyes | Glaucoma eyes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ED | Total | P-value** | AD | ED | Total | P-value** | AD | ED | Total | P-value** | P-value*** | |
| Global MRW(μm/year) | −1.68 (−2.8, −0.08)* | −1.55 (−2.6, −0.01)* | −1.61 (−2.7, −0.03) | 0.11 | −2.2 (−3.8, −0.1)* | −1.82 (−2.7, −0.05)* | −2.01 (−3.3, −0.06)* | 0.03 | −2.87 (−4.3, −0.2)* | −2.91 (−4.5, −0.03)* | −2.89 (−4.4, −0.11)* | 0.21 | <0.001 |
|
Nasal MRW (μm/year) |
−0.06 (−2.1, 1.3) | 0.04 (−3.1, 2.8) |
0.01 (−2.1, 27) |
0.17 | −0.17 (−2.5, 1.8) | 0.31 (−1.7, 2.5) |
0.07 (−2.1, 2.2) |
0.15 | −1.06 (−2.5, −0.5)* |
−1.14 (−3.2, −0.18)* |
−1.1 (−2,92, −0.32)* |
0.18 | <0.001 |
|
Temporal MRW (μm/year) |
−1.88 (−2.9, −0.3)* |
−1.95 (−2.4, −0.05)* |
−1.91 (−2.5, −0.07) |
0.22 | −2.61 (−3.7, −0.1)* |
−2.15 (−2.3, −0.11)* |
−2.38 (−3.1,−0.1)* |
0.01 | −2.77 (−4.2, −0.1)* |
−2.29 (−3.29, −0.15)* |
−2.53 (−3.46, −0.12)* |
0.06 | <0.001 |
|
Inferior MRW (μm/year) |
−1.44 (−2.3, −0.4)* |
−1.53 (−2.4, −0.03)* |
−1.48 (−2.4, −0.21) |
0.07 | −1.99 (−2.4, −0.2)* |
−1.61 (−2.1, −0.05)* |
−1.8 (−2.2, −0.12)* |
0.03 | −3.14 (−4.7, −1.2)* |
−3.23 (−4.14, −0.23)* |
−3.18 (−4.37, −0.66)* |
0.35 | <0.001 |
|
Superior MRW (μm/year) |
−0.55 (−1.5, 1.4) | −0.95 (−1.3, 1.2)* |
−0.75 (−1.4, 1.3) | 0.22 | −0.71 (−1.2, 1.6)* |
−0.32 (−1.5, 1.4) | −0.52 (−1.3, 1.6) | 0.26 | −1.77 (−2.85, −0.14)* |
−1.85 (−2.23, −0.7)* |
−1.79 (−2.61, −0.32)* |
0.12 | <0.001 |
Abbreviations: MRW, minimum rim width
significant slope (P-value < 0.05),
P-value evaluates differences in the mean rate of change between AD and ED eyes,
(ANOVA) P-values between “total” mean values among diagnostic groups (without respect to race).
Figure 2.
Distributions of the global Bruch’s membrane minimum rim width (BMO-MRW) estimated rates of change over time (μm/year) for African descent (AD) and European descent (ED) eyes in healthy (left), glaucoma suspect (center) and glaucoma (right) eyes. Change in MRW is significant between AD and ED eyes in suspect eyes only.
Figure 3.
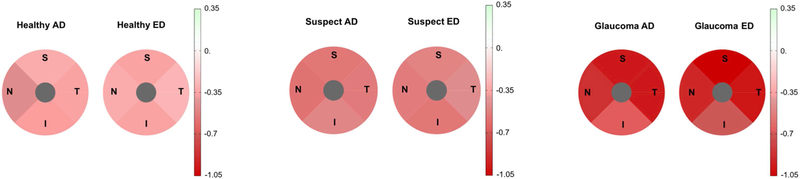
Pie charts showing Bruch’s membrane minimum rim width (BMO-MRW) estimated rates of change over time (μm/year) by sector for African descent (AD) and European descent (ED) eyes in healthy (left), glaucoma suspect (center) and glaucoma (right) eyes. Change in MRW is significant between AD and ED eyes in suspect eyes only. Change location is primarily temporal and inferior in all diagnostic groups.
Baseline global and sectoral RNFLT derived from the RNFL circle scans are presented in Table 4. Healthy eyes had thicker global and sectoral RNFL compared to glaucoma suspect and glaucoma eyes at baseline in all sectors (all comparisons p < 0.001 using ANOVA) except the temporal sector where the RNFLT was similar between healthy eyes and glaucoma suspect eyes (p = 0.23). There was no statistically significant difference in baseline RNFL measurement between AD and ED eyes in any diagnostic group (all comparisons p ≥ 0.07).
Table 4.
Baseline retinal nerve fiber layer (RNFL) thickness from African descent and European descent individuals within diagnostic groups
| Healthy eyes | Glaucoma suspect eyes | Glaucoma eyes | ANOVA* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ED | Total | P- value |
AD | ED | Total | P- value |
AD | ED | Total | P- value |
P- value |
|
|
Global RNFL (um) |
96.1 ± 11.5 | 96.1 ± 12.1 | 96.1 ± 11.7 | 0.50 | 90.2 ± 12.6 | 88.5 ± 10.9 | 89.35 ± 11.3 | 0.28 | 72.0 ± 17.8 | 73.4 ± 20.9 | 72.7 ± 19.1 | 0.31 | <0.001 |
|
Nasal RNFL (um) |
75 ± 14.9 | 75.9 ± 13.4 | 75.45 ± 13.9 | 0.59 | 68.4 ± 13.9 | 67.1 ± 12.7 | 67.75 ± 13.2 | 0.32 | 59.9 ± 16.2 | 60.9 ± 21.3 | 60.4 ± 18.5 | 0.63 | <0.001 |
| Temporal RNFL (um) | 62.1 ± 11.9 | 66 ± 13.1 | 64 ± 12.7 | 0.07 | 64.9 ± 12.4 | 67.0 ± 11.7 | 65.95 ± 11.92 | 0.09 | 55.7 ± 11.9 | 59.0 ± 16.7 | 57.35 ± 12.33 | 0.07 | <0.001 |
| Inferior RNFL (um) | 127.2 ± 19.1 | 123.7 ± 18.0 | 125.95 ± 18.7 | 0.14 | 116.4 ± 20.9 | 112.6 ± 17.5 | 114.7 ± 18.9 | 0.12 | 85.4 ± 32.7 | 88.2 ± 31.6 | 86.8 ± 31.97 | 0.27 | <0.001 |
| Superior RNFL (um) | 117.5 ± 18.3 | 116.6 ± 17.7 | 117.01 ± 18.2 | 0.4 | 111.1 ± 19.5 | 107.3 ± 17.9 | 109.4 ± 18.2 | 0.08 | 87.4 ± 23.6 | 85.7 ± 27.0 | 86.55 ± 25.12 | 0.65 | <0.001 |
Abbreviations: RNFL, retinal nerve fiber layer. All values are mean (±SD) and Wilcoxon rank sum test P-values reported representing the differences between AD and ED eyes.
P-values represent differences across diagnostic groups.
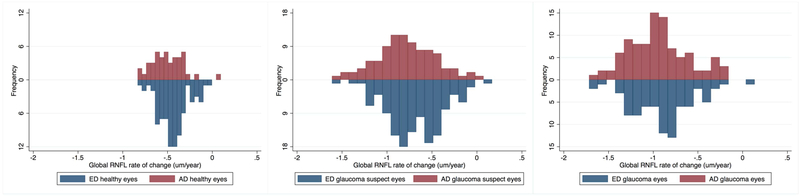
Estimated rates of structural loss of global and sectoral RNFL in healthy, glaucoma suspect and glaucoma eyes adjusted for age, mean IOP during follow-up, CCT, axial length and BMO area are presented in Table 5. Global, nasal, temporal, inferior, and superior RNFLT showed a significant change over time (change greater than 0 μm/year) for all diagnostics groups We observed no race-related differences in rate of change of RNFLT in any of the diagnostic groups (all comparisons p ≥ 0.16). Distributions of rates of change in RNFLT (μm/year) in ED and AD eyes for healthy, suspect and glaucoma groups are shown in Figure 4. Pie charts showing RNFLT rates of change over time by sector in ED and AD eyes for healthy, suspect and glaucoma groups are shown in Figure 5. Values shown also are adjusted for age, mean IOP during follow-up, CCT, axial length, and BMO area.
Table 5.
Estimated rate of change (and 95 percent confidence intervals) in retinal nerve fiber layer (RNFL) thickness for African descent and European descent individuals within diagnostic groups. Values shown are adjusted for age, mean IOP during follow-up, CCT, axial length, and Bruch’s membrane opening area.
| Healthy eys | Glaucoma suspect eyes | Glaucoma eyes | ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ED | Total | P- Value** |
AD | ED | Total | P- Value** |
AD | ED | Total | P- Value** |
P- value*** |
|
|
Global RNFL (um/year) |
−0.51 (−0.7,−0.1) * |
−0.44 (−0.68,−0.01) |
−0.44 (−0.68,−0.01) * |
0.35 | −0.75 (−0.97,−0.52) * |
−0.64 (−0.74,−0.35) * |
−0.75 (−0.97,−0.52) |
0.75 | −0.94 (−1.2,−0.6) * |
−0.89 (−1.19,−0.54) |
−0.91 (−1.19,−0.52) * |
0.4 | 0.02 |
|
Nasal RNFL (um/year) |
−0.49 (−0.6,−0.24) * |
−0.35 (−0.51,−0.07) |
−0.42 (−0.55,−0.12) * |
0.3 | −0.59 (−0.92,−0.24) * |
−0.55 (−0.88,−0.02) * |
−0.57 (−0.91,−0.13) |
0.9 | −0.88 (−1.05,−0.61) * |
−0.91 (−1.12,−0.55) |
−0.9 −1.11,−0.57) * |
0.27 | 0.01 |
|
Temporal RNFL (um/year) |
−0.38 (−0.6,−0.06) * |
−0.31 (−0.41,−0.01) |
−0.34 (−0.52,−0.03) * |
0.8 | −0.56 (−0.78,−0.34) * |
−0.49 (−0.82,−0.18) * |
−0.52 (−0.81,−0.21) |
0.58 | −0.97 (−1.1,−0.46) * |
−0.99 (−1.09,−0.35) |
−0.98 (−1.09,−0.37) * |
0.81 | 0.02 |
|
Inferior RNFL (um/year) |
−0.41 (−0.56,−0.02) * |
−0.38 (−0.52,−0.01) |
−0.39 (−0.54,−0.01) * |
0.39 | −0.51 (−0.72,−0.19) * |
−0.57 (−0.79,−0.34) * |
−0.54 −0.74,−0.22) |
0.27 | −0.69 (−0.92,−0.42) * |
−0.71 (−1.04,−0.41) |
−0.70 (−0.89,−0.45) * |
0.64 | 0.01 |
|
Superior RNFL (um/year) |
−0.35 (−0.52,−0.12) * |
−0.39 (−0.61,−0.09) |
−0.37 (−0.57,−0.11) * |
0.33 | −0.58 (−0.75,−0.15) * |
−0.53 (−0.76,−0.24) * |
−0.55 (−0.76,−0.21) |
0.16 | −0.97 (−1.35,−0.54) * |
−1.05 (−1.28,−0.65) |
−1.01 (−1.29,−0.62) * |
0.38 | 0.004 |
Abbreviations: RNFL, retinal nerve fiber layer thickness.
significant slope (P-value < 0.05),
P-value evaluates differences in the mean rate of change between AD and ED eyes,
(ANOVA) P-values between “total” mean values among diagnostic groups (without respect to race).
Figure 4.
Distributions of the global restinal nerve fiber layer (RNFL) thickness estimated rates of change over time (μm/year) for African descent (AD) and European descent (ED) eyes in healthy (left), glaucoma suspect (center) and glaucoma (right) eyes. No significant differences between AD and ED eyes were observed in any diagnostic category.
Figure 5.
Pie charts showing global retinal nerve fiber layer (RNFL) thickness estimated rates of change over time (μm/year) by sector for African descent (AD) and European descent (ED) eyes in healthy (left), glaucoma suspect (center) and glaucoma (right) eyes. No significant differences between AD and ED eyes were observed in any diagnostic category. Patterns of change vary across all diagnostic groups.
DISCUSSION
The current study showed a faster estimated decrease in global and sectoral minimum neuroretinal rim width over time in glaucoma suspect eyes of AD compared to ED individuals. No such race related differences were observed in healthy or glaucomatous eyes. In addition, no race-related differences in estimated rate of change of RNFL thickness were observed in any of the three diagnostic groups selected. We also showed a significant rate of change in BMO-MRW and RNFL thickness over our relatively short follow up in healthy as well as suspect and glaucoma eyes.
Previous cross-sectional studies have reported that the BMO-MRW has a higher sensitivity for detecting early to moderate glaucoma eyes than RNFL thickness at a fixed specificity of 95%.25 In addition, Rhodes and colleagues reported no cross-sectional difference in BMO-MRW between healthy AD and ED eyes.27 Similarly, our group recently reported that longitudinal change in neuroretinal rim area (measured globally and by quadrant using confocal scanning laser ophthalmoscopy, CSLO) was similar over 5 years in AD and ED eyes with known progressive glaucomatous optic neuropathy (PGON; by masked stereoscopic photograph assessment) at study endpoint.14 The current results are consistent with our previous results because PGON eyes in our previous report were glaucomatous at baseline with glaucomatous appearance of the optic nerve head or parapapillary retina in conjunction with repeatable abnormal VF results (i.e., eyes were similar to the eyes in our glaucoma group that showed no race-related differences). In addition, CSLO neuroretinal rim measurements are not comparable to SDOCT measurements because they are calculated using a standard reference plane placed 50 μm posterior to the optic disc margin along a user drawn contour line while SDOCT neuroretinal rim measurements, in the current study, were made relative to the BMO automatically identified based on z-axis a-scans. It is possible, then, that the current findings represent the detection of earlier structural change in AD eyes with suspicion of glaucoma than in ED eyes with suspicion of glaucoma. That is, the rate of change in BMO-MRW might be more rapid prior to the development of visual field defects in AD eyes than in ED eyes providing earlier evidence of disease related structural change resulting in the previously reported earlier age of glaucoma onset in individuals of AD.12
Other possibilities that may explain our results exist but likely can be ruled out. First, longitudinal structural change is more likely and more rapid in AD eyes compared to ED eyes at higher levels of IOP, but not at lower levels.28 Because of this, mean IOP during follow-up was included in mixed effects models used to calculate rates of change.
It has been shown that there is an interaction between age and race in laminar position in healthy eyes, with laminar position being more anterior with increased age in ED eyes and more posterior with increased age in AD eyes.29 This may have contributed to race-related differences in rate of change in BMO-MRW between races, although we also included age in our mixed effects models (and this interaction has not been demonstrated in glaucoma suspect or glaucomatous eyes). Secondly, CCT is an accepted risk factor for the development of glaucoma in suspect eyes,30 the progression of glaucomatous visual field defects in glaucomatous eyes31 and the development of sustained, significant loss of VF sensitivity in advanced glaucoma32, and is thinner in AD compared to ED eyes.33 For these reasons, we also included CCT as a covariate in our mixed effects models. In addition, because CCT in our sample was thinner on average in AD compared to ED eyes (as expected), we conducted a post hoc analysis in which we compared rate of change of BMO-MRW and RNFL thickness globally and by sector for all diagnostic groups relative to the median CCT in all eyes (median CCT = 549 μm, CCT > 549 μm was compared to CCT < 549 μm). All comparisons resulted in p ≥ 0.29 (i.e., none were significant).
Healthy eyes were younger than glaucoma and glaucoma suspect eyes at baseline in our sample. It is possible that the observed difference in rate of change in BMO-MRW and RNFL thickness is attributable in part to the effect of natural aging and we therefore adjusted for aging in the models.
Admittedly, the significant rates of change reported in the current study are relatively small. To further address this issue and put the magnitude of the difference into perspective, we conducted a post hoc analysis that compared the rate of BMO-MRW thinning in glaucoma eyes with the thickest (top 20th percentile) baseline global MRW and the rate of BMO-MRW thinning in the healthy eyes with the thinnest (lowest 20th percentile) global BMO-MRW. We then compared the mean (95% CI) difference in the rate of change between the glaucoma thickest 20% vs thinnest healthy 20% (−0.43 (−0.53, −0.24) μm /yr), to the BMO-MRW difference in the rate of change in AD vs ED in suspect eyes (−0.38 (−0.51, −0.23) μm /yr); and found almost complete overlap in the 95% CI of these differences with p = 0.18. This overlap suggests that the magnitude of the racial differences in rate of BMO-MRW thinning in suspect eyes is similar to the magnitude of the differences in the rates between healthy and glaucoma eyes.
Finally, classification of AD and ED eyes in the present study was based on self-report. It is known that prevalence of glaucoma in individuals of AD varies somewhat by geographical location likely due in part to genetic diversity and environmental exposure.21 A recent biogeographic ancestry analysis from the ADAGES indicated that based on SNP genotyping of 297 self-reported AD individuals, the mean percentage of African admixture in the ADAGES cohort was 79.6%. Genotyping of 359 self-reported ED individuals indicated the mean percentage of African admixture was 3.5%. 22 As the previous analysis of our ADAGES data suggest, self-reported race is an accurate reflection of biogeographic ancestry.
We did not directly compare rate of change of BMO-MRW to rate of change of RNFL thickness over time among diagnostic groups or between races, although this is a consideration for future research. Recent results presented by Gardiner et al.34, suggest that RNFL thickness may be more useful than MRW or minimum rim area for monitoring glaucomatous change. These authors studied 157 eyes of non-end stage glaucoma patients or high-risk ocular hypertensives over a minimum of approximately 2.5 years. Disease-related change was described as the longitudinal decrease in signal-to-noise ratio (LSNR, defined as the rate of change over time defined by ordinary least squared linear regression divided by the standard deviation of the residuals of rate of change over time, in order to equate measurement scales for all parameters investigated). With this model a more negative LSNR indicates a change in signal greater than observed variability (i.e., a significant disease-related change in tissue measurement).35
In conclusion, these results suggest that Bruch’s membrane opening minimum rim width decreases significantly faster in glaucoma suspect eyes of AD individuals than in glaucoma suspect eyes of ED individuals. In addition, over a relatively short period of time, both SDOCT-measured minimum rim width and retinal nerve fiber layer thickness decrease, even in healthy eyes. These results confirm the need to consider strongly the influence of race and the need to dissociate the effects of natural aging and disease related change in the assessment of glaucoma progression.
ACKNOWLEDGEMENTS/DISCLOSURE
a. Funding/Support
This work was supported by the National Eye Institute of the National Institutes of Health [grant numbers EY022039, EY027945, EY11008, P30EY022589, EY026590, EY027510, EY026574, EY023704, EY026574, EY018926]; Eyesight Foundation of Alabama; Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; Edith C. Blum Research Fund, Columbia University Medical Center, New York, NY; participant retention incentive grants in the form of glaucoma medication at no cost from Alcon Laboratories Inc, Allergan, Alcon, Pfizer Inc, and Santen Inc. and an unrestricted grant from Research to Prevent Blindness, New York, New York.
b. Financial Disclosures C Bowd: None.
LM Zangwill: Financial Support: Carl Zeiss Meditec, Heidelberg Engineering, Optovue, Topcon Medical Systems. Recipient: Optovue, Topcon Medical Systems.
RN Weinreb: Consultant: Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Eyenovia, Novartis, Sensimed, Unity, Valeant; Financial Support: Heidelberg Engineering, Carl Zeiss Meditec, Genentech, Konan, Optovue, Topcon, Optos, Centervue.
MA Fazio: Financial Support: EyeSight Foundation of Alabama, Research to Prevent Blindness, Heidelberg Engineering.
CA Girkin: Financial Support: EyeSight Foundation of Alabama, Research to Prevent Blindness, Heidelberg Engineering.
JM Liebmann: Consultant: Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Reichert, Valeant Pharmaceuticals; Financial Support: Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Optovue, Reichert, Topcon.
A Belghith: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Friedman DS, Jampel HD, Munoz B, West SK. The prevalence of open-angle glaucoma among blacks and whites 73 years and older: the Salisbury Eye Evaluation Glaucoma Study. Arch Ophthalmol 2006;124(11):1625–1630. [DOI] [PubMed] [Google Scholar]
- 2.Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol 2000;118(6):819–825. [DOI] [PubMed] [Google Scholar]
- 3.Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996; 103(11):1721–1726. [DOI] [PubMed] [Google Scholar]
- 4.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. The New England journal of medicine. 1991. ;325(20):1412–1417. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MR. Glaucoma in blacks: where do we go from here? JAMA. 1989;261(2):281–282. [PubMed] [Google Scholar]
- 6.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 7.Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol 1994;112(6):821–829. [DOI] [PubMed] [Google Scholar]
- 8.Mason RP, Kosoko O, Wilson MR, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies. Part I. Prevalence findings. Ophthalmology. 1989;96(9):1363–1368. [DOI] [PubMed] [Google Scholar]
- 9.Martin MJ, Sommer A, Gold EB, Diamond EL. Race and primary open-angle glaucoma. Am J Ophthalmol 1985;99(4):383–387. [DOI] [PubMed] [Google Scholar]
- 10.Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci 2006;47(10):4254–4261. [DOI] [PubMed] [Google Scholar]
- 11.Wilensky JT, Gandhi N, Pan T. Racial influences in open-angle glaucoma. Ann Ophthalmol 1978;10(10):1398–1402. [PubMed] [Google Scholar]
- 12.Wilson R, Richardson TM, Hertzmark E, Grant WM. Race as a risk factor for progressive glaucomatous damage. Ann Ophthalmol 1985;17(10):653–659. [PubMed] [Google Scholar]
- 13.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol 2010;128(5):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammel N, Belghith A, Bowd C, et al. Rate and Pattern of Rim Area Loss in Healthy and Progressing Glaucoma Eyes. Ophthalmology. 2016;123(4):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 2010;128(5):541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girkin CA, McGwin G Jr., Sinai MJ, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118(12):2403–2408. [DOI] [PubMed] [Google Scholar]
- 17.Kashani AH, Zimmer-Galler IE, Shah SM, et al. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol 2010;149(3):496–502 e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racette L, Boden C, Kleinhandler SL, et al. Differences in visual function and optic nerve structure between healthy eyes of blacks and whites. Arch Ophthalmol 2005;123(11):1547–1553. [DOI] [PubMed] [Google Scholar]
- 19.Tjon-Fo-Sang MJ, de Vries J, Lemij HG. Measurement by nerve fiber analyzer of retinal nerve fiber layer thickness in normal subjects and patients with ocular hypertension. Am J Ophthalmol 1996;122(2):220–227. [DOI] [PubMed] [Google Scholar]
- 20.Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. Journal of the National Medical Association. 2006;98(10):1626–1629. [PMC free article] [PubMed] [Google Scholar]
- 21.Girkin CA, Nievergelt CM, Kuo JZ, et al. Biogeographic ancestry in the African Descent and Glaucoma Evaluation Study (ADAGES): Association with corneal and optic nerve structure. Invest Ophthalmol Vis Sci 2015;56:2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belghith A, Bowd C, Yousefi S, et al. Measurement of the peripapillary retinal layer thickness in glaucoma and healthy eyes using San Diego Automated Layer Segmentation Algorithm. Association for Research in Vision and Ophthalmology 2015 Imaging in the Eye Conference; 2015; Denver, CO. . [Google Scholar]
- 24.Belghith A, Medeiros FA, Bowd C, et al. Structural change can be detected in advanced-glaucoma eyes. Invest Ophth Vis Sci 2016;57(9):Oct511–Oct518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan BC, O'Leary N, Almobarak FA, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120(3):535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reis AS, Sharpe GP, Yang H, Nicolela MT, Burgoyne CF, Chauhan BC. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012;119(4):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes LA, Huisingh CE, Quinn AE, et al. Comparison of Bruch's Membrane Opening Minimum Rim Width Among Those With Normal Ocular Health by Race. American journal of ophthalmology. 2017;174:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khachatryan N, Medeiros FA, Sharpsten L, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): Predictors of Visual Field Damage in Glaucoma Suspects. Am J Ophthalmol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodes LA, Huisingh C, Johnstone J, et al. Variation of laminar depth in normal eyes with age and race. Invest Ophthalmol Vis Sci 2014;55(12):8123–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):714–720; discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 31.Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study G. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 2001;131(6):699–708. [DOI] [PubMed] [Google Scholar]
- 32.The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Ophthalmol 2002;134(4):499–512. [DOI] [PubMed] [Google Scholar]
- 33.La Rosa FA, Gross RL, Orengo-Nania S. Central corneal thickness of Caucasians and African Americans in glaucomatous and nonglaucomatous populations. Archives of ophthalmology. 2001;119(1):23–27. [PubMed] [Google Scholar]
- 34.Gardiner SK, Boey PY, Yang H, Fortune B, Burgoyne CF, Demirel S. Structural Measurements for Monitoring Change in Glaucoma: Comparing Retinal Nerve Fiber Layer Thickness With Minimum Rim Width and Area. Invest Ophthalmol Vis Sci 2015;56(11):6886–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardiner SK, Fortune B, Demirel S. Signal-to-noise ratios for structural and functional tests in glaucoma. Transl Vis Sci Technol 2013;2(6):3. [DOI] [PMC free article] [PubMed] [Google Scholar]