Abstract
Purpose of review:
This review examines what is known about the FGF-23/α-Klotho co-dependent and independent pathophysiological effects, and whether FGF-23 and/or α-Klotho are potential therapeutic targets.
Recent findings:
FGF-23 is a hormone derived mainly from bone, and α-Klotho is a transmembrane protein. Together they form a trimeric signaling complex with FGFRs in target tissues to mediate the physiological functions of FGF-23. Local and systemic factors control FGF-23 release from osteoblast/osteocytes in bone, and circulating FGF-23 activates FGFR/α-Klotho complexes in kidney proximal and distal renal tubules to regulate renal phosphate excretion, 1,25(OH)2D metabolism, sodium and calcium reabsorption, and ACE2 and α-Klotho expression. The resulting bone-renal-cardiac-immune networks provide a new understanding of bone and mineral homeostasis, as well as identify other biological effects FGF-23. Direct FGF-23 activation of FGFRs in the absence of α-Klotho is proposed to mediate cardiotoxic and adverse innate immune effects of excess FGF-23, particularly in chronic kidney disease, but this FGF-23, α-Klotho independent signaling is controversial. In addition, circulating soluble Klotho (sKl) released from the distal tubule by ectodomain shedding is proposed to have beneficial health effects independent of FGF-23.
Summary:
Separation of FGF-23 and α-Klotho independent functions has been difficult in mammalian systems and understanding FGF-23/α-Klotho co-dependent and independent effects are incomplete. Antagonism of FGF-23 is important in treatment of hypophosphatemic disorders caused by excess FGF-23, but its role in chronic kidney disease is uncertain. Administration of recombinant sKl is an unproven therapeutic strategy that theoretically could improve the healthspan and lifespan of patients with α-Klotho deficiency.
Keywords: Fibroblast growth factor 23 (FGF-23), α-Klotho, fibroblast growth factor receptors, hypophosphatemia, innate immunity, cardiovascular, kidney, bone
Introduction
FGF-23 is a member of the hormonal FGF subfamily that also includes FGF-19 and FGF-21 that emerged during the evolution of vertebrates to activate FGF receptors complexed with Klotho co-receptors [1]. FGF-23 activates FGFR 1c, −3c and −4, but not FGFR-2, in the presence of cell surface membrane α-Klotho [2].
α-Klotho is a single-pass transmembrane cell surface protein with extracellular KL1 (Glu-34 to Phe-506) and KL2 (Leu-515 to Ser-950) domains [3]. The N-terminus of FGF-23 and the KL2 domain of α-Klotho interact with FGFRs and the C-terminus of FGF-23 binds to a pocket created by the KL1 and KL2 domains to form the active ternary receptor complex [3]. This canonical FGF-23/FGFR/α-Klotho co-dependent signaling limits FGF-23 effects to tissues expressing α-Klotho (Figure 1, middle circle) [2].
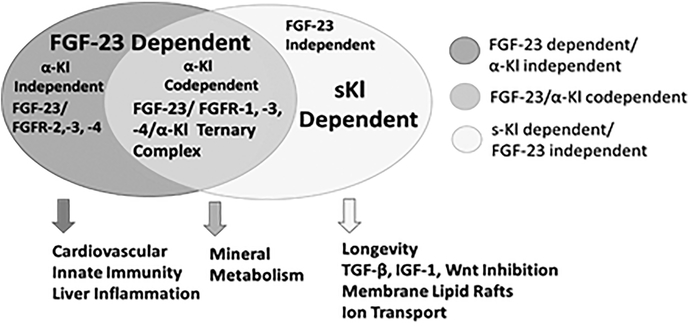
Figure 1. Venn diagram of FGF-23 and α-Kl signaling mechanisms and pathophysiological functions.
FGF-23/α-Kl co-dependent canonical signaling (middle circle), α-Kl dependent/FGF-23 independent sKL signaling (right circle), and FGF-23 dependent/ α-Kl independent non-canonical signaling (left circle).
α-Klotho orthologues with a single KL1-like domain emerged during evolution before FGF-23, indicating the presence of FGF-23 independent functions [4]. In mammalians, α-Klotho undergoes ectodomain shedding and subsequent proteolytic cleavage to generate soluble KL1 and KL2 (sKl) circulating protein fragments. Circulating sKl imparts a multitude of purported FGF-23 independent functions [5] (Figure 1, left circle). Recent studies suggest that FGF-23 can directly activate FGFs in the absence of membrane α-Klotho [6] (Figure 1, right circle).
Canonical FGF-23-α-Klotho dependent functions.
FGF-23 is expressed predominately in osteoblasts/osteocytes (Obs/Ocys) in bone, at low levels in venous sinusoids of bone marrow, thymus, and lymph nodes, and in ventral lateral thalamic nuclei of the brain; in disease states, FGF-23 may be ectopically expressed in tissues that do not normally express FGF-23 [7]. Established physiological functions of FGF-23 are mediated by activation of cell surface FGFR/α-Klotho binary receptor complexes located in in proximal and distal tubules of the kidney to regulate renal phosphate, sodium, and calcium reabsorption, 1,25(OH)2D metabolism, angiotensin converting enzyme 2 and α-Klotho expression [7]. The co-dependent functions of FGF-23 and α-Klotho are supported by the fact that FGF-23−/− and α-Kl−/− mice are exact phenocopies, α-Kl−/− mice are refractory to FGF-23 effects, and compound mutant FGF-23 and α-Klotho have non-additive effects [8–12].
Normal mineral homeostasis requires FGF-23 levels in a narrow range of 50 to 200 pg/ml. Elevations of FGF-23 beyond this range result in hypophosphatemia, low 1,25(OH)2D levels and rickets/osteomalacia, while FGF-23 deficiency causes hyperphosphatemia, excess 1,25(OH)2D, and soft tissue calcifications (i.e., tumoral calcinosis).
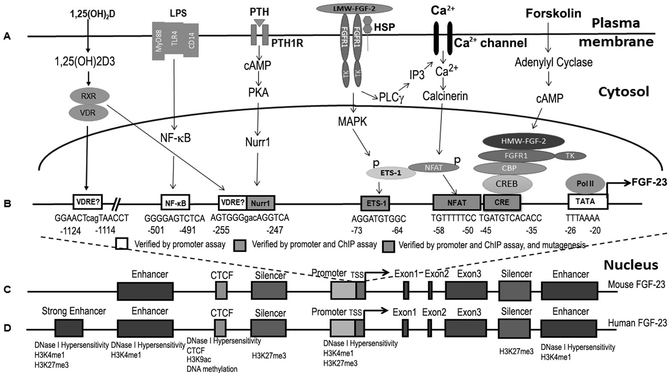
Transcriptional regulation plays a key role in determining circulating levels of FGF-23. The FGF-23 gene locus has a proximal promoter, four enhancers and two silencer regions that are conserved between mice and humans [13]. Local and systemic factors coordinately regulate FGF-23 gene transcription in Obs/Ocys to increase circulating FGF-23 that create afferent limbs of several endocrine networks [7]. Posttranslational processing of FGF-23, including phosphorylation, glycosylation, and proteolysis, also contributes to its circulating bioactivity [14]. FGF-23 contains a R(176)XXR(179)/S(180)AE subtilisin-like proprotein convertase motif between its N- and C-terminus. Proprotein convertases cleave FGF-23 into inactive fragments in a pre-release degradative pathway. Polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3) mediates O-glycosyation of FGF-23 prevents cleavage, while the family with sequence similarity 20, member C (Fam20C), phosphorylates FGF-23 to prevent O-glycosylation and promote FGF-23 cleavage by unknown subtilisin-like proprotein convertases [15]. Recently, plasminogen activator inhibitor-1 (PAI-1), a serine protease inhibitor (SERPIN), inhibits cleavage of FGF-23, presumably through inhibition of tPA and uPA [16].
This FGF23/α-Klotho co-dependent functions have been implicated in: 1) mineral metabolism (i.e., local factors linked to mineralization of extracellular matrix, and systemic effects of PTH, 1,25(OH)2D, and calcium) [17], 2) inflammation (i.e., LPS and TNFα)[18], 3) cardiovascular (CV) homeostasis (i.e., Renin-Angiotensin-Aldosterone and Sympathetic Nervous System)[19,20], 4) energy metabolism (i.e., insulin) [21,22], and 5) hematopoiesis (i.e., Hif1α, iron deficiency and erythropoietin) [23,24].
Bone and mineral metabolism.
One established function of the FGF-23/FGFR/α-Klotho ternary complex in all vertebrates from fish to humans is to coordinate bone mineral metabolism with renal handling of phosphate. FGF-23 production in bone is regulated by factors involved in extracellular matrix mineralization [17], including mutations in Phex, dentin matrix protein 1 (Dmp1), Enpp1, and FAM20C.
Phex and Dmp1 work through a common pathway involving FGFR1, which also contributes to increased FGF-23 in chronic kidney disease (CKD) models [25–27]. In addition, HMW-FGF2, which is derived from alternative translation, functions as an intracellular ligand for FGFR1 in Hyp mice [28]. Transgenic HMW-FGF2 reproduce the phenotype of Hyp mice [26,29].
Other pathways linking bone mineralization and FGF-23 involve pyrophosphate (PPi) metabolism [30,31]. ENPP1 generates extracellular PPi, a physiochemical inhibitor of mineralization through the hydrolysis of extracellular ATP, and PPi is metabolized to phosphate (P) by tissue non-specific alkaline phosphatase (TNAP) to provide phosphate form mineralization. Inactivation of ENPP1 would be predicted to decrease local concentrations of PPi and P and increase local ATP levels [32], which might regulate FGF-23 through purinergic receptors [33] or unknown metabolic (intracellular) or endocrine (extracellular) phosphate sensor in osteoblasts [34–37].
Multiple systemic factors also converge to regulate FGF-23 gene transcription. 1,25(OH)2D, extracellular calcium, and PTH/cAMP dependent signaling pathways, but surprisingly not phosphorus, stimulate FGF-23 production in Obs/Ocys [38]. The store operated Ca2+ entry (SOCE) also regulates FGF-23 expression in osteocytes [20]. The PTH receptor and GαS signaling regulates FGF-23 transcription in osteocytes [39]. Although serum phosphate levels positively correlate with elevations in FGF23 levels in ESRD [40], phosphate restriction has variable, limited, and delayed effects to lower elevated FGF23 levels animal models and patients with CKD [41,42].
FGF-23 targets FGFR/α-Klotho complexes in both the proximal and distal tubule. Recent studies show that conditional deletion of α-Klotho in the proximal tubules results in loss of FGF-23 effects on inhibit Npt2a and Npt2c sodium-dependent phosphate co-transporters and suppression of Cyp27b1 and upregulation of Cyp24a1 [43]. FGFR3 and FGFR4 preferentially regulate vitamin D metabolism in the proximal tubule [44], whereas FGFR1 is most important in mediating the effects of FGF-23 on phosphate and calcium transport in the proximal and distal tubules [45]. The mechanisms underlying these differential renal tubule effects of FGFR1, 3 and 4 are unknown.
Physiologically, local factors linked to mineralization of extracellular matrix in conjunction with PTH, 1,25(OH)2D and circulating calcium, regulate FGF-23, which targets the kidney to match the phosphate buffering capacity/mineralization of bone [46]. There is also a FGF-23 vitamin D counter-regulatory loop, whereby 1,25(OH)2D stimulates FGF-23 and FGF-23 suppresses 1,25(OH)2D levels by inhibiting Cyp27B1 and stimulating Cyp24A1 in the renal proximal tubule [47]. In addition, there is a calcium-FGF-23 endocrine loop, whereby calcium stimulates FGF-23 in bone and FGF-23 stimulates calcium reabsorption in the distal tubule [45,48].
Originally it was proposed that PTH stimulates FGF-23 in bone and FGF-23 suppresses PTH production/secretion in the PTG to create a feed-back loop [49]. Recent studies that conditionally deleted FGFRs and α-Klotho in the PTG found that FGF-23 stimulates PTH secretion and cell proliferation [50]. Clinical disorders of excess FGF-23 are typically characterized by elevated PTH.
Canonical FGF-23/FGFR/α-Klotho signaling is important in several diseases. Excess FGF-23 causes rare hereditary hypophosphatemic disorders in humans and mouse disease homologues, including X-linked hypophosphatemic rickets (XLH)/Hyp mice, caused by inactivating mutations of Phex [51], autosomal recessive hypophosphatemic rickets 1 (ARHR1), caused by inactivating mutations of Dmp1 [52], ARHR2, caused by inactivating mutations in Enpp1 [30], and Raine Syndrome (RNS), caused by inactivation mutations in FAM20C [53]. Tumor-induced osteomalacia (TIO) is an acquired paraneoplastic disorder caused by excess FGF-23 produced by tumors [40].
Increased FGF-23 also plays an adaptive role in maintaining phosphate homeostasis [40], and also suppresses 1,25(OH)2D as an additional adaptive response to alter mineral metabolism in in early chronic kidney disease (CKD) [46,54]. It appears that increased FGF-23 gene transcription in bone is the initial event leading to reductions in 1,25(OH)2D and secondary elevations in PTH in CKD.
There are several clinical disorders caused by defects in FGF-23 processing [16]. GalNAc-T3 inactivating mutations cause autosomal recessive familial tumoral calcinosis by reducing O-glycosylation leading to increased FGF-23 degradation [55]. In contrast, Raines syndrome, a disorder of FGF-23 excess, is caused by inactivation mutations in FAM20C, leading to decreased phosphorylation of FGF-23, enhanced O-glycosylation and decreased degradatation of FGF-23 [14]. In some studies [56], but not others [40], FGF-23 degradation is reported to be inhibited in CKD.
Immunity and inflammation.
Elevated FGF-23 is linked to inflammation and adverse infection outcomes in CKD [57]. FGF-23 is proposed to impair host responses to infection and impair innate immune responses [58,59]. FGF-23 is expressed in endothelial cells in the venous sinusoid of bone marrow, as well as in the thymus and spleen [9]. The function of FGF-23 in these sites is not clear, but this location implicates a role of FGF-23 in regulating macrophage, neutrophils, T-cell and/or B-cell functions. FGF-23 effects to activate immune cells in the inflammatory milieu [7,18] may lead to adverse outcomes associated with bacterial infections.
A paracrine role for FGF-23 in regulation of innate immune responses has been described that consists of the ectopic production of FGF-23 and α-Kl in activated macrophages to reconstitute canonical FGFR/α-Klotho signaling in the inflammatory milieu [7,18] (Figure 3). Resting macrophages do not normal express either FGF-23 or α-Klotho. Inflammation stimulates the production of FGF-23 locally in M1 activated macrophages [18]. As noted above, circulating FGF-23 is stimulated in both animal models and humans in response to infection, inflammation, and oxidative stress. TNFα also stimulates FGF-23 gene transcription in osteoblasts in vitro [18].
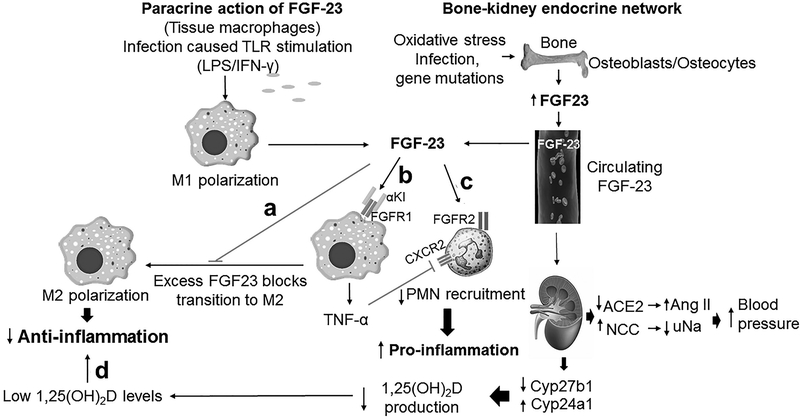
Figure 3. FGF-23 regulates innate immune responses.
Inflammation stimulates FGF-23 expression in bone, and ectopic expression of FGF-23 in M1 macrophages to create both systemic and paracrine signaling. a. FGF-23 blocks macrophage transition to M2 and resolution of inflammation. b. FGF-23 activates FGFR/α-Kl complexes in macrophages to stimulate TNF-α and promote inflammation. c. FGF-23 may activate FGFR2 to impair PMN recruitment. d. FGF-23 through FGFR/α-Kl in the kidney suppresses 1,25(OH)2D, creating networks that further modulate host responses to infection.
Systemic elevations of FGF-23 may indirectly impact innate immune responses through suppression of 1,25(OH)2D production by the kidney. Low vitamin D and high FGF-23 serum levels are associated with infectious and cardiac deaths in a large cohort of patients with end stage renal disease (ESRD) [60,61]. Microbiota induced inflammation in the germ free mice inhibits FGF-23 and suppresses TNF-α to modulate vitamin D homeostasis [62].
Cardiovascular homeostasis.
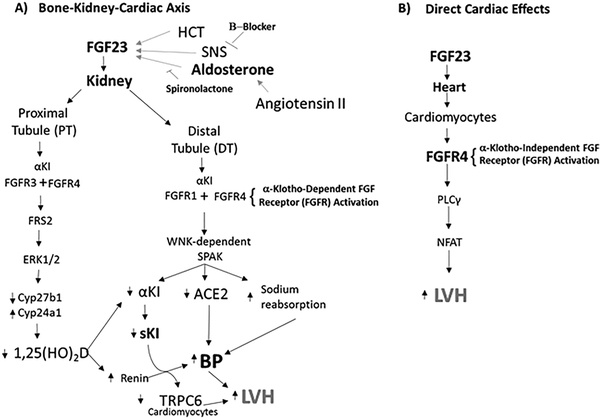
Chronic elevations of FGF-23 are linked to increased mortality and cardiovascular disease in CKD and in the normal population [63]. The cardiac effects of FGF-23 are potentially explained by a bone-renal-cardiac axis created by FGF-23 “on-target” activation of FGFR/α-Klotho binary complexes in the kidney. The afferent limb of this bone-renal-cardiac axis consists of aldosterone, Ang II, and β-adrenergic pathways stimulate FGF-23 gene expression in bone [19,20]. The efferent limb consists of FGF-23 activation of FGFRs/α-Kl in renal tubules through at least four mechanisms (Figure 4). First, FGF-23 could activate RAAS through either suppression of 1,25 (OH)2D, which would increase renin expression [64], and/or reduction of ACE2 expression. Both RAAS activation and ACE2 insufficiency are linked to cardiac hypertrophy and myocardial fibrosis, and deletion of ACE2 in the renal proximal tubule (PT) in mice results in HTN. Ang II and excess FGF-23 have additive effects on induction of LVH in mouse models [19,65]. Second, FGF-23 administration to mice induces HTN and LVH through stimulation of sodium-chloride cotransporter (NCC) in the renal distal tubule (DT) leading to sodium retention [66]. Third, FGF-23 and α-Klotho, which are not expressed in the normal heart, are ectopically expressed in the stressed myocardium [67], which may potential reconstitute canonical signaling in a paracrine manner. Fourth, FGF-23 suppresses kidney expression of α-Kl [65], which may lead to LVH through loss of soluble Klotho (sKl) cardioprotective effect (i.e., α-Klotho independent effects, vide infra) [68].
Figure 4. Indirect and Direct Mechanisms of FGF-23 cardiac effects.
A) Canonical mechanisms whereby FGF-23 regulates cardiovascular functions through a bone-kidney-cardiac endocrine network. LVH could be due to effects of FGF-23 to suppress ACE2 expression or stimulate distal tubular sodium reabsorption. Alternatively, FGF-23 induced LVH could be mediated by suppression of sKL release by the kidney and the effect of sKl to increase TRP6 in the heart. B) Proposed non-canonical direct effects of FGF-23 on FGFR4 in the heart.
Other canonical effects.
Insulin suppresses FGF-23 production [21], which should lead to renal phosphate conservation to match insulin effects on cellular glucose and phosphate uptake homeostasis. FGF-23 may also induce insulin-resistance and glucose intolerance via effects on bone, liver, and kidney [69]. The role of FGF-23 in regulating energy metabolism, however, is not supported by clinical studies that show normal glucose levels in XLH patients [70].
FGF23/FGFR/α-Klotho signaling has also been implicated in prostate cancer progression [71]. FGF-23 is ectopically expressed in prostate cancer cell lines, and FGF-23 stimulates prostate cancer cell proliferation in vitro and in vivo [71]. Release of FGF-23 from prostate cancer cells is a cause of paraneoplastic hypophosphatemia [72].
Factors that regulate erythropoiesis, including erythropoietin, iron deficiency, and HIF1α, also stimulate FGF-23 expression in bone and bone marrow [23,24]. In mice, rhEPO is variably reported to induced FGF23 mRNA in bone and bone marrow in erythroid precursors, a site that does not normally express FGF-23 [73]. Parenteral iron administration transiently stimulates FGF-23 production and inhibits degradation, whereas iron deficiency also stimulates FGF23 production and bone FGF-23 mRNA expression, and correction of iron deficiency can suppress FGF-23 in ADHR [23].
FGF-23 is expressed in the ventrolateral thalamic nucleus in the brain and α-Klotho is expressed in the choroid plexus and hippocampal synaptosomes [74]. The function of FGF-23/FGFR/α-Klotho in the central nervous system is not clear [75]. Phosphate concentrations are very low in the CSF, and it is tempting to speculate that a possible function is for FGF-23 to promote phosphate removal from the CSF via activation FGFR/α-Klotho mediated phosphate transport. FGF-23 may also play a role in the maturation of hippocampal neurons and in cognition [76].
FGF-23 effect independent of α-Klotho (non-canonical effects of FGF-23).
Several groups have proposed that FGF-23 can activate FGFRs in the absence of α-Klotho. High concentrations of FGF-23 do activate FGFR signaling in the absence of α-Kl in vitro, under some conditions, but the effect is small compared to FGF-23 activation of FGFRs in the presence of α-Klotho [77]. Non-canonical effects of FGF-23 have been reported in the heart, PMNs and liver that do not normally express α-Klotho. Several studies suggest that FGF-23 directly activates FGFR4 in the myocardium to stimulate LVH [6,77] and in hepatocytes to stimulate cytokine production [78], and FGFR2 in PMNs to impair host response to infection [58].
Direct activation of FGFRs by FGF-23 in the absence of α-Klotho is inconsistent with the functional and structural data regarding the obligate requirement of α-Klotho for FGF-23 tissue restricted activation of FGFRs [3]. It is difficult to conceive how non-specific activation of FGFRs by FGF-23 would be limited to only a few tissues. Low expression of α-Klotho, below the detection limit of immunohistochemistry, has been shown to impart canonical signaling in the proximal tubule and osteoblasts, and confounds attributing FGF-23 to direct activation of FGFRs [43,79]. It is also difficult to exclude α-Klotho dependent effects due to the ectopic expression of α-Klotho in the heart and immune cells and because sKl130 may be able to substitute for membrane α-Klotho in FGF-23 dependent FGFR activation [3,18].
The evidence that FGFR4 is directly activated by FGF-23 in the myocardium and liver lacks rigor [77,78]. There are weaknesses in study designs, including failure to conditionally ablate FGFR4 in the heart or hepatocytes; and reliance on global FGFR4−/− mice, which could have effects due to loss of FGFR4 in the kidney or other tissues. With regard to the cardiac effects, existing studies did not administer rFGF-23 to FGFR4−/− mice to test if loss of FGFR4 prevents FGF-23 induced LVH; rather studies used dietary phosphate loading to secondarily elevate FGF-23 levels without controlling for cofounding effects of hyperphosphatemia [77]. Circulating concentrations of FGF-21 are also increased in CKD, yet activation of FGFR4 signaling through FGF-21 is purported to have cardioprotective effects [80]. It is not clear why FGF-23, but not FGF-21, activation of FGFR4 would have different direct effects on the myocardium. If FGFR2 and FGFR4 activation by FGF-23 can occur in the absence of membrane α-Kl and/or presence of circulating s-Kl, new concepts are needed to explain the observed tissue restrictive functions of FGF-23 [2].
α-Klotho-independent effects.
α-Klotho orthologues emerged earlier in evolution than FGF-23, indicating the presence of FGF-23 independent functions. C50F7.10 and E02H9.5, the Klotho orthologues present in Caenorhabditis elegans, encode only the K1 domain and lacks the structural features necessary to form binary complexes with FGFRs. Klotho orthologues in these invertebrates inhibit DAF-2, which is homologous to IGF1 signaling in mammals. There is compelling evidence the α-Klotho has effects independent of FGF-23. For example, transgenic overexpression of α-Kl is associated with increased longevity [5], a phenotype distinct from FGF-23 excess [4]. Moreover, injection of sKl130 elicits biological responses distinct from FGF-23. Enhancing Klotho levels in the brain are proposed to enhance cognition and prevent age-associated neurodegenerative, demyelinating [76].
α-Klotho is highly expressed in the distal tubule of the kidney; where its level of expression is more than what is needed to impart FGF-23 signaling, indicating that the distal tubule of the kidney is an endocrine organ that releases sKl into the circulation [12]. α-Klotho undergoes ADAM 10 and 17 dependent ectodoman shedding to release soluble Kl1+Kl2 proteins (sKl130). In addition, a 176Arg-His-Thr-Arg179 proteolytic cleavage site allows genesis of distinct Kl1 (Glu-34 to Phe-506) and Kl2 (Leu-515 to Ser-950) fragments. There is also an alternatively spliced α-Klotho mRNA that may not be translated into a protein in mammalian systems [81,82]. The relative concentrations of the sKl130 (sKl1+ Kl2) and sKl65 (sKl1) proteins and their respective biological contributions remain unclear [83]. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17 [84]. α-Klotho gene transcription in the kidney is also stimulated by 1,25(OH)2D [85] and suppressed by FGF-23 [65]. Reductions in sKl are observed in CKD [86].
There are several possible mechanisms mediating the FGF-23 independent effects of α-Klotho. The sKl soluble isoforms may act as a decoy receptor to disrupt IGF1, Wnt and TGF-β signaling [87]. sKl is reported to exert cardioprotective effects by downregulating TRPC6 channels in cardiomyocytes [88], and to inhibit RAAS and normalize blood pressure in CKD mouse models [89]. Other FGF-23 independent functions are likely due to α-Klotho sialidase activity that stabilizes membrane expression of transient receptor potential cation channel, subfamily V, member 5 (TRPV5) [90,91] and renal outer medullary potassium channel 1 (ROMK1) [92]. Finally, sKl is reported to bind to membrane lipid rafts to disrupt lipid organization and down-regulate raft-dependent PI3K/Akt signaling [93]. The biological effects of sKl differ from FGF-23. Moreover, FGF-23 mediated suppression of sKl, rather than direct effects of FGF-23 on FGFR4, may account for the cardiotoxic effects of FGF-23 on the heart [94].
Treatment considerations.
The ultimate goal of understanding the co-dependent and independent effects of FGF-23 and α-Klotho is to develop new treatments to modulate the activity of these signaling networks to correct the adverse effects observed in various disease states. These putative FGF-23 independent effects of α-Klotho, however, are difficult to quantify and separate from the co-dependent actions of FGF-23 using mouse genetic approaches [5]. The best evidence for separate functions of FGF-23 and sKl are derived from pharmacological studies that show that the administration of FGF-23 or sKl produce opposite cardiotoxic and cardioprotective effects [94], suggesting that potential therapies might be designed to inhibit FGF-23 and to administer recombinant sKl.
Treatment of the rare hereditary hypophosphatemic disorders traditionally consisted of 1,25(OH)2D and phosphate supplements, which does not cure the disease, and is associated with toxicities related to excess phosphate and 1,25(OH)2D, including nephrocalcinosis. Recently, KRN23 (Crysvita, burosumab) has been approved for treatment of XLH [95]. KRN23 is an FGF-23 blocking antibody. KRN23 subcutaneously administration improves rickets and increases serum phosphate levels in XLH [95]. The disadvantages of KRN23 are systemic administration, long-half life, and cost. Small molecule inhibitors of FGF-23 have also been identified that may offer advantage over a blocking antibody [96].
An open question is whether inhibition of FGF-23 has a role to prevent the cardiotoxic and impairment of immune response attributed to excess FGF-23. One problem is that a causative role of FGF-23 in cardiovascular complications remains controversial. Indeed, recent studies failed to identify LVH in either animal homologues of XLH [97] or patients with XLH [70]. Even if inhibition of elevated FGF-23 proves to be cardioprotective in diseases, such as CKD, the therapeutic window is narrow, and preclinical studies in CKD models show that inhibiting FGF-23 with a potent, long acting blocking antibody increases mortality due to oversuppression of FGF-23 [98].
sKl is suppressed in CKD, and administration of sKl prevents cardiovascular complications in mouse models. To date, sKl130 has not been pursued as a therapy, possibly because of the potential of off-target effects from stimulating canonical FGF-23 signaling. Preclinical studies show that Ang II cardiotoxicity is exacerbated in transgenic α-Klotho mice [99], whereas other studies show that sKl130 administration attenuates cardiovascular toxicity [100]. Studies are lacking that examine the therapeutic effects of the “ancestral” Kl65 isoform that is most likely to have FGF-23 independent functions.
Conclusion.
A new understanding of mineral metabolism, cardiovascular homeostasis and innate immunity has been discovered by investigating the FGF-23 and α-Klotho co-dependent and independent physiological effects. Abnormalities of these signaling pathways underlie bone and mineral disorders as well as cardiovascular and infectious diseases. While the therapeutic role of FGF-23 inhibition has been established for hypophosphatemic disorders caused by excess FGF-23, the role of FGF-23 antagonists and recombinant sKl administration to prevent cardiovascular and innate immune disorders that occur CKD remain to be determined.
Figure 2. Transcriptional control of FGF-23.
A) Cell signaling pathways and B) transcription factors and cis-elements in the proximal promoter regulating FGF-23 gene transcription. C) Mouse FGF-23 gene showing proximal promoter, silencer region, transcriptional repressor CTCF and four enhancers. D) Location of the potential enhancer regions by histone modifications and CTCF binding at −38, −16, −10, and + 7kb from the TSS across the FGF-23 genetic locus.
Key points:
Canonical FGF-23 and α-Klotho codependent signaling defines unexpected bone-renal endocrine networks regulating phosphate, calcium, and vitamin D metabolism.
FGF-23 canonical signaling in the kidney creates a bone-renal-cardiac axis that regulates blood pressure and leads to left ventricular hypertrophy through effects to stimulate sodium reabsorption, suppress ACE2 expression and reduce α-Klotho transcription by the kidney.
Ectodomain shedding of α-Klotho from the kidney generates sKl that acts as a circulating hormone with actions distinct from those of FGF-23.
Antagonism of FGF-23 is a proven treatment for hypophosphatemic disorders caused by excess FGF-23.
Chronic kidney disease is characterized by FGF-23 excess and sKl deficiency, but whether FGF-23 antagonists or sKl administration improve outcomes in CKD remains to be established.
Acknowledgements
Financial support and sponsorship
This work was supported by the National Institutes of Health (NIH) grant number 1R01AR045955 from NIAMS.
Footnotes
Conflicts of interest
Dr. Quarles has received honoraria from Amgen for serving on a scientific advisory board.
Reference Section
- 1.Itoh N, Ornitz DM: Functional evolutionary history of the mouse fgf gene family. Dev Dyn (2008) 237(1):18–27. [DOI] [PubMed] [Google Scholar]
- 2. *.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical fgf receptor into a specific receptor for fgf23. Nature (2006) 444(7120):770–774. The seminial observations showing that α-Klotho is the obligate co-receptor required for FGF-23 activation of FGFRs in target tissues. [DOI] [PubMed] [Google Scholar]
- 3. **.Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M: Alpha-klotho is a non-enzymatic molecular scaffold for fgf23 hormone signalling. Nature (2018) 553(7689):461–466. This study provives the structural basis for formation of the FGF-23/α-Klotho/FGFR complexes, but overstates the conclusion regarding the “on demand” function of sKl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hum JM, O’Bryan L, Smith RC, White KE: Novel functions of circulating klotho. Bone (2017) 100(36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I et al. : Suppression of aging in mice by the hormone klotho. Science (2005) 309(5742):1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. *.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P et al. : Fgf23 induces left ventricular hypertrophy. J Clin Invest (2011) 121(11):4393–4408. The manuscript raises the controversial and yet to be established non-canonical actions of FGF-23 to activate FGFR4 in the heart in the absence of Klotho [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Quarles LD: Multiple faces of fibroblast growth factor-23. Current opinion in nephrology and hypertension (2016) 25(4):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of fgf23 demonstrates an essential physiological role of fgf23 in phosphate and vitamin d metabolism. J Clin Invest (2004) 113(4):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. *.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD: Regulation of fibroblastic growth factor 23 expression but not degradation by phex. J Biol Chem (2003) 278(39):37419–37426. This is the seminal paper showing that FGF-23 is expressed in bone and transcriptionally regulated. [DOI] [PubMed] [Google Scholar]
- 10.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H et al. : Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature (1997) 390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 11.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS: In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology (2009) 23(2):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE: The kidney is the principal organ mediating klotho effects. Journal of the American Society of Nephrology : JASN (2014) 25(10):2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onal M, Carlson AH, Thostenson JD, Benkusky NA, Meyer MB, Lee SM, Pike JW: A novel distal enhancer mediates inflammation-, pth-, and early onset murine kidney disease-induced expression of the mouse fgf23 gene. JBMR plus (2018) 2(1):32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE: Dynamic regulation of fgf23 by fam20c phosphorylation, galnac-t3 glycosylation, and furin proteolysis. Proceedings of the National Academy of Sciences of the United States of America (2014) 111(15):5520–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto H, Ramos-Molina B, Lick AN, Prideaux M, Albornoz V, Bonewald L, Lindberg I: Posttranslational processing of fgf23 in osteocytes during the osteoblast to osteocyte transition. Bone (2016) 84(120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. *.Eren M, Place AT, Thomas PM, Flevaris P, Miyata T, Vaughan DE: Pai-1 is a critical regulator of fgf23 homeostasis. Science advances (2017) 3(9):e1603259 This paper points to a novel mechanism whereby FGF-23 metabolism (cleavage) is regulated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quarles LD: Skeletal secretion of fgf-23 regulates phosphate and vitamin d metabolism. Nat Rev Endocrinol (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. *.Han X, Li L, Yang J, King G, Xiao Z, Quarles LD: Counter-regulatory paracrine actions of fgf-23 and 1,25(oh)2 d in macrophages. FEBS letters (2016) 590(1):53–67. This manuscript was the first to show that FGF-23 is upregulted in activated macrophages and provide a potential mechanism for paracrine actions of FGF-23 to regulate immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi M, Ye R, Han X, Armstrong B, Liu X, Chen Y, Sun Y, Quarles LD: Cardiovascular interactions between fibroblast growth factor-23 and angiotensin ii. Scientific reports (2018) 8(1):12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Umbach AT, Chen H, Yan J, Fakhri H, Fajol A, Salker MS, Spichtig D, Daryadel A, Wagner CA, Foller M et al. : Up-regulation of fgf23 release by aldosterone. Biochem Biophys Res Commun (2016) 470(2):384–390. [DOI] [PubMed] [Google Scholar]
- 21.Bar L, Feger M, Fajol A, Klotz LO, Zeng S, Lang F, Hocher B, Foller M: Insulin suppresses the production of fibroblast growth factor 23 (fgf23). Proceedings of the National Academy of Sciences of the United States of America (2018) 115(22):5804–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N: Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin d3 synthesis in leptin-deficient mice. J Bone Miner Res (2010) 25(8):1711–1723. [DOI] [PubMed] [Google Scholar]
- 23.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ et al. : Iron deficiency drives an autosomal dominant hypophosphatemic rickets (adhr) phenotype in fibroblast growth factor-23 (fgf23) knock-in mice. Proceedings of the National Academy of Sciences of the United States of America (2011) 108(46):E1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinkenbeard EL, Hanudel MR, Stayrook KR, Appaiah HN, Farrow EG, Cass TA, Summers LJ, Ip CS, Hum JM, Thomas JC, Ivan M et al. : Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica (2017) 102(11):e427–e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan A, Durlacher K, Silver J, Naveh-Many T, Levi R: The fibroblast growth factor receptor mediates the increased fgf23 expression in acute and chronic uremia. American journal of physiology Renal physiology (2016) 310(3):F217–221. [DOI] [PubMed] [Google Scholar]
- 26. **.Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD: Osteocyte-specific deletion of fgfr1 suppresses fgf23. PloS one (2014) 9(8):e104154 This reference along with the next three estabishes the importance of FGFR1 signaling in the regulation of FGF23 gene transcription in bone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X, Xiao Z, Quarles LD: Membrane and integrative nuclear fibroblastic growth factor receptor (fgfr) regulation of fgf-23. J Biol Chem (2015) 290(33):20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chlebova K, Bryja V, Dvorak P, Kozubik A, Wilcox WR, Krejci P: High molecular weight fgf2: The biology of a nuclear growth factor. Cellular and molecular life sciences : CMLS (2009) 66(2):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao L, Homer-Bouthiette C, Hurley MM: Fgf23 neutralizing antibody partially improves bone mineralization defect of hmwfgf2 isoforms in transgenic female mice. J Bone Miner Res (2018) 33(7):1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM: Loss-of-function enpp1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. American journal of human genetics (2010) 86(2):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie NC, Zhu D, Milne EM, van ‘t Hof R, Martin A, Darryl Quarles L, Millan JL, Farquharson C, MacRae VE: Altered bone development and an increase in fgf-23 expression in enpp1(−/−) mice. PloS one (2012) 7(2):e32177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murali SK, Roschger P, Zeitz U, Klaushofer K, Andrukhova O, Erben RG: Fgf23 regulates bone mineralization in a 1,25(oh) d and klotho-independent manner. J Bone Miner Res (2015). [DOI] [PubMed] [Google Scholar]
- 33.Wang N, Robaye B, Gossiel F, Boeynaems JM, Gartland A: The p2y13 receptor regulates phosphate metabolism and fgf-23 secretion with effects on skeletal development. FASEB journal : official publication of the Federation of American Societies for Experimental Biology (2014) 28(5):2249–2259. [DOI] [PubMed] [Google Scholar]
- 34.Bergwitz C, Juppner H: Phosphate sensing. Advances in chronic kidney disease (2011) 18(2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenertz LY, Baughman CJ, Waldschmidt NV, Thaler R, van Wijnen AJ: Control of bone development by p2x and p2y receptors expressed in mesenchymal and hematopoietic cells. Gene (2015) 570(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyagawa K, Yamazaki M, Kawai M, Nishino J, Koshimizu T, Ohata Y, Tachikawa K, Mikuni-Takagaki Y, Kogo M, Ozono K, Michigami T: Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic hyp mice. PloS one (2014) 9(4):e93840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M et al. : Type iic sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol (2009) 20(1):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. *.David V, Dai B, Martin A, Huang J, Han X, Quarles LD: Calcium regulates fgf-23 expression in bone. Endocrinology (2013) 154(12):4469–4482. This comprehesive paper examines the interactions between calcium, phosphorus and PTH in regulating FGF-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T: Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone (2011) 49(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber TJ, Liu S, Indridason OS, Quarles LD: Serum fgf23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res (2003) 18(7):1227–1234. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Gillihan R, He N, Fields T, Liu S, Green T, Stubbs JR: Dietary phosphate restriction suppresses phosphaturia but does not prevent fgf23 elevation in a mouse model of chronic kidney disease. Kidney international (2013) 84(4):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, Wolf M: Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association (2011) 26(2):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeshita A, Kawakami K, Furushima K, Miyajima M, Sakaguchi K: Central role of the proximal tubular alphaklotho/fgf receptor complex in fgf23-regulated phosphate and vitamin d metabolism. Scientific reports (2018) 8(1):6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Martin A, David V, Quarles LD: Compound deletion of fgfr3 and fgfr4 partially rescues the hyp mouse phenotype. American journal of physiology Endocrinology and metabolism (2011) 300(3):E508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han X, Yang J, Li L, Huang J, King G, Quarles LD: Conditional deletion of fgfr1 in the proximal and distal tubule identifies distinct roles in phosphate and calcium transport. PloS one (2016) 11(2):e0147845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quarles LD: Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest (2003) 112(5):642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin d. J Am Soc Nephrol (2006) 17(5):1305–1315. [DOI] [PubMed] [Google Scholar]
- 48.Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG: Fgf23 promotes renal calcium reabsorption through the trpv5 channel. The EMBO journal (2014) 33(3):229–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro OM, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for fgf23 in rats. J Clin Invest (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawakami K, Takeshita A, Furushima K, Miyajima M, Hatamura I, Kuro OM, Furuta Y, Sakaguchi K: Persistent fibroblast growth factor 23 signalling in the parathyroid glands for secondary hyperparathyroidism in mice with chronic kidney disease. Scientific reports (2017) 7(40534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD: Pathogenic role of fgf23 in hyp mice. American journal of physiology Endocrinology and metabolism (2006) 291(1):E38–49. [DOI] [PubMed] [Google Scholar]
- 52.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H et al. : Loss of dmp1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature genetics (2006) 38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whyte MP, McAlister WH, Fallon MD, Pierpont ME, Bijanki VN, Duan S, Otaify GA, Sly WS, Mumm S: Raine syndrome (omim #259775), caused by fam20c mutation, is congenital sclerosing osteomalacia with cerebral calcification (omim 259660). J Bone Miner Res (2016). [DOI] [PubMed] [Google Scholar]
- 54.Quarles LD: Role of fgf23 in vitamin d and phosphate metabolism: Implications in chronic kidney disease. Experimental cell research (2012) 318(9):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. **.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H: Polypeptide galnac-transferase t3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires o-glycosylation. J Biol Chem (2006) 281(27):18370–18377. This report established the mechanism of post-translational regulation of FGF-23. [DOI] [PubMed] [Google Scholar]
- 56.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H: Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab (2010) 95(2):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL et al. : Fibroblast growth factor 23 and inflammation in ckd. Clinical journal of the American Society of Nephrology : CJASN (2012) 7(7):1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, Unruh M, Zarbock A: Fgf23 signaling impairs neutrophil recruitment and host defense during ckd. J Clin Invest (2016) 126(3):962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzpatrick EA, Han X, Xiao Z, Quarles LD: Role of fibroblast growth factor-23 in innate immune responses. Frontiers in endocrinology (2018) 9(320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK: Low vitamin d and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the hemo study. J Am Soc Nephrol (2016) 27(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Ding XY, Xiang DM, Xu J, Huang XL, Hou FF, Zhou QG: Enhanced m1 and impaired m2 macrophage polarization and reduced mitochondrial biogenesis via inhibition of amp kinase in chronic kidney disease. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology (2015) 36(1):358–372. [DOI] [PubMed] [Google Scholar]
- 62. *.Bora SA, Kennett MJ, Smith PB, Patterson AD, Cantorna MT: The gut microbiota regulates endocrine vitamin d metabolism through fibroblast growth factor 23. Frontiers in immunology (2018) 9(408 A provocative paper liking the microbiome with FGF-23 regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. The New England journal of medicine (2008) 359(6):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaidya A, Williams JS: The relationship between vitamin d and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism: clinical and experimental (2012) 61(4):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD: A comparative transcriptome analysis identifying fgf23 regulated genes in the kidney of a mouse ckd model. PloS one (2012) 7(9):e44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG: Fgf23 regulates renal sodium handling and blood pressure. EMBO molecular medicine (2014) 6(6):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richter M, Lautze HJ, Walther T, Braun T, Kostin S, Kubin T: The failing heart is a major source of circulating fgf23 via oncostatin m receptor activation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation (2015) 34(9):1211–1214. [DOI] [PubMed] [Google Scholar]
- 68.Xie J, Yoon J, An SW, Kuro-o M, Huang CL: Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol (2015) 26(5):1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garland JS, Holden RM, Ross R, Adams MA, Nolan RL, Hopman WM, Morton AR: Insulin resistance is associated with fibroblast growth factor-23 in stage 3–5 chronic kidney disease patients. Journal of diabetes and its complications (2014) 28(1):61–65. [DOI] [PubMed] [Google Scholar]
- 70. *.Chesher D, Oddy M, Darbar U, Sayal P, Casey A, Ryan A, Sechi A, Simister C, Waters A, Wedatilake Y, Lachmann RH et al. : Outcome of adult patients with x-linked hypophosphatemia caused by phex gene mutations. Journal of inherited metabolic disease (2018). This is an important contribution to understanding the long-term clinical complications related to excess FGF-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng S, Wang J, Zhang Y, Creighton CJ, Ittmann M: Fgf23 promotes prostate cancer progression. Oncotarget (2015) 6(19):17291–17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee EK, Martinez MC, Blakely K, Santos KD, Hoang VC, Chow A, Emmenegger U: Fgf23: Mediator of poor prognosis in a sizeable subgroup of patients with castration-resistant prostate cancer presenting with severe hypophosphatemia? Medical hypotheses (2014) 83(4):482–487. [DOI] [PubMed] [Google Scholar]
- 73.Daryadel A, Bettoni C, Haider T, Imenez Silva PH, Schnitzbauer U, Pastor-Arroyo EM, Wenger RH, Gassmann M, Wagner CA: Erythropoietin stimulates fibroblast growth factor 23 (fgf23) in mice and men. Pflugers Archiv : European journal of physiology (2018). [DOI] [PubMed] [Google Scholar]
- 74.Laszczyk AM, Fox-Quick S, Vo HT, Nettles D, Pugh PC, Overstreet-Wadiche L, King GD: Klotho regulates postnatal neurogenesis and protects against age-related spatial memory loss. Neurobiology of aging (2017) 59(41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q, Vo HT, Wang J, Fox-Quick S, Dobrunz LE, King GD: Klotho regulates ca1 hippocampal synaptic plasticity. Neuroscience (2017) 347(123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR: The neuroprotective effect of klotho is mediated via regulation of members of the redox system. J Biol Chem (2014) 289(35):24700–24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A et al. : Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell metabolism (2015) 22(6):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M et al. : Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney international (2016) 90(5):985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Komaba H, Kaludjerovic J, Hu DZ, Nagano K, Amano K, Ide N, Sato T, Densmore MJ, Hanai JI, Olauson H, Bellido T et al. : Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney international (2017) 92(3):599–611. [DOI] [PubMed] [Google Scholar]
- 80.Lin Z, Zhou Z, Liu Y, Gong Q, Yan X, Xiao J, Wang X, Lin S, Feng W, Li X: Circulating fgf21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in chinese. PloS one (2011) 6(4):e18398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y: Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun (1998) 242(3):626–630. [DOI] [PubMed] [Google Scholar]
- 82.Mencke R, Harms G, Moser J, van Meurs M, Diepstra A, Leuvenink HG, Hillebrands JL: Human alternative klotho mrna is a nonsense-mediated mrna decay target inefficiently spliced in renal disease. JCI Insight (2017) 2(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted klotho protein in sera and csf: Implication for post-translational cleavage in release of klotho protein from cell membrane. FEBS letters (2004) 565(1–3):143–147. [DOI] [PubMed] [Google Scholar]
- 84.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of klotho by adam10 and adam17. Proceedings of the National Academy of Sciences of the United States of America (2007) 104(50):19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G: Vitamin d receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun (2011) 414(3):557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim HJ, Kang E, Oh YK, Kim YH, Han SH, Yoo TH, Chae DW, Lee J, Ahn C, Oh KH: The association between soluble klotho and cardiovascular parameters in chronic kidney disease: Results from the know-ckd study. BMC nephrology (2018) 19(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS et al. : Augmented wnt signaling in a mammalian model of accelerated aging. Science (2007) 317(5839):803–806. [DOI] [PubMed] [Google Scholar]
- 88. *.Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL: Cardioprotection by klotho through downregulation of trpc6 channels in the mouse heart. Nature communications (2012) 3(1238 An important observation regarding the cardiovascular effecst of sKl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y: Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol (2015) 185(12):3211–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL: Removal of sialic acid involving klotho causes cell-surface retention of trpv5 channel via binding to galectin-1. Proceedings of the National Academy of Sciences of the United States of America (2008) 105(28):9805–9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG: The beta-glucuronidase klotho hydrolyzes and activates the trpv5 channel. Science (2005) 310(5747):490–493. [DOI] [PubMed] [Google Scholar]
- 92.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL: Regulation of renal outer medullary potassium channel and renal k(+) excretion by klotho. Molecular pharmacology (2009) 76(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dalton G, An SW, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, Hilgemann DW, Xie J, Luby-Phelps K, Kohler JJ, Birnbaumer L et al. : Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proceedings of the National Academy of Sciences of the United States of America (2017) 114(4):752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han X, Ross J, Kolumam G, Pi M, Sonoda J, King G, Quarles LD: Cardiovascular effects of renal distal tubule deletion of the fgf receptor 1 gene. J Am Soc Nephrol (2018) 29(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. **.Carpenter TO, Whyte MP, Imel EA, Boot AM, Hogler W, Linglart A, Padidela R, Van’t Hoff W, Mao M, Chen CY, Skrinar A et al. : Burosumab therapy in children with x-linked hypophosphatemia. The New England journal of medicine (2018) 378(21):1987–1998. The definive clinical trial showing that blocking FGF-23 is an effective treatment of XLH. [DOI] [PubMed] [Google Scholar]
- 96.Xiao Z, Riccardi D, Velazquez HA, Chin AL, Yates CR, Carrick JD, Smith JC, Baudry J, Quarles LD: A computationally identified compound antagonizes excess fgf-23 signaling in renal tubules and a mouse model of hypophosphatemia. Science signaling (2016) 9(455):ra113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu ES, Thoonen R, Petit E, Yu B, Buys ES, Scherrer-Crosbie M, Demay MB: Increased circulating fgf23 does not lead to cardiac hypertrophy in the male hyp mouse model of xlh. Endocrinology (2018) 159(5):2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM et al. : Fgf23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest (2012) 122(7):2543–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu X, Chen Y, McCoy CW, Zhao T, Quarles DL, Pi M, Bhattacharya SK, King G, Sun Y: Differential regulatory role of soluble klothos on cardiac fibrogenesis in hypertension. American journal of hypertension (2016) 29(10):1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro OM, Moe OW: Recombinant alpha-klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney international (2017) 91(5):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]