Abstract
Background
Although studies involving preterm infants ≤34 weeks gestation report a decreased incidence of patent ductus arteriosus after antenatal betamethasone, studies involving younger gestation infants report conflicting results.
Methods
We used preterm baboons, mice and humans (≤276/7 weeks gestation) to examine betamethasone’s effects on ductus gene expression and constriction both in vitro and in vivo.
Results
In mice, betamethasone increased the sensitivity of the premature ductus to the contractile effects of oxygen without altering the effects of other contractile or vasodilatory stimuli. Betamethasone’s effects on oxygen sensitivity could be eliminated by inhibiting endogenous prostaglandin/nitric oxide signaling. In mice and baboons, betamethasone increased the expression of several developmentally-regulated genes that mediate oxygen-induced constriction (K+ channels) and inhibit vasodilator signaling (phosphodiesterases). In human infants, betamethasone increased the rate of ductus constriction at all gestational ages. However, in infants born ≤256/7 weeks gestation, betamethasone’s contractile effects were only apparent when prostaglandin signaling was inhibited, whereas, at 26-27 weeks gestation betamethasone’s contractile effects were apparent even in the absence of prostaglandin inhibitors.
Conclusions
We speculate that betamethasone’s contractile effects may be mediated through genes that are developmentally regulated. This could explain why betamethasone’s effects vary according to the infant’s developmental age at birth.
Introduction
Postnatal closure of the ductus arteriosus takes place through a process that involves initial vasoconstriction followed by anatomic remodeling. In contrast with full term newborn infants, preterm infants frequently fail to constrict their ductus arteriosus after birth and therefore fail to initiate the remodeling process (for review see (1)). The initial vasoconstriction depends on a balance between developmentally regulated pathways that promote constriction (these involve endothelin, smooth muscle calcium channels, Rho-kinase related calcium sensitization, and myosin and cytoskeletal proteins) and those that oppose it (prostaglandins, nitric oxide, carbon monoxide, potassium channels, and cyclic AMP and cyclic GMP).
Antenatal betamethasone accelerates the maturation of a number of fetal organs and decreases the incidence of death, respiratory distress syndrome, necrotizing enterocolitis, and severe grades of intraventricular hemorrhage in preterm infants. Whether it affects the incidence of patent ductus arteriosus is still unclear. A limited number of studies performed in fetal sheep (2), rats (3), and humans (4,5) suggest that antenatal corticosteroids increase the contractile tone of the premature fetal ductus. Although several observational (6–8) and controlled studies (9) have reported a decreased incidence of patent ductus arteriosus (PDA) in premature infants after antenatal betamethasone administration (especially in those exposed to chorioamnionitis (10)), other studies have reported contradictory findings (11,12). It is interesting to note that the infants studied in the reports (6–9) that found a higher incidence of spontaneous ductus closure after antenatal betamethasone, were born at a more advanced gestational age than the infants in the studies that failed to observe this effect (11,12).
We hypothesized that antenatal betamethasone does indeed promote ductus constriction but that its effects may be mediated by genes that are developmentally regulated. If this is the case, then betamethasone’s effects on postnatal constriction may depend on the infant’s developmental age at the time of birth.
Therefore, we examined the effects of antenatal betamethasone (BMZ) on both spontaneous and indomethacin-induced ductus constriction at each advancing gestational week in babies born before 28 weeks gestation. We also used nonhuman primates and rodent models to see how antenatal BMZ might affect genes that are both developmentally regulated and are also important in determining ductus patency and constriction.
Methods
This study was performed in human infants, baboons and mice.
Human Patient Population and PDA treatment protocol
The Institutional Review Board of the University of California San Francisco approved this project. Infants were eligible for the study if they were born between January 2004 and June 2017, delivered at ≤276/7 weeks gestation, and admitted to the intensive care nursery at the University of California San Francisco within 24 hours of birth. Gestational age was determined by the date of last menstrual period and early ultrasounds (before 24 weeks gestation). Detailed descriptions of our approach to respiratory and hemodynamic support have been previously published (13).
Unless delivery was felt to be imminent, all mothers delivering before 34 weeks gestation were given antenatal BMZ (two 12-mg doses administered 24 hours apart). Prior in vitro (14) and in vivo (15–17) studies have shown that antenatal corticosteroids can improve lung function and decrease the risk of severe IVH as early as 7 hours after dosing, however, these effects are reversible and begin to wane by 9 days after the first dose (14,17). Therefore, the infants in our study were divided into two groups depending on the interval between the first dose of antenatal BMZ and delivery: Group A (“inadequate BMZ treatment”) included infants who were either never treated, delivered before 7 hours, or delivered more than 9 days after the first dose of BMZ. Group B (“adequate BMZ treatment”) included infants who delivered during the interval between 7 hours and 9 days following the first BMZ dose. There were no differences between Group A and Group B in the incidence of perinatal factors reported to be associated with persistent ductus patency: birth weight (A=886±195 gm; B=787±196 gm), gestational age at delivery (A=26.3±1.1 weeks; B+25.9±1.1 weeks), incidence of male sex (A=45%; B=52%), Caucasian race (A=40%; B=44%), maternal diabetes (A=7%; B=10%), or chorioamnionitis (A=21%; B=22%).
During the 14-year period of the study there were two distinct epochs of PDA management that enabled us to examine the relationships between antenatal BMZ exposure and the rates of spontaneous and indomethacin-induced ductus constriction (13). During the first epoch, prior to May 2011, infants were treated with prophylactic indomethacin (PINDO) starting within 15 hours of birth. Six potential PINDO doses (0.2, 0.1, 0.1, 0.1, 0.1 and 0.1 mg/kg) were given at 24 hours intervals (doses 4-6 were given only if there was evidence of ductus patency on the echocardiogram performed before the third dose). An echocardiogram was repeated at the end of the first week. During the PINDO epoch, 284 infants were admitted to the nursery, 16 did not receive PINDO because of initial oliguria, elevated creatinine, or coagulopathy, and 28 died before the echocardiogram could be performed at 7 days. Therefore 240 infants were available to examine the relationship between BMZ exposure and the rate of indomethacin-induced ductus constriction on day 7.
In May 2011, we changed to a more “Conservative” treatment approach. During epoch 2 (May 2011 through June 2017) PINDO was no longer used. PDAs were no longer treated with indomethacin until at least 8 postnatal days to allow for spontaneous closure. During epoch 2, 191 infants were admitted to the nursery; 20 died before the echocardiogram could be performed. Therefore 171 infants were available to examine the relationship between BMZ exposure and the rate of spontaneous ductus constriction by day 7.
The echocardiographic studies have been previously described (13). A “moderate-to-large” PDA was defined by a ductus internal diameter ≥ 1.5mm (or PDA:left pulmonary artery diameter ratio ≥0.5) in addition to one or more of the following echocardiographic criteria: a) left atrium-to-aortic root ratio ≥1.6, b) ductus flow velocity ≤2.5 m/sec or mean pressure gradient across the ductus ≤8 mm, c) left pulmonary artery diastolic flow velocity > 0.2 m/sec, and/or d) reversed diastolic flow in the descending aorta. Ductus that failed to meet these criteria were considered to be “constricted” (small or closed).
Baboons
We previously examined and reported the effects of BMZ on 27 genes that were developmentally regulated in the baboon (Papio papio) ductus arteriosus (18,19). In the current study we used the RNA from the same animals to examine the effects of BMZ on an additional 36 developmentally regulated genes (Supplemental Table S1 (online)).
Mice
CD-1 mice were maintained at Vanderbilt University. All protocols were approved by the Institutional Animal Care and Use Committee. We used the isolated mouse ductus as a model for studying the effects of BMZ exposure on ductus contractility. Timed matings were performed and BMZ-treated pregnant dams were injected subcutaneously with 0.2mg BMZ (Celestone, American Regent) twice daily on days 14 and 15 of gestation (term=day 19) (20). Treated and untreated preterm litters were delivered by cesarean section on day 17. We determined the adequacy of our BMZ dosing regimen by examining its ability to produce known BMZ-induced effects in other organ systems. Consistent with previous reports (20,21) BMZ-treated mouse pups were smaller and had a lower birth weight when compared to untreated, age matched controls (Control (n=70): 0.704±0.059 gm, BMZ (n=67): 0.674±0.074 gm, p<0.02).
Lung development was significantly accelerated in BMZ-treated mouse pups (Figure 1 d-f). Lung morphometry was performed as previously reported (22). Airspace volume density was calculated by dividing the sum of the airspace area by the total area. Six serial sections from two mice at each gestational age were measured. BMZ-treated lungs had increased airspace density (Control=5.3±1.9%, BMZ=33.0±6.2% of volume density, p<0.01) and airspace diameter (Control=34±6μm, BMZ=59±6μm, p<0.05) compared to untreated day 17 lungs.
Figure 1. Betamethasone exposure enhanced lung maturation.
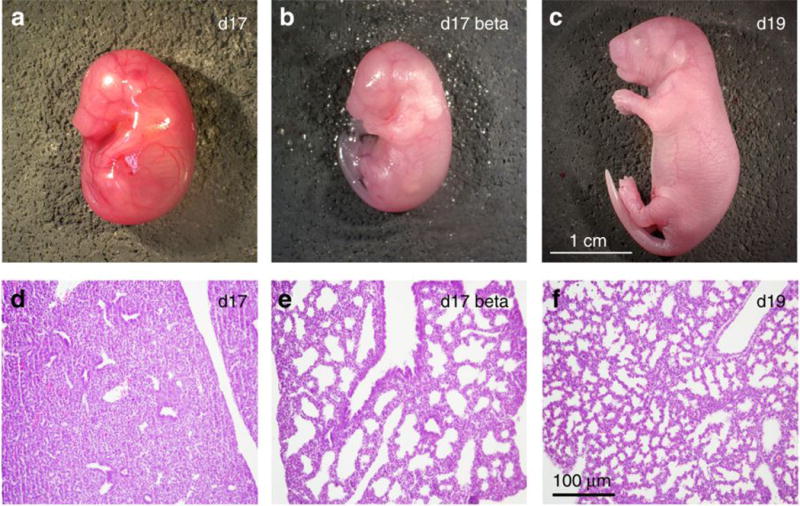
Mice were fixed and paraffin embedding. 8μm sections of lung (d, e, f) were stained with hematoxylin and eosin. Representative day 17 control (a and d), day 17 BMZ-treated (b and e), and day 19 control (c and f) pups are shown. Scalar bar refers to panels d-f.
BMZ has previously been shown to alter the expression of epithelial sodium channel (SCNN1A) and surfactant associated protein-B (SFTPB) mRNA both in vivo and in vitro (23). Quantitative polymerase chain reaction (PCR) (see Methods below) indicated that BMZ-treated mouse lungs had significantly higher expression of SCNN1A and SFTPB RNA than untreated Control lungs (SCNN1A ΔCT (see Table 1 for definition of ΔCT): Control=−3.26±0.85, BMZ=−2.87±0.61, p<0.05; SFTPB ΔCT: Control=−1.17±0.82, BMZ=+0.30±0.53, p<0.05). Lower doses of BMZ (0.1 mg once a day, or 0.1 mg twice a day (on days 14 and 15) did not produce any significant effects on body weight or SCNN1A and SFTPB RNA expression (data not shown).
Table 1. Effects of antenatal betamethasone exposure on Real Time polymerase chain reaction (PCR) measurements of genes involved with ductus arteriosus constriction in fetal mice.
Fetal mouse ductus “samples” from 17 day gestation untreated mice were compared with ductus from 17 day gestation mice exposed to 72 hours of betamethasone prior to necropsy. Genes that were affected by BMZ in the baboon (see Supplemental Table S1 (online)) (that are also involved with ductus contractility) (in addition to KCNMA1/BKCa and PTGS1/COX1) were examined in the mouse ductus “samples”. Each fetal ductus “sample” contained 4-6 ductus, pooled together, from a single litter. Number of separate “samples” (litters) used: 17 days gestation (untreated) (n=12), 17 days gestation + betamethasone (BMZ) (n=13). ΔCT represents the difference in cycle threshold (CT) between the expression of the housekeeping gene Malate dehydrogenase (MDH) and the gene of interest. Each unit of ΔCT represents a 2-fold change in a gene’s mRNA. The more negative the ΔCT, the fewer the number of starting copies of a gene (mRNA).
| Mouse | Mouse | Effects of BMZ | ||||
|---|---|---|---|---|---|---|
| GENE/Alias | 17d gestation | 17d gestation + BMZ | Mouse | Baboon | ||
| ΔCT | ΔCT | |||||
| mean | sd | mean | sd | p<0.05 | p<0.05 | |
| Ca++ signaling | ||||||
| CACNA1G/Ca-alpha1G | −3.21 | 0.58 | −3.24 | 0.64 | – |
|
| CACNB2/CaLbeta2 | −2.29 | 0.66 | −2.18 | 0.25 | – |
|
| ATP2A3/SERCA3 | −5.04 | 0.47 | −4.76 | 0.56 | – |
|
| SLC8A1/NCX-1 | −4.38 | 0.58 | −4.07 | 0.75 | – |
|
| ROCK1 | −1.87 | 0.48 | −1.94 | 0.74 | – |
|
| K+ channels | ||||||
| KCNA2/Kv1.2 | −3.99 | 0.71 | −3.48* | 0.52 |
|
|
| KCNS3/Kv9.3 | −0.89 | 0.56 | −0.78 | 0.46 | – |
|
| KCNAB2/Kvbeta1.2 | −2.87 | 0.56 | −2.39* | 0.44 |
|
|
| KCNMA1/BKCa | −6.52 | 0.91 | −6.01 | 0.60 | – | – |
| KCNMB1/BKCa-betal | −2.90 | 0.50 | −2.43* | 0.55 |
|
|
| Contractile proteins | ||||||
| MYOCD/Myocardin | 0.77 | 0.52 | −0.68 | 0.60 | – |
|
| Vasoactive signaling | ||||||
| AGTR1/Angiotensin II receptor type 1 | −2.83 | 0.71 | −2.43* | 0.43 |
|
|
| HIF1A/HIF 1 alpha | −1.79 | 0.39 | −1.56 | 0.38 | – |
|
| HMOX1/hemeoxygenase 1 | −0.44 | 0.82 | 0.01 | 0.85 | – |
|
| NOS3/eNOS | −1.26 | 0.57 | −1.18 | 0.47 | – |
|
| PTGS1/COX1 | −5.07 | 0.54 | −4.98 | 0.46 | – | – |
| PTGS2/COX2 | −5.88 | 0.77 | −5.79 | 0.72 | – |
|
| PDE1A | −2.97 | 0.68 | −2.73 | 0.47 | – |
|
| PDE1B | −2.58 | 0.88 | −1.54* | 0.53 |
|
|
| PDE3B | −2.51 | 0.28 | −2.29* | 0.28 |
|
|
| PDE5A | −1.33 | 0.70 | −1.03 | 0.67 | – |
|
| PDGFB/PDGF-B chain | −2.64 | 0.62 | −2.70 | 0.52 | – |
|
| VEGFA | −1.78 | 0.51 | −1.44 | 0.38 | – |
|
 , = p<0.05, ACT of BMZ treated ductus is significantly greater (in a positive direction) than ACT of untreated ductus;
, = p<0.05, ACT of BMZ treated ductus is significantly greater (in a positive direction) than ACT of untreated ductus;
 , = p<0.05, ACT is significantly less; −= p>0.05, ACT is not significantly different.
, = p<0.05, ACT is significantly less; −= p>0.05, ACT is not significantly different.
Quantitative Real Time RT-PCR
Total RNA was isolated from each individual baboon ductus and from fetal mouse ductus “samples” and from fetal mouse lungs (day 17) using the RNeasy Mini Kit (Qiagen). For mouse ductus gene expression studies, the fetal ductus from a single litter (4-6 fetuses/litter) were isolated and pooled together as a single mouse ductus “sample”. We used 13 BMZ-treated mouse ductus “samples” (n=13 litters) and 12 untreated mouse ductus “samples” (n=12 litters). For the mouse lung gene expression studies, each lung sample came from a single fetus. Three fetal mouse lung samples were examined per litter (BMZ: n=39 samples, 13 litters; untreated: n=36 samples, 12 litters).
Relative levels of gene expression were analyzed using TaqMan Universal PCR master mix and TaqMan probes. Cycle threshold (CT) values were determined using the ABI PRISM 7500 Sequence detection system (Applied Biosystems). Reactions were run in triplicate. The degree of expression of the gene of interest was determined using the relative gene expression method. The housekeeping gene (malate dehydrogenase (MDH)) was used as an internal control to normalize the data (24,25).
Myography
Fetal day 17 mouse ductus (with and without BMZ treatment) were isolated and mounted on glass pipet tips in microvessel perfusion chambers equipped with a digital image capture system (26). Non-recirculating, deoxygenated Krebs buffer (36.5-37.5°C) perfused the chambers at 6 ml/min. The intra-luminal diameter was measured at the point of maximum constriction. Mouse vessels were pressurized to physiological neonatal mouse mean arterial pressure (15 mmHg, preterm) using a column of deoxygenated Krebs buffer. Mouse vessels were challenged with two doses of 50 mM KCl in Krebs buffer (with KCl substituted for NaCl) to test vessel reactivity and to determine maximum constriction values.
Non-contractile vessels were excluded from further study. Vessels were then exposed to one of the following protocols: 1) increasing oxygen concentrations (2%, 5%, 12%, 21%, and 95% O2; 5%CO2, balance N2) with or without pre-treatment with N(G)-nitro-L-arginine methylester (L-NAME) (10−4 M, Cayman Chemical) and indomethacin (5.6×10−6 M, Sigma) in Krebs buffer; 2) increasing KCl concentrations (12.5, 25, and 50 mM) in deoxygenated Krebs buffer; 3) increasing U-46619 (a thromboxane receptor agonist) concentrations (10−9 M - 10−6 M, Cayman Chemical) in deoxygenated Krebs buffer; 4) increasing concentrations of prostaglandin E2 (PGE2) (10−11 M - 10−7 M Cayman Chemical) in 95% oxygenated Krebs buffer; 5) increasing concentrations of sodium nitroprusside (SNP) (10−9 M - 10−3 M, Sigma) in 95% oxygenated Krebs buffer. For each protocol, lumen diameters were allowed to plateau (20-40 minutes) before the next dose was added. At the conclusion of each experiment, vessels were treated with papaverine (10−4 M, Sigma) to determine the lumen diameter at maximal relaxation. More than 10 vessels from at least 5 different litters were used for each experimental condition.
Prostaglandin Metabolite Assay
Ductus from day 17 mouse fetuses with and without antenatal BMZ exposure were isolated and placed in serum-free Dulbecco’s modified Eagle’s medium with 1% penicillin-streptomycin. A total of 5 ductus from a single mouse litter were added to each chamber. Vessels from 18 untreated and 17 BMZ-treated mouse litters were used for analysis. Vessels were incubated at 37°C for 40 minutes, after which the conditioned media was collected and analyzed for prostaglandin metabolite production by the stable isotope dilution assay utilizing gas chromatography/negative ion chemical ionization-mass spectrometry using an Agilent 5973 Inert Mass Selective Detector coupled with an Agilent 6890n Network GC system (Agilent Labs, Torrance, CA) as previously described (27).
Statistics
Values are expressed as mean ± standard deviation. The Student t test was used to compare means. When appropriate, results were analyzed by a Mann-Whitney U test or ANOVA and post-hoc analysis. Chi square tests were used to compare categorical variables between the two clinical treatment groups (“adequate BMZ” and “inadequate BMZ” treatment). P <0.05 was considered statistically significant.
Results
When infants in the Conservative period (epoch 2) were examined as a group (≤276/7 weeks gestation), antenatal BMZ exposure was only associated with a non-significant increase in the rate of spontaneous ductus constriction. The incidence of persistent moderate-to-large PDA at the end of the first week was 84/128 (66%) in infants who were “adequately” treated with BMZ, and 36/43 (84%) in those who were “inadequately” treated. Similarly, BMZ was not associated with a significant increase in the rate of indomethacin-induced constriction during the PINDO treatment period (epoch 1) (incidence of moderate-to-large PDA at the end of the first week after receiving prophylactic indomethacin: 31/146 (21%) in “adequately” treated infants; 25/94 (27%) in those who were “inadequately” treated).
Although there was no significant association between BMZ exposure and ductus constriction in the entire group of immature infants (≤276/7 weeks gestation), there was a significant association when we examined the relationship at each advancing gestational week (Figure 2). The rates of spontaneous constriction were not significantly affected by BMZ in babies ≤256/7 weeks gestation, however, after 256/7 weeks, BMZ exposure was associated with a significant increase in the rate of spontaneous constriction (open bars Figure 2). As expected, prophylactic indomethacin increased the rate of postnatal constriction (1,28). In babies ≤256/7 weeks gestation, BMZ exposure was associated with an increased rate of indomethacin-induced constriction, whereas after 256/7 weeks, BMZ exposure had no effect on indomethacin-induced constriction (dark filled bars Figure 2).
Figure 2. The effects of antenatal betamethasone on both spontaneous and indomethacin-induced ductus constriction at 7 days after birth.
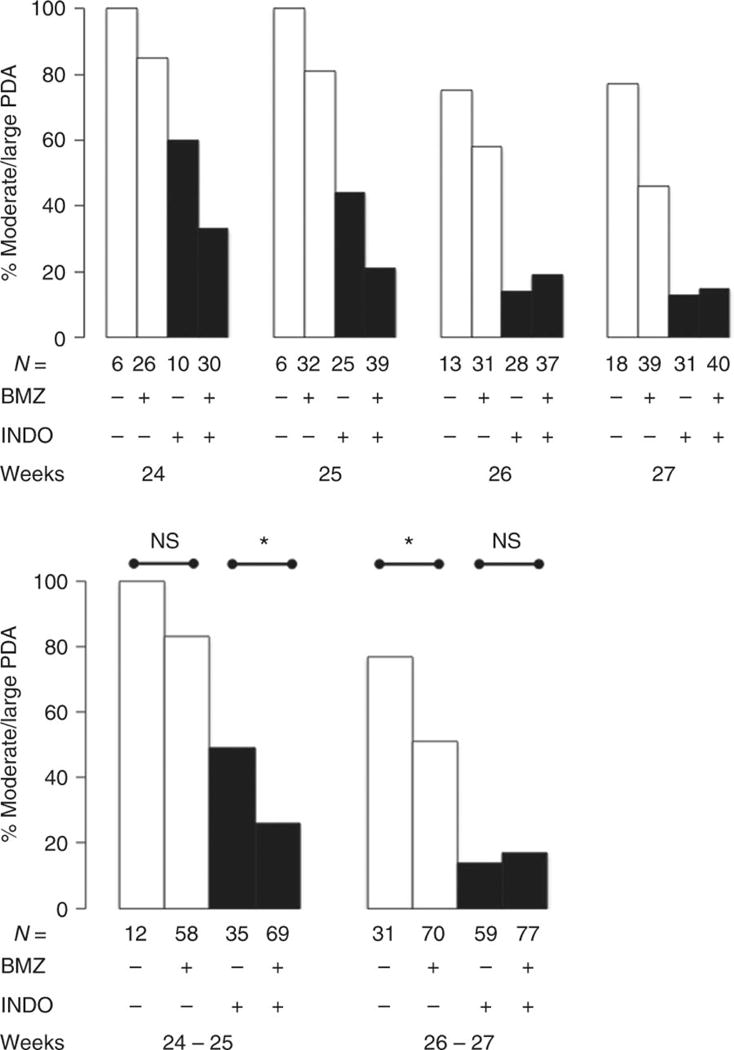
Bars represent the incidence of moderate-to-large PDA that persist beyond 7 days. Open bars = Conservative era; Dark bars = Prophylactic indomethacin era. The top panel shows the effects of betamethasone at each gestational week: ≤246/7, 250/7-256/7, 260/7-266/7, and 270/7-276/7 weeks. Statistical analyses were performed on the two gestational age groups presented in the bottom panel (≤256/7) and (260/7-276/7).
BMZ+, “Adequate BMZ exposure” see Methods for definition. N, number of infants examined by echocardiogram at 7 days. *, p<0.05; NS, not significant.
To examine how antenatal BMZ exposure might play a role in increasing the rate of ductus constriction, we first examined its effects on RNA expression in the ductus of both baboon and mouse fetuses. We were interested in identifying a set of genes that were developmentally regulated in the ductus and whose expression was also affected by antenatal BMZ exposure. Developmentally regulated candidate genes were chosen because: 1) their expression in the ductus had previously been shown to differ from their expression in the aorta, 2) their expression in the ductus was developmentally regulated, and 3) their pharmacologic inhibition (or mutations or polymorphisms) had been shown to affect ductus closure (24,25). In the baboon, BMZ alters the mRNA expression of several genes that are altered by advancing gestation. These include genes involved with oxygen-induced constriction (calcium channels, calcium pumps, and potassium channels), vasoactive signaling (prostaglandins, nitric oxide, angiotensin, cyclic nucleotides), and contractile protein regulation as well as genes involved with ductus remodeling and permanent closure (Supplemental Table S1 (online)).
Due to the small amount of RNA in our mouse ductus “samples”, we chose to examine only those mouse genes involved with ductus contractility that were also affected by BMZ in the baboon ductus (Supplemental Table S1 (online)). BMZ affected a more limited number of genes in the mouse (Table 1). In particular, BMZ altered mRNA expression of angiotensin II receptor-R1 (ATII-R1), potassium channels (BKCa-beta1, Kv1.2, and Kvbeta1.2), and phosphodiesterases (Pde1b and Pde3b) (Table 1).
We next examined the effects of antenatal BMZ exposure on ductus contractility using isolated pressurized ductus from fetal untreated Control and BMZ-exposed mice. When incubated at the same pressures, under baseline conditions (buffer bubbled with 0% O2), ductus obtained from Control and BMZ-treated mice had similar size lumina (lumen diameter: BMZ exposed = 272±77 μm, n=26; Control = 268±54 μm, n=28) suggesting no change in initial resting tone at low O2. Similarly, when the ductus were maximally dilated by papaverine, the lumina of Control and BMZ-treated mice were similar in size (lumen diameter: BMZ exposed = 361±42 μm, n=26; Control = 378±73 μm, n=28).
BMZ (in concentrations from 10−9 M-10−4 M), when added to the bath solution of Control ductus, had no acute effect on ductus tone in vitro (data not shown). We used three in vitro contractile stimuli (O2, K+, and U46619) to investigate the effects of in utero BMZ exposure on ductus tone (Figure 3). Ductus from both Control and BMZ-exposed mice constricted with increasing concentrations of all three stimuli. BMZ exposure increased the sensitivity of the ductus to O2 induced constriction (EC50 (O2%): Control = 8.5±6.0 O2%; BMZ-exposed=3.6±2.5 O2, p<0.05) without affecting its sensitivity to the other stimuli (K+ or U46619) (Figure 3 a, c, d). BMZ did not alter the maximum contractile effects of the three stimuli since their efficacies were similar in both Control and BMZ-exposed ductus.
Figure 3. Betamethasone exposure increased the sensitivity of the mouse ductus to O2.
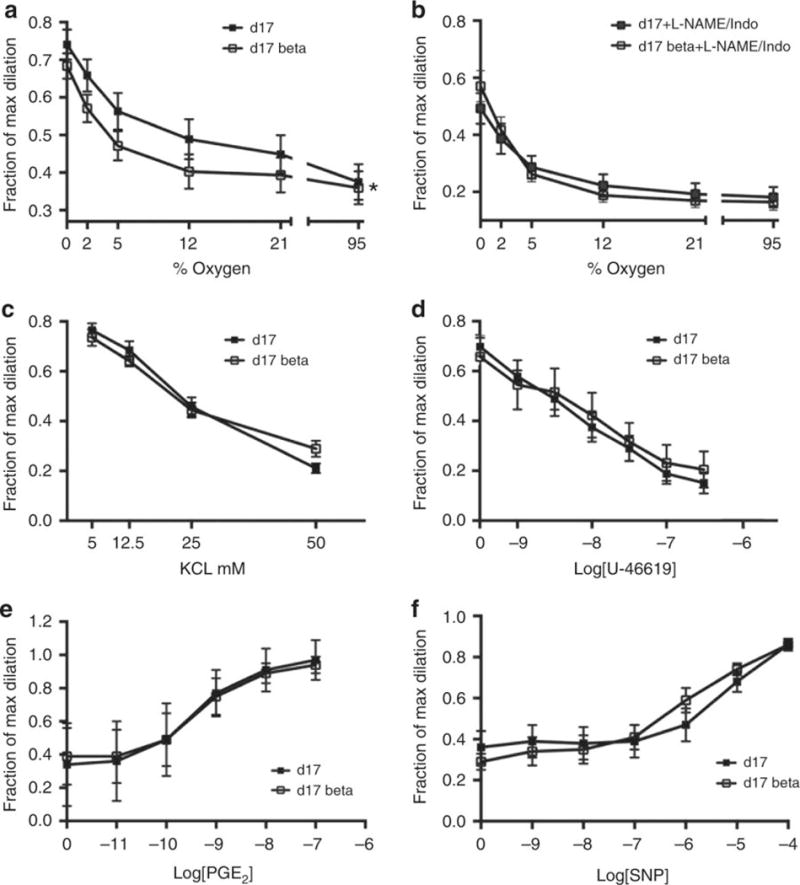
PO2 when the baths were bubbled with 0, 2, 5, 12, and 21% oxygen were 42.5 ± 2, 57.3 ± 2.1, 75.1 ± 1.8, 118.8 ± 1.7, and 181.0 ± 5.6 mm Hg, respectively. BMZ-treated vessels were significantly more sensitive to O2 than untreated-Control ductus (EC50 (concentration which produces 50% of maximal response): Control 8.5±6.0% O2; BMZ 3.6±2.5% O2, *p<0.05) (a). L-NAME/Indomethacin treatment increased the sensitivity of the Control ductus to O2 (Control EC50 (O2%)= 3.5±2.2 O2%). There was no additional increase in O2 sensitivity when BMZ-exposed ductus were treated with L-NAME/Indomethacin (BMZ 2.5±1.1% O2) (b). The K+ and U-46619 dose-response experiments were performed in 0% oxygen. BMZ treatment did not alter contractile responses to KCl (EC50×10−3 M: Control 22.6±5.1; BMZ 21.1±6.9) (c) or U-46619 (EC50×10−9 M: Control 12.2±13.8; BMZ 18.2±20.3) (d). Isolated vessels were pre-constricted with O2 prior to addition of increasing concentrations of PGE2 (EC50×10−10 M: Control 4.9±2.5; BMZ 5.9±4.9) (e) or the NO donor, SNP (EC50×10−6 M: Control 8.7±7.9; BMZ 2.6±2.7) (f). Error bars = standard deviation. Maximum dilation was produced with papaverine (10−4 M).
In parallel experiments, we pretreated ductus from both Control and BMZ-exposed mice with L-NAME (10−4 M) and indomethacin (5.6×10−6 M) to block nitric oxide (NO) and prostaglandin signaling prior to oxygen exposure. The lumina of both Control and BMZ-exposed ductus constricted significantly (Figure 3b) when incubated with indomethacin and L-NAME under baseline conditions (0% O2). Inhibition of prostaglandin and NO production increased the sensitivity of the Control ductus to O2 (Control EC50 (O2%)= 3.5±2.2 O2%) but did not affect the sensitivity of the BMZ-exposed ductus (whose sensitivity to O2 had already been increased by BMZ) (BMZ EC50 (O2)= 2.5±1.1 O2%). After treatment with indomethacin and L-NAME, both Control and BMZ-exposed ductus had similar sensitivities to oxygen (Figure 3b).
We pre-constricted Control and BMZ-exposed ductus with 95% oxygen and challenged them with increasing concentrations of PGE2 or sodium nitroprusside (SNP) (an NO donor) to examine the effects of BMZ on the ability of exogenous PGE2 and SNP to dilate the ductus. Antenatal exposure to BMZ did not affect the ability of either exogenous PGE2 or SNP to dilate the ductus (Figure 3e,f).
BMZ exposure also did not appear to affect mouse ductus prostaglandin production since the prostaglandin metabolites were not different between the Control and BMZ-exposed mouse ductus (Supplemental Table S2 (online)).
Discussion
We found that betamethasone significantly increased the rate of ductus constriction in preterm infants, however, the means by which this was accomplished varied according to the infant’s gestational age at birth (Figure 2). Among infants born at ≤256/7 weeks gestation, betamethasone’s effect could only be appreciated after prostaglandin production had been eliminated; whereas, at 26-27 weeks gestation the increased rate of constriction was apparent even in the absence of prostaglandin inhibitors (Figure 2). Our findings are consistent with prior studies that reported ductus constriction in utero after BMZ administration (3–5) and after birth following postnatal corticosteroid administration (29).
Several factors may account for the differences between our results and those of prior clinical studies (11,12). We examined the relationship between BMZ and ductus patency at individual gestational ages; in contrast, prior studies examined the entire population of immature infants together as a group. The later approach ignores the potential effects of development on the interaction between BMZ and ductus constriction. In addition, our goal was to examine the relationship between BMZ and the elimination of persistent, hemodynamically important PDA shunts. Therefore, we defined spontaneous ductus constriction as the infant’s ability to eliminate a moderate-to-large left-to-right ductus shunt by the end of the first week. Previous studies have been concerned only with whether the PDA was present or absent, without considering the magnitude of the shunt when present. While the persistence of a moderate-to-large PDA shunt is significantly associated with serious neonatal morbidities, there is no association between the presence of a small PDA shunt and neonatal morbidity (30).
We also used different criteria to determine if an infant was “adequately” or “inadequately” exposed to antenatal BMZ. Antenatal corticosteroids have beneficial effects on other organ systems as early as 7 hours after dosing. However, these effects are reversible and begin to wane after 9 days (14–17). Prior studies either considered infants to be “inadequately” treated if they delivered less than 24 hours after the first BMZ dose (even though some infants may have derived potential benefits from delivery between 7 and 24 hours) (31), or considered them to be “adequately” treated if they delivered any time after the first BMZ dose - no matter how long the interval between the first dose and delivery (14,17). In contrast, we considered infants to be “adequately” treated with BMZ only if they delivered between 7 hours and 9 days after the first dose. All other infants were considered “inadequately” treated. It is worth noting that while prior single-course BMZ trials (that considered infants to be “adequately” treated with BMZ even if they delivered more than 9 days after the first dose of BMZ) did not observe any effects of BMZ on PDA constriction (11,12), studies that used repeated courses of BMZ to offset the waning effects of BMZ found that repeated courses of BMZ lowered the incidence of PDA (32).
Betamethasone alters gene expression by interacting with glucocorticoid response elements on the gene’s promoter regions or by regulating the expression of other transcription factors that regulate target gene expression. We hypothesized that by increasing the expression of developmentally regulated genes related to contractility, BMZ might enhance the ductus’ potential for constriction at birth. In the preterm baboon, BMZ altered the mRNA expression of a large number of developmentally regulated ductus genes that interact in complex ways to promote both ductus patency and closure (Supplemental Table S1 (online)). We used the preterm fetal mouse model to see if BMZ caused similar changes in ductus gene expression and to examine BMZ’s effects on ductus contractility. We found a smaller number of developmentally regulated genes that were affected by BMZ in the mouse ductus than in the baboon ductus (Table 1). The more limited effect of BMZ on mouse gene expression may be due either to species differences or to the more advanced developmental age of the mouse ductus at the time of collection and analysis (mouse: 89% of term gestation; baboon: 67% term gestation). At 89% of term gestation some of the mouse ductus’ developmentally regulated genes may have already increased to a point where betamethasone no longer enhances their effect.
BMZ increased the sensitivity of the mouse ductus to oxygen without altering the maximal contractile effects of oxygen (or other contractile stimuli like K+ or U-46619) (Figure 3). Several possible explanations can be postulated for BMZ’s effects on the mouse ductus’ sensitivity to oxygen based on BMZ’s effects on mouse ductus gene expression. BMZ increased the expression of the phosphodiesterases (Pde1b and Pde3b), K+ channel genes, and the angiotensin II receptor type 1 (ATII-R1) (Table 1). Pde1b and Pde3b hydrolyze the second messangers cAMP and cGMP (33), which are increased in the presence of endogenous prostaglandins and nitric oxide. Since inhibitors of Pde3b have been shown to dilate the preterm ductus (34,35), upregulation of Pde1b and Pde3b by BMZ might increase the ductus’ sensitivity to oxygen by inhibiting cGMP/cAMP activity.
Similarly, BMZ’s effects on K+ channel gene expression could contribute to the ductus’ increased sensitivity to oxygen. The voltage-gated K+ channels regulate vascular cell membrane potential (and subsequent Ca2+ flux) and have oxygen-sensing capabilities (36,37). Preterm ductus smooth muscle cells have reduced numbers of O2-sensitive K+ channels and diminished O2-sensitive K+ currents. Thebaud et al found that increasing O2-sensitive K+ channel gene expression can “rescue” this developmental deficiency and confer O2 responsiveness to the preterm ductus (38).
BMZ also increased ductus AGTR1/angiotensin II receptor type 1 (ATII-R1) expression. ATII-R1 inhibits adenylate cyclase, activates phospholipase C, and causes vasoconstriction in other vascular beds (39). However, its role in ductus closure is still in question (40).
It is interesting to note that the effects of BMZ on oxygen sensitivity were no longer apparent if the ductus were also treated with indomethacin and L-NAME (Figure 3). BMZ does not appear to alter the rate of prostaglandin production in mice (Supplemental Table S2 (online)) or other species (2), nor does it alter the expression of genes responsible for prostaglandin and NO synthesis (Ptgs1, Ptgs2, or eNOS) (Table 1). Similarly, BMZ does not appear to alter the sensitivity of the mouse ductus to exogenous prostaglandin E2 or SNP (Figure 3). We speculate that BMZ may affect endogenous prostaglandin and NO signaling by increasing the expression of Pde1b and Pde3b (Table 1) (see above).
These findings in mice are similar to what we observed in human infants born at 26-27 weeks gestation. At this more advanced gestational age BMZ exposure was associated with a significant increase in the rate of spontaneous ductus constriction, whereas postnatal indomethacin treatment eliminated the difference between BMZ-exposed and inadequately exposed infants (Figure 2).
There are several limitations to our study. We used a prospectively collected, single center, observational data set. Since the incidence of moderate-to-large PDA and neonatal morbidities differ by center, our results may not be generalizable to centers where the rates differ from ours. As an observational study, the reason for the non-administration of antenatal BMZ or for the timing of delivery after the BMZ course could not be controlled and there is the possibility of unmeasured residual confounding. Corticosteroids have multiple genomic and non-genomic effects on vascular tissues. Our study focused on the genomic effects of BMZ and did not address any of its potential non-genomic actions. We also were primarily concerned with the effects of BMZ on the initial ductus constriction and ignored its potential effects on ductus remodeling.
On the other hand, there are also strengths to our study. The single center aspect of the study meant that the same consensus-driven, standardized approaches to respiratory, hemodynamic, fluid, nutrition and PDA evaluation and management were consistent among the infants. The same neonatologist reviewed all of the infants’ echocardiograms in addition to prospectively following the clinical course of all of the study infants and recording all of the study data.
In conclusion, we found that BMZ affects genes that both promote and oppose ductus constriction. We speculate that since these genes are also developmentally regulated, BMZ’s effects on postnatal constriction may vary depending on the gestational age at the time of birth. Among infants born at ≤256/7 weeks gestation betamethasone appears to affect pathways that promote constriction with less of an effect on those (like prostaglandin signaling) that oppose it. At this gestation we were only able to detect BMZ’s effect on constriction after indomethacin treatment (Figure 2). On the other hand, at 26-27 weeks gestation, betamethasone increased the rate of spontaneous ductus constriction in the absence of prostaglandin inhibitors. At this gestation, betamethasone appears to have more of an effect on pathways (like prostaglandin signaling) that oppose constriction and less of an effect on pathways that promote it since no additional beneficial effects of betamethasone could be observed when prostaglandin inhibitors were added (Figure 2). We speculate that at 26-27 weeks gestation the ductus’ developmentally regulated genes that promote constriction may have already increased to a point where betamethasone no longer enhances their effect. Future studies will be needed to unravel these observations.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by a grant from U.S. Public Health Service NHLBI (HL109199, HL46691, HL56061, HL52636 BPD Resource Center and P51RR13986 Primate Center facility support) and by a gift from the Jamie and Bobby Gates Foundation.
U.S. Public Health Service NHLBI (HL109199, HL46691, HL56061, HL52636, P51RR13986) and by a gift from the Jamie and Bobby Gates Foundation.
Footnotes
Disclosures: None
Conflict of interests: We have no conflict of interests.
References
- 1.Clyman R. Mechanisms Regulating Closure of the Ductus Arteriosus. In: Polin R, Abman S, Rowitch D, Benitz W, Fox W, editors. Fetal and Neonatal Physiology. 5th. Philadelphia, PA: Elsevier; 2017. pp. 592–598. [Google Scholar]
- 2.Clyman RI, Mauray F, Roman C, et al. Effects of antenatal glucocorticoid administration on the ductus arteriosus of preterm lambs. Am J Physiol. 1981;241:H415–H420. doi: 10.1152/ajpheart.1981.241.3.H415. [DOI] [PubMed] [Google Scholar]
- 3.Momma K, Nishihara S, Ota Y. Constriction of the fetal ductus arteriosus by glucocorticoid hormones. Pediatr Res. 1981;15:19–21. doi: 10.1203/00006450-198101000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Wasserstrum N, Huhta JC, Mari G, Sharif DJ, Willis R, Neal NK. Betamethasone and the human fetal ductus arteriosis. Obstet Gynecol. 1989;74:897–900. [PubMed] [Google Scholar]
- 5.Kahler C, Schleussner E, Moller A, Seewald HJ. Doppler measurements in fetoplacental vessels after maternal betamethasone administration. Fetal Diagn Ther. 2004;19:52–57. doi: 10.1159/000074261. [DOI] [PubMed] [Google Scholar]
- 6.Clyman RI, Ballard PL, Sniderman S, et al. Prenatal administration of betamethasone for prevention of patient ductus arteriosus. J Pediatr. 1981;98:123–126. doi: 10.1016/s0022-3476(81)80557-4. [DOI] [PubMed] [Google Scholar]
- 7.Doyle LW, Kitchen WH, Ford GW, Rickards AL, Lissenden JV, Ryan MM. Effects of antenatal steroid therapy on mortality and morbidity in very low birth weight infants. J Pediatr. 1986;108:287–292. doi: 10.1016/s0022-3476(86)81006-x. [DOI] [PubMed] [Google Scholar]
- 8.Waffarn F, Siassi B, Cabal L, Schmidt PL. Effect of antenatal glucocorticoids on clinical closure of the ductus arteriosus. Am J Dis Child. 1983;137:336–338. doi: 10.1001/archpedi.1983.02140300018005. [DOI] [PubMed] [Google Scholar]
- 9.Eronen M, Kari A, Pesonen E, Hallman M. The effect of antenatal dexamethasone administration on the fetal and neonatal ductus arteriosus. A randomized double-blind study. Am J Dis Child. 1993;147:187–192. doi: 10.1001/archpedi.1993.02160260077026. [DOI] [PubMed] [Google Scholar]
- 10.Park HW, Choi YS, Kim KS, Kim SN. Chorioamnionitis and Patent Ductus Arteriosus: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0138114. doi: 10.1371/journal.pone.0138114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onland W, de Laat MW, Mol BW, Offringa M. Effects of antenatal corticosteroids given prior to 26 weeks’ gestation: a systematic review of randomized controlled trials. Am J Perinatol. 2011;28:33–44. doi: 10.1055/s-0030-1262509. [DOI] [PubMed] [Google Scholar]
- 12.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–335. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 13.Liebowitz M, Clyman RI. Prophylactic Indomethacin Compared with Delayed Conservative Management of the Patent Ductus Arteriosus in Extremely Preterm Infants: Effects on Neonatal Outcomes. J Pediatr. 2017;187:119–126. doi: 10.1016/j.jpeds.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidaeff AC, Ramin SM, Gilstrap LC, 3rd, Alcorn JL. Characterization of corticosteroid redosing in an in vitro cell line model. Am J Obstet Gynecol. 2004;191:1403–1408. doi: 10.1016/j.ajog.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 15.Ring AM, Garland JS, Stafeil BR, Carr MH, Peckman GS, Pircon RA. The effect of a prolonged time interval between antenatal corticosteroid administration and delivery on outcomes in preterm neonates: a cohort study. Am J Obstet Gynecol. 2007;196:457 e451–456. doi: 10.1016/j.ajog.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Costa S, Zecca E, De Luca D, De Carolis MP, Romagnoli C. Efficacy of a single dose of antenatal corticosteroids on morbidity and mortality of preterm infants. Eur J Obstet Gynecol Reprod Biol. 2007;131:154–157. doi: 10.1016/j.ejogrb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Liebowitz M, Clyman RI. Antenatal Betamethasone: A Prolonged Time Interval from Administration to Delivery Is Associated with an Increased Incidence of Severe Intraventricular Hemorrhage in Infants Born before 28 Weeks Gestation. J Pediatr. 2016;177:114–120 e111. doi: 10.1016/j.jpeds.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 19.Waleh N, Hodnick R, Jhaveri N, et al. Patterns of gene expression in the ductus arteriosus are related to environmental and genetic risk factors for persistent ductus patency. Pediatr Res. 2010;68:292–297. doi: 10.1203/PDR.0b013e3181ed8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart JD, Gonzalez CL, Christensen HD, Rayburn WF. Impact of multiple antenatal doses of betamethasone on growth and development of mice offspring. Am J Obstet Gynecol. 1997;177:1138–1144. doi: 10.1016/s0002-9378(97)70030-9. [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir H, Guvenal T, Cetin M, Kaya T, Cetin A. A placebo-controlled comparison of effects of repetitive doses of betamethasone and dexamethasone on lung maturation and lung, liver, and body weights of mouse pups. Pediatr Res. 2003;53:98–103. doi: 10.1203/00006450-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Plosa EJ, Young LR, Gulleman PM, et al. Epithelial beta1 integrin is required for lung branching morphogenesis and alveolarization. Development. 2014;141:4751–4762. doi: 10.1242/dev.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- 24.Waleh N, Barrette AM, Dagle JM, et al. Effects of Advancing Gestation and Non-Caucasian Race on Ductus Arteriosus Gene Expression. J Pediatr. 2015;167:1033–1041 e1032. doi: 10.1016/j.jpeds.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelton EL, Ector G, Galindo CL, et al. Transcriptional profiling reveals ductus arteriosus-specific genes that regulate vascular tone. Physiol Genomics. 2014;46:457–466. doi: 10.1152/physiolgenomics.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese J, Waleh N, Poole SD, Brown N, Roman C, Clyman RI. Chronic in utero cyclooxygenase inhibition alters PGE2-regulated ductus arteriosus contractile pathways and prevents postnatal closure. Pediatr Res. 2009;66:155–161. doi: 10.1203/PDR.0b013e3181aa07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Khuffash A, Jain A, Corcoran D, et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr Res. 2014;76:238–244. doi: 10.1038/pr.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coceani F, Baragatti B. Mechanisms for ductus arteriosus closure. Semin Perinatol. 2012;36:92–97. doi: 10.1053/j.semperi.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014:CD001146. doi: 10.1002/14651858.CD001146.pub4. [DOI] [PubMed] [Google Scholar]
- 30.Schena F, Francescato G, Cappelleri A, et al. Association between Hemodynamically Significant Patent Ductus Arteriosus and Bronchopulmonary Dysplasia. J Pediatr. 2015;166:1488–1492. doi: 10.1016/j.jpeds.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt B, Seshia M, Shankaran S, et al. Effects of prophylactic indomethacin in extremely low-birth-weight infants with and without adequate exposure to antenatal corticosteroids. Arch Pediatr Adolesc Med. 2011;165:642–646. doi: 10.1001/archpediatrics.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltoniemi OM, Kari MA, Hallman M. Repeated antenatal corticosteroid treatment: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90:719–727. doi: 10.1111/j.1600-0412.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Manganiello VC, Clyman RI. Expression, activity and function of cAMP and cGMP phosphodiesterases in the mature and immature ductus arteriosus. Pediatr Res. 2008;64:477–481. doi: 10.1203/PDR.0b013e3181827c2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyoshima K, Momma K, Imamura S, Nakanishi T. In vivo dilatation of the fetal and postnatal ductus arteriosus by inhibition of phosphodiesterase 3 in rats. Biol Neonate. 2006;89:251–256. doi: 10.1159/000089954. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa Y, Yokoyama U, Iwamoto M, et al. Inhibition of phosphodiesterase type 3 dilates the rat ductus arteriosus without inducing intimal thickening. Circ J. 2012;76:2456–2464. doi: 10.1253/circj.cj-12-0215. [DOI] [PubMed] [Google Scholar]
- 36.Waleh N, Reese J, Kajino H, et al. Oxygen-induced tension in the sheep ductus arteriosus: effects of gestation on potassium and calcium channel regulation. Pediatr Res. 2009;65:285–290. doi: 10.1203/PDR.0b013e31819746a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelakis E, Rebeyka I, Bateson J, Olley P, Puttagunta L, Archer S. Voltage-gated potassium channels in human ductus arteriosus. Lancet. 2000;356:134–137. doi: 10.1016/S0140-6736(00)02452-1. [DOI] [PubMed] [Google Scholar]
- 38.Thebaud B, Michelakis ED, Wu XC, et al. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: implications for infants with patent ductus arteriosus. Circulation. 2004;110:1372–1379. doi: 10.1161/01.CIR.0000141292.28616.65. [DOI] [PubMed] [Google Scholar]
- 39.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segar JL. Late-gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R80–88. doi: 10.1152/ajpregu.00421.2003. [DOI] [PubMed] [Google Scholar]
- 40.Pagni E, Baragatti B, Scebba F, Coceani F. Functional closure of the ductus arteriosus at birth: evidence against an intermediary role of angiotensin II. Pharmacology. 2014;93:120–125. doi: 10.1159/000358013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


