Abstract
Background
Intravascular imaging with intravascular ultrasound (IVUS) and optical coherence tomography (OCT) is an important adjunct to invasive coronary angiography.
Objectives
The primary objective was to examine the frequency of intravascular coronary imaging, trends in imaging use, and outcomes of patients undergoing angiography and/or PCI in the United States.
Methods
Adult patients ≥18 years of age undergoing in-hospital cardiac catheterization from January 2004 to December 2014 were identified from the National Inpatient Sample (NIS). International Classification of Diseases, Ninth Revision (ICD-9) diagnosis and procedure codes were used to identify IVUS and OCT use during diagnostic angiography and PCI.
Results
Among 3,211,872 hospitalizations with coronary angiography, intracoronary imaging was performed in 88,775 cases (4.8% of PCI and 1.0% of diagnostic procedures), with IVUS in 98.9% and OCT in 1.1% of cases. Among patients undergoing PCI, the rate of intravascular coronary imaging increased from 2.1% in 2004-2005 to 6.6% in 2013-2014 (p<0.001 for trend). Use of intravascular coronary imaging was associated with lower in-hospital mortality in patients undergoing PCI (adjusted OR 0.77; 95% CI 0.71-0.83). There was marked variability in intravascular imaging by hospital, with 63% and 13% of facilities using intravascular imaging in <5% and >15% of PCIs, respectively.
Conclusions
In a large administrative database from the United States, intravascular imaging use was low, increased over time, and imaging was associated with reduced in-hospital mortality. Substantial variation in the frequency of intravascular imaging by hospital was observed. Additional investigation to determine clinical benefits of IVUS and OCT are warranted.
Keywords: Coronary Angiography, Intravascular Imaging, Intravascular Ultrasound, Mortality, Optical Coherence Tomography, Percutaneous Coronary Intervention, Revascularization
Background
Coronary angiography in the cardiac catheterization laboratory is the standard approach to evaluate coronary artery anatomy in patients suspected to have coronary artery disease (CAD).(1) In the vast majority of patients, coronary angiography may be sufficient to identify coronary pathology. However, in complex or indeterminate coronary lesions, angiography alone may not be adequate.(2) Intravascular ultrasound (IVUS) is an imaging technology that can be used to identify plaque rupture, endothelial disruption, the presence of thrombus, and coronary artery dissection. IVUS can also determine vessel diameter, percent plaque burden, and minimal luminal area of intermediate coronary lesions, facilitate optimal stent sizing, and confirm stent apposition after percutaneous coronary intervention (PCI).(3) Optical coherence tomography (OCT), an imaging modality that uses near-infrared light to generate high-resolution axial images of the coronary arteries, may also be used to complement angiography for similar indications.(4,5) Although there are many procedural advantages to coronary angiography with intravascular coronary imaging with IVUS or OCT, large observational studies and randomized controlled trials of intravascular imaging have not consistently demonstrated a clinical benefit in patients undergoing PCI.(6-11)
Despite the wide availability of intravascular coronary imaging in cardiac catheterization laboratories nationwide, patterns of intravascular imaging use in contemporary clinical practice are not well characterized.(12,13) In the present study, we examined trends in the use of intravascular imaging in United States hospitals over the span of a decade, and the outcomes of patients undergoing invasive coronary angiography with and without intravascular coronary imaging.
Methods
Study Population
Adult patients ≥18 years of age undergoing in-hospital cardiac catheterization from 2004 to 2014 were identified from the Healthcare Cost and Utilization Project's (HCUP) National Inpatient Sample (NIS). The NIS is a national administrative database that includes discharge-level data from a 20% stratified sample of U.S. hospitals through 2011 and 20% of discharges from all HCUP participating hospitals from 2012 onward. Clinical Classifications Software (CCS) procedure codes, aggregates of relevant International Classifications of Diseases, Ninth Revision (ICD-9) procedure codes, were used to identify patients who underwent diagnostic coronary angiography (CCS procedure code 47) and/or percutaneous coronary intervention with or without stent placement (CCS procedure code 45) during hospital admission, excluding hospitalizations with codes for right heart catheterization without coronary angiography. Intravascular ultrasound use was identified by ICD-9 procedure code 00.24 and OCT use was identified by ICD-9 procedure code 38.24. Acute myocardial infarction was identified using ICD-9 diagnosis codes for STEMI (410.01 to 410.61, 410.81, and 410.91) and non-ST-segment elevation myocardial infarction (NSTEMI) (410.71). Bare metal and drug eluting stent placement were identified by ICD-9 procedure codes 36.06 and 36.07, respectively. Demographic, clinical, and hospital characteristics were recorded for all patients. The primary outcome was in-hospital all-cause mortality.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) and compared using the Independent-samples t test for univariate analyses. Categorical variables are presented as percentages and were compared by Chi-Squared tests. Multivariable logistic regression was used to estimate adjusted odds ratios (aOR) and 95% confidence intervals (CI) for the use of intravascular imaging and in-hospital outcomes. Adjusted in-hospital mortality was determined from multivariable models with age as a continuous variable, sex, race, tobacco use, obesity, hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, CAD, congestive heart failure, anemia, malignancy, elective hospitalization, acute myocardial infarction, STEMI, primary expected payer, bed size, location, and geographic region as covariates. Unadjusted testing of trends over time were performed using the Cochran-Armitage test. In order to determine variability in the use of intravascular imaging by hospital in contemporary practice, data were analyzed for hospitals with ≥75 PCI recorded in 2014. This time frame corresponds to period in which 20% of discharges from all participating hospitals were included in the NIS.
Sampling weights were applied for trend analyses and to determine national incidence estimates, as per HCUP guidance. Unless otherwise specified, unweighted data were used in all analyses. Statistical analyses were performed using SPSS 20 (IBM SPSS Statistics, Armonk, NY, USA) and R (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests are two-sided and P-values <0.05 were considered to be statistically significant.
Results
Characteristics of Patients with Coronary Angiography with and without Intravascular Imaging
A total of 3,211,872 hospitalizations in which cardiac catheterization and coronary angiography were performed were identified between 2004 and 2014, corresponding to an estimated 15,378,851 hospitalizations in the United States, after applying sampling weights. Intracoronary imaging was performed in 88,775 cases (2.8%), with intravascular ultrasound performed in 87,804 of the cases (2.7%) and OCT performed in 1,052 of the cases (0.03%). Overall, presentation with acute coronary syndrome, elective hospitalization, private insurance status, admission to an urban teaching hospital, and hospital admission in the western region of the United States were associated with intravascular coronary imaging after multivariable adjustment (Supplemental Table 1). Patients undergoing intravascular imaging were more likely to undergo PCI (81.0% vs. 45.2%, p<0.001) and less likely to be referred for coronary artery bypass grafting (6.6% vs. 9.3%, p<0.001) than those in whom intravascular imaging was not performed.
Among 1,727,792 patients undergoing diagnostic coronary angiography without PCI, intravascular imaging was performed in 16,901 (1.0%) cases, with IVUS in 16,749 and OCT in 166 cases. In the cohort of 1,484,080 patients undergoing PCI, intravascular imaging was performed in 71,874 (4.8%) cases, with IVUS in 71,055 and OCT in 886 cases. Patients undergoing diagnostic coronary angiography and PCI with intravascular imaging were more likely to have a history of prior PCI and present electively to the hospital than patients in whom intravascular imaging was not performed. Clinical characteristics of patients undergoing diagnostic coronary angiography and PCI with and without intravascular imaging are shown in Supplemental Table 2.
Trends in Intravascular Coronary Imaging
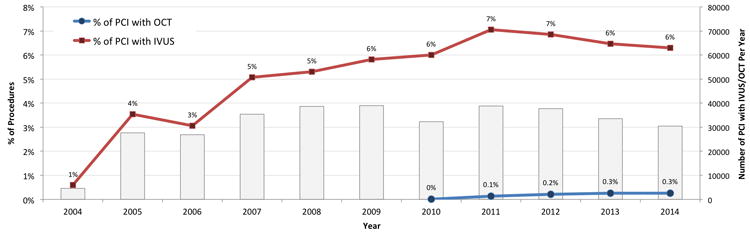
Overall, the frequency of coded intravascular imaging use was low and increased over time, from 1.2% of procedures in 2004-2005 to 3.5% of procedures in 2013-2014 (p<0.001 for trend). In the cohort of patients undergoing PCI, the frequency of coded intravascular coronary imaging use in PCI increased from 2.1% of procedures in 2004-2005 to 6.6% of procedures in 2013-2014 (p<0.001 for trend), with increases in both IVUS and OCT use (Figure 1). In the contemporary period, IVUS was used in 93.4% and OCT in 6.6% of PCI with intravascular coronary imaging.
Figure 1. Frequency and proportion of OCT and IVUS over time in patients undergoing percutaneous coronary intervention.
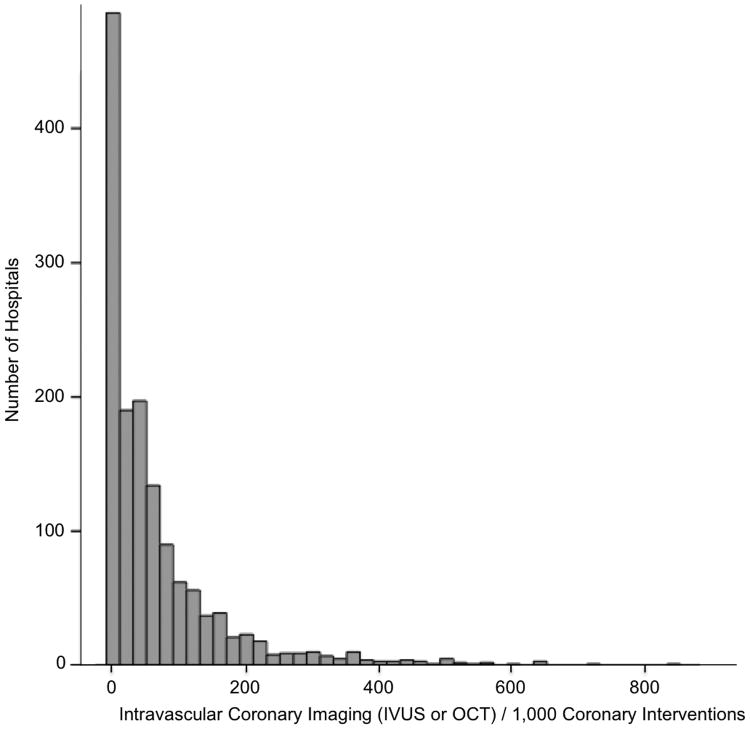
Intravascular Coronary Imaging Use By Hospital
Among 1,445 hospitals that documented at least ≥75 hospitalizations with PCI in 2014, 910 (63.0%) hospitals used intravascular imaging in <5% of all PCI, 1144 (79.2%) of hospitals performed intravascular imaging in <10% of PCI, and 1262 (87.3 %) performed intravascular imaging in <15% of PCI. The distribution of the frequency of intravascular imaging in patients undergoing PCI is shown in Figure 2.
Figure 2. Distribution of the frequency of intravascular imaging during percutaneous coronary intervention by hospital in 2014.
• Includes hospitals with ≥75 PCI in 2014
Outcomes associated with IVUS Imaging
Intravascular coronary imaging was associated with lower all-cause, in-hospital mortality among patients undergoing cardiac catheterization with or without PCI in unadjusted and multivariable adjusted analyses (0.9% versus 1.8%, aOR 0.68, 95% CI 0.63 – 0.73). This association was also observed in subgroups undergoing PCI (0.9% versus 1.7%, aOR 0.77, 95% CI 0.71 – 0.83) and among patients with and without acute coronary syndromes at the time of inpatient hospitalization. (Table 1)
Table 1. Outcomes of patients undergoing coronary angiography and/or PCI in patients with and without intravascular imaging.
| No Intravascular Imaging | Intravascular Imaging (IVUS + OCT) | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) | p-value | |
|---|---|---|---|---|---|
| In Hospital Mortality | |||||
| All Patients (Diagnostic or PCI) | 1.8% | 0.9% | 0.51 (0.47-0.55) | 0.68 (0.63-0.73) | <0.001 |
| Diagnostic Angiography Only | 1.9% | 1.1% | 0.57 (0.49-0.65) | 0.77 (0.67-0.89) | 0.001 |
| Percutaneous Coronary Intervention | 1.6% | 0.9% | 0.53 (0.49-0.57) | 0.77 (0.71-0.83) | <0.001 |
| Elective Hospitalization | 1.0% | 0.5% | 0.53 (0.44-0.62) | 0.79 (0.67-0.94) | <0.001 |
| Stable Coronary Artery Disease | 0.9% | 0.4% | 0.42 (0.37-0.48) | 0.69 (0.60-0.78) | <0.001 |
| NSTEMI | 2.4% | 1.3% | 0.56 (0.49-0.64) | 0.71 (0.62-0.81) | <0.001 |
| STEMI | 5.7% | 3.1% | 0.53 (0.48-0.59) | 0.67 (0.60-0.74) | <0.001 |
Adjusted for age, sex, race, tobacco use, obesity, hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, coronary artery disease, congestive heart failure, anemia, malignancy, elective hospitalization, acute myocardial infarction, STEMI, primary payer, hospital bed size, location, and geographic region.
Abbreviations: NSTEMI: Non-ST segment elevation myocardial infarction, PCI: Percutaneous Coronary Intervention, STEMI: ST segment elevation myocardial infarction.
Overall, coronary angiography with intravascular coronary imaging (with or without PCI) was associated with fewer diagnoses of acute kidney injury during hospitalization in comparison to coronary angiography without intravascular imaging (5.9% versus 7.5%, p<0.001; adjusted OR [aOR] 0.90 (95% CI 0.88 – 0.93). Similar findings were observed for IVUS (aOR 0.90, 95% 0.87-0.93), but not OCT (aOR 1.01, 95% 0.77-1.31). In the subgroup of patients undergoing PCI, intravascular imaging was associated with an increased frequency of acute kidney injury (aOR 1.06, 95% CI 1.02-1.10).
Discussion
In a large administrative database from the United States from 2004 to 2014, intravascular imaging was used during coronary angiography (with or without PCI) in only 2.8% of patients, including 1.0% of all diagnostic procedures and 4.8% of all PCI. The vast majority of intravascular coronary imaging was performed with IVUS, with relatively few OCT-guided procedures. Over time, the proportion of procedures with intravascular imaging increased from 2004 to 2011, but plateaued thereafter. In the current analysis, there was substantial variability in the frequency of use of intravascular coronary imaging reported between hospitals. This variability by hospital facility may reflect differences in imaging device availability, individual practice patterns, regional training and/or culture, physician comfort with image interpretation, time pressures in the cardiac catheterization laboratory, sensitivity to additional procedural costs, and uncertainty regarding the clinical benefits of intravascular imaging during PCI. Additional investigation is necessary to fully understand the rationale for this substantial variation in intravascular coronary imaging in clinical practice.
Despite its relatively low utilization in clinical practice, intravascular coronary imaging provides obvious procedural benefits in the appropriate setting (Table 2). Intravascular imaging can be used to characterize plaque morphology, facilitate the diagnosis of coronary dissection and intramural hematoma, and confirm optimal stent apposition to the arterial wall, especially in ostial stenoses, left main and bifurcation interventions. Although intravascular imaging can be used to determine the anatomic severity of coronary lesions prior to PCI, its role is in this regard has been diminished by substantial outcomes data supporting PCI guided by coronary physiology with fractional flow reserve (FFR), and more recently, iFR. In most cases, coronary physiology is preferred to intravascular imaging to determine the severity of angiographically intermediate coronary stenoses. However, in patients with acute coronary syndromes, FFR may underestimate lesion severity in an infarct vessel and its use in this setting is discouraged. Thus, in limited circumstances intracoronary imaging may be preferred to coronary physiology to guide management of angiographically intermediate coronary artery stenoses.
Table 2. Advantages and disadvantages of intravascular imaging.
| Advantages / Clinical Utility of Intravascular Imaging: | Disadvantages of Intravascular Imaging: |
|---|---|
Diagnostic Use:
|
Diagnostic & Interventional Use:
|
Interventional Use:
|
The risks of intravascular coronary imaging are low, particularly among patients planned for PCI and for whom systemic anticoagulation, guide catheter, and guide wire placement are already required. However, OCT does require the additional administration of a contrast bolus during image acquisition to eliminate blood swirl artifact, increasing risks of contrast-induced nephropathy. IVUS images can be obtained without use of any additional contrast material. Table 2 lists key advantages and disadvantages of using intravascular imaging as an adjunct to coronary angiography.
In this retrospective analysis, intravascular imaging was associated with lower risk of in-hospital death among patients undergoing PCI or diagnostic angiography, with or without acute coronary syndromes. This may be related to lower rates of stent malapposition or edge dissection associated with intravascular imaging during PCI. While hypothesis generating, these findings must be interpreted with caution, as the lower mortality observed in this study may be confounded by severity of presentation or by indication for PCI, and prior randomized trials have not consistently demonstrated a reduction in stent thrombosis associated with intravascular imaging.(14,15) Alternatively, the use of intravascular imaging may reflect the presence of intermediate, rather than severe CAD and may simply identify a population with more favorable outcomes.(16)
Despite multiple randomized trials and registry studies comparing PCI with IVUS guidance versus conventional angiography alone, the clinical benefit of IVUS imaging remains controversial.(6-11) In the IVUS-XPL trial, 1400 patients with long coronary lesions were randomized to receive IVUS-guided PCI versus angiography-guided PCI. Intravascular ultrasound was associated with lower rates of major adverse cardiovascular events at 1 year, driven largely by reduced ischemia-driven target lesion revascularization.(9) No differences in cardiac death and myocardial infarction at 1 year were observed. Similar reductions in target lesion and target vessel revascularization associated with IVUS-guided PCI have been reported in meta-analyses of randomized trials.(15) Data from large observational studies are mixed, with some studies supporting a benefit of IVUS-guided PCI,(6) while others report no improvement in patient outcomes.(7)
In contrast, there are relatively few studies evaluating clinical outcomes associated with OCT-guided PCI. The Illumien III: OPTIMIZE PCI randomized clinical trial demonstrated that OCT-guided PCI is safe and non-inferior to IVUS-guided PCI and angiography with regard to the primary efficacy endpoint of minimum stent area post-PCI, but no benefits were reported.(5,17,18) The DOCTORS trial, which randomized 240 patients with non-ST-segment elevation acute coronary syndromes to OCT-guided PCI or angiography-guided PCI demonstrated no differences in periprocedural complications and higher post-procedure fractional flow reserve in favor of OCT.(19) Consequently, additional investigation is required to identify the long-term clinical benefits of intravascular coronary imaging with OCT.
Study Limitations
There are a number of limitations to this study. First, the present data are derived from procedure codes recorded in a large national administrative inpatient database and are consequently subject to reporting bias and/or coding errors. In particular, under-coding may have affected the reported frequency of intravascular imaging during diagnostic angiography and PCI. In accordance with HCUP, all hospitals were de-identified, and validation of the OCT and IVUS procedure codes reported in the NIS could not be performed. Second, only procedures performed on hospital inpatients were available for analysis. The frequency of intravascular coronary imaging in outpatient interventional cardiology procedures may vary substantially due to differences in indications for angiography and the severity of illness, and there may be significant residual confounding despite multivariable adjustment. Third, coronary angiographic findings, intravascular imaging findings, and discrete measures of left ventricular function were not recorded in this dataset. Fourth, details of the in-hospital medical management were not available for review, and use of newer antiplatelet agents, high-intensity statins, and other guideline directed medical therapies associated with improved clinical outcomes could not be determined. Fifth, although OCT was FDA-approved for intravascular coronary imaging in 2010, only a small number of OCT procedures were identified in the current analysis. OCT use may have increased substantially since 2014, the last year of data available from the NIS. Sixth, data were analyzed as a simple random sample for the analyses without trends (i.e., without using weights). This may introduce empirical weighting and thus may not be reflective of the overall population. Similarly, hospitals were not part of the NIS sampling frame in later years and are not representatively sampled. Finally, long-term outcomes after diagnostic angiography and PCI were not available from this in-hospital dataset.
Conclusions
In a large administrative inpatient database from the United States, overall utilization of intravascular imaging with invasive coronary angiography was low. Intravascular imaging was performed in only 2.8% of cases overall and only 4.8% of PCIs. There was substantial variation in the frequency of use of intravascular imaging during coronary procedures among US hospitals. Intravascular imaging was associated with reduced in-hospital mortality in patients undergoing diagnostic angiography and PCI. Additional investigation to determine the short and long-term clinical benefits of IVUS and OCT using large-scale randomized controlled trials are warranted.
Supplementary Material
Acknowledgments
Nathaniel R. Smilowitz was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award T32HL098129.
Sponsor / Funding: None.
Footnotes
Disclosures: Dr. Weisz serves on the medical advisory boards of Angioslide, Corindus, Filterlex, Medvisor, and Trisol. The reminder of the authors report no relationships that could be construed as a conflict of interest.
References
- 1.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 2.Mintz GS. Clinical utility of intravascular imaging and physiology in coronary artery disease. J Am Coll Cardiol. 2014;64:207–22. doi: 10.1016/j.jacc.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 4.Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 5.Ali ZA, Maehara A, Genereux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 6.Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–70. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 7.Frohlich GM, Redwood S, Rakhit R, et al. Long-term survival in patients undergoing percutaneous interventions with or without intracoronary pressure wire guidance or intracoronary ultrasonographic imaging: a large cohort study. JAMA Intern Med. 2014;174:1360–6. doi: 10.1001/jamainternmed.2014.1595. [DOI] [PubMed] [Google Scholar]
- 8.Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107:374–82. doi: 10.1016/j.amjcard.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Hong SJ, Kim BK, Shin DH, et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JAMA. 2015;314:2155–63. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 10.Agostoni P, Valgimigli M, Van Mieghem CA, et al. Comparison of early outcome of percutaneous coronary intervention for unprotected left main coronary artery disease in the drug-eluting stent era with versus without intravascular ultrasonic guidance. Am J Cardiol. 2005;95:644–7. doi: 10.1016/j.amjcard.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369–76. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60:2337–9. doi: 10.1016/j.jacc.2012.08.990. [DOI] [PubMed] [Google Scholar]
- 13.Kim LK, Feldman DN, Swaminathan RV, et al. Rate of percutaneous coronary intervention for the management of acute coronary syndromes and stable coronary artery disease in the United States (2007 to 2011) Am J Cardiol. 2014;114:1003–10. doi: 10.1016/j.amjcard.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes With Intravascular Ultrasound-Guided Stent Implantation: A Meta-Analysis of Randomized Trials in the Era of Drug-Eluting Stents. Circ Cardiovasc Interv. 2016;9:e003700. doi: 10.1161/CIRCINTERVENTIONS.116.003700. [DOI] [PubMed] [Google Scholar]
- 15.Bavishi C, Sardar P, Chatterjee S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: Meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. doi: 10.1016/j.ahj.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijns W, Shite J, Jones MR, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36:3346–55. doi: 10.1093/eurheartj/ehv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maehara A, Ben-Yehuda O, Ali Z, et al. Comparison of Stent Expansion Guided by Optical Coherence Tomography Versus Intravascular Ultrasound: The ILUMIEN II Study (Observational Study of Optical Coherence Tomography [OCT] in Patients Undergoing Fractional Flow Reserve [FFR] and Percutaneous Coronary Intervention) JACC Cardiovasc Interv. 2015;8:1704–14. doi: 10.1016/j.jcin.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Meneveau N, Souteyrand G, Motreff P, et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting) Circulation. 2016;134:906–17. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.