Abstract
Purpose
Chemical exchange saturation transfer (CEST) MRI has been used for quantitative assessment of dilute metabolites and/or pH in disorders such as acute stroke and tumor. However, routine asymmetry analysis (MTRasym) may be confounded by concomitant effects such as semisolid macromolecular magnetization transfer (MT) and nuclear overhauser enhancement (NOE). Resolving multiple contributions is essential for elucidating the origins of in vivo CEST contrast.
Methods
Here we used a newly proposed Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting on densely sampled Z-spectrum to quantify multi-pool contribution from water, NOE, MT, guanidinium, amine and amide protons in adult male Wistar rats before and after global ischemia.
Results
Our results revealed the major contributors to in vivo T1-normalized MTRasym (3.5 ppm) contrast between white and gray matters (WM/GM) in normal brain (−1.96%/s) are pH-insensitive macromolecular MT (−0.89%/s) and NOE (−1.04%/s). Additionally, global ischemia resulted in significant changes of MTRasym, being −2.05%/s and −1.56%/s in WM and GM, which are dominated by changes in amide (−1.05%/s, −1.14%/s) and MT (−0.88%/s, −0.62%/s). Notably, the pH-sensitive amine and amide effects account for nearly 60% and 80% of the MTRasym changes seen in WM and GM, respectively after global ischemia, indicating MTRasym is predominantly pH-sensitive.
Conclusion
Combined amide and amine effects dominated the MTRasym changes after global ischemia, indicating MTRasym is predominantly pH-sensitive and suitable for detecting tissue acidosis following acute stroke.
Keywords: amide proton transfer (APT), magnetization transfer (MT), nuclear overhauser enhancement (NOE), chemical exchange saturation transfer (CEST), global ischemia, Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting
Introduction
Amide proton transfer (APT) MRI, a specific type of chemical exchange saturation transfer (CEST) MRI, generates enhanced contrast from labile amide protons which undergo chemical exchange with bulk water (1–5). In vivo APT MRI utilizes base-catalyzed exchange of amide protons from endogenous mobile proteins/peptides (2). It allows pH-weighted imaging with substantially higher pH-sensitivity than conventional MR spectroscopy (MRS)-based techniques (6–8). Therefore, APT MRI has been used for detecting tissue pH changes such as in acute ischemic stroke, providing additional metabolic information to routine diffusion and perfusion MRI for differentiation of benign oligemia and metabolic penumbra and prediction of stroke outcomes (9–13).
Despite the increasing interest in pH mapping with APT MRI, in vivo pH quantification remains challenging due to the complex origin of CEST effect as assessed by the commonly used asymmetry analysis of magnetization transfer (MTRasym). While RF spillover (direct water saturation) is accounted for in MTRasym, other concomitant effects such as semisolid macromolecular magnetization transfer (MT) and nuclear overhauser enhancement (NOE) effects also contribute towards the MTRasym contrast, undermining the pH-specificity of APT MRI (4,14). Therefore, it is essential to resolve individual contribution for elucidating the origins of the contrast in APT MRI and for improved pH quantification. Least-squares fitting of CETS Z-spectrum using multi-pool Lorentzian models is one of the extensively used methods to resolve APT, MT and NOE effects (15–19). A recent comparison study showed that multi-pool Lorentzian fitting provides more accurate quantification of CEST and NOE effects particularly at low irradiation powers, which can be overestimated by the Lorentzian difference (LD) analysis or underestimated by the three-offset method (20). Despite its accuracy over the other two quantification methods, the fitting reliability can be constrained by image signal-to-noise ratio (SNR). In addition, the fitting results can be biased if the initial values and boundary values are not properly chosen. Recently, we proposed an Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting algorithm that quantifies CEST images based on initial values from multi-Lorentzian fitting of iteratively less downsampled images (21). The IDEAL approach provides fitting results with smaller coefficient of variation and higher contrast-to-noise ratio at a faster fitting speed compared to the voxel-wise fitting method, desirable whereas SNR is suboptimal. In addition, acquisition of high spectral resolution CEST spectrum is crucial to resolving multiple exchangeable pools. In this study, we aimed to determine the major contributors to the MTRasym contrast between white and gray matters as well as to the changes in MTRasym after global ischemia using the IDEAL analysis of densely sampled Z-spectrum.
Methods
Animal Stroke Model
The animal experiments were approved by the Institutional Animal Care and Use Committee, Massachusetts General Hospital (IACUC, MGH). Adult male Wistar rats (Charles River Laboratory, Wilmington, MA) were anesthetized with 1.5-2.0% isoflurane/air mixture throughout the study. Heart rate and oxygen content of blood (SpO2) were monitored throughout the experiment (Nonin Pulse Oximeter 8600, Plymouth, MN). An animal model of global cerebral ischemia was used by potassium chloride injection through the femoral artery. Multiparametric MRI scans were performed on eight animals before and after global cerebral ischemia.
MRI
MRI scans were performed on a 4.7 Tesla small-bore scanner (Bruker Biospec, Erlangen Germany) with a dual RF coil setup, including a 70 mm volume transmitter coil and an actively-decoupled 20 mm surface receiver coil, to simultaneously achieve homogeneous B1 field and sensitive detection. Multi-slice MRI (5 slices, slice thickness/gap=1.8/0.2 mm, field of view=20x20 mm2, image matrix=48x48) was acquired with echo-planar imaging (EPI). CEST Z-spectrum was acquired from −6 ppm to 6 ppm with intervals of 0.05 ppm and RF irradiation power level of 0.75 μT. We set the recovery time to 8,500 ms, primary RF saturation duration (3,500 ms) and secondary RF saturation duration (500 ms), echo time (TE) = 27 ms, 1 average and scan time = 34 min (22). Water saturation shift referencing (WASSR) map was collected with RF irradiation power level of 0.5 μT TR/TS=1,500/500 ms for frequency offsets ranging between ±0.5 ppm with intervals of 0.05 ppm. In addition, T1-weighted images were acquired using inversion recovery EPI, with seven inversion delays ranging from 250 ms to 3,000 ms (TR/TE = 6,500/15 ms, 4 averages, scan time=3 min); T2-weighted SE images were obtained with two TE of 30 and 100 ms (TR = 3,250 ms, 16 averages; scan time=2 min) (23). Diffusion MRI was obtained using single-shot isotropic diffusion-weighted MRI with two b-values of 250 and 1,000 s/mm2 (repetition time (TR)/echo time (TE) = 3250/54 ms, 16 averages, scan time=2 min) (24).
Data Analysis
Data were processed in MATLAB (MathWorks, Natick, MA). Parametric T1w map was obtained with mono-exponential fitting of the signal intensities as a function of the inversion time ( ), where η is the inversion efficiency and TIi is the ith inversion time. T2w and apparent diffusion coefficient (ADC) maps were calculated as and , where TE1,2 and b1,2 are two TEs and diffusion b values, respectively, with ΔTE and Δb being their differences.
Z-spectra (I) were corrected for B0 field inhomogeneity using a WASSR map and normalized by the signal without RF irradiation (I0) (25,26). The conventional MTRasym was calculated as . T1-normalized asymmetry analysis was performed by MTRasym/T1w (27). The Z-spectra were flipped as 1-I/I0 and fitted using our newly proposed Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting algorithm (21). The IDEAL approach quantifies CEST images based on initial values from multi-pool Lorentzian fitting of iteratively less downsampled images until the original resolution. Briefly, a global Z-spectrum was first obtained by averaging the Z-spectra from all voxels within the brain region. Then a seven-pool Lorentzian model including water (0 ppm), semisolid macromolecular MT (−2 ppm), amide (3.5 ppm), amine (2.75 ppm), guanidinium (2 ppm) and Nuclear Overhauser enhancement (NOE) effects at 1.6 ppm and 3.5 ppm upfield from water was applied to estimate the CEST effects from different pools (18,28–30). The Z-spectrum was fitted as a sum of multiple Lorentzian functions with the following equation
| [2] |
where ω is the frequency offset from the water resonance, Ai, ωi and σi are the amplitude, frequency offset and linewidth of the CEST peak for the ith proton pool, respectively. We used loosely constrained bounds for the multi-pool Lorentzian fitting of the global Z-spectrum, which were between 10% and 10 times of the initial values for amplitude and linewidth, and ±20% of the corresponding linewidth for frequency offset of each pool. The B0 inhomogeneity-corrected CEST images were downsampled to a matrix size of 4×4. Then the voxel-wise multi-pool Lorentzian fitting were performed on the downsampled images with initial values of each voxel determined by the fitting results of the global Z-spectrum and tightly constrained bounds of ±10% the initial values for amplitude and linewidth, and ±5% of the corresponding linewidth for frequency offset. The same processing step was repeated sequentially for the iteratively less downsampled CEST images till the original image size of 48×48. For each iteration, the initial values of each voxel were determined by nearest-neighbor interpolation of the fitted amplitude/linewidth/offset maps of the last downsampled images.
Results
We compared the apparent diffusion coefficient (ADC) and cerebral blood flow (CBF) in rat brains (N=8) before and after global ischemia. Global ischemia resulted in significant reductions in ADC (from 0.73 ± 0.14 μm2/ms to 0.53 ± 0.13 μm2/ms, p<0.001) and CBF (from 0.88 ± 0.15 to 0.06 ± 0.05, p<0.001). Figure 1a shows multi-pool Lorentzian fitting of the globally averaged flipped Z-spectra (1-I/I0) at B1 = 0.75 T from a representative rat brain before and after global ischemia. Note this RF saturation power has been optimized for sensitizing amide proton exchange of endogenous proteins/peptides during focal ischemia (31). Figure 1b compares the raw Z-spectra after subtraction of fitted water and MT effects with the fitted individual CEST effects from the normal and ischemic brain. Apparent peaks can be found at 3.5 ppm, 2.75 ppm, 2 ppm, −1.6 ppm and −3.5 ppm of the water- and MT-corrected Z-spectra. These fitting results of the globally averaged Z-spectra were subsequently used as the initial values for the iterative IDEAL fitting. Figure 2 shows the fitted amplitude maps of each pool using the IDEAL fitting approach. Significant contrasts between white matter (WM) and gray matter (GM) were found in the amplitude maps of amide (3.5 ppm), water, semisolid MT and NOE (−3.5 ppm) (P<0.01 for each pool), with GM appearing hyperintensity in amide and water maps and hypointensity in MT and NOE maps. Upon global ischemia, such contrasts between WM and GM persisted in water, MT and NOE maps (P<0.001). Meanwhile, the fitted amplitude maps of amide (3.5 ppm) and amine (2.75 ppm) displayed a substantial signal reduction across the whole brain and such changes were significant for all eight animals with P<0.001. In addition, the fitted water and guanidinium (2.0 ppm) maps showed significant signal increase after global ischemia with P<0.005.
Figure 1.
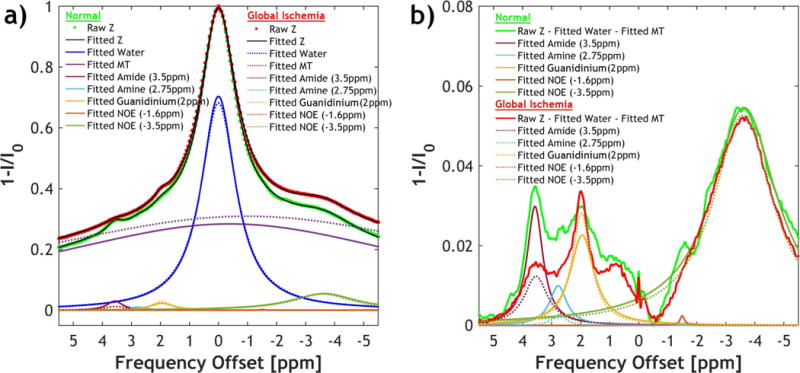
a) Multi-pool Lorentzian fitting of the Z-spectra before (solid lines) and after global ischemia (dotted lines) decouples the contribution from different pools, including saturation transfer effects from amide (3.5 ppm), amine (2.75 ppm), guanidinium (2.0 ppm), NOE (−1.6 ppm), NOE (−3.5 ppm), as well as direct water saturation and MT. b) The fitted water and MT effects were subtracted from the raw Z-spectra, showing apparent CEST contrasts between normal and ischemic tissues at amide (3.5 ppm), amine (2.75 ppm) and guanidinium (2.0 ppm).
Figure 2.
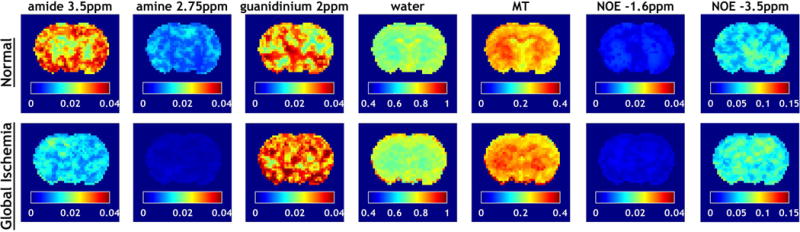
Fitted amplitude maps of each pool using IDEAL fitting in a representative rat brain before and after global ischemia.
To further evaluate the contribution from each pool to the composite contrast observed in the MTRasym (3.5ppm) map, asymmetry analysis at 3.5 ppm was performed on each fitted pool. Pronounced T1 and T2 contrasts can be found between WM and GM in both normal and ischemic brains. In addition, small yet significant T1 changes were induced by global ischemia, predominately in GM (−3.5 ± 1.7%, P<0.001). To account for the change in T1 relaxation, the MTRasym and decoupled contribution of each pool at 3.5ppm were normalized by T1 and the results were summarized in Table 1. In the normal brains, the average difference of −1.96%/s (P<0.001) in T1-normalized MTRasym (3.5ppm) between WM and GM is dominated by contributions from semisolid macromolecular MT (MTCasym) effects (−0.89%/s, P<0.05) and NOE-3.5ppm (−1.04%/s, P<0.001). After global ischemia, the MTRasym contrast between WM and GM still existed, showing an average difference of −2.44%/s (P<0.001) with similar major contributions from MTCasym (−1.16%/s, P<0.001) and NOE-3.5ppm (−1.30%/s, P<0.001). Note the relative difference in MTRasym between WM and GM remained stable after global ischemia (P=0.18). While the IDEAL approach identified amide, amine, guanidinium and water pools as showing significant changes in peak amplitude upon global ischemia, further asymmetry analysis of individual fitted pool showed that the average T1-normalized MTRasym changes of −2.05%/s and −1.56%/s in WM and GM have two major contributors, i.e. the changes in MTCasym (−0.88%/s, −0.62%/s) and amide 3.5 ppm (−1.05%/s, −1.14%/s), respectively. In WM, MTCasym and amide effects accounted for 43.0% and 51.4%, respectively of the observed MTRasym change induced by ischemia. Their contributions became 39.5% and 72.6% in GM. The rest fitted pools had less than 10% contributions individually, with amine (2.75 ppm) effect being the largest (6.0% in WM and 7.8% in GM).
Table 1.
Comparison of T1, T2, T1-normalized MTRasym and contributions from MTR asymmetry in T1-normalized decoupled amide, amine, guanidinium, NOE and semisolid macromolecular MT (MTCasym) effects toward T1-normalized MTRasym at 3.5 ppm in white matter (WM) and gray matter (GM) of normal and ischemic brains.
| Normal | Ischemia | ||
|---|---|---|---|
| T1 (s) | WM | 1.363 ± 0.009 | 1.367 ± 0.016 |
|
| |||
| GM | 1.656 ± 0.017*** | 1.713 ± 0.018***††† | |
|
| |||
| T2 (ms) | WM | 50.9 ± 0.5 | 49.2 ± 0.7††† |
|
| |||
| GM | 53.4 ± 0.5*** | 54.4 ± 0.9***††† | |
|
| |||
| MTRasym (%/s) | WM | −3.88 ± 0.28 | −5.93 ± 0.34††† |
|
| |||
| GM | −1.92 ± 0.52*** | −3.49 ± 0.71***††† | |
|
| |||
| Amide_3.5ppm (%/s) | WM | 2.08 ± 0.20 | 1.02 ± 0.09††† |
|
| |||
| GM | 2.00 ± 0.23 | 0.90 ± 0.10**††† | |
|
| |||
| Amine_2.75ppm (%/s) | WM | 0.15 ± 0.04 | 0.02 ± 0.01††† |
|
| |||
| GM | 0.14 ± 0.04 | 0.02 ± 0.01††† | |
|
| |||
| Guanidinium _2.0ppm (%/s) | WM | 0.24 ± 0.03 | 0.30 ± 0.04†† |
|
| |||
| GM | 0.17 ± 0.02*** | 0.20 ± 0.03***† | |
|
| |||
| NOE_−1.6ppm (%/s) | WM | −0.001 ± 0.000 | −0.003 ± 0.002†† |
|
| |||
| GM | −0.001 ± 0.000 | −0.002 ± 0.001† | |
|
| |||
| NOE_−3.5ppm (%/s) | WM | −4.42 ± 0.58 | −4.62 ± 0.18 |
|
| |||
| GM | −3.38 ± 0.42*** | −3.33 ± 0.13*** | |
|
| |||
| MTCasym (%/s) | WM | −1.79 ± 0.88 | −2.67 ± 0.38† |
|
| |||
| GM | −0.89 ± 0.87* | −1.51± 0.44*** | |
Mean ± standard deviation were shown. Paired t-tests were performed with
p<0.001,
p<0.01 indicating significant difference between WM and GM, and
p<0.001,
p<0.01,
p<0.05 indicating significant difference between normal and ischemic tissues.
Discussion
In this study, we used a newly proposed IDEAL fitting algorithm that exploits the high SNR of downsampled images for iterative fitting that avoids arbitrary selection of initial values which is prone to operator bias, enabling automated and adaptive fitting for reliable estimation of individual CEST effects. The fitted amplitude maps from IDEAL approach revealed larger amide and smaller NOE effects in GM than in WM, similar to previous finding using three offset measurement (32), extrapolated MT reference approach (33) or five-pool Lorentzian Z-spectral fitting method (29). In addition to the significant WM/GM contrast seen in the decoupled amide and NOE effects, we were able to determine that the T1-normalized MTRasym (3.5 ppm) contrast between normal white and gray matters predominantly arise from MT and NOE effects. Furthermore, we found such WM/GM contrast remained unchanged after global ischemia. Given that MT is typically considered pH-insensitive and normal WM and GM have similar intracellular pH (34), it becomes necessary to correct for this inherent heterogeneity in the MTRasym image to improve the pH-specificity of APT MRI. Recently, we have demonstrated the magnetization transfer and relaxation-normalized APT (MRAPT) analysis can regress out the inherent heterogeneity in normal brain MTRasym image which are associated with semisolid MT, NOE and longitudinal relaxation (13). The MRAPT measurement leads to improved pH specificity of in vivo APT MRI, allowing semi-automatic pH lesion segmentation for demarcating graded tissue acidification.
Previous studies have shown that side-chain amine protons of mobile proteins and peptides (2-3 ppm) may contribute to the downfield APT-weighted signal (3.5ppm), especially at low field strength where significant overlapping of the amide and amine peaks occurs (17,33,35). The IDEAL analysis allows us to isolate these sources by modeling systems with multiple pools and determine their contribution towards the MTRasym change seen in ischemic brain. After global ischemia, the amplitude of fitted amide (3.5 ppm) and amine (2.75 ppm) pools significantly decreased, which can be attributed to their base-catalyzed proton exchange (2,36,37) and the tissue acidosis associated with lactate buildup (38). Despite the intermediate exchanging amine protons at 2.75 ppm showed a small contribution to the T1-normalized MTRasym contrast between normal and ischemic brains, amide (3.5 ppm) and amine (2.75 ppm) together accounted for approximately 2/3 of the total contrast in WM and nearly 80% in GM. With both amine and amide protons being pH-sensitive (10,39), the MTRasym measurement is predominantly sensitized to pH change associated with tissue acidosis in the ischemic brain despite its complex sources.
Interestingly, we detected a significant increase in the CEST effect at 2.0 ppm as compared to the reduced amide (3.5 ppm) and amine (2.75 ppm) signals. The cerebral CEST contrast near 2.0 ppm has been previously attributed to amine or guanidinium protons, which are abundant in side chains of mobile proteins/peptides (37,40,41). The opposite changes in this signal and amide effect possibly arise from the different chemical exchange rates between amine/guandinium and amide protons, which have been exploited by McVicar et al. for concentration-independent pH measurement during cerebral ischemia (5). Recently, Jin et al. combined amide and guanidinium CEST effects to enhance the pH mapping of ischemic insult at 9.4 Tesla (41). Besides the guanidinium protons, the increased CEST effect at 2.0 ppm after global ischemia may have other contributors. An earlier MT study using selective water-exchange (WEX)-filter spectroscopy found the peak at 2.0 ppm with relative to water resonance became more pronounced upon global ischemia (42), which was assigned to increased mobile lipids (43). In addition, the guanidinium protons of creatine are known to contribute to the 2.0 ppm CEST effect despite its relatively low concentration in brain and base-catalyzed property (28,44). Using a multi-pool decoupling approach, our previous study found that the fitted guanidinium CEST effect at 2.0 ppm reduced in focal ischemic lesion (45). However, this finding can be limited by the less densely sampled Z-spectrum obtained at a larger saturation power (1.5μT) and fitted the CEST effect between 2 ppm and 3 ppm with a single exchanging pool whereas two peaks (2.0 ppm and 2.75 ppm) can be clearly detected with finer spectral resolution in current study. Herein, the multi-pool IDEAL fitting of densely sampled Z-spectrum allows us to resolve the opposite changes of CEST effects at 2.0 ppm and 2.75 ppm at 4.7 Tesla.
The saturation power for APT MRI of ischemia used in this study was relatively weak (0.75 μT used in this study) (31), which is desired for fitting multi-pool CEST effects (20). At this power level, the MTRasym maps are generally negative, largely attributable to the strong NOE effect upfield from water and asymmetric semisolid macromolecular MT effects. In previous studies by Jin et al. and Zhang et al. (29,32), they showed the no significant changes in NOE (−3.5ppm) effect following acute stroke with relatively weak B1 power (<1.25 μT). Similarly, we found that the decoupled NOE effect remained unchanged and had no significant contribution towards the MTRasym change induced by global ischemia. It should be mentioned that, the relayed-NOE (rNOE) effect via exchangeable protons has been demonstrated by WEX studies (42,46) and its change after acute ischemic stroke has been reported by others (47). However, the discrepancy can arise from the different saturation power B1 used, the stroke onset time, and the fitting approaches. While WEX study in protein solution phantoms and tissue homogenates showed only slight pH dependence of upfield NOE effect (46), further investigation of the pH sensitivity of NOE effect, especially for in vivo imaging, is needed. Indeed, besides amide, the MTRasym change after global ischemia can be traced to a second major source of MT. However, the fitted amplitude of MT pool showed no significant change but its offset shifted (Figure 1a), indicating the contribution from MT pool stems from increased asymmetry. The altered MTCasym has also been reported in rat brain tumors (48), attributed to varied distribution of the immobile macromolecule population under pathological conditions. On the other hand, the MT should be mainly pH insensitive due to the dominant contrast stemming from direct dipolar transfer from protons in the semi-solid macromolecules to bound water. Indeed, the contribution of MTCasym towards MTRasym is non-negligible, being 46.1% and 46.4% in normal GM and WM, respectively. Such contribution remained to be 45.0% and 43.2% in GM and WM immediately after acute focal ischemia. The small to little changes of MTCasym contribution after ischemia, especially in GM, confer high pH specificity to APT-weighted MRI.
There are a few issues that bear discussion. First, the brain temperature following global ischemia might gradually decrease after global ischemia although the animal was kept on the heating pad. Given the chemical exchange rate can be affected by the temperature, we postulate that stronger T1-normalized MTRasym change (−2.05%/s in WM and −1.56%/s in GM) observed in the global ischemia over previously reported −1.0%/s in focal ischemia (10) may include contributions from a small temperature change. In contrast, acute ischemic stroke may be accompanied by cerebral hyperthermia, which can lead to an elevated chemical exchange rate. Therefore, the true pH-dependent MTRasym change in acute ischemic stroke shall be between the apparent MTRasym changes detected in global ischemia and in focal ischemia. Second, the IDEAL approach fitted a total of seven peaks given their visibility in the acquired Z-spectra. The visibility of a CEST peak depends not only on the proton concentration, exchange rate and frequency offset of the exchangeable protons, but also on the field strength, saturation condition, B0 field homogeneity, and Z-spectral resolution. Without sufficient prior knowledge, taking an invisible peak into account for fitting or fitting of overlapping peaks may lead to errors even strong R2 can be achieved. Indeed, it is possible that the fitted MT pool may have partial contribution from NOE signal, which is a broad resonance between −2 ppm and −5 ppm with several discrete peaks indicating its composite nature rather than a single exchanging pool. In addition, other relatively fast exchanging pools such as hydroxyl protons may also be partially saturated but were treated as part of the MT or water peak in the current fitting, resulting in a shift of the apparent MT effect.
Conclusion
Our study identified the major contributors of MTRasym contrast between WM and GM in normal and ischemic brains as NOE and pH-insensitive MT, the correction of which is indispensable to improve the pH-specificity of in vivo APT MRI. We further showed that combined amide and amine effects dominated the MTRasym changes after global ischemia, indicating MTRasym is predominantly pH-sensitive for detecting tissue acidosis following acute stroke.
Acknowledgments
This study was supported by grants from National Institutes of Health R21NS085574 (to Sun), R01NS083654 (to Sun) and P51OD011132 (to Yerkes National Primate Research Center).
References
- 1.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature medicine. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 3.Sheth VR, Li Y, Chen LQ, Howison CM, Flask CA, Pagel MD. Measuring in vivo tumor pHe with CEST-FISP MRI. Magn Reson Med. 2012;67(3):760–768. doi: 10.1002/mrm.23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun PZ, Wang E, Cheung JS. Imaging acute ischemic tissue acidosis with pH-sensitive endogenous amide proton transfer (APT) MRI–correction of tissue relaxation and concomitant RF irradiation effects toward mapping quantitative cerebral tissue pH. Neuroimage. 2012;60(1):1–6. doi: 10.1016/j.neuroimage.2011.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McVicar N, Li AX, Goncalves DF, Bellyou M, Meakin SO, Prado MA, Bartha R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab. 2014;34(4):690–698. doi: 10.1038/jcbfm.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. The Journal of biological chemistry. 1973;248(20):7276–7278. [PubMed] [Google Scholar]
- 7.Chang LH, Shirane R, Weinstein PR, James TL. Cerebral metabolite dynamics during temporary complete ischemia in rats monitored by time-shared 1H and 31P NMR spectroscopy. Magn Reson Med. 1990;13(1):6–13. doi: 10.1002/mrm.1910130103. [DOI] [PubMed] [Google Scholar]
- 8.Ojugo A, McSheehy P, McIntyre D, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous 19F and 31P probes. NMR Biomed. 1999;12(8):495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27(6):1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 10.Sun PZ, Cheung JS, Wang E, Lo EH. Association between pH-weighted endogenous amide proton chemical exchange saturation transfer MRI and tissue lactic acidosis during acute ischemic stroke. J Cereb Blood Flow Metab. 2011;31(8):1743–1750. doi: 10.1038/jcbfm.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tietze A, Blicher J, Mikkelsen IK, Ostergaard L, Strother MK, Smith SA, Donahue MJ. Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR Biomed. 2014;27(2):163–174. doi: 10.1002/nbm.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaiss M, Xu J, Goerke S, Khan IS, Singer RJ, Gore JC, Gochberg DF, Bachert P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI–application to pH-weighted MRI of acute stroke. NMR Biomed. 2014;27(3):240–252. doi: 10.1002/nbm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Zhou IY, Chan ST, Wang Y, Mandeville ET, Igarashi T, Lo EH, Ji X, Sun PZ. pH-sensitive MRI demarcates graded tissue acidification during acute stroke - pH specificity enhancement with magnetization transfer and relaxation-normalized amide proton transfer (APT) MRI. Neuroimage. 2016;141:242–249. doi: 10.1016/j.neuroimage.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jokivarsi KT, Grohn HI, Grohn OH, Kauppinen RA. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magn Reson Med. 2007;57(4):647–653. doi: 10.1002/mrm.21181. [DOI] [PubMed] [Google Scholar]
- 15.Zaiss M, Schmitt B, Bachert P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J Magn Reson. 2011;211(2):149–155. [Google Scholar]
- 16.Jones CK, Huang A, Xu J, Edden RA, Schar M, Hua J, Oskolkov N, Zaca D, Zhou J, McMahon MT, Pillai JJ, van Zijl PC. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage. 2013;77:114–124. doi: 10.1016/j.neuroimage.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desmond KL, Moosvi F, Stanisz GJ. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn Reson Med. 2014;71(5):1841–1853. doi: 10.1002/mrm.24822. [DOI] [PubMed] [Google Scholar]
- 18.Windschuh J, Zaiss M, Meissner JE, Paech D, Radbruch A, Ladd ME, Bachert P. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015;28(5):529–537. doi: 10.1002/nbm.3283. [DOI] [PubMed] [Google Scholar]
- 19.Zaiss M, Windschuh J, Paech D, Meissner JE, Burth S, Schmitt B, Kickingereder P, Wiestler B, Wick W, Bendszus M, Schlemmer HP, Ladd ME, Bachert P, Radbruch A. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage. 2015;112:180–188. doi: 10.1016/j.neuroimage.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XY, Wang F, Li H, Xu J, Gochberg DF, Gore JC, Zu Z. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed. 2017;30(7) doi: 10.1002/nbm.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou IY, Wang E, Cheung JS, Zhang X, Fulci G, Sun PZ. Quantitative chemical exchange saturation transfer (CEST) MRI of glioma using Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting. Scientific reports. 2017;7(1):84. doi: 10.1038/s41598-017-00167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun PZ, Cheung JS, Wang E, Benner T, Sorensen AG. Fast multi-slice pH-weighted chemical exchange saturation transfer (CEST) MRI with unevenly segmented RF irradiation. Magn Reson Med. 2011;65(2):588–594. doi: 10.1002/mrm.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung JS, Wang E, Zhang X, Mandeville E, Lo EH, Sorensen AG, Sun PZ. Fast radio-frequency enforced steady state (FRESS) spin echo MRI for quantitative T2 mapping: minimizing the apparent repetition time (TR) dependence for fast T2 measurement. NMR Biomed. 2012;25(2):189–194. doi: 10.1002/nbm.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori S, van Zijl PCM. Diffusion weighting by the trace of the diffusion tensor within a single scan. Magn Reson Med. 1995;33:41–52. doi: 10.1002/mrm.1910330107. [DOI] [PubMed] [Google Scholar]
- 25.Stancanello J, Terreno E, Castelli DD, Cabella C, Uggeri F, Aime S. Development and validation of a smoothing-splines-based correction method for improving the analysis of CEST-MR images. Contrast media & molecular imaging. 2008;3(4):136–149. doi: 10.1002/cmmi.240. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Liu CM, Liu PK, Sun PZ. Improved measurement of labile proton concentration-weighted chemical exchange rate (k(ws)) with experimental factor-compensated and T(1) -normalized quantitative chemical exchange saturation transfer (CEST) MRI. Contrast Media Mol Imaging. 2012;7(4):384–389. doi: 10.1002/cmmi.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai K, Singh A, Poptani H, Li W, Yang S, Lu Y, Hariharan H, Zhou XJ, Reddy R. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed. 2015;28(1):1–8. doi: 10.1002/nbm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XY, Wang F, Afzal A, Xu J, Gore JC, Gochberg DF, Zu Z. A new NOE-mediated MT signal at around -1.6ppm for detecting ischemic stroke in rat brain. Magn Reson Imaging. 2016;34(8):1100–1106. doi: 10.1016/j.mri.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XY, Wang F, Jin T, Xu J, Xie J, Gochberg DF, Gore JC, Zu Z. MR imaging of a novel NOE-mediated magnetization transfer with water in rat brain at 9.4 T. Magn Reson Med. 2017;78(2):588–597. doi: 10.1002/mrm.26396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun PZ, Zhou J, Huang J, van Zijl P. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia. Magn Reson Med. 2007;57(2):405–410. doi: 10.1002/mrm.21151. [DOI] [PubMed] [Google Scholar]
- 32.Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med. 2013;69(3):760–770. doi: 10.1002/mrm.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heo HY, Zhang Y, Lee DH, Hong X, Zhou J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 Tesla. Magn Reson Med. 2016;75(1):137–149. doi: 10.1002/mrm.25581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Back T, Hoehn M, Mies G, Busch E, Schmitz B, Kohno K, Hossmann KA. Penumbral tissue alkalosis in focal cerebral ischemia: relationship to energy metabolism, blood flow, and steady potential. Annals of neurology. 2000;47(4):485–492. [PubMed] [Google Scholar]
- 35.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50(6):1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 36.Sun PZ, Benner T, Copen WA, Sorensen AG. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke. 2010;41(10 Suppl):S147–151. doi: 10.1161/STROKEAHA.110.595777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin T, Wang P, Zong X, Kim SG. Magnetic resonance imaging of the Amine-Proton EXchange (APEX) dependent contrast. Neuroimage. 2012;59(2):1218–1227. doi: 10.1016/j.neuroimage.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part II: Mechanisms of damage and treatment. Journal of neurosurgery. 1992;77(3):337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- 39.Sun PZ, Sorensen AG. Imaging pH using the chemical exchange saturation transfer (CEST) MRI: Correction of concomitant RF irradiation effects to quantify CEST MRI for chemical exchange rate and pH. Magn Reson Med. 2008;60(2):390–397. doi: 10.1002/mrm.21653. [DOI] [PubMed] [Google Scholar]
- 40.Zong X, Wang P, Kim SG, Jin T. Sensitivity and source of amine-proton exchange and amide-proton transfer magnetic resonance imaging in cerebral ischemia. Magn Reson Med. 2014;71(1):118–132. doi: 10.1002/mrm.24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin T, Wang P, Hitchens TK, Kim SG. Enhancing sensitivity of pH-weighted MRI with combination of amide and guanidyl CEST. Neuroimage. 2017;157:341–350. doi: 10.1016/j.neuroimage.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Zijl PC, Zhou J, Mori N, Payen JF, Wilson D, Mori S. Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn Reson Med. 2003;49(3):440–449. doi: 10.1002/mrm.10398. [DOI] [PubMed] [Google Scholar]
- 43.Hakumaki JM, Kauppinen RA. 1H NMR visible lipids in the life and death of cells. Trends Biochem Sci. 2000;25(8):357–362. doi: 10.1016/s0968-0004(00)01614-5. [DOI] [PubMed] [Google Scholar]
- 44.Goerke S, Zaiss M, Bachert P. Characterization of creatine guanidinium proton exchange by water-exchange (WEX) spectroscopy for absolute-pH CEST imaging in vitro. NMR Biomed. 2014;27(5):507–518. doi: 10.1002/nbm.3086. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Zhou IY, Lu D, Manderville E, Lo EH, Zheng H, Sun PZ. pH-sensitive amide proton transfer effect dominates the magnetization transfer asymmetry contrast during acute ischemia-quantification of multipool contribution to in vivo CEST MRI. Magn Reson Med. 2017 doi: 10.1002/mrm.26829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaiss M, Windschuh J, Goerke S, Paech D, Meissner JE, Burth S, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Ladd ME, Bachert P, Radbruch A. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med. 2017;77(1):196–208. doi: 10.1002/mrm.26100. [DOI] [PubMed] [Google Scholar]
- 47.Heo HY, Zhang Y, Burton TM, Jiang S, Zhao Y, van Zijl PCM, Leigh R, Zhou J. Improving the detection sensitivity of pH-weighted amide proton transfer MRI in acute stroke patients using extrapolated semisolid magnetization transfer reference signals. Magn Reson Med. 2017;78(3):871–880. doi: 10.1002/mrm.26799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua J, Jones CK, Blakeley J, Smith SA, van Zijl PC, Zhou J. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med. 2007;58(4):786–793. doi: 10.1002/mrm.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]


