Abstract
Pilocytic astrocytomas are typically grade I astrocytomas, only rarely progressing to anaplastic counterparts [1]. In the case of anaplastic pilocytic astrocytomas, some are associated with neurofibromatosis type 1 (NF1) [2], others are associated with radiation treatment [2], and the remainder appear de novo. These de novo tumours can be particularly challenging to distinguish from glioblastomas, which are grade IV and carry a worse prognosis. Here we report an unusual case of malignant transformation of a pilocytic astrocytoma in the absence of NF1 alterations or radiation treatment.
Keywords: Astrocytoma, Glioma, Mutation
A two -year-old girl presented with several weeks of emesis in the morning and right eye deviation. Head magnetic resonance imaging (MRI) demonstrated a 6 cm heterogeneous right cerebellar mass (Figure 1) with tonsillar herniation and obstructive hydrocephalus. She underwent gross total tumour resection but developed MRI findings concerning for recurrence 10 months later. A 6 mm nodule, near the resection cavity, was subsequently resected 12 months from the initial surgery. After both resections, no adjunct therapy was given.
Figure 1.
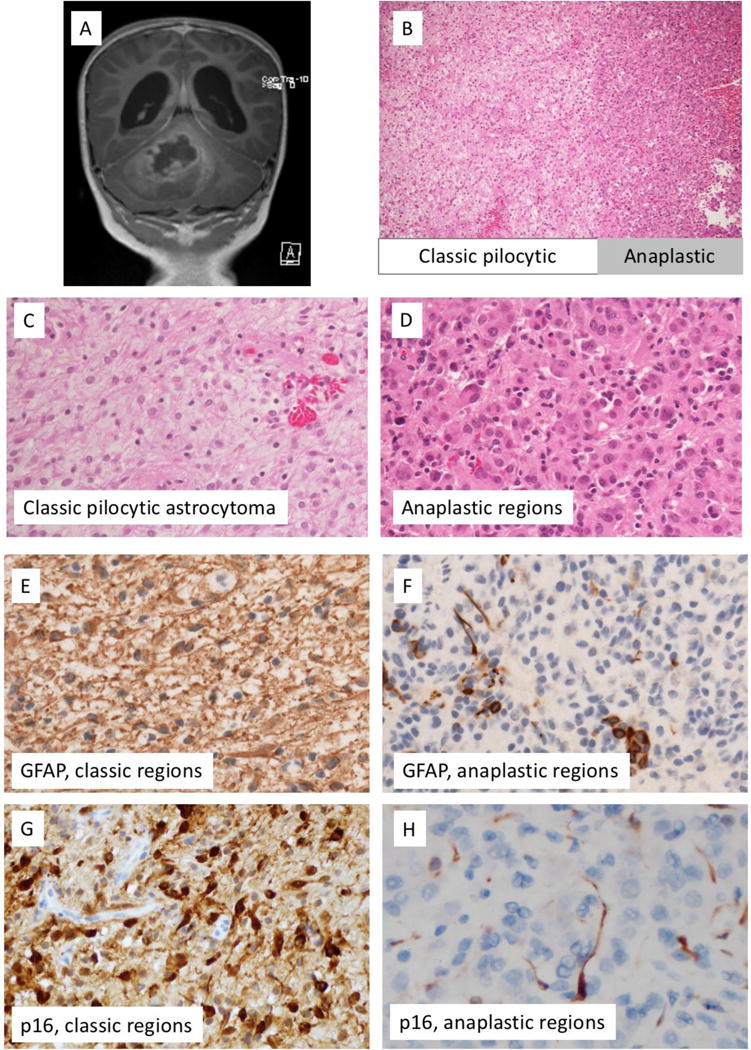
Findings at time of initial resection. A) Pre-resectionT1-postgadolinium magnetic resonance imaging, B) H&E-stained section, interface of classic-appearing and anaplastic-appearing regions. C) Higher resolution H&E-stained section, region with classic features of pilocytic astrocytoma. D) Higher resolution H&E-stained section, anaplastic region. E) GFAP immunohistochemical staining shows strong diffuse positivity in classic pilocytic regions (E), but only scattered positive cells in anaplastic regions (F). On p16 immunohistochemical stains, the regions with classic features of pilocytic astrocytoma demonstrate diffuse positivity (G), while anaplastic regions demonstrate loss of p16 expression (H).
On histology, the initial resection specimen demonstrated some areas with classic features of pilocytic astrocytoma, including oval to piloid cells, biphasic architecture, and occasional Rosenthal fibres and eosinophilic granular bodies. Other areas demonstrated increased cellularity with anaplastic features, including a focal pseudo-papillary structure (Supplemental Figure 1). Within these anaplastic areas, the cells demonstrated a more epithelioid appearance (Figure 1), with moderate eosinophilic cytoplasm and increased mitotic activity (focally up to 8 mitoses/10 high power fields).
The differential diagnosis was broad: Immunohistochemical stains did not show INI-1 loss or BRG-1 loss, and were negative for inhibin, Melan-A, cytokeratin, and transthyretin, arguing against atypical teratoid/rhabdoid tumour, hemangioblastoma, melanoma, or choroid plexus neoplasm. A CD45 stain showed scattered lymphocytes but was negative in tumour cells, excluding lymphoma.
Immunohistochemical stains demonstrated staining for S-100 in both parts. There was strong GFAP staining in the classic-appearing areas, with decreased staining in the regions of anaplasia (Figure 1), supporting a glial neoplasm. Synaptophysin stain showed weak to moderate positivity throughout but did not identify any ganglion cell or neurocytic component. Neurofilament protein stain demonstrated a predominantly solid growth pattern with entrapped axons noted only at the periphery. The MIB-1 labelling index was estimated at up to 1% in classic areas, and up to 7% in the anaplastic regions.
To better assess the two distinct areas of the tumour, next generation sequencing (NGS) was performed on the tumour specimen, with the classic pilocytic area and the anaplastic part of the tumour tested separately at the Children’s Hospital of Philadelphia utilizing a custom-designed targeted NGS panel that includes sequencing of the coding regions of 237 cancer genes for mutations and copy number variations and RNA-sequencing of 106 fusion gene partners for cancer associated fusion genes (https://www.testmenu.com/chop/ Tests/785964, last accessed 3/22/2018). Both regions demonstrated the presence of a novel KANK1- NTRK2 fusion. In addition to the fusion, the regions with classic features of pilocytic astrocytoma showed a gain of several genes on chromosome 9p (defined as the presence of greater than two copies of the given region), including JAK2, CD274, CDKN2A/B and PAX5. Interestingly, the regions with an anaplastic appearance demonstrated complicated copy number changes on chromosome 9p including gains of JAK2, CD274, and PAX5, and losses of CDKN2A/B with homozygous deletion identified in a fraction of cells, as well as gain of additional genes on chromosome 9q, including GNAQ, ABL1, NTRK2, and TSC1 (Supplemental Table 1). Correspondingly, a p16 stain showed strong staining in classic regions and loss of staining in the majority of tumour cells in the anaplastic regions (Figure 1). Of note, no mutations in NF1, H3F3A, or HIS1H3B were identified. Additional testing was performed separately for MGMT promoter methylation status. Both the classic pilocytic areas and the anaplastic-appearing regions of the initial tumour lack MGMT promoter methylation.
At the time of recurrence, the tumour demonstrated histologic features similar to the anaplastic areas of the initial resection (Supplemental Figure 2). The KANK1-NTRK2 fusion was still present, as well as the gains on chromosome 9 (Supplemental Table 1), and a heterozygous CDKN2A/2B deletion, suggesting expansion of an anaplastic clone. To our knowledge, other than NTRK2 fusion, none of the chromosome 9 gains have been previously reported in pilocytic astrocytoma, including those examined with whole genome sequencing analysis [3]. It remains unclear which, if any, may have played a role in the rapid recurrence of this tumour.
The most common genetic alteration in pilocytic astrocytoma is BRAF-KIAA fusion; however, it was not identified in this case. Instead, the tumour had a unique alteration, in the form of a KANK1-NTRK2 fusion; NTRK2 fusion can occur in a subset of pilocytic astrocytomas without BRAF-KIAA fusions [4, 5]. This fusion likely provides an alternative mechanism for MAPK pathway activation, which has been observed in virtually all pilocytic astrocytomas [3, 6].
Loss of p16 expression, as would occur in the setting of CDKN2A/2B deletion, has been correlated with more aggressive behaviour in pilocytic astrocytomas [7], and one study identified homozygous CDKN2A deletions in 20% of histologically anaplastic pilocytic astrocytomas [8]. Broader studies across all paediatric low-grade gliomas have identified CDKN2A inactivation/deletion in over half of secondary high-grade gliomas arising from paediatric low-grade gliomas [9-10], as well as 17% of paediatric low grade gliomas without BRAF V600E mutations [11], although the implications for prognosis are not always as clear in patients with tumours lacking BRAF V600E mutation [12]. A recent report focusing solely on anaplastic astrocytomas with piloid features identified CDKN2A/B deletion in 80% of their cohort [13]. In our case, the presence of this deletion solely in anaplastic areas, in combination with atypical cytology and increased mitotic index, suggests anaplastic transformation within a pilocytic astrocytoma. In one case report of a pilocytic astrocytoma with spontaneous anaplastic transformation, BRAF V600E mutation was combined with a heterozygous CDKN2A deletion [14], also suggesting CDKN2A deletion as a potential driver for anaplastic transformation. Additionally, through the use of the comprehensive NGS-based testing in both components, this case provides evidence supporting CDKN2A inactivation as a mechanism for anaplastic transformation in pilocytic astrocytomas, including those with rarer initiating mutations or fusions.
Interestingly, a model system for progression of PXA identified a combination of CDKN2A deletion combination with a broader gain of chromosome 9 in mouse xenografts studying PXA progression, and more studies are needed to identify how each may play role in progression of low grade gliomas [9].
ATRX mutations have been correlated with more aggressive behaviour in NF1-associated gliomas, including pilocytic astrocytomas [15 ]. NGS revealed wildtype ATRX in the initial tumour (both low and high-grade regions), as well as the recurrence. Additionally, immunohistochemical staining performed on the initial resection confirmed the presence of ATRX expression. Another study identified PTEN mutations in approximately one third of cases [8]. In that study, the three cases identified with p16 mutation additionally harboured PTEN mutations [8]; however, no PTEN mutations were identified in the current case.
In many previously reported pilocytic astrocytomas with malignant transformation, there was a prior history of pilocytic astrocytoma treated with radiotherapy. Interestingly, a recent report looking at anaplastic astrocytoma with piloid features identified a history of radiation in only 5% of such cases within their cohort [13], suggesting that radiation is not a requirement for anaplastic transformation. Our case represents anaplastic transformation at initial presentation, with no prior history of radiotherapy. Through analysis of both classic-appearing and anaplastic-appearing regions of the tumour, we are able to provide unique evidence for features present at tumour initiation versus those implicated in tumour progression. This case demonstrates a novel KANK1-NTRK2 fusion, which likely provides a mechanism for activation of the MAPK pathway. Finally, this case demonstrates CDKN2A/B deletion in the anaplastic-appearing areas and recurrence, providing unique evidence to support the hypothesis that loss of CDKN2A can serve as a mechanism of anaplastic transformation.
Supplementary Material
Acknowledgments
Drs. Mariarita Santi, Brian Harding, Arie Perry, and Giselle López performed histologic and immunohistochemical analysis of the tumour. Dr. Marilyn Li performed the genomic analysis of the tumour. Dr. Giselle López wrote the manuscript. All authors reviewed the manuscript and provided edits.
Funding
This work was supported by the National Institutes of Health National Cancer Institute Training Program in Translational Brain Tumor Research, T32 CA151022, and by the University of California-San Francisco Department of Pathology Residents’ Teaching and Research Endowments.
Footnotes
DR GISELLE YVETTE LOPEZ (Orcid ID : 0000-0001-5435-6668)
Conflicts of Interest
The authors have no conflicts of interest to disclose. The study was carried out according to the ethical requirements of the Children’s Hospital of Philadelphia and UCSF.
Works Cited
- 1.Collins VP, Tihan T, VandenBerg SR, Burger PC, Hawkins C, Jones D, Giannini C, Rodriguez F, Figarella-Branger D. Pilocytic astrocytoma. In: Louis D, Ohgaki Wiestler O, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4. Lyon: International Agency for Research on Cancer; 2016. pp. 80–89. [Google Scholar]
- 2.Rodriguez FJ, Scheithauer BW, Burger PC, Jenkins S, Giannini C. Anaplasia in pilocytic astrocytoma predicts aggressive behavior. Am J Surg Pathol. 2010;34:147–160. doi: 10.1097/PAS.0b013e3181c75238. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW, St Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome Project Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nats Genet. 2013;45(6):602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones DT, Hutter B, Jäger N, Korshunov A, Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA, Fontebasso AM, Stütz AM, Hutter S, Zuckermann M, Sturm D, Gronych J, Lasitschka B, Schmidt S, Seker-Cin H, Witt H, Sultan M, Ralser M, Northcott PA, Hovestadt V, Bender S, Pfaff E, Stark S, Faury D, Schwartzentruber J, Majewski J, Weber UD, Zapatka M, Raeder B, Schlesner M, Worth CL, Bartholomae CC, von Kalle C, Imbusch CD, Radomski S, Lawerenz C, van Sluis P, Koster J, Volckmann R, Versteeg R, Lehrach H, Monoranu C, Winkler B, Unterberg A, Herold-Mende C, Milde T, Kulozik AE, Ebinger M, Schuhmann MU, Cho YJ, Pomeroy SL, von Deimling A, Witt O, Taylor MD, Wolf S, Karajannis MA, Eberhart CG, Scheurlen W, Hasselblatt M, Ligon KL, Kieran MW, Korbel JO, Yaspo ML, Brors B, Felsberg J, Reifenberger G, Collins VP, Jabado N, Eils R, Lichter P, Pfister SM, International Cancer Genome Consortium PedBrain Tumor Project Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathak P, Kumar A, Jha P, Purkait S, Faruq M, Suri A, Suri V, Sharma MC, Sarkar C. Genetic alterations related to BRAF-FGFR genes and dysregulated MAPK/ERK/mTOR signaling in adult pilocytic astrocytoma. Brain Pathol. 2017;27:580–589. doi: 10.1111/bpa.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG, Nikkhah G, Maciaczyk J, Kahlert U, Jain D, Bar E, Cohen KJ, Eberhart CG. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 2011;17:3590–3599. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, Gilmer-Flynn H, Sarkaria JN, Jenkins S, Long J, Rodriguez FJ. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121:407–420. doi: 10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kogiso M, Qi L, Lindsay H, Huang Y, Zhao X, Liu Z, Braun FK, Du Y, Zhang H, Bae G, Zhao S, Injac SG, Sobieski M, Brunell D, Mehta V, Tran D, Murray J, Baxter PA, Yuan XJ, Su JM, Adesina A, Perlaky L, Chintagumpala M, Parsons DW, Lau CC, Stephan CC, Lu X, Li XNl. Xenotransplantation of Pediatric Low Grade Gliomas Confirms the Enrichment of BRAF V600E Mutation and Preservation of CDKN2A Deletion in a Novel Orthotopic Xenograft Mouse Model of Progressive Pleomorphic Xanthoastrocytoma. Oncotarget. 2017;8(50):87455–87471. doi: 10.18632/oncotarget.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, Stavropoulos J, Alon N, Pole JD, Ray PN, Navickiene V, Mangerel J, Remke M, Buczkowicz P, Ramaswamy V, Stucklin AG, Li M, Young EJ, Zhang C, Castelo-Branco P, Bakry D, Laughlin S, Shlien A, Chan J, Ligon KL, Rutka JT, Dirks PB, Taylor MD, Greenberg M, Malkin D, Huang A, Bouffet E, Hawkins CE, Tabori U. BRAF Mutation and CDKN2A Deletion Define a Clinically Distinct Subgroup of Childhood Secondary High-Grade Glioma. J Clin Oncol. 2015;33(9):1015–1022. doi: 10.1200/JCO.2014.58.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassaletta A, Zapotocky M, Mistry M, Ramaswamy V, Honnorat M, Krishnatry R, Stucklin AG, Zhukova N, Arnoldo A, Ryall S, Ling C, McKeown T, Loukides J, Cruz O, de Torres C, Ho C, Packer RJ, Tatevossian R, Qaddoumi I, Harreld JH, Dalton JD, Mulcahy-Levy J, Foreman N, Karajannis MA, Wang S, Snuderl M, Rao AN, Giannini C, Kieran M, Ligon KL, Garre ML, Nozza P, Mascelli S, Raso A, Mueller S, Nicolaides T, Silva K, Perbet R, Vasiljevic A, Conter CF, Frappaz D, Leary S, Crane C, Chan A, Ng H, Shi Z, Mao Y, Finch E, Eisenstat D, Wilson B, Carret AS, Hauser P, Sumerauer D, Krskova L, Larouche V, Fleming A, Zelcer S, Jabado N, Rutka JT, Dirks P, Taylor MD, Chen S, Bartels U, Huang A, Ellison DW, Bouffet E, Hawkins C, Tabori U. Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J Clin Oncol. 2017;35(25):2934–3941. doi: 10.1200/JCO.2016.71.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones DTW, Witt O, Pfister SM. BRAF V600E Status Alone Is Not Sufficient as a Prognostic Biomarker in Pediatric Low-Grade Glioma. J Clin Oncol. 2018;36(1):96. doi: 10.1200/JCO.2017.75.8987. [DOI] [PubMed] [Google Scholar]
- 13.Reinhardt A, Stichel D, Schrimpf D, Sahm F, Korshunov A, Reuss DE, Koelsche C, Huang K, Wefers AK, Hovestadt V, Sill M, Gramatzki D, Felsberg J, Reifenberger G, Koch A, Thomale UW, Becker A, Hans VH, Prinz M, Staszewski O, Acker T, Dohmen H, Hartmann C, Mueller W, Tuffaha MSA, Paulus W, Heß K, Brokinkel B, Schittenhelm J, Monoranu CM, Kessler AF, Loehr M, Buslei R, Deckert M, Mawrin C, Kohlhof P, Hewer E, Olar A, Rodriguez FJ, Giannini C, NageswaraRao AA, Tabori U, Nunes NM, Weller M, Pohl U, Jaunmuktane Z, Brandner S, Unterberg A, Hänggi D, Platten M, Pfister SM, Wick W, Herold-Mende C, Jones DTW, von Deimling A, Capper D. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018 doi: 10.1007/s00401-018-1837-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Yeo YH, Byrne NP, Counelis GJ, Perry A. Adult with cerebellar anaplastic pilocytic astrocytoma associated with BRAF V600E mutation and p16 loss. Clin Neuropathol. 2013;32:159–164. doi: 10.5414/NP300564. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez FJ, Vizcaino MA, Blakeley J, Heaphy CM. Frequent alternative lengthening of telomeres and ATRX loss in adult NF1-associated diffuse and high-grade astrocytomas. Acta Neuropathol. 2016;132:761–763. doi: 10.1007/s00401-016-1619-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


