Abstract
Background
Women with a history of gestational diabetes (GDM) are at risk for development of both overt Type 2 diabetes (T2DM) and cardiovascular disease (CVD) at higher rates and at earlier ages than control women. Current guidelines recommend longitudinal testing of glucose tolerance for women with prior GDM, but no formal assessments of cardiovascular disease are suggested. This study estimated the prevalence of metabolic syndrome in women with GDM in recent pregnancy who were followed for at least 1 year postpartum to quantify their cardiovascular risks.
Methods
This is a retrospective study of women who were diagnosed with GDM in a public hospital and followed for at least 1 year after delivery and who had tests performed at a minimum 4–12 weeks postpartum and 6 and 12 months postpartum. Primary outcomes were prevalence of glucose tolerance abnormalities and metabolic syndrome (MetS) defined by two prevailing sets of diagnostic criteria.
Results
One hundred fifty-one indigent, primarily Latina women who had been diagnosed in their last pregnancy with GDM comprised the study population. At the first visit postpartum, 4.7% were found to have overt diabetes and between 24 and 31% met the criteria for MetS. By the end of 12 months, another 14.5% were diagnosed with overt diabetes, and 38.5% had prediabetes. An additional 12–25% of the woman who had not had MetS at baseline developed MetS by the end of the 1-year follow-up.
Conclusions
Given the high prevalence of MetS among women with recent history of GDM immediately postpartum and its rapid development in the following year, further research is needed to enable the development of practice guidelines that will define appropriate short and long-term evaluations needed to assess risk for cardiovascular disease in these women.
Keywords: Gestational diabetes, Metabolic syndrome, Practice guidelines, Longitudinal screening, Cardiovascular risk factors
Background
Gestational diabetes (GDM) has long been recognized as a strong predictor for the early development of Type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [1–6]. Women with a history of GDM have up to a 70% chance of developing overt diabetes in their lifetimes [1, 7–9]. The risk of cardiovascular disease in women with a history of GDM is at least twice that of women without that history, even after controlling for age, body mass index, smoking, Townsend (deprivation) quintile, baseline lipid-lowering medication and baseline hypertension [3, 6, 10–13]. Some studies show that this increased CVD risk is independent of the development of Type 2 diabetes mellitus [10].
Routine testing for glucose tolerance 4–12 weeks after delivery with a 2-h 75-g oral glucose tolerance test is standard of care following a pregnancy complicated by GDM [9]. Early postpartum testing is done to distinguish women who had undiagnosed pre-existing diabetes from those who only had gestational diabetes [9]. For those with normal oral glucose tolerance test results postpartum, the American College of Obstetricians and Gynecologists (ACOG) recommends assessment of glycemic status every 1–3 years [9]. The American Diabetes Association (ADA) recommends repeat testing of glucose metabolism every 3 years [14].The Endocrine Society calls for “periodic glucose assessment” at unspecified intervals [15].In Sweden, England and other countries, annual repeat testing is recommended [7].
Adherence to postpartum testing guidelines is notoriously poor. Only 50–60% of women diagnosed with GDM, who are seen for postpartum care, are administered any postpartum glucose testing in the year following delivery [6, 16]. Even more do not get tested because they do not return for postpartum care. One recent study found that almost half of all postpartum women are not seen for any postpartum care within 99 days of delivery, even when such services were available for free [17]. Patients may not return for postpartum care because they are anxious about their condition or the visit costs [16]. Low testing rates may also be explained by the fact that significant gaps exist in clinician knowledge and practice relating to postpartum care for women who have had GDM [18].The complexity of the oral glucose tolerance test itself or a failure of clinicians to order the test may also contribute. Inadequate coordination of care between the woman’s obstetrician and her primary care provider has also been identified as a barrier to timely postpartum glucose tolerance testing [6, 16]. This lack of testing is so prevalent that some experts have suggested oral glucose tolerance testing be done while the patient is still hospitalized on postpartum day two [19].
Longer term adherence to screening test recommendations is also not common, even though abnormalities in glucose tolerance can develop rapidly in the months and years following delivery [20]. In a 15-year follow-up study of women with prior GDM, the authors observed that most women did not seek medical care until they developed clinical symptoms of diabetes [7].
Most professional attention has been focused on the high lifetime risks that women with a history of GDM face for developing T2DM. Even though these women are also at high lifetime risk for cardiovascular disease, partial or complete formal testing for cardiovascular risks for women with history of GDM is not routinely recommended by any professional organization [9, 14, 15]. O’Higgins found that women previously diagnosed with gestational diabetes mellitus are not even routinely screened for cardiovascular risk factors [21].Metabolic syndrome, which is commonly used as a marker for cardiovascular risk in the general population, is not listed in any postpartum practice guidelines for women following pregnancy complicated by GDM. The latest guidance documents from both ACOG and ADA make no mention of postpartum CVD risk assessment [9, 14] That lack of direction is reflected in practice; screening for cardiovascular risk factors such a smoking, high body mass index, hypertension and dyslipidemia occurs to be no more often among women who had GDM than it is among control women [6].
In face of the rapid deterioration of glucose tolerance in women with a history of CVD in the months immediately following delivery, it was hypothesized that metabolic syndrome might also develop among women with GDM in their recent pregnancy. This study examines the prevalence of MetS immediately postpartum and within 12 months of delivery. [22].
Methods
This is a retrospective study of the medical records of women cared for in a clinic at Harbor-UCLA Medical Center, which provided gynecological care to women, who in their last pregnancy had been diagnosed with gestational diabetes using prevailing National Diabetes Data Group criteria at the time of diagnosis. Those criteria included glucose concentrations (fasting and 3 hourly levels following a 100 g oral glucola load) with upper limits normal being 105/190/165/145 mg/dL [23]. Throughout the study period, the protocols for care of women with GDM were stable. The cost of postpartum care for these patients was completely covered for at least 6 weeks postpartum by the pregnancy-only emergency MediCal (California Medicaid) program. Beyond that initial Medicaid-funded postpartum period, many women were enrolled into the California Medicaid-waiver program that provided them free contraceptive services. Others continued to be seen in the clinic under a Los Angeles County ability-to-pay program that was available at the time.
The clinic was overseen by an endocrinologist (diabetologist) and staffed by two women’s health care nurse practitioners who also specialized in gestational diabetes. The patients were scheduled to be seen at or before 6 weeks postpartum and every 3 months thereafter. Laboratory testing was extensive; glucose tolerance tests, lipid panels, C-peptide levels were obtained at least every 6 months. Blood pressure, weight, BMI, waist and hip circumferences were routinely measured at each visit, although patients could decline any measurement. In addition to diabetes counseling, lifestyle promotion and breastfeeding support, social services, trained dietary counseling and contraception were all provided. Once a woman was diagnosed with overt diabetes, her care was transferred to another diabetes clinic.
Approval to conduct this study was obtained from the John F. Wolfe Institutional Review Board and the Research Committee at the Los Angeles BioMedical Research Institute at Harbor-UCLA Medical Center (project number 20547–01). Approval was granted on an exempt basis because it was determined that the risk of harm to the subjects was no greater than minimal and no personal identifiers were to be used.
Data were extracted by the authors directly from the clinic summary sheets that were filled out by the provider at the time of the visit. These sheets recorded for each patient all of the information collected to follow GDM-related outcomes over time. If questions arose, it was possible to consult the medical records for clarification.
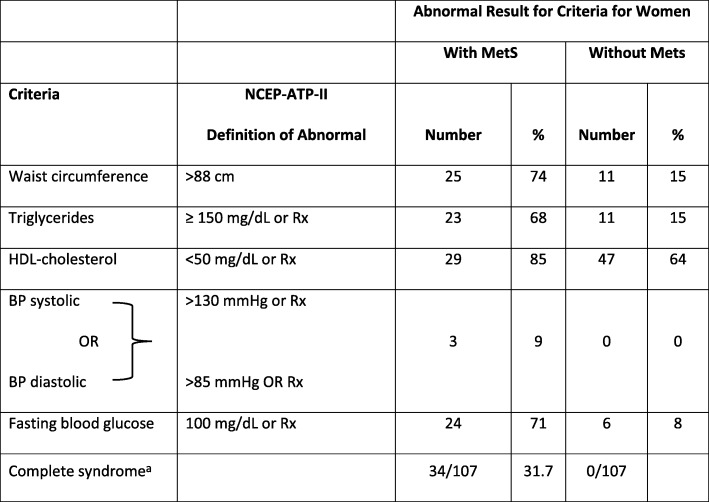
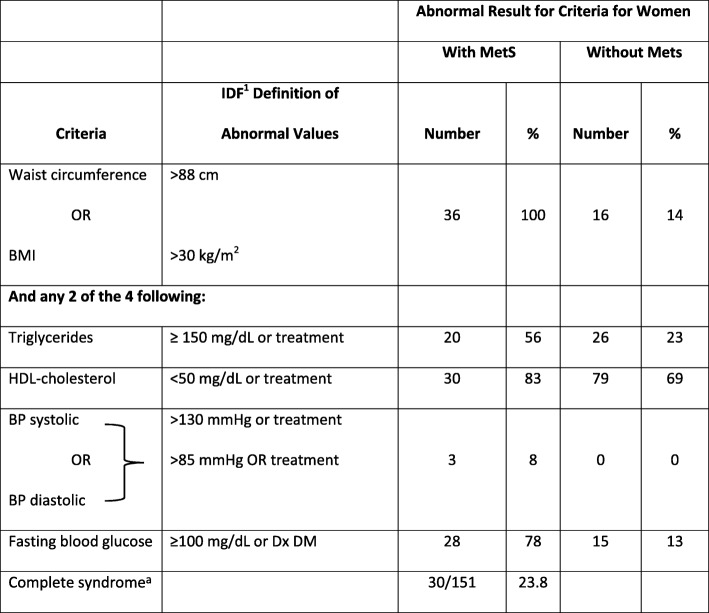
Diagnosis of metabolic syndrome (MetS) was made using the two most recognized definitions of “metabolic syndrome” including the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria and the International Diabetes Foundation (IDF) criteria to enable comparison with other studies [24, 25]. See Tables 1 and 2 for specific criteria used to define each MetS in each system. Both these criteria consider blood pressure, dyslipidemia and glucose abnormalities and measures of obesity. One difference between NCEP-ATP III and IDF is that the former uses BMI and latter uses waist circumference as a measure of central obesity although BMI > 30 can be used in IDF if waist measurements are lacking. More significantly, NCEP-ATP III allows for any 3 of 5 abnormalities to diagnose MetS, whereas IDF requires central obesity, but uses any 2 of the remaining 4 criteria to complete the diagnosis. Definitions of postpartum diabetes and prediabetes were compatible with the American Diabetes Association’s Diagnosis and Classification of Diabetes Mellitus [14]. The frequencies of abnormal patterns were calculated using descriptive statistics. A p value of < 0.05 was selected as the threshold for statistical significance.
Table 1.
Number and Percent of Women with Abnormal Test Results at 4–12 Weeks Following GDM Delivery using NCEP-ATPIIIb Criteria for Metabolic Syndrome (MetS)
aDiagnosis requires any 3 of the 5 criteria
bNCEP-ATP III National Cholesterol Education Program – Adult Treatment Panel III, Rx any medication prescribed to treat abnormalities
Table 2.
Number and Percent of Women with GDM with Abnormal Test Results at 4–12 Weeks Following Delivery using IDFa Criteria for Metabolic Syndrome (MetS)
*Diagnosis requires any 3 of 5 criteria = 30
aIDF International Diabetes Federation, Tx Any specific treatment for condition, Dx DM Diagnosis of diabetes
Results
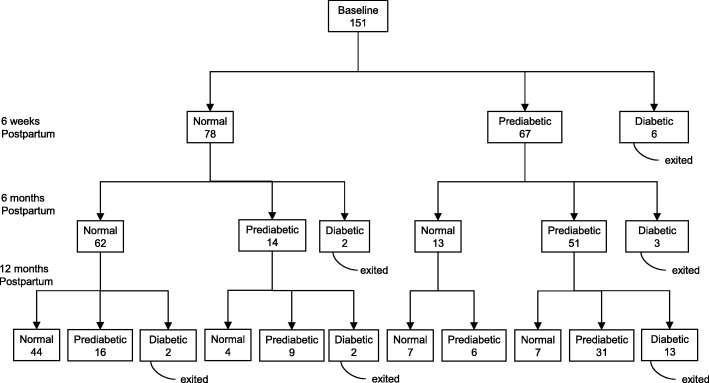
The study population included the 151 women who had been seen in the postpartum GDM clinic for at least the following 3 visits: at 6-weeks and approximately 6 and 12 months later. The women studied were indigent and primarily Latina, with a mean age of 32 (range of 20–42 years) and a mean parity of 2.6. The mean body mass index (BMI) was 30.0 kg/m2 (range 20.0–46.5 kg/m2). Figure 1 (entitled Outcomes of testing for glucose tolerance in women gestational diabetes in recent pregnancy (n = 151)) displays the frequency with which prediabetes and overt diabetes developed in these women in the first 12 months. At the initial 6 weeks postpartum evaluation, 44.4% had prediabetes and 4.7% were excluded from later analysis because they were diagnosed with overt diabetes. Over the one-year study period, another 14.5% of the remaining subjects developed overt diabetes and another 38.5% who initially had normal glucose tolerance, became prediabetic.
Fig. 1.
Outcomes of testing for glucose tolerance in women with gestational diabetes in recent pregnancy (n = 151)
Tables 1 and 2 show the percentage of women who had abnormal values in the variables used to diagnose metabolic syndrome at 4–12 weeks by criteria used for diagnoses of MetS. By that postpartum visit, nearly 1 in 3 postpartum women with recent GDM was diagnosed as having metabolic syndrome by either classification system. The major reason that the numbers of women included in the ATP III group were lower (107) than those in the IDF group (151) is that the latter allowed either BMI or waist circumference to be used as a measure of central obesity. Information about BMI was more readily available for more women than was waist circumference.
The value of considering the syndrome as a whole can be seen in the prevalence of individual abnormalities among those without MetS in Tables 1 and 2. By definition, all women with MetS in the IDF group had central obesity, but 14% of those without MetS by IDF criteria at baseline also had central obesity. By the NCEP-ATP III criteria, a similar pattern was seen; 74% of those with MetS had obesity, but 15% of those without MetS had BMI > 30 kg/m2. The other variables were similar between the groups. Abnormal fasting blood glucose was found in 71–78% of subjects with MetS, but 8–13% of those without MetS also had elevated FBS. Low HDL was fairly common in all subjects (83–85% with MetS and 64–69% without MetS), but hypertriglyceridemia was much more prevalent among women with MetS compared to those without MetS (56–68% vs 15–23%). Blood pressure elevations were uncommon in any of our patients. The high rates of abnormalities discovered at the first test cannot be attributed to physiologic changes of pregnancy, since, in general, they persisted throughout the 12-month follow-up period, with the exception of hypertriglyceridemia.
Prevalence of MetS increased in our subjects over time. See Table 3. By 12 months, of the women who did not have MetS initially, 18 (24.7%) met the ATP III criteria for MetS by 12 months, 14 (11.6%) met the IDF criteria. For those women who developed MetS, elevated FBS was the most frequent element to change, but hypertriglyceridemia also developed quite frequently. Waist circumference increase was also prevalent among those who developed MetS during the follow-up period (data not shown). Of note, nearly one-third of the GDM women who developed new onset MetS in the year following initial testing had normal FBS.
Table 3.
Results of Testing of Women with GDM Who Had No MetS at Initial Postpartum Testing, but Were Diagnosed with MetS by 12 Months, Displayed by Criteria and Composite Elements
| NCEP-ATP III Criteria | IDF Criteria | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| High density lipoprotein | 0 | 0 | 2 | 14.3 |
| Fasting blood sugar | 13 | 72.2 | 11 | 78.6 |
| Triglycerides | 11 | 61.1 | 6 | 42.9 |
| Blood Pressure | 5 | 27.8 | 2 | 14.3 |
| Body Mass Index (BMI) | 8 | 44.4 | 0 | 0 |
| Body Mass Index (BMI)/waist | 0 | 0 | 1 | 7.1 |
| Total | 18 | 24.7 | 14/121 | 11.6 |
Discussion
Gestational diabetes affects an estimated 7% of pregnancies in the United States and approximately 86% of those cases are GDM [9]. That amounts to approximately a quarter million cases each year [26]. The problem is increasing; the older age of women experiencing pregnancy and the increasing prevalence of both obesity and physical inactivity all contribute to this growth. The long-term health consequences of GDM may also be influenced by such factors [27].We found that over 25.5% of women with recent GDM experienced worsening of glucose tolerance in the first year following their deliveries; these rates that are higher than prior reports [22].
GDM is a marker of compromised pancreatic reserve, but also has been thought to represent insulin resistance [6, 28]. Stern et al. have described a “common soil hypothesis” underlying mechanism for the simultaneous increases in risks for both CVD and T2DM in women with GDM [29]. Whatever the underlying pathophysiology is, GDM is also closely associated with increased risk for early cardiovascular disease [3, 10, 30]. Our study shows that metabolic syndrome can frequently be diagnosed at the first postpartum visit; 24–31% of subjects had MetS diagnosed by of the prevailing diagnostic criteria. This may represent previously undiagnosed MetS, just as the postpartum oral glucose tolerance test can reveal previously undiagnosed diabetes, but it does underscore the fact that women with a history of GDM are at high risk for MetS and warrant testing. The prevalence of MetS in women who had experienced GDM in the last pregnancy continued to grow in the months following delivery. Another 12–25% of subjects developed MS by the end of the 12 months of follow-up. Some women diagnosed with MetS had normal fasting blood glucose levels, suggesting the need to monitor all women with prior GDM, not just those with DM.
The long term cardiovascular risks of women who have experienced gestational diabetes are very well documented [3, 6, 12, 13]. However, professional screening guidelines for women with a history of GDM focus almost entirely on initial postpartum and longitudinal tests of glucose tolerance, not for CVD [9, 15, 31–35]. Similarly, treatment recommendations for women with GDM are designed to delay the development of overt diabetes [36–41]. In particular, long term studies of potential lifestyle interventions make little or no explicit mention of other elements of metabolic syndrome or the risk factors for cardiovascular disease [42].
In most studies in which BMI and sometimes dyslipidemia were measured at different times following delivery, no formal analyses were performed for development of metabolic syndrome [22, 43]. Pallardo et al. found in the postpartum assessment that postpartum glucose intolerance was positively associated with abnormalities in BMI, waist circumference, waist-to-hip ratio; triglycerides and blood pressure, but not with total cholesterol or HDL cholesterol [44]. O’Higgins et al. reported 52% of postpartum women with prior GDM had dyslipidemia; 80% of women with abnormal postpartum oral GTT values had abnormal lipids [21]. Costacou et al. found an association between a history of GDM and excessive waist circumference [45]. Stuebe et al. and Meyers-Seifer found that women who had had GDM 3–5 years postpartum developed dyslipidemias at higher rates [46, 47]. O’Sullivan found that women with previous GDM followed for 22–28 years had increased risk for dyslipidemia, higher blood pressure and more abnormal electrocardiograms [48].
A few studies have specifically studied the association between a history of GDM and MetS in either the short term or with longer follow-up. At 3 months postpartum an association between GDM and metabolic syndrome was reported [49]. The incidence of MetS at 20 months was 37% compared to a 10% prevalence in controls [50]. At 8–10 years, women with history of GDM were 2–4 times more likely to have metabolic syndrome than controls; that risk was even higher among women with BMI > 30 kg/mL [51, 52]. Our study found even in a one-year follow-up that 10–25% of women with GDM in the most recent pregnancy who did not have MetS immediately postpartum developed it by 12 months.
This retrospective study has several limitations that may impact the generalizability of our findings. It reflects the experiences of subjects in one clinic. Our subjects were indigent, primarily Hispanic, women. Many potential candidates were excluded because they failed to return to the hospital-based clinic or they failed to follow-up for 12 months. Information about other variables that may increase cardiovascular risk, such as smoking, was not collected, but historically smoking was very rare in this population. Breastfeeding data over time were not collected, so no correction could be made for the impact of breastfeeding or stopping it can have on weight gain or other components of MetS. However, in previous studies of breastfeeding continuation patterns in women who delivered at Harbor UCLA, over half of women who said in labor that they planned to exclusively breastfeed had discontinued that practice by 6 weeks postpartum [53]. This means that the absence of this information would not likely have influenced our findings results greatly. Prior GDM or multifetal pregnancies were not controlled for; it was assumed that the impacts those factors might have had on the diagnosis of GDM would have resolved by the time of the postpartum testing.
While the high prevalence of MetS diagnosed at the time of postpartum testing following a GDM-complicated pregnancy may not represent causation, it certainly does identify a high-risk population in need of formal evaluation. However, considering the numbers of women who developed MetS by 1 year postpartum, guidelines for women with a history of GDM should include recommendations for assessment of cardiovascular risk factors. Those assessments should be at least as detailed for women with prior GDM as they are for women with PCOS who faced far lower risk of developing either overt diabetes or CVD. [54–67]. Active and ongoing monitoring for MetS among women with histories of GDM might motivate them to adopt lifestyle changes needed to prevent Type 2 diabetes as well as to reduce their risks for cardiovascular disease [37].
Conclusions
Women who have experienced GDM represent a high-risk group that deserves formal evaluation over time not only for glucose tolerance, but also for cardiovascular disease risk factors. MetS is a convenient measure. Additional research is needed to help formulate guidelines to more thoroughly address the long-term cardiovascular health risks of women with a history of GDM.
Acknowledgements
Special thanks to Brian Jean, MS, Professor of Mathematics at Taft College for his efforts, commitment and expertise in analyzing the data. We thank Monica Hau Hien Le, MD, Zuhra Musherraf, DO and Anje VanBerckelaur, MD for their previous contribution to our understanding of this population. We extend our gratitude to Joan Kuboshigi, RNP, Eli Ipp, MD and Marilyn Williams, RNP, and the other clinicians who provided care for these women and generously allowed us to analyze their data.
Funding
No external funding was utilized. The authors donated their time and efforts.
Availability of data and materials
Please contact authors for data requests.
Abbreviations
- ACOG
American college of obstetricians and gynecologists
- ADA
American diabetes association
- ATPIII
Adult treatment Panel III of the national cholesterol education program
- BMI
Body mass index
- CVD
Cardiovascular disease
- GDM
Gestational diabetes mellitus
- HDL
High density lipoprotein
- IDF
International diabetes foundation
- Met(S)
Metabolic syndrome
- PCOS
Polycystic ovary syndrome
- T2DM
Type 2 diabetes mellitus
Authors’ contributions
NKS organized the data, helped analyze the results, helped draft the manuscript and approved the final manuscript. ALN provided the data, designed the study, helped analyze the results, helped draft the manuscript and approved the final version of the manuscript.
Ethics approval and consent to participate
Approval to conduct this study was obtained on an exempt basis from the John F. Wolfe Human Subjects Committee of the Los Angeles BioMedical Research Institute at Harbor-UCLA Medical Center (project number 20547–01).
Consent for publication
Not applicable.
Competing interests
Neetu K. Sodhi, D.O., M.P.H. declares that they have no competing interests.
Anita L. Nelson, MD declares the following competing interests: Grants/Research: Agile, ContraMed, Estetra PRL, Evofem Inc., FHI (MonaLisa), Mathra Pharma, Merck; Honoraria/Speakers Bureau: Allergan, Bayer, Merck; Consultant/Advisory Board: Agile, AMAG Pharma, Bayer, ContraMed, Merck, Pharmanest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Neetu K. Sodhi, Email: drsodhi@bloomobgyn.com
Anita L. Nelson, Email: anitalnelson1@gmail.com
References
- 1.Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durnwald Celeste. Gestational diabetes: Linking epidemiology, excessive gestational weight gain, adverse pregnancy outcomes, and future metabolic syndrome. Seminars in Perinatology. 2015;39(4):254–258. doi: 10.1053/j.semperi.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31:1668–1669. doi: 10.2337/dc08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;18:371–376. doi: 10.1503/cmaj.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archambault C, Arel R, Filion KB. Gestational diabetes and risk of cardiovascular disease: a scoping review. Open Med. 2014;8:e1–e9. [PMC free article] [PubMed] [Google Scholar]
- 6.Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018;15:e1002488. doi: 10.1371/journal.pmed.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linné Y, Barkeling B, Rössner S. Natural course of gestational diabetes mellitus: long term follow-up of women in the SPAWN study. BJOG. 2002;109:1227–1231. doi: 10.1046/j.1471-0528.2002.01373.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. 2007;30:878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Practice Bulletins—Obstetrics ACOG Practice Bulletin No. 190: Gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 10.Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29:2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 11.Hopmans TE, van Houten C, Kasius A, Kouznetsova OI, Nguyen LA, Rooijmans SV, et al. Increased risk of type II diabetes mellitus and cardiovascular disease after gestational diabetes mellitus: a systematic review. Ned Tijdschr Geneeskd. 2015;159:A8043. [PubMed] [Google Scholar]
- 12.Goueslard K, Cottenet J, Mariet AS, Giroud M, Cottin Y, Petit JM, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15:15. doi: 10.1186/s12933-016-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177:1735–1742. doi: 10.1001/jamainternmed.2017.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association Position Statement 13. Management of diagetes in pregnancy: Standards of medical care in diabetes 2018. Diabetes Care. 2018;41(Supplement1):S137–S143. doi: 10.2337/dc18-S013. [DOI] [PubMed] [Google Scholar]
- 15.Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez NG, Niznik CM, Yee LM. Optimizing postpartum care for the patient with gestational diabetes mellitus. Am J Obstet Gynecol. 2017;217:314–321. doi: 10.1016/j.ajog.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz EB, Braughton MY, Riedel JC, Cohen S, Logan J, Howell M, et al. Postpartum care and contraception provided to women with gestational and preconception diabetes in California’s Medicaid program. Contraception. 2017;96:432–438. doi: 10.1016/j.contraception.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Van Ryswyk E, Middleton P, Hague W, Crowther C. Clinician views and knowledge regarding healthcare provision in the postpartum period for women with recent gestational diabetes: a systematic review of qualitative/survey studies. Diabetes Res Clin Pract. 2014;106:401–411. doi: 10.1016/j.diabres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Werner EF, Has P, Tarabulsi G, Lee J, Satin A. Early postpartum glucose testing in women with gestational diabetes mellitus. Am J Perinatol. 2016;33:966–971. doi: 10.1055/s-0036-1583193. [DOI] [PubMed] [Google Scholar]
- 20.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 21.O’Higgins AC, O’Dwyer V, O’Connor C, Daly SF, Kinsley BT, Turner MJ. Postpartum dyslipidaemia in women diagnosed with gestational diabetes mellitus. Ir J Med Sci. 2017;186:403–407. doi: 10.1007/s11845-016-1474-y. [DOI] [PubMed] [Google Scholar]
- 22.Nelson AL, Le MH, Musherraf Z, Vanberckelaer A. Intermediate-term glucose tolerance in women with a history of gestational diabetes: natural history and potential associations with breastfeeding and contraception. Am J Obstet Gynecol. 2008;198:699. doi: 10.1016/j.ajog.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 23.National Diabetes Data Group. Classification and Diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979:(12):1039-57. [DOI] [PubMed]
- 24.National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III), Executive Summary of the third report. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 26.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 28.Clark CM, Jr, Qiu C, Amerman B, Porter B, Fineberg N, Aldasouqi S, et al. Gestational diabetes: should it be added to the syndrome of insulin resistance? Diabetes Care. 1997;20:867–871. doi: 10.2337/diacare.20.5.867. [DOI] [PubMed] [Google Scholar]
- 29.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44:369–374. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 30.Harreiter J, Dovjak G, Kautzky-Willer A. Gestational diabetes mellitus and cardiovascular risk after pregnancy. Womens Health. 2014;10:91–108. doi: 10.2217/whe.13.69. [DOI] [PubMed] [Google Scholar]
- 31.Hunt KJ, Conway DL. Who returns for postpartum glucose screening following gestational diabetes mellitus? Am J Obstet Gynecol. 2008;198:404. doi: 10.1016/j.ajog.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tovar A, Chasan-Taber L, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis. 2011;8:A124. [PMC free article] [PubMed] [Google Scholar]
- 33.Blatt AJ, Nakamoto JM, Kaufman HW. Gaps in diabetes screening during pregnancy and postpartum. Obstet Gynecol. 2011;117:61–68. doi: 10.1097/AOG.0b013e3181fe424b. [DOI] [PubMed] [Google Scholar]
- 34.Shah BR, Lipscombe LL, Feig DS, Lowe JM. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study. BJOG. 2011;118:1484–1490. doi: 10.1111/j.1471-0528.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 35.Benhalima K, Leuridan L, Calewaert P, Devlieger R, Verhaeghe J, Mathieu C. Glucose intolerance after a recent history of gestational diabetes. Int J Endocrinol. 2014;2014:727652. doi: 10.1155/2014/727652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchanan TA, Page KA. Approach to the patient with gestational diabetes after delivery. J Clin Endocrinol Metab. 2011;96:3592–3598. doi: 10.1210/jc.2011-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicklas JM, Zera CA, Seely EW, Abdul-Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a hisotry of gestational diabetes. BMC Pregnancy Childbirth. 2011;11:23. [DOI] [PMC free article] [PubMed]
- 38.Gabbe SG, Landon MB, Warren-Boulton E, Fradkin J. Promoting health after gestational diabetes: a national diabetes education program call to action. Obstet Gynecol. 2012;119:171–176. doi: 10.1097/AOG.0b013e3182393208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Chen L, Horswell R, Xiao K, Besse J, Johnson J, et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Womens Health (Larchmt). 2012;21:628–633. doi: 10.1089/jwh.2011.3318. [DOI] [PubMed] [Google Scholar]
- 40.Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010;59:2625–2630. doi: 10.2337/db10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ACOG Committee Opinion No. 666 Optimizing Postpartum Care. Obstet Gynecol. 2016;127:e187–e192. doi: 10.1097/AOG.0000000000001487. [DOI] [PubMed] [Google Scholar]
- 42.Aroda VR, Christophi CA, Edelstein SL, Zhang P, Herman WH, Barrett-Connor E, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the diabetes prevention program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon JH, Kwak SH, Jung HS, Choi SH, Lim S, Cho YM, et al. Weight Gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015;100:3548–3555. doi: 10.1210/JC.2015-1113. [DOI] [PubMed] [Google Scholar]
- 44.Pallardo F, Herranz L, Garcia-Ingelmo T, Grande C, Martin-Vaquero P, Jañez M, et al. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care. 1999;22:1053–1058. doi: 10.2337/diacare.22.7.1053. [DOI] [PubMed] [Google Scholar]
- 45.Costacou T, Bosnyak Z, Harger GF, Markovic N, Silvers N, Orchard TJ. Postpartum adiponectin concentration, insulin resistance and metabolic abnormalities among women with pregnancy-induced disturbances. Prev Cardiol. 2008;11:106–115. doi: 10.1111/j.1751-7141.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 46.Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Seely EW, et al. Gestational glucose tolerance and maternal metabolic profile at 3 years postpartum. Obstet Gynecol. 2011;118:1065–1073. doi: 10.1097/AOG.0b013e3182325f5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care. 1996;19:1351–1356. doi: 10.2337/diacare.19.12.1351. [DOI] [PubMed] [Google Scholar]
- 48.O’Sullivan JB. The interaction between pregnancy, diabetes and long-term maternal outcome. In: Reece EA, Custan DR, editors. Diabetes Mellitus in Pregnancy. 2. New York: Churchill Livingston; 1995. pp. 389–398. [Google Scholar]
- 49.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670–677. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kousta E, Efstathiadou Z, Lawrence NJ, Jeffs JA, Godsland IF, Barrett SC, et al. The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia. 2006;49:36–40. doi: 10.1007/s00125-005-0058-6. [DOI] [PubMed] [Google Scholar]
- 51.Bo S, Monge L, Macchetta C, Menato G, Pinach S, Uberti B, et al. Prior gestational hyperglycemia: a long-term predictor of the metabolic syndrome. J Endocrinol Invest. 2004;27:629–635. doi: 10.1007/BF03347494. [DOI] [PubMed] [Google Scholar]
- 52.Lauenborg J, Mathiesen E, Hansen T, Glümer C, Jørgensen T, Borch-Johnsen K, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004–4010. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 53.Halderman LD, Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol. 2002;186:1250–1256. doi: 10.1067/mob.2002.123738. [DOI] [PubMed] [Google Scholar]
- 54.Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan JL, Kar S, Vanky E, Morin-Papunen L, Piltonen T, Puurunen J, et al. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am J Obstet Gynecol. 2017;217:189.e1–189.e8. doi: 10.1016/j.ajog.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Lerchbaum E, Schwetz V, Rabe T, Giuliani A, Obermayer-Pietsch B. Hyperandrogenemia in polycystic ovary syndrome: exploration of the role of free testosterone and androstenedione in metabolic phenotype. PLoS One. 2014;9:e108263. doi: 10.1371/journal.pone.0108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. Clinical review: Guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - PART 2. Endocr Pract. 2015;21:1415–1426. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- 58.Mehrabian F, Khani B, Kelishadi R, Kermani N. The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females. J Res Med Sci. 2011;16:763–769. [PMC free article] [PubMed] [Google Scholar]
- 59.Sung YA, Oh JY, Chung H, Lee H. Hyperandrogenemia is implicated in both the metabolic and reproductive morbidities of polycystic ovary syndrome. Fertil Steril. 2014;101:840–845. doi: 10.1016/j.fertnstert.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 60.Melo AS, Vieira CS, Romano LG, Ferriani RA, Navarro PA. The frequency of metabolic syndrome is higher among PCOS Brazilian women with menstrual irregularity plus hyperandrogenism. Reprod Sci. 2011;18:1230–1236. doi: 10.1177/1933719111414205. [DOI] [PubMed] [Google Scholar]
- 61.Guo J, Chen JL, Whittemore R, Whitaker E. Postpartum lifestyle interventions to prevent type 2 diabetes among women with history of gestational diabetes: A systematic review of randomized clinical trials. J Womens Health. 2016;25:38–49. doi: 10.1089/jwh.2015.5262. [DOI] [PubMed] [Google Scholar]
- 62.Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol. 2007;66:513–517. doi: 10.1111/j.1365-2265.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 63.Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007;88:1389–1395. doi: 10.1016/j.fertnstert.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 64.ACOG Committee on Practice Bulletin No. 108 Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936–949. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- 65.McCartney CR, Marshall JC. Clinical Practice. Polycystic ovary syndrome. N Engl J Med. 2016;375:54–64. doi: 10.1056/NEJMcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexander CJ, Tangchitnob EP, Lepor NE. Polycystic ovary syndrome: a major unrecognized cardiovascular risk factor in women. Rev Obstet Gynecol. 2009;2:232–239. [PMC free article] [PubMed] [Google Scholar]
- 67.Díaz M, Chacón MR, López-Bermejo A, Maymó-Masip E, Salvador C, Vendrell J, et al. Ethinyl estradiol-cyproterone acetate versus low-dose pioglitazone-flutamide-metformin for adolescent girls with androgen excess: divergent effects on CD163, TWEAK receptor, ANGPTL4, and LEPTIN expression in subcutaneous adipose tissue. J Clin Endocrinol Metab. 2012;97:3630–3638. doi: 10.1210/jc.2012-1754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact authors for data requests.