Abstract
Currently, there is still a lack of appropriate in vitro model for studying lung cancers, especially for recapitulating their invasion and metastasis properties. To develop an appropriate in vitro model for lung cancer research, low-temperature molding principle of biological manufacturing and 3D bioprinting was used in this study to fabricate a cell-laden hydrogel grid scaffold structure, using gelatin–sodium alginate-lung cancer cell A549/95-D suspension as the bio-ink. Cells distributed evenly in this model with high viability, and can be cultured sustainably. This model can be cultured for up to 28 days and maintained its structural integrity. Histology, gene analysis, and scratch test showed that 3D printed cells had enhanced invasion and migration capability compared to those cultured in 2D environment, indicating that the in vitro model developed in this study was more biomimetic compared to 2D models, and it is highly valuable in biomedical research.
Keywords: 3D bioprinting, Lung cancer, Tumor model, Invasion and metastasis
Introduction
Three-dimensional (3D) printing, originally named rapid prototyping, is now referred to as additive manufacturing technology. In 3D printing, discrete layer representations, with its height much smaller (order of magnitude) than other dimensions, are stacked layer-by-layer to form the 3D structure. It is a highly digitized process, which helps to reproduce the 3D structure quickly, efficiently, and accurately. Based on these technical features, 3D printing technology has a great potential in biomedical applications, including medical model printing and non-biological medical implant fabrication (Rengier et al. 2010; Mironov et al. 2003). In the field of biomedical research, 3D bioprinting develops rapidly in the recent years, and has become one of the most popular technologies in tissue engineering. With the control of computer, the 3D bioprinter can stack the mixture of cells and biological materials layer-by-layer to build tissue-like or organ-like structures in vitro. These 3D structures with complex shapes composed of living cells, growth factors, and matrix materials, which provide suitable microenvironment for most cell activities such as adhesion, growth, migration, differentiation, and communication. With the improvement in structure and cell environment compared to two-dimensional (2D) culture environment, 3D bioprinting can hopefully help to control cell growth and differentiation in vitro, and eventually to form tissues or organs with normal, physiological activities, and functions, which can be used in most medical research such as drug screening, function analysis of gene expression, and even transplantation of human organ and tissue (Zein et al. 2013; Sochol et al. 2016; Shafiee et al. 2016; Datta et al. 2017; Ozbolat et al. 2016; Knowlton et al. 2015).
Lung cancer, as a type of malignant tumor, is the leading cause of cancer death in the world. Non-small cell lung cancer, with high metastasis rate and high recurrence rate being the main reason of risk, accounts for about 80–85% of lung cancer (Hirschhaeuser et al. 2010), and still lacks effective and ideal clinical treatment approaches. An important part of cancer treatment is to develop effective anti-tumor drugs. Over the past few decades, a variety of studies on tumor occurrence, progress, and anti-tumor drug evaluation have been performed, but they are mostly based on 2D tumor model, in which cancer cells were cultured in vitro in a single layer (Mironov et al. 2003). However, most types of cells, when cultured in 2D microenvironment, will lose or change some of their original features and functions (Ozbolat and Hospodiuk 2016) such as cell–cell and cell–matrix interactions (biochemical signals, metabolite concentration gradients, and mechanistic constraints). Different characteristics of in vitro 3D tumor model and 2D culture of single-layer tumor model are also reflected in many aspects, such as the level of gene transcription and protein expression, concentration gradient of protein, cell signaling, morphology, and proliferation, cell activity, tissue structure, and response to drug (Mironov et al. 2003). Many drugs that have good pharmacological activity in 2D in vitro models may have poor efficacy or even cause severe adverse reactions in animals or clinical trials (Wienkers and Heath 2005; Brandon et al. 2003), which is leading to the failure of drug development. Therefore, there is an urgent need for a more reliable and effective tumor model that is more biomimetic of the actual in vivo environment to help the study of cancer occurrence and development, drug screening, and other aspects of tumor research.
For most of highly metastatic tumors such as the non-small cell lung cancer, one of the most important issues current studies which wants to solve is to limit the invasion and metastasis of cancer to realize patient survival with tumor. This study aims to develop a 3D bioprinted lung cancer model and to evaluate the feasibility of utilizing it in biomedical applications, using lung cancer cell A549 and 95-D as target cells.
Materials and methods
Cell culture
Human lung cancer cells A549 and 95-D were purchased from American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Gibco), 100 µg/ml penicillin, and 100 µg/ml streptomycin (Gibco). The cells were cultured at 37 °C in humidified atmosphere with 5% CO2, and cells at passage 4–5 were used in experiments in this study.
Matrix material
Gelatin powder (Sigma-Aldrich, St. Louis, MO, USA) and sodium alginate powder (WaKo Pure Chemical Industries, Ltd, Osaka, Japan) were sterilized by gamma ray radiation and then dissolved in 0.9% sterile sodium chloride saline to obtain 20% and 2% (w/v) concentration, respectively (Gaetani et al. 2012; Skardal and Atala 2015; Wu et al. 2016).
Preparation for printing process
A549/95-D cells were detached from cell culture plate, respectively, collected in suspension at a density of about 3 × 106/ml, and mixed with gelatin–alginate solution at the volumetric ratio of 1:2 to obtain the bio-ink for printing, resulting a final cell density in hydrogel of 106/ml. To remove air bubbles in the bio-ink, the solution was centrifuged at 1000 rpm for 10 s before printing. The temperature of the printing chamber of the 3D bioprinter (Livprint Norm, Medprin, China) was pre-cooled to 8 °C.
Printing, gel formation, and gel dissociation
The bio-ink (with cells) was extruded through a needle to obtain the 3D cell-hydrogel multilayer grid scaffold structure. More specifically, the extruding speed was set at 0.15 ml/s and the moving speed of the needle was set at 3.5 mm/s. the printing needle used for this experiment was standard dispensing needle with an inner diameter of 260 µm. The structure had eight layers and measured to be 12 mm × 12 mm in width and length. After printing, this structure was placed in 3% (w/v) sterile calcium chloride solution for 3 min to crosslink sodium alginate with calcium ion to increase the cross-linking intensity (Sarker et al. 2015). Printed structures were then cultured in DMEM medium-containing 10% FBS after being washed in phosphate buffered saline (PBS) for twice to minimize cytotoxicity of calcium ion, and the structures can be cultured for more than 2 weeks.
In the following studies, these structures need to be dissociated to obtain cells for further experiment. They can be dissociated by immersing in a solution of 55 mM sodium citrate and 20 mM ethylenediamine tetra-acetic acid (EDTA) for 3 min to de-crosslink the calcium alginate (Datta et al. 2017), and to obtain cells for further experiment.
Cell survival and proliferation
Live/dead staining (KeyGEN BioTECH, Nanjing, China) was performed on printed cellular structures to determine cell viability after printing. Briefly, printed scaffolds were first incubated in a solution containing 8 µM propidium iodide and 2 µM calcein-AM for 10 min. Then, the scaffolds were washed three times with PBS and observed under fluorescence microscope; live and dead cells (stained green and red) were counted in nine different fields for each sample.
Alamar blue assay (YEASEN, Shanghai, China) was performed every 2 days during the culturing period to show cell proliferation in printed 3D environment. Briefly, A549 or 95-D cells were cultured in individual cell wells (2D group) or in 3D printed scaffold. For cell proliferation test, each cell well or scaffold was incubated in 2 ml Alamar Blue working solution (one part of Alamer Blue with nine parts of fresh medium) for 2 h. After reaction, 100 µl of the resulting solution of each sample was added in individual wells of a 96-well plate, and the optical density (OD) values of each well, at 570 nm and 630 nm were obtained with a microplate reader. This value was normalized to that of day 1 for each type of cells, and the normalized values were plotted against time.
Cell morphological characterization
Immediately after printing and at every 2 days during the culturing period, the 3D cultured model was imaged under optical microscope and scanning electron microscope (SEM, Ultra 55, ZEISS, Germany) at 5 kV to record the cell growth and changes in the structure of the model. At the same time, morphology and distribution of the A549/95-D cells in 2D culture model were recorded and compared with those of cells in 3D printed model.
One day after printing, cell-containing model samples were collected and fixed with 4% paraformaldehyde, paraffin-embedded, and sliced for hematoxylin–eosin (H&E) stain.
Cell invasion and migration
Using fluorescence-based quantitative polymerase chain reaction (PCR), the expression of the target genes (MMP-2 and MMP-9) that are related to cell invasion was analyzed to show the differences on cell invasion and migration at the gene expression level between cells cultured in 3D printed model (cultured for 1 week) and 2D environment.
Cell migration was evaluated by scratch test. Briefly, 3D printed cells isolated as mentioned in Sect. 2.4 or 2D cultured cells were seeded in individual wells of 6-well plates at a density of 2 × 105 cells per well. After incubating for 12 h, scratches were gently made with a 1 ml pipette tip (about 150 µm in diameter). At 0 h, 12 h, and 24 h after scratching, the area covered by migrated cells was measured.
The rate of gap coverage was related to the migration capability of the cells: the faster the gap was filled by cells, the better migration capability that cells have. This test was conducted on A549 and 95-D cells, respectively. The in vitro migration capacity of different groups of cells was evaluated by the coverage of the scratch in the serum-free culture. Results between cells from 3D printing model (cultured for 1 week) and 2D cultured cells were compared to show the change in cell migration capability in vitro after 3D printing and culturing.
Tumor invasion assay
Transwell assay was implemented to evaluate if the invasion capability was enhanced after cells were cultured in 3D environment. In this assay, the Transwell, a special type of cell culture well, is separated into upper and lower chambers by an insert that permits cells to migrate through. Specifically, in this study, Transwell chambers were placed in the wells of 24-well plates. 3D printed and 2D cultured cells on day 7 were harvested and re-suspended in medium at a density of 2 × 105 cells/ml, respectively. 200 µl cell suspension was added in the upper well of each Transwell chamber. After incubating for 24 h, cells migrated to the lower well through the insert were stained with crystal violet and the number of stained cells was counted. The number of cells attached to the lower chamber side of the membrane through the membrane pores from the upper chamber can indirectly reflect the in vitro invasion capability of the tumor cells. In this study, this test was performed with both A549 and 95-D cells.
Statistical analysis
All results are presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA with a Bonferroni post hoc test to determine the degree of significance. Statistical significance was defined as *p < 0.01, **p < 0.005.
Results
Model structure and cell survival
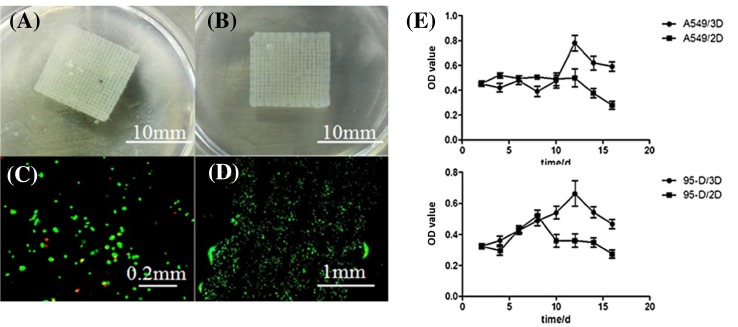
Tumor-like cell-laden hydrogel grid scaffold model with cells was successfully printed (Fig. 1a, b). Live/dead assay showed that the majority of cells remained viable after printing, as the survival rate of A549 and 95-D cells in the model was 90.8 ± 2.2% and 92.1 ± 2.0%, respectively (Fig. 1c, d).
Fig. 1.
Structure a, b of printed model and live/dead images of cells cultured in this model (c, d). It can be seem that a well-defined shape can be printed, and cells maintained high viability after 3D bioprinting. Proliferation profile of cells (e top: A549 cell line, bottom: 95-D cell line) cultured in 2D and 3D environment, from day 2 to day 16 of culturing. OD of the Y-axis is directly related to the number of cells. It can be seen that cells cultured in 2D or 3D environment had distinct proliferation profile
Cell proliferation
From the cell proliferation curve (Fig. 1e), it can be seen that, in the early culturing period (2–8 days), there was no significant difference between 3D cultured and 2D cultured cells for both A549 and 95-D cells. After day 8, the 3D cultured cells showed a significant increase in the proliferation rate, and the 2D cultured cells began to decline at the corresponding time for both types of cells. From day 12 to 14, the proliferation rate of 3D cultured cells reached the peak and began to decline. The differences showed that proliferation of both types of cells after 3D printing was normal and even slightly better than that of 2D cultured group. Cell proliferation assay showed that A549 and 95-D cells can maintain their proliferation capability when cultured after 3D printing. This normal cell growth and proliferation provided a base for the follow-up research and application of the in vitro tumor model.
Cell morphological characterization
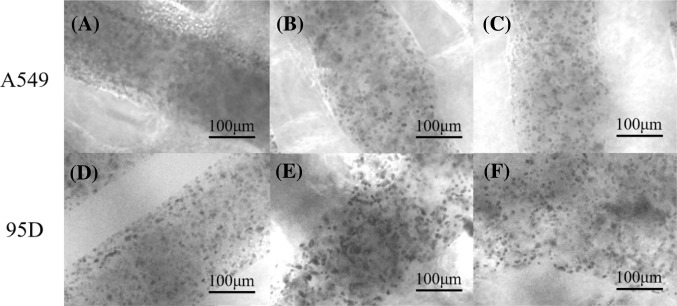
Figure 2 shows the microstructure of printed models and cells within these models, for each type of cell (A549 or 95-D), under optical microscope on day 1, day 8, and day 15 of culturing after printing. On day 1 (Fig. 2a, d) for both kinds of cell, cell distribution within the model was relatively uniform, and the model structure was relatively well defined. On day 8 (Fig. 2b, e), cells began to aggregate, formed clusters composed of several cells, the cell distribution was no longer uniform, and the model structure began to change, but it remained relatively well defined. On day 15 (Fig. 2c, f), the model structure became blur, cell distribution changed little compared with that on day 8, and became relatively stable. After continued culturing (more than 15 days), some model samples began to break down, so the culturing time in the following experiments was set to no more than 15 days.
Fig. 2.
Microstructure and encapsulated cells of printed models containing A549 (a–c) or 95-D (d–f) cells, observed under optical microscope on day 1 (a, d), day 8 (b, e), and day 15 (c, f) of culturing. Cells distributed evenly within the printed structure
On day 8 of culturing after printing, the cell-containing model was dissociated by sodium citrate/EDTA solution to remove the scaffold material, and the cells within were collected via centrifugation, cultured in 2D dish (3D-to-2D group), and compared with original 2D culture model (2D group). The results are shown in Fig. 3. Both A549 and 95-D cells in 3D-to-2D group (Fig. 3a, c) showed clearly the formation of cell aggregates, which was significantly different from the cells in the original 2D culture group (Fig. 3b, d). After excluding the influence of extracellular scaffold structure, the agglomeration of cells showed that 3D printing might have “priming” effect on the cells tested, as the behavior of these cells was different compared to those cultured in 2D, even after they were no longer cultured in 3D environment.
Fig. 3.
Comparison of 3D-to-2D (a, c) and continuous 2D (b, d) cultured A549 (a, b) and 95-D (c, d) cells. It can be seen that 3D printed culturing condition had a “priming” effect on both types of cells
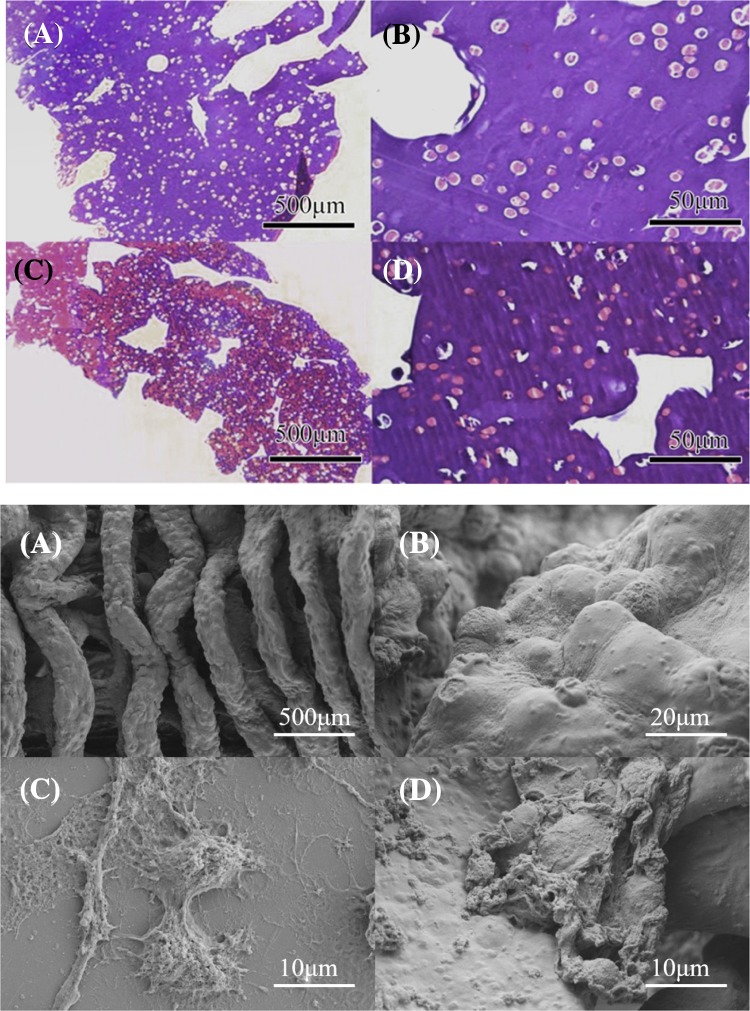
Hematoxylin–eosin (HE) staining of cell-containing model for both types of cells was performed and shown in Fig. 4. The model was on day 1 of culturing after printing and the cells within were getting stabilized. The results showed that cells were discrete and uniformly distributed in the model after printing, indicating that the aggregates were formed by spontaneous aggregation of cells being cultured in 3D environment rather than uneven mixing and printing.
Fig. 4.
Top: H&E staining of the printed model of A549 cell at × 40 (a) and × 400 (c), and of 95-D cells at × 40 (b) and × 400 (d); bottom: The microstructure of printed model (a), cells in the 3D printed model at different magnification (b, d), and cell morphology when cultured in 2D condition (c) observed under SEM
Figure 4 shows the morphology of the printed model and original 2D cultured cells observed under SEM. The structure of the supporting grid can be clearly seen in Fig. 4a. Bending of the linear structure was caused by dehydration when preparing sample for SEM, rather than deformation in the actual model structure. A large number of uniformly distributed protrusions are showed in Fig. 4a, which should be cells near the surface of the scaffold. Figure 4b is at a higher magnification and it clearly shows the protrusions containing cells. The distribution of protrusions was dense and uniform, suggesting that the cells within the printed structure had a trend of crawling to the surface of the scaffold where there was a higher nutrient concentration, indicating that the model might impair the transportation of nutrients and metabolic wastes in and out of the model. Figure 4c, d shows 2D cultured cells and the surface layer cells of the 3D printed model, respectively. The secretion, synapse, and connection of cells in two groups can be seen respectively. It showed that the cell secretion was more in the 3D model of Fig. 4d than 2D model, and the synapse and connection was also more complicated and closer.
Cell invasion and migration
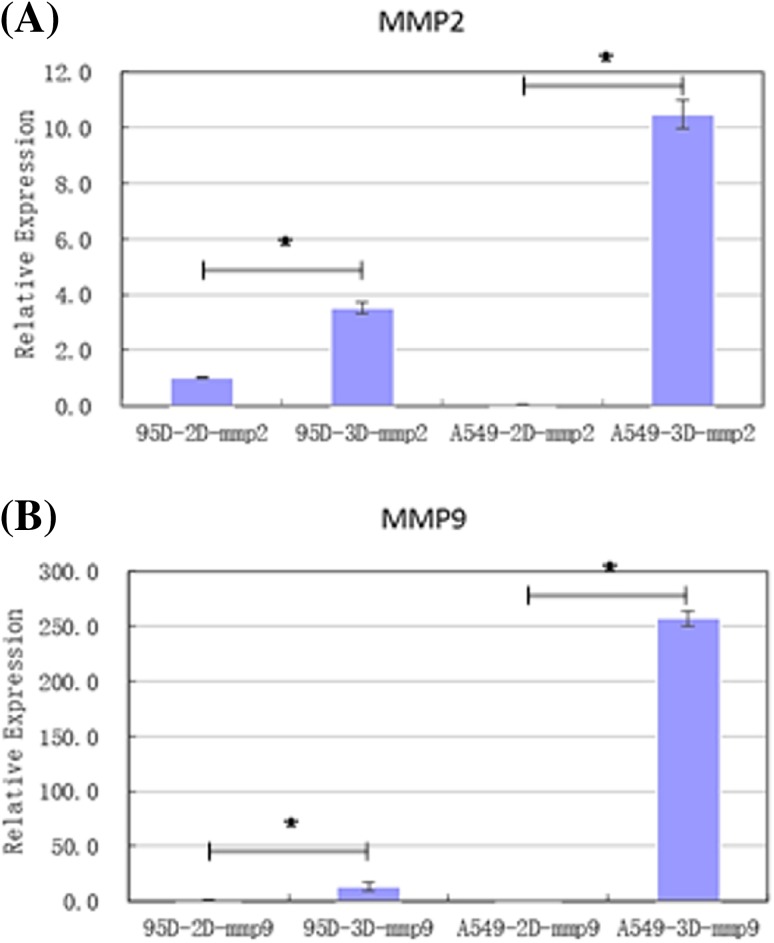
Invasiveness migration-related genes matrix metalloproteinases 2 (MMP2) and MMP9 were selected as target genes for quantitative PCR (qPCR) detection. qPCR was performed on 3D and 2D groups for both types of cells to investigate changes in cell invasion and migration capability under different environments at the gene expression level. The sample 95D-2D-mmp2 was selected as a reference, which was the expression level of MMP2 in 2D cultured 95-D cells that was set as 1, and the relative expression of genes in other groups was normalized to this value. Relative expression of the two genes in each group of samples was mapped and showed in Fig. 5.
Fig. 5.
Relative expression of MMP2 and MMP9 in A549 and 95-D cells when cultured in 3D or 2D environment (value was normalized to expression of MMP2 gene in 2D cultured 95-D cells, *p < 0.05). These two genes were expressed differently in 2D and 3D conditions
For both genes, expression in 3D cultured group was higher than that of 2D cultured group, respectively, for both types of cells. The relative gene expression of 95-D cells in the 3D group increased several to more than ten times after printing and culturing, while, for A549 cells, it was even higher. Although gene expression level might not completely determine the actual relevant function of the cells, this result indicates that, in the constructed 3D in vitro tumor model, A549 and 95-D cells both had higher invasion and migration potential than in 2D culture.
On the other hand, the lateral comparison results between A549 and 95-D cells in gene expression are shown in Fig. 5. In the 2D cultured group, the relative expression of MMP2 and MMP9 genes in 95-D cells was slightly higher than that of A549 cells, while, in the 3D printing group, oppositely, the relative expression of MMP2 and MMP9 genes in A549 cells was higher than that of 95-D cells. This suggested that, at the genetic level, the effects of 3D culturing environment are different for different types of cells.
Cell scratch test
Cell scratch test was used to reflect the in vitro migration capability of A549 and 95-D cells cultured in 3D and 2D environment, by evaluating and comparing the covered area after scratch by cell migration during the process of culturing. The images of each group at day 0, 1, and 2 after scratch were analyzed and then change in scratch area was calculated. The results are showed in Fig. 6.
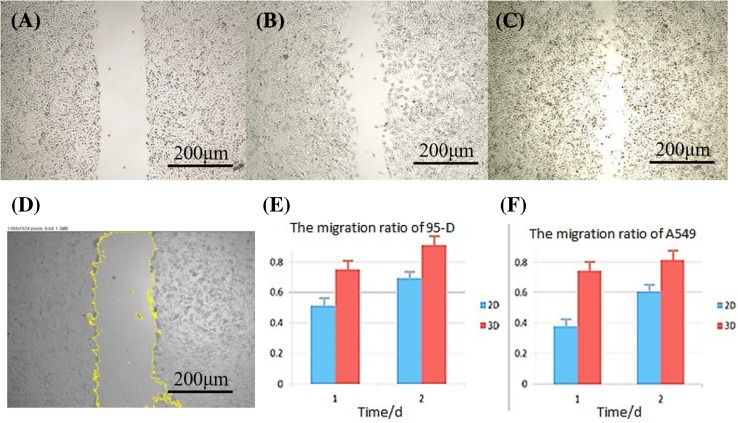
Fig. 6.
Scratch test. The scratch at day 0, 1, 2 of 2D cultured A549 cells (a–c). d Scratch region selected for calculation. There was a trend of higher migration ratio of 3D cultured cells, as shown in panels E&F (the total scratch area at day 0 was used as reference and set to 1)
The images of scratch in Fig. 6a–c showed that cells had obvious migration capability. It can be seen in Fig. 6e, f; the migration ratio of 95-D and A549 cells in 3D group was significantly higher than that in 2D group. This indicated that cells in the 3D model were more capable of migrating than 2D cultured cells.
Tumor invasion assay
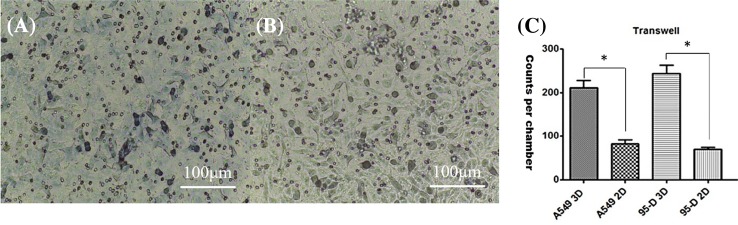
Results of this test are shown in Fig. 7. It can be seen from Fig. 7a, b that there were significantly more cells invaded through the membrane in 3D group compared to 2D group, and cell distribution was more uniform. Figure 7c shows cell invasion data of each group; there were more invasion counts in 3D group than in 2D group for both type of cells, indicating that the cells in the 3D tumor-like model had higher in vitro invasion capability than the cells in the traditional 2D culture model.
Fig. 7.
Comparison of cell invasion between 2D (a) and 3D (b) cultured A549 cells. Compared with 2D culture, there were more cells migrated through the membrane for 3D printed group, indicating higher invasiveness
Discussion
In in vitro medical research such as tumor research, a suitable and accurate model is the basis of reliability and effectiveness. In the past decades, 2D cell culture models were mostly used for tumor related in vitro studies. The cells were deposited on a petri dish in the form of a single layer, which was significantly different from the actual in vivo 3D environment and affects the characteristics, functions, and gene expression of cells. A 3D tumor model that is more biomimetic of the in vivo microenvironment is needed.
In this study, bioprinting was used to construct an in vitro 3D lung cancer tumor-like model using lung cancer cell lines A549 and 95-D, respectively. The general principle of material selection of bio-ink is that, in addition to biocompatibility, it needs to have good fluidity and appropriate viscosity when being printed, and can be easily solidified after it is printed, so the structure can maintain its shape for a certain period of time (Chung et al. 2013). To fulfill this requirement, we performed a series of experiments to evaluate the best concentration for each component.
Alginate sodium solution was selected, because it could turn into hydrogel when mixed with calcium ion. We have observed the mobility of alginate solution at different concentrations of 1%, 2%, 3%, and 4%. When the concentration of alginate was above 3%, the solution lacked mobility; when it was below 1%, the solution lacked viscosity. Therefore, we chose alginate solution with 1% final concentration as a component of the bio-ink to serve as extracellular matrix and support the structure. Gelatin is a commonly used material for biomedical research; its viscosity is sensitive to external temperature: at 20–25 °C, it remains as solid, while it starts to become liquid when temperature reaches 30 °C. During culturing, gelatin dissolves gradually, leaving the scaffold material porous, which is suitable for adhesion and proliferation of cells, as well as diffusion of nutrients and metabolic wastes. Furthermore, gelatin could enhance cell attachment. We have tested gelatin solution at different concentrations, and found that, at 10%, gelatin solution became solid at 20 °C, and it became liquid at 37 °C, which is suitable for bio-ink application.
By combining 1% alginate and 10% gelatin solution, the bio-ink can be appropriately extruded from the print-head while maintaining its intended structure in the printing chamber at lower temperature, and to be cross-linked to remain solid during normal culturing. Therefore, it was used as bio-ink for this study.
We conducted related studies to evaluate cell biology in such model. The printed tumor-like model had a clear and regular structure, and the survival rate of cells in the structure maintained higher than 90% after printing, proving that temperature and pressure changes in the printing process caused a little damage to the cell. Optical microscopy and HE staining showed that cells distributed uniformly in the model after printing and they had the tendency to aggregate in the model. SEM showed that the cells in the model had more cell–cell contact than 2D cultured cells, indicating that there may be more intra-cellular interactions and communication. Cell proliferation assay showed that, in the 3D model, for both types of cells, cell proliferation was normal and even slightly better than when cultured in 2D as with the same initial cell density, cell number in the 3D printed hydrogel tended to be higher than that of 2D cultured cells and this trend maintained through the later stage of culturing. The main reason might be that cells had more space in 3D structure and experienced contact inhibition later than when cultured in 2D, which lead to a relatively longer proliferation period. Furthermore, it was found that different cells responded differently to 3D culturing environment, indicating that there is cell-specific sensitivity to the change of 2D to 3D culturing environment.
A preliminary study was conducted on cell invasion and migration with this in vitro lung cancer tumor-like model. The results showed that the invasion and migration capabilities of cells in 3D printed group were higher than those of 2D cultured cells, reflected by the level of related gene expression and in vitro cell performance. It was verified that the in vitro tumor-like model of lung cancer could better reflect the high metastatic and high invasion characteristics of cells. Therefore, further research and application of this model were expected to provide some help for the treatment of related cancers.
On the other hand, there were some limitations in this research. SEM results showed that, in the 3D printed model, cells within had a tendency to move to the surface of the scaffold where a higher concentration of nutrients existed, indicating that the hydrogel used in this study had insufficient nutrient transport capacity, so it might be difficult to form a working model that is larger in size.
Although gelatin–sodium alginate system could simulate the extracellular structure and environment to a certain extent, it was difficult for the constructed model to be maintained in vitro for more than 3 weeks, as the structure would become disintegrated. At this time, there was not enough newly formed extracellular matrix to support this disintegrating structure. Therefore, further research and improvements are needed for the hydrogel system used in 3D bioprinting.
Conclusion
In this study, a tumor-like lung cancer model was successfully created with 3D printing using the alginate–gelatin bio-ink, with either A549 or 95-D lung cancer cells. These cells distributed evenly in the printed hydrogel and remained highly viable after printing, suggesting that the change in temperature and pressure during the printing process had minimal damage to the cells. Cell number of the 3D printed hydrogel increased up to day 12 of culturing, and the number remained higher compared to 2D cultured cells at the same time point thereafter.
We performed preliminary evaluation of biological properties of the printed cells, mainly on cell invasion and migration capabilities, using scratch test and gene analysis of MMP2 and MMP9 genes. Results showed that both properties were improved in 3D printed cells compared to 2D cultured cells, at in vitro and gene levels. These results support the feasibility of constructing tumor-like lung cancer model with 3D bioprinting.
Funding
This project is funded by the Shenzhen Special Fund for Global Experts Team, China (no. KQTD201209) and the Key Research and Development Projects of People’s Liberation Army (no. BWS17J036).
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Brandon E, Raap C, Meijerman I, Beijnen J, Schellensa J. An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicol Appl Pharmacol. 2003;189:233–246. doi: 10.1016/S0041-008X(03)00128-5. [DOI] [PubMed] [Google Scholar]
- Chung J, Naficy S, Yue Z, et al. Bio-ink properties and printability for extrusion printing living cells. Biomater Sci. 2013;1:763–773. doi: 10.1039/c3bm00012e. [DOI] [PubMed] [Google Scholar]
- Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017;51:1–20. doi: 10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Gaetani R, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148(1):3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 2015;33(9):504–513. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Mironov V, Boland T, Trusk T, et al. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- Rengier F, Mehndiratta A, Von TH, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- Sarker B, Rompf J, Silva R, Lang N, Detsch R, Kaschta J, Fabry B, Boccaccini AR. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int J Biol Macromol. 2015;78:72–78. doi: 10.1016/j.ijbiomac.2015.03.061. [DOI] [PubMed] [Google Scholar]
- Shafiee A, Atala A. Printing technologies for medical applications. Trends MolMed. 2016;22(3):254–265. doi: 10.1016/j.molmed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Skardal A, Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015;43(3):730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- Sochol RD, Gupta NR, Bonventre JV. A role for 3D printing in kidney-on-a-chip platforms. Curr Transplant Rep. 2016;3(1):82–92. doi: 10.1007/s40472-016-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienkers L, Heath T. Predicting in vivo drug interactions from in vitro drug discovery data Larry C. & Timothy G. Heath. Nat Rev Drug Discov. 2005;4:825–833. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep. 2016;6:24474. doi: 10.1038/srep24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304–1310. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]