Abstract
Abstract
Cystinosis, a rare autosomal recessive disease caused by intracellular cystine accumulation, occurs in an estimated 1/100,000–200,000 live births. Ocular non-nephropathic cystinosis is typically diagnosed during adulthood, when patients present with corneal crystal deposition and no systemic involvement. Due to the rarity of the condition, diagnosis is often delayed and can have a significant impact on the overall prognosis of the disease. Early diagnosis is therefore imperative to ensure successful treatment and improve quality of life, as most of its clinical manifestations can be prevented or delayed. Early detection strategies and practical approaches for the ocular management of cystinosis were discussed during the Ophthalmology Cystinosis Forum, a 1-day meeting held in Berlin, Germany during June 2017. Recommendations for early detection comprise ophthalmic assessment, including self- and clinician-assessed recording of photophobia, and visual acuity, slit-lamp examination and tonometry ophthalmic examinations. In vivo confocal microscopy and anterior segment optical coherence tomography were highlighted as valuable techniques in evaluating cystine crystals in the cornea, in vivo and non-invasively. The mainstay of ocular cystinosis treatment is the cystine-depleting aminothiol cysteamine. Indeed, early treatment with and strict adherence to cysteamine therapy has a considerable impact on the long-term prognosis of ocular cystinosis. In rare diseases such as ocular cystinosis, standardised guidelines and recommendations for detection, patient care and follow-up assessments are essential. Such guidelines provide a support tool for healthcare professionals caring for ocular cystinosis patients. Multidisciplinary teams (MDTs) are essential for delivering gold standard care and improving quality of life for patients and their families. This review paper highlights current early detection policies, clinical treatment strategies and practical approaches for the ocular management of cystinosis, including implementing a cystinosis MDT. Additionally, discussions of the Ophthalmology Cystinosis Forum held in 2017 are summarised.
Funding
Orphan Europe.
Plain Language Summary
Plain language summary available for this article.
Keywords: Infantile nephropathic cystinosis, Juvenile nephropathic cystinosis, Multidisciplinary team, Ocular cystinosis
Plain Language Summary
Cystinosis results from the build-up of a chemical known as cystine within certain cells of your body, as a consequence of a rare genetic disorder. It can affect your eyes and is not usually noticeable until the build-up of cystine crystals causes problems with your vision. This normally happens when you are an adult.
Early recognition of cystinosis in your eyes is important, as early treatment can achieve better results, and can prevent problems with your vision interfering with your everyday activities. You should tell your eye doctor if you are having problems with your eyes, particularly if they hurt in bright light or sunshine. The doctor will test your eyesight and may also use special cameras and other equipment to check the build-up of cystine crystals in your eyes. Cystinosis within the eyes is often treated with a medication that reduces the build-up of cystine crystals. This medication may be taken by mouth or as eye drops or eye gel.
Cystinosis can affect other parts of the body as well as the eyes, so you might also need to see other types of doctors. These can include specialists dealing with your kidneys, heart, hormones, bones and joints, nervous system and heart. They will work together as a team to help you manage your cystinosis.
Introduction
Cystinosis, a rare autosomal recessive disorder which affects the lysosomal storage system [1, 2], is caused by mutations in the CTNS gene encoding the lysosomal membrane transport protein, cystinosin, which is responsible for cystine egress [3, 4]. Such mutations result in cystine accumulation within cellular lysosomes to crystal-forming levels [5]. As the disorder progresses, cystine crystal deposition occurs in multiple organs, with the kidneys and eyes being affected first [4, 6, 7]. The prevalence of cystinosis is approximately 1 case in 100,000–200,000 live births, with cases found worldwide in all ethnic groups [4].
Clinical Description
Three clinical types of cystinosis have been described based on symptoms and age at presentation: infantile nephropathic, juvenile nephropathic and ocular non-nephropathic cystinosis [2, 8]. Infantile nephropathic cystinosis (NC) is the most common form and accounts for over 95% of all cystinosis cases; it is usually diagnosed during the first 2 years of life [2, 8]. If left untreated, NC can lead to poor growth, failure to thrive, renal tubular Fanconi syndrome, renal failure and other non-renal complications [4, 9]. Although untreated NC is the most common identifiable cause of renal Fanconi syndrome in young children, diseases such as Wilson’s disease, Lowe syndrome, Dent’s disease, and other metabolic diseases including classic galactosaemia, tyrosinaemia and glycogen storage diseases, should be considered in the differential diagnosis of renal Fanconi syndrome [6]. Juvenile NC has similar features to the infantile nephropathic form; however, progression is slower, and the onset of symptoms is typically in late childhood or during adolescence [2, 8, 10]. Patients with this form of cystinosis may retain renal function well into their 30s, and growth is only moderately impaired [2]. Ocular non-nephropathic cystinosis is typically diagnosed during adulthood, when patients present only with corneal crystal deposition and no systemic involvement [8, 11, 12].
Renal Manifestations
As previously mentioned, the most common initial clinical manifestation of infantile NC is Fanconi syndrome (growth retardation, electrolyte imbalance, dehydration, rickets, polyuria, polydipsia), which typically occurs at 6–12 months of age, due to the inability of the renal tubules to reabsorb small molecules [2, 13, 14]. If left untreated, glomerular function gradually deteriorates, and renal failure, which can lead to end-stage renal disease (ESRD) by 10 years of age, can develop. The rate of ESRD development does, however, vary among patients [13, 15]. Juvenile NC manifests similar renal symptoms, but progression is typically much slower [2, 6], with renal function maintained until patients are in their in fourth decade [2]. As the disease does not recur in kidney grafts, the therapy of choice in cystinosis patients is renal transplantation [6, 7]. Interstitial deposits of cystine crystals, originating from the host mononuclear cells, can be observed following transplantation, but they are not of pathological significance [6, 7]. Haemodialysis and peritoneal dialysis are temporary measures while patients await renal replacement [4, 6, 7].
Ocular Manifestations
Photophobia (glare sensitivity, blepharospasm) is one of the most frequently reported ocular symptoms in patients with cystinosis [9, 12]. Patients present with crystal accumulation affecting the cornea and conjunctiva [11, 12], but crystals can also be deposited in the iris, ciliary body, choroid and retinal pigment epithelium (RPE), and lens capsule [12]. Corneal crystal deposition starts in infancy in the anterior periphery of the cornea, and progresses centrally and posteriorly [12]. The crystals, which have a fine, uniform needle-shaped structure, are diffusely distributed within the corneal tissues. RPE hypopigmented mottling has also been seen in cystinosis patients; this can develop as early as 5 weeks of age, but more typically occurs during the second decade of life [12, 16]. A pigmentary retinopathy may result from accumulated damage to the retina and RPE [17]. The differential diagnosis of photophobia and corneal crystals includes multiple myeloma [18] and Bietti crystalline corneal dystrophy [9].
Diagnosis
Cystinosis is diagnosed by measuring levels of free non-protein cystine within polymorphonuclear leukocytes [2, 3]. This diagnosis is supported by slit-lamp examination showing corneal crystals, which are typically present in all affected patients by 16 months of age [2, 4]. Due to the rarity of cystinosis, diagnosis is often delayed and only occurs in some patients who present with ESRD; this has a significant impact on the overall prognosis of the disease [19]. Early diagnosis of cystinosis is therefore imperative to ensure successful treatment and improve quality of life, as most of its clinical manifestations can be prevented or delayed [2, 19].
This review paper highlights early detection strategies and practical approaches for the ocular management of cystinosis, as presented at the Ophthalmology Cystinosis Forum, a 1-day meeting held in Berlin, Germany in June 2017. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Early Detection Strategies for Ophthalmic Complications of Cystinosis
Due to improved treatment and renal transplantation, cystinosis patients are now living longer and are more prone to non-renal complications, such as those affecting the eyes [7]. As previously mentioned, early identification and optimised management of these complications helps ensure that patients not only live longer but have a better quality of life [7]. A range of early detection strategies are discussed below.
Diagnostic Testing
Early diagnosis is typically made by a paediatric nephrologist when patients present with growth retardation and symptoms of Fanconi syndrome [7, 8]. Early referral to the ophthalmologist is recommended, as patients will inevitably develop cystine crystals, and their clinical appearance often gives a strong indication of diagnosis [7]. Ophthalmic assessment involves recording symptoms, such as photophobia and visual disturbances, as well as any ocular surface discomfort and epiphora (watering eyes). Examination should include visual acuity measurements, careful slit-lamp examination to detect anterior segment crystals, most notably in cornea and conjunctiva, and tonometry [7, 9]. Best-corrected visual acuity (BCVA) is assessed for both distance (6 m) and near (33 cm) using a consistent scale, e.g. Snellen or LogMAR [9]. Slit-lamp examination can often reveal the presence of crystals in young patients with subtle crystal deposition in the cornea, and tonometry is performed to measure intraocular pressure using either Goldman tonometry in older co-operative children, or rebound tonometry (e.g. iCare® tonometer) [9].
Images of the corneal crystals can be compared against a scale of library images presented by Gahl et al. [3], which shows corneas with corneal cystine crystal scores (CCCS) of 0–3.00, in increments of 0.25. Additionally, specific guidelines are provided in order to obtain consistent slit-lamp images; these include: (1) using 16X magnification ensuring that the patient looks straight ahead and the slit beam is off-centre by greater than 60 degrees (measured to the front bar); (2) using a narrow slit-light beam − 1.0–2.0 mm (w) × 10 mm (l); (3) ensuring light reflection is distant from the slit beam; (4) positioning the slit beam such that the posterior beam hits the lateral edge of the iris/pupil aperture and the anterior beam is in the centre of the cornea; the anterior cornea should be most in focus [20]. It is further hoped that a standardised guideline for cystine crystal imaging with traditional slit-lamp examination will be developed [9].
Self- and Clinician-Assessed Photophobia
Photophobia can be graded using the grading system published by Liang et al. (and previously published for vernal keratoconjunctivitis or other inflammatory ocular surface diseases) [21, 22]; the system includes both self-assessed and clinician-assessed grading for photophobia (Table 1). These grades are useful in standardising the approach for detecting cystinosis and monitoring patients [9].
Table 1.
Self- and clinician-assessed evaluation of photophobia [9]
| Grade | Self-assessed photophobia | Clinician-assessed photophobia |
|---|---|---|
| 0 | No photophobia | No photophobia under the slit-lamp beam even with the largest slit beam |
| 1 | Slight difficulty with light causing occasional eye blinking | Photophobia to moderate slit-lamp beam light |
| 2 | Slight difficulty causing regular eye blinking | Photophobia to dimmest slit-lamp beam light |
| 3 | Moderate difficulty with light requiring sunglasses | Inability to tolerate the blue light |
| 4 | Severe difficulty requiring almost permanent sunglasses | Photophobia needing dark glasses and unable to open eyes inside the illuminated consulting room |
| 5 | Extreme difficulty with light requiring patient to stay inside—cannot even tolerate natural light with sunglasses | Unable to open eyes in darkened room |
Anterior Segment of the Eye: Imaging the Cornea
Anterior Segment Optical Coherence Tomography (AS-OCT)
Anterior segment optical coherence tomography is a non-invasive imaging technology that produces detailed cross-sectional images in biological systems, using low-coherence interferometry [23]. This technique is now widely available and is commonly used for imaging the cornea and anterior segment. The depth of crystal deposition can be estimated with the callipers on the system’s software, and can provide an objective marker for the severity of crystal deposition (Fig. 1) [24]. Corneal pachymetry mapping software can be used to measure corneal thickness [24]. Crystal deposition may also occur in the ciliary body, but this cannot be imaged as effectively with AS-OCT. Nevertheless, such deposition may cause an anterior rotation of the ciliary body and ciliary processes, and forward movement of the peripheral iris with progressive narrowing of the angles, which can be measured with the AS-OCT [12, 25]. This is an essential analysis, as narrow angles may impact the subsequent development of glaucoma in some cystinosis patients.
Fig. 1.
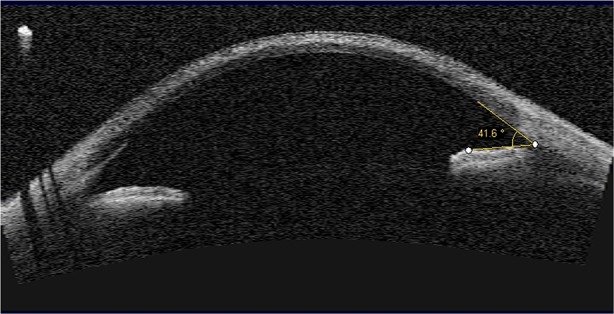
Anterior segment optical coherence tomography (AS-OCT) of the eye in cystinosis showing measurement of the anterior chamber angle. Measurement of the depth of corneal crystal deposition using the software's calipers can provide an objective marker for the severity of crystal deposition.
Original image provided by S. Biswas, Manchester Royal Eye Hospital, Manchester, UK
AS-OCT is well tolerated, as it uses an infrared light source, which is not uncomfortable for cystinosis patients; however, the machine is expensive, and tissue penetration can be limited due to its inability to visualise through the iris and sclera [23, 26] (Table 2).
Table 2.
Advantages and disadvantages of diagnostic techniques for imaging the anterior segment of the eye
| Technique | Advantages | Disadvantages |
|---|---|---|
| Anterior segment optical coherence tomography |
Well tolerated, as it uses an infrared light source, which is not uncomfortable for cystinosis patients [23, 26] Non-invasive [23] Rapid image acquisition (0.25 s) [24] Semi-automated image analysis [24] |
Machine is expensive [23] Tissue penetration can be limited, as it is unable to visualise through the iris and sclera [23] Unable to accurately quantify the amount of deposits within the cornea [34, 36] |
| Ultrasound biomicroscopy |
Provides high-resolution visualisation of iris position [28] Enables visualisation of morphological and topographical changes in the anterior chamber angle [28] |
May cause discomfort, as it uses a probe that requires an immersion technique (a saline-filled eye cap can, however, be adapted to the probe) [28] May cause abrasion and infection, and potential anterior segment deformation [28] Patients must be in a more supine position and may require general or local anaesthetics [28] Time-consuming [28] Requires a trained operator [28] |
| In vivo confocal microscopy |
High-resolution with details at a cellular level [29] Tissue components can be easily visualised, e.g. nerves, cells, blood vessels and connective tissue [29, 31] Gives a precise localisation of the depth of crystal deposition in the cornea [24] Only way to analyse crystal deposits at a cellular level in the cornea, limbus and conjunctiva [24] Most useful for quantitative analysis of therapeutic interventions for corneal disease [24] Able to simultaneously assess corneal co-pathology: neuropathy and inflammatory changes such as number of dendritic cells [31] |
Machine is expensive [31] Requires an experienced ophthalmologist or well trained technician to acquire good quality images [31] |
Ultrasound Biomicroscopy (UBM)
UBM is a non-invasive technique which uses high-frequency ultrasound, typically 35–50 MHz, to visualise the anterior segment of the eye in detail [27]. Ultrasound waves have the ability to travel through the iris and ciliary body pigment epithelia, allowing the capture of high-resolution images of the anterior segment, particularly the ciliary body and ciliary processes, which are not otherwise visualised using AS-OCT [27, 28]. UBM enables visualisation of morphological and topographical changes in the anterior chamber angle, but tolerance may be an issue, as it requires an immersion technique [28] (Table 2).
In Vivo Confocal Microscopy (IVCM)
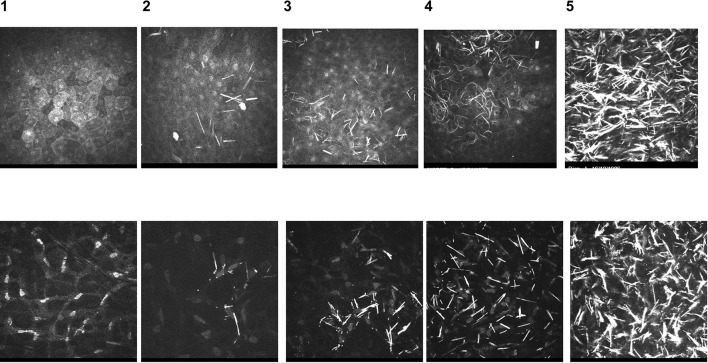
In vivo confocal microscopy uses the principle of a light source that is confocal with the microscope objective lens and is focused onto a plane of the tissue being imaged [29]. It provides very high-resolution images at a specified depth within the cornea, blurring out areas that are separate from the specific plane being imaged [29]. IVCM can be used to scan a tissue area and allow construction of an image field in a specific tissue plane or depth [29]. Labbé et al. have published work analysing crystal deposition in the cornea using IVCM [24]. A series of standardised images is provided to allow the comparison and grading of images of the patient’s corneal layers; a score of 0–4 is assigned to individual layers to grade the amount of crystals within the cornea: superficial epithelium, basal epithelium, Bowman’s membrane, anterior stroma, mid-stroma, posterior stroma and endothelium (Fig. 2). IVCM can provide information on crystal density, inflammatory cell infiltration and corneal nerve damage [21], and has proved useful for assessing the efficacy of topical ocular cystinosis treatment [24, 30]. Tissue components such as nerves, cells, blood vessels and connective tissue can be easily visualised with IVCM; however, the machine is expensive [31] (Table 2).
Fig. 2.
In vivo confocal microscopy (IVCM) standardised images used to compare and score images from cystinosis patients. Standardised IVCM images (400 × 400 μm) used to compare and grade images of patient corneal layers, represented in percentages to indicate the number of deposits in the field of each image: 0, no crystal; 1, < 25%; 2, 25–50%; 3, 50–75%; 4, > 75%. Upper panel: superficial epithelium; lower panel: stroma.
Original images provided by H. Liang, Quinze-Vingts National Ophthalmology Hospital, Paris, France
Posterior Segment of the Eye: Imaging the Retina
Widefield Retinal Imaging Using Optos Optomap®
Widefield retinal imaging produces a 200-degree view of the retina (~ 82% of the surface area) and combines scanning laser ophthalmoscopy with an ellipsoidal mirror to obtain images of the retinal periphery [32]. This is achieved with one capture and does not need bright illumination lighting or a contact lens.
Widefield retinal imaging is well tolerated, particularly in children, as it uses scanning laser rather than incandescent light; therefore, photosensitivity is not a challenge [32]. However, one disadvantage of this technique is the obvious distortion and decreased resolution of the far temporal and nasal peripheral retina [33] (Table 3).
Table 3.
Advantages and disadvantages of diagnostic techniques for imaging the posterior segment of the eye
| Technique | Advantages | Disadvantages |
|---|---|---|
| Widefield retinal imaging using Optos Optomap® |
Enables a large area of the retina to be captured rapidly [32] Can be magnified to a high depth—macula and optic disc [33, 52] Well tolerated, particularly in children, as it uses scanning laser rather than incandescent light; therefore, photosensitivity is not a challenge [32] Can be used to obtain an autofluorescence capture of the fundus to measure RPE function and damage, particularly to help identify areas of stressed photoreceptor RPE [53] |
Imaging of the far superior and inferior peripheral retina is less complete compared with that of the temporal and nasal retina [33] Obvious distortion and decreased resolution of the far temporal and nasal peripheral retina [33] |
| Optical coherence tomography (OCT) |
Rapid image acquisition of the structural view of the retina [54] Spectral-domain or swept-source OCT scans (10,000 images/s) enable highly detailed anatomical analysis of the retinal layers [34, 36] |
Tissue penetration can be limited, as it is unable to visualise through the iris and sclera [23] Unable to accurately quantify the amount of deposits within the cornea [34, 36] |
RPE retinal pigment epithelium
Optical Coherence Tomography (OCT)
Optical coherence tomography can be used to check the integrity of the retinal layers, particularly the posterior retina, and also to measure retinal thickness [34]. Crystal deposition within the retinal layers can be observed using OCT. This technique is also very helpful in monitoring the optic nerve (disc volume and retinal nerve fibre layer) to provide evidence of progressive optic neuropathy or increased intracranial pressure over time [24, 34, 35].
Spectral-domain or swept-source OCT scans (10,000 images/s) enable highly detailed anatomical analysis of the retinal layers; however, OCT is unable to accurately quantify the amount of deposits within the retina [34, 36] (Table 3).
Other Diagnostic Techniques
There are several other useful techniques, including visual field testing and electroretinography. With increasing age, cystinosis patients show evidence of increasing loss of visual field, and most experience moderate to severe constriction of the visual field towards their later years [16]. Electroretinography (ERG) can be used to measure attenuated rod- and cone-mediated signals, which indicate widespread retinopathy and correlate with the symptoms of nyctalopia and/or visual field loss [16].
Summary
Table 4 presents a summary of the diagnostic techniques performed in cystinosis patients. Depending upon the age of the patient, an annual investigation protocol is suggested [20]. During the first year following diagnosis, three visits are recommended to establish baselines and optimise treatment. ERG and visual evoked potentials can be performed if there is evidence of reduced visual field or nyctalopia. Standardised protocols can help with understanding the progression of the disease and allow treatment adjustment [20].
Table 4.
Example of an investigation schedule for techniques performed in cystinosis patients [20]
| Age (years) | VA and refraction | Motility | S/L | S/L imaging | AS-OCT | IVCM | Optos® | OCT retina | VF |
|---|---|---|---|---|---|---|---|---|---|
| 0–3 | X | X | X | ± | ± | ± | |||
| 3–7 | X | X | X | X | X | ± | X | X | |
| 7–16 | X | X | X | X | X | X | X | X | X |
| > 16 | X | X | X | X | X | X | X | X | X |
VA visual acuity, S/L slit lamp, AS-OCT anterior segment optical coherence tomography, IVCM in vivo confocal microscopy, OCT optical coherence tomography, VF visual field
X denotes techniques that are performed; ± denotes techniques that may or may not be performed due to the patient’s age
Modern Treatment Strategies and Practical Approaches for Ocular Cystinosis
The mainstay of cystinosis treatment is the cystine-depleting aminothiol cysteamine, or mercaptoethylamine [3]. Cysteamine reacts with lysosomal cystine to form cysteamine–cysteine mixed disulphide, which exits the lysosome via the lysine cationic transport system, consequently lowering intracellular cystine concentrations [3, 37]. Thus, cysteamine can deplete cells of more than 90% of their cystine content [3, 37]. Early initiation of, and strict adherence to, cysteamine treatment has a considerable impact on long-term prognosis, as it has been shown to retard or prevent renal deterioration, improve growth, delay non-renal complications and improve patient life expectancy [3, 11, 19, 38]. Cysteamine treatment is available as oral, ophthalmic solution and lubricant gel formulations [30, 37, 39–41].
Oral Cysteamine
Oral cysteamine (Cystagon®; Orphan Europe, Puteaux, France), which was approved by the European Medicines Agency (EMA) in 1997, is administered on the basis of body surface area; for children ≤ 12 years of age, the recommended dose is 1.30 g/m2/day of the free base divided four times daily. For patients aged > 12 years and > 50 kg weight, the recommended dose is 2 g/day, divided four times daily [42]. Although oral cysteamine is effective in preventing pigmentary retinopathy, it has no effect on corneal cystine crystals, as the concentration achieved in corneal tissue is inadequate [2, 3, 10, 43]. Topical cysteamine hydrochloride (CH) is therefore administered to dissolve cystine crystals [2, 3, 10]. The most common adverse events (AEs) reported for oral cysteamine include vomiting, nausea, diarrhoea, loss of appetite, fever and somnolence [42].
One of the main challenges with oral cysteamine therapy is long-term compliance due to the need for frequent dosing. In a Spanish study of 34 paediatric and adult patients, 89% of those under 11 years of age received the correct daily dosing schedule, compared with 56% of older patients [44].
Topical Cysteamine Eye Drops: Ophthalmic Solution
Cysteamine ophthalmic solution (Cystaran™; Sigma-Tau Pharmaceuticals, Gaithersburg, MD, USA) was approved by the United States Food and Drug Administration in 2012 [37, 40]. The formulation contains 6.5 mg/ml (0.65%) of CH, which is equivalent to 4.4 mg/ml (0.44%) of cysteamine, as the active ingredient [37, 40]. The main disadvantage of topical cysteamine eye drops is the requirement to administer either every waking hour, or 6–12 times per day [11, 40]. In addition, cysteamine oxidises to its disulphide form, cystamine, at room temperature; thus, the formulation requires cold storage to ensure stability [11, 37]. These drawbacks make patient compliance difficult [37]. The instability of topical cysteamine eye drops at room temperature makes packaging and distribution difficult; as such, cysteamine eye drops have not been licensed in Europe [11]. Off-licence formulations containing various concentrations, composition and buffers are the only options available in Europe; these formulations are prepared by hospitals or local pharmacies [41]. The most common AEs reported for topical cysteamine eye drops include sensitivity to light, redness, eye pain and irritation, headache and visual field defects [37]. However, these AEs are believed to result mainly from the primary condition rather than the cysteamine drops [37].
Real-world experience with oral and topical cysteamine formulations.
Patient 1: A 5-year-old female, who had been diagnosed with infantile NC at 12 months of age, presented with growth retardation, poor appetite, polydipsia, polyuria and metabolic acidosis, hypophosphataemia and hypokalaemia. Ophthalmic manifestations included the presence of bilateral iridescent crystals in the anterior stroma of the cornea (observed using AS-OCT) and discrete photophobia. Upon diagnosis, the patient was treated with oral cysteamine and 0.10% topical cysteamine ophthalmic solution (administered five times per day). At follow-up, the patient did not complain of photophobia or other ocular symptoms: bilateral corneal crystals were more densely dispersed in the periphery, and there was no evidence of crystals in other anterior segment structures or the posterior segment. However, despite being on treatment with topical cysteamine for 4 years, there has been no obvious reduction in corneal crystals on slit-lamp examination. Following the initiation of treatment, no evidence of biomicroscopic reduction in the density of corneal crystal deposits was observed. However, this may be due in part to the centre not having the technology to quantify the density of crystal deposits. The patient complained about the dosage of the topical cysteamine solution and preservation method.
Topical Cysteamine Eye Drops: Lubricant (Gel) Formulation
In an attempt to reduce frequency of application, novel cysteamine-containing gels have been developed. The suitability of these formulations for delivery of cysteamine to the cornea has been tested using rheology, bioadhesion, dissolution stability and optical clarity evaluations [45].
One such CH formulation (Cystadrops®; Orphan Europe, Puteaux, France) was approved by the EMA in 2017 [41, 46]. This formulation contains 5.5 mg/ml (0.55%) of CH, equivalent to 3.8 mg/ml (0.38%) of cysteamine, as the active ingredient [41, 46], with a recommended dose of one drop to be inserted into each eye four times daily [46]. The gel formulation contains carboxymethylcellulose sodium as a viscous agent, which prolongs the contact of the active ingredient with the eye, thereby allowing a reduction in dosing frequency [40, 41]. Before opening, the formulation must be refrigerated (2–8 °C), but after opening, it can be stored at room temperature for up to 7 days [46].
In a randomised open-label phase 3 study, cystinosis patients ≥ 2 years old were randomised 1:1 to receive 0.55% CH or 0.10% CH, administered in both eyes, four times per day for 90 days [41]. The primary objective of the study was to compare the efficacy of 0.55% CH gel eye drops with 0.10% CH eye drops in reducing corneal cystine crystal density as assessed using IVCM total score. After 90 days, corneal crystal density was reduced by 40% in the 0.55% CH group compared with the 0.10% CH group; this reduction was evident after 30 days [41]. A decrease in crystal density in all corneal layers was also observed in the 0.55% group. The higher concentration of the CH gel eye drops, together with the viscosity of the formulation, which allows for an increase in cornea contact time and consequently deeper penetration of cysteamine into the interior layers of the cornea, is likely to contribute to the observed efficacy [41].
Compared with the CH ophthalmic solution, patient compliance is expected to improve with the CH gel formulation due to its lower dosage and storage convenience [30, 41]. The most common AEs for this gel formulation are stinging and burning; however, these reactions are typically mild or moderate and commonly occur with all CH formulations, which require an acidic pH to maintain stability [41, 47]. In the future, a study comparing the efficacy of the 0.55% CH gel formulation and the 0.44% CH solution (when it becomes available in Europe) in decreasing corneal crystals in ocular cystinosis will be useful.
Other developmental gel formulations for the ocular delivery of cysteamine have been evaluated using physicochemical and animal studies. In particular, the use of ion-sensitive and hyaluronic acid-based hydrogels and those formulated with carbomer 934 have been shown to achieve a high retention time and control the release of cysteamine over a period of several hours [45, 48, 49].
Cysteamine-Preloaded Contact Lenses
Several early preclinical studies have investigated the feasibility of cysteamine-preloaded contact lenses for ocular cystinosis, predominantly to reduce dosing frequency. In two separate studies, commercially available contact lenses were preloaded with 50 mg/ml cysteamine and vitamin E, which prolongs the release of other ocular drugs and also retards cysteamine oxidation [50, 51]. Silicone hydrogel contact lenses demonstrated a favourable duration of drug elution, which equated to exposure for 4–5 h daily to deliver an effective dose of cysteamine.
Real-world experiences with topical cysteamine formulation.
Patient 2: A 32-year-old woman who had suffered infantile NC, had growth retardation and had undergone a kidney transplant at 10 years of age. She had been referred to Centro Hospitalar Universitário de Coimbra, Coimbra, Portugal at 13 years of age, due to severe photophobia and blepharospasm associated with severe corneal cystine crystal deposition. Ophthalmic manifestations included severe photophobia and diffuse corneal cystine crystal deposition (corneal images obtained using the IVCM Rostock Cornea Module with the Heidelberg® Retina Tomograph and AS-OCT). The cystine crystals, which were hyperreflective and had various shapes, e.g. rectangular or fusiform, occurred mainly in the anterior and mid-stroma, and were absent in the surface epithelium and basal cells of the central cornea. The posterior stroma showed lower crystal density; no deposits were found in the endothelium. BVCA was 20/32 oculus dextrum (OD) and 20/40 oculus sinistrum (OS). The patient was initially treated with topical cysteamine ophthalmic solution 0.1136%, prepared in the hospital pharmacy and used 10 times a day for 8 years, with substantial improvement in photophobia and visual acuity (20/25 OD and 20/32 OS). The patient has subsequently commenced treatment with CH gel 3.8 mg/ml, four times per day. Three and six months after starting treatment with CH gel, a reduction in the density of crystals in the anterior stroma was observed. The patient reported an improvement in photophobia and contrast sensitivity. BCVA remained 20/25 OD and 20/32 OS, and the patient is satisfied with the current treatment, highlighting the convenience of the CH gel formulation. This case study points to the usefulness of cysteamine eye drops in treating visual complaints associated with crystal deposition in the cornea, especially photophobia. The CH gel formulation has been well tolerated, and its convenient dosage has contributed to increased patient compliance, thus improving quality of life. Confocal microscopy and AS-OCT have also been valuable techniques in evaluating cystine crystals in the cornea in vivo and non-invasively.
Real-world experiences with topical cysteamine formulation.
Patient 3: A 12-year-old Hispanic boy, with consanguineous parents, was diagnosed with infantile NC at 7 years of age. His first symptoms were growth retardation and Fanconi syndrome; the patient had renal failure from 3 years of age and was placed on dialysis. Other associated medical conditions included hypertension, hyperlipidaemia, hypothyroidism and left ventricular hypertrophy. The patient required kidney transplantation when he was 8 years old. His ophthalmic manifestations included photophobia and foreign body sensation, dry eye and irritation. AS-OCT images showed diffuse corneal cystine crystal deposition with hyperreflective crystals mainly in the anterior stroma. Crystal deposits were also found in the iris, conjunctiva, the angle and the limbus. BCVA was 20/60, and the patient’s slit-lamp CCCS score was 3. He had a normal retinal exam, and no pigmentary retinopathy was observed. Corneal pachymetry map confirmed thinner corneas in this patient; a mean central corneal thickness (CCT) of 470 μm was observed (normal mean CCT 540 μm) [24, 55]. The patient was initially treated with oral cysteamine and has since commenced treatment with CH gel formulation. Treatment with cysteamine has not reduced photophobia; however, there have been no side effects. The patient is only able to visit an ophthalmologist twice a year, as he lives a long distance from the city, and follow-up is therefore difficult. It is believed that an earlier diagnosis and treatment in this patient could have changed his treatment outcome.
Strategies for Delivering Gold Standard Care and Follow-up of Patients with Ocular Cystinosis
There is a lack of standardised guidelines and recommendations for detecting ocular cystinosis, patient care and follow-up assessments [9]. In 2017, Pinxten et al. produced a document to guide scheduling of follow-up visits and a protocol for ophthalmological examination based on the experiences of the authors [9]; however, international gold standard guidelines and recommendations are still needed. Such guidelines and recommendations will provide a support tool for healthcare professionals who care for ocular cystinosis patients.
In considering the care and follow-up of cystinosis patients, there are certain challenges that need to be addressed. These include which specialties should be involved, profiling the services according to the age of the patient, prioritising investigations, and deciding who takes overall responsibility for leading the multidisciplinary assessment and information sharing. Much of this can be achieved through local agreement and negotiation. The nephrologist may be viewed as the key clinician, and other specialties such as endocrinology, orthopaedics, neurology and cardiology can be consulted as necessary. As ocular involvement in cystinosis is universal, the presence of an ophthalmologist should be a prerequisite for any cystinosis (MDT). Naturally, the clinicians involved with the care of patients should have a specific interest and experience of the condition.
One of the difficulties with ocular cystinosis is managing the transition from childhood to adulthood. It is during this difficult period that patients may become dissociated from the expertise previously available to them in managing their condition. The period around adolescence to early adulthood often coincides with a deterioration in treatment compliance, as affected individuals, moving out of the sphere of influence of their parents or guardians, gain more independence but not necessarily enough confidence to manage their own condition. It is therefore important, in our opinion, to establish robust transitional arrangements, which may involve setting up joint clinics with both adult and paediatric clinicians seeing the patient together prior to handing over care entirely into the adult setting.
Upon diagnosis, new patients with cystinosis may require up to three visits in their first year to establish baseline measurements of their ocular status. Depending on age and co-operation of the patient, this should include imaging of the cornea using slit-lamp, AS-OCT and IVCM modalities. Posterior segment imaging with Optomap® and OCT of the macula and optic disc should also be undertaken. Additionally, formal visual field assessments at baseline are also recommended in those patients with the appropriate developmental age. Once baselines are established, patients with cystinosis are followed up annually within an MDT. In the Manchester cystinosis MDT, this is led by the paediatric nephrologist, and patients’ appointments are co-ordinated with endocrinology and ophthalmology. Patients are initially reviewed by the ophthalmology team, which assesses visual acuity and completes initial questionnaires addressing visual symptoms such as photophobia, ocular irritation and compliance with topical medication. It is possible to organise a number of technician-delivered investigations and imaging in patients, such as visual fields, and anterior and posterior segment imaging, as outlined in the sections above. Additional specialised tests such as electrodiagnostics could be requested depending on whether symptoms of visual field loss or night blindness are revealed, but would not be routinely organised. Slit-lamp examination represents the most fundamental and essential component of the assessment and the easiest way to assess the presence of corneal crystals. It is also essential to ascertain the presence of ocular surface disease, presence of corneal neovascularisation and any evidence of anterior chamber inflammation. Intraocular pressure measurement is also important, as secondary glaucoma risk increases with increasing age.
Quantitative assessment of corneal crystals is most accurately achieved using IVCM of the cornea based on the method described by Labbé et al. [24]. However, this requires additional equipment, skilled technicians and co-operative subjects. In the absence of the ready availability of confocal corneal imaging, AS-OCT and/or slit-lamp photographic imaging can be performed, both of which are less invasive and are rapidly acquired. Posterior segment examination and imaging, and in particular volume scans of the optic disc, are important to document the presence of papilloedema, as patients with cystinosis are known to be at risk of increased intracranial pressure. Volume scans and nerve fibre layer analysis may be helpful to monitor the status of such patients longitudinally. Patients can expect to be in hospital for most of the day, having assessments, imaging and blood investigations in the morning and clinical review and review of investigation and imaging results in the afternoon with the MDT clinicians. A pro forma of ophthalmic assessment can be used to record all findings including colour vision, anterior segment slit-lamp exam, anterior and posterior segment imaging and fundus, as well as details of the treatment advice given and a cystinosis eye symptoms questionnaire.
Conclusions
This review paper highlights early detection strategies and practical approaches for the ocular management of patients with cystinosis. Early detection, treatment, monitoring and follow-up are essential in ensuring patients not only live longer, but have a better quality of life. As highlighted in this paper, several devices and techniques can be used for the ocular assessment of patients with cystinosis; in addition, this paper supports available data on the treatment of ocular manifestations resulting from cystinosis. Early treatment with, and strict adherence to, cysteamine has a considerable impact on the long-term prognosis of ocular cystinosis. In diseases such as ocular cystinosis, MDTs are essential for delivering gold standard care and improving quality of life for patients and their families.
Acknowledgements
Funding
Editorial assistance, which was provided by Kunbi Ayo-Okanlawon of Cello Health MedErgy Europe, and article processing charges were funded by Orphan Europe.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors contributed to the draft of the manuscript, critically reviewed for intellectual content and approved the final version submitted for publication. Martha Gaviria, Luisa Malheiro and João Pedro Marques contributed to the acquisition of data. Susmito Biswas, Vincenzo Giordano and Hong Liang contributed to (or were involved in) the study design and interpretation of data.
Disclosures
Vincenzo Giordano is an employee of Orphan Europe. Susmito Biswas, Martha Gaviria, Luisa Malheiro, João Pedro Marques and Hong Liang have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7021994.
References
- 1.Cherqui S. Cysteamine therapy: a treatment for cystinosis, not a cure. Kidney Int. 2012;81:127–129. doi: 10.1038/ki.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 3.Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71:100–120. doi: 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- 4.Nesterova G, Gahl WA. Cystinosis: the evolution of a treatable disease. Pediatr Nephrol. 2013;28:51–59. doi: 10.1007/s00467-012-2242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anikster Y, Lucero C, Guo J, Huizing M, Shotelersuk V, Bernardini I, et al. Ocular nonnephropathic cystinosis: clinical, biochemical, and molecular correlations. Pediatr Res. 2000;47:17–23. doi: 10.1203/00006450-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Wilmer MJ, Schoeber JP, Van Den Heuvel LP, Levtchenko EN. Cystinosis: practical tools for diagnosis and treatment. Pediatr Nephrol. 2011;26:205–215. doi: 10.1007/s00467-010-1627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariceta G, Camacho JA, Fernandez-Obispo M, Fernandez-Polo A, Gamez J, Garcia-Villoria J, et al. Cystinosis in adult and adolescent patients: recommendations for the comprehensive care of cystinosis. Nefrologia Spain. 2015;35:304–321. doi: 10.1016/j.nefro.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Greenbaum L. Nephropathic cystinosis: diagnosis, management, and challenges in long-term treatment. Intern Med News. 2015;1–8.
- 9.Pinxten A-M, Hua M-T, Simpson J, Hohenfellner K, Levtchenko E, Casteels I. Clinical practice: a proposed standardized ophthalmological assessment for patients with cystinosis. Ophthalmol Ther. 2017;6:93–104. doi: 10.1007/s40123-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmonem MA, Veys KR, Soliman NA, Van Dyck M, Van Den Heuvel LP, Levtchenko E. Cystinosis: a review. Orphanet J Rare Dis. 2016;11:1–17. doi: 10.1186/s13023-016-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shams F, Livingstone I, Oladiwura D, Ramaesh K. Treatment of corneal cystine crystal accumulation in patients with cystinosis. Clin Ophthalmol. 2014;8:2077–2084. doi: 10.2147/OPTH.S36626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsilou E, Zhou M, Gahl WA, Sieving P, Chan C. Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Surv Ophthalmol. 2007;52:97–105. doi: 10.1016/j.survophthal.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherqui S, Courtoy PJ. The renal Fanconi syndrome in cystinosis: pathogenic insights and therapeutic perspectives. Nat Rev Nephrol. 2017;13:115–131. doi: 10.1038/nrneph.2016.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niaudet P. Cystinosis. Orphanet Encycl. 2004;1–6. http://www.orpha.net/data/patho/GB/uk-cystin.pdf.
- 15.Geelen JM, Monnens LA, Levtchenko EN. Follow-up and treatment of adults with cystinosis in the Netherlands. Nephrol Dial Transplant. 2002;17:1766–1770. doi: 10.1093/ndt/17.10.1766. [DOI] [PubMed] [Google Scholar]
- 16.Tsilou ET, Rubin B, Reed G, Caruso R, Iwata F, Balog J, et al. Nephropathic cystinosis: posterior segment manifestations and effects of cysteamine therapy. Ophthalmology. 2006;113:1002–1009. doi: 10.1016/j.ophtha.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Bishop R. Ocular complications of infantile nephropathic cystinosis. J Pediatr. 2017;183S:S19–S21. doi: 10.1016/j.jpeds.2016.12.055. [DOI] [PubMed] [Google Scholar]
- 18.Kleta R, Blair SC, Bernardini I, Kaiser-Kupfer MI, Gail WA. Keratopathy of multiple myeloma masquerading as corneal crystals of ocular cystinosis. Mayo Clin Proc. 2004;79:410–412. doi: 10.4065/79.3.410. [DOI] [PubMed] [Google Scholar]
- 19.Emma F, Nesterova G, Langman C, Labbe A, Cherqui S, Goodyer P, et al. Nephropathic cystinosis: an international consensus document. Nephrol Dial Transplant. 2014;29:iv87–iv94. doi: 10.1093/ndt/gfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas S. Approaches for early recognition and modern management strategies for ophthalmic complications of cystinosis (oral presentation). 2nd Ophthalmol Cystinosis Forum. 2017.
- 21.Liang H, Baudouin C, Tahiri Joutei Hassani R, Baudouin FB, Labbe A. Photophobia and corneal crystal density in nephropathic cystinosis: an in vivo confocal microscopy and anterior-segment optical coherence tomography study. Investig Ophthalmol Vis Sci. 2015;56:3218–3225. doi: 10.1167/iovs.15-16499. [DOI] [PubMed] [Google Scholar]
- 22.Keklikci U, Soker S, Sakalar Y, Unlu K, Ozekinci S, Tunik S. Efficacy of topical cyclosporin A 0.05% in conjunctival impression cytology specimens and clinical findings of severe vernal keratoconjunctivitis in children. Jpn J Ophthalmol. 2008;52:357–362. doi: 10.1007/s10384-008-0577-z. [DOI] [PubMed] [Google Scholar]
- 23.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;22:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labbe A, Niaudet P, Loirat C, Charbit M, Guest G, Baudouin C. In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the cornea in nephropathic cystinosis. Ophthalmology. 2009;116:870–876. doi: 10.1016/j.ophtha.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Mungan N, Nischal K, Héon E, MacKeen L, Balfe J, Levin AV. Ultrasound biomicroscopy of the eye in cystinosis. Arch Ophthalmol [Internet]. 2000;118:1329–1333. doi: 10.1001/archopht.118.10.1329. [DOI] [PubMed] [Google Scholar]
- 26.Lin S. How valuable is anterior segment imaging? Glaucoma Today [Internet]. 2014;53–8. http://glaucomatoday.com/pdfs/gt0914_F_lin.pdf.
- 27.Silverman RH, Cannata J, Shung KK, Gal O, Patel M, Lloyd HO, et al. 75 MHz ultrasound biomicroscopy of anterior segment of eye. Ultrason Imaging [Internet]. 2006;28:179–188. doi: 10.1177/016173460602800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filipe HP, Carvalho M, Freitas L. Ultrasound biomicroscopy and anterior segment optical coherence tomography in the diagnosis and management of glaucoma. Vis Pan-Am. 2016;15:37–42. [Google Scholar]
- 29.Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. Br J Ophthalmol [Internet] 2003;87:225–236. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labbe A, Baudouin C, Deschenes G, Loirat C, Charbit M, Guest G, et al. A new gel formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis: the Cystadrops OCT-1 study. Mol Genet Metab. 2014;111:314–320. doi: 10.1016/j.ymgme.2013.12.298. [DOI] [PubMed] [Google Scholar]
- 31.Villani E, Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr Eye Res [Internet]. 2014;39:213–231. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoughy SS, Arevalo JF, Kozak I. Update on wide- and ultra-widefield retinal imaging. Indian J Ophthalmol. 2015;63:575–581. doi: 10.4103/0301-4738.167122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witmer MT, Parlitsis G, Patel S, Kiss S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis® noncontact ultra-widefield module versus the Optos® Optomap®. Clin Ophthalmol. 2013;7:389–394. doi: 10.2147/OPTH.S41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhi M, Duker JS. Optical coherence tomography–current and future applications. Curr Opin Ophthalmol [Internet] 2013;24:213–221. doi: 10.1097/ICU.0b013e32835f8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson JW, Aleman TS, Xu W, et al. Evaluation of optical coherence tomography to detect elevated intracranial pressure in children. JAMA Ophthalmology. 2017;135(4):320–328. doi: 10.1001/jamaophthalmol.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozak I, Arevalo J, Shoughy S. Intraretinal crystals in nephopathic cystinosis and fanconi syndrome. JAMA Ophthalmol [Internet]. 2017;135:e165169. doi: 10.1001/jamaophthalmol.2016.5169. [DOI] [PubMed] [Google Scholar]
- 37.Huynh N, Gahl WA, Bishop RJ. Cysteamine ophthalmic solution 0.44% for the treatment of corneal cystine crystals in cystinosis. Expert Rev Ophthalmol. 2013;8:341–345. doi: 10.1586/17469899.2013.814885. [DOI] [Google Scholar]
- 38.Brodin-Sartorius A, Tête MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–189. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 39.Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med. 2007;147:242–250. doi: 10.7326/0003-4819-147-4-200708210-00006. [DOI] [PubMed] [Google Scholar]
- 40.Radojkovic B. Cysteamine eye drops in the treatment of cystinosis—an Australian perspective. J Pharm Pract Res. 2015;45:440–445. doi: 10.1002/jppr.1148. [DOI] [Google Scholar]
- 41.Liang H, Labbé A, Le Mouhaër J, Plisson C, Baudouin C. A new viscous cysteamine eye drops treatment for ophthalmic cystinosis: an open-label randomized comparative phase III pivotal study. Investig Ophthalmol Vis Sci. 2017;58:2275–2283. doi: 10.1167/iovs.16-21080. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Cystagon Summary of Product Characteristics. 2007.
- 43.Ling C, Liu X, Chen Z, Jiang Y, Fan J, Meng Q, et al. Corneal cystine crystals in cystinosis. Arch Dis Child [Internet]. 2017; http://adc.bmj.com/content/early/2017/03/21/archdischild-2016-312456.abstract. [DOI] [PubMed]
- 44.Ariceta G, Lara E, Camacho JA, Oppenheimer F, Vara J, Santos F, et al. Cysteamine (Cystagon®) adherence in patients with cystinosis in Spain: successful in children and a challenge in adolescents and adults. Nephrol Dial Transplant. 2015;30:475–480. doi: 10.1093/ndt/gfu329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenzie B, Kay G, Matthews KH, Knott R, Cairns D. Preformulation of cysteamine gels for treatment of the ophthalmic complications in cystinosis. Int J Pharm. 2016;515:575–582. doi: 10.1016/j.ijpharm.2016.10.044. [DOI] [PubMed] [Google Scholar]
- 46.European Medicines Agency. Cystadrops Summary of Product Characteristics. Cystadrops EPAR-Prod. Inf. 2017.
- 47.Lyseng-Williamson KA. Cystadrops® (cysteamine hydrochloride 0.55% viscous eye-drops solution) in treating corneal cystine crystal deposits in patients with cystinosis: a profile of its use. Drugs Ther Perspect. 2017;33:1–7. doi: 10.1007/s40267-016-0361-y. [DOI] [Google Scholar]
- 48.Buchan B, Kay G, Heneghan A, Matthews KH, Cairns D. Gel formulations for treatment of the ophthalmic complications in cystinosis. Int J Pharm. 2010;392:192–197. doi: 10.1016/j.ijpharm.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 49.Luaces-Rodriguez A, Díaz-Tomé V, González-Barcia M, Silva-Rodríguez J, Herranz M, Gil-Martínez M, et al. Cysteamine polysaccharide hydrogels: study of extended ocular delivery and biopermanence time by PET imaging. Int J Pharm. 2017;528:714–722. doi: 10.1016/j.ijpharm.2017.06.060. [DOI] [PubMed] [Google Scholar]
- 50.Hsu K-H, Fentzke RC, Chauhan A. Feasibility of corneal drug delivery of cysteamine using vitamin E modified silicone hydrogel contact lenses. Eur J Pharm Biopharm. 2013;85:531–540. doi: 10.1016/j.ejpb.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Dixon P, Fentzke RC, Bhattacharya A, Konar A, Hazra S, Chauhan A. In vitro drug release and in vivo safety of vitamin E and cysteamine loaded contact lenses. Int J Pharm. 2018;544:380–391. doi: 10.1016/j.ijpharm.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 52.Ghasemi Falavarjani Khalil, Wang Kang, Khadamy Joobin, Sadda Srinivas R. Ultra-wide-field imaging in diabetic retinopathy; an overview. Journal of Current Ophthalmology. 2016;28(2):57–60. doi: 10.1016/j.joco.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retin Vitr [Internet]. 2016;2:12. doi: 10.1186/s40942-016-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2:9–25. doi: 10.1038/sj.neo.7900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rashid RF, Farhood QK. Measurement of central corneal thickness by ultrasonic pachymeter and oculus pentacam in patients with well-controlled glaucoma: hospital-based comparative study. Clinical Ophthalmology. 2016;10:359–364. doi: 10.2147/OPTH.S96318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.