Abstract
Background
Implementation of new practice guidelines for statin use was very poor.
Objective
To test a multi-component quality improvement intervention to encourage use of new guidelines for statin use.
Design
Cluster-randomized, usual-care controlled trial.
Participants
The study population was primary care visits for patients who were recommended statins by the 2013 guidelines, but were not receiving them. We excluded patients who were over 75 years old, or had an ICD9 or ICD10 code for end-stage renal disease, muscle pain, pregnancy, or in vitro fertilization in the 2 years prior to the study visit.
Interventions
A novel quality improvement intervention consisting of a personalized decision support tool, an educational program, a performance measure, and an audit and feedback system. Randomization was at the level of the primary care team.
Main Measures
Our primary outcome was prescription of a medium- or high-strength statin. We studied how receiving the intervention changed care during the quality improvement intervention compared to before it and if that change continued after the intervention.
Key Results
Among 3787 visits to 43 primary care providers, being in the intervention arm tripled the odds of patients being prescribed an appropriate statin (OR 3.0, 95% CI 1.8–4.9), though the effect resolved after the personalized decision support ended (OR 1.7, 95% CI 0.99–2.77).
Conclusions
A simple, personalized quality improvement intervention is promising for enabling the adoption of new guidelines.
ClinicalTrials.gov Identifier
Electronic supplementary material
The online version of this article (10.1007/s11606-018-4681-6) contains supplementary material, which is available to authorized users.
Key Words: cardiovascular disease, implementation science, clinical trials
INTRODUCTION
In 2013 and 2014, new clinical practice guidelines by major clinical organizations changed the recommended use of cholesterol-lowering statin drugs to be based on estimates of a patient’s chance of developing atherosclerotic cardiovascular disease (ASCVD), rather than cholesterol level.1–5 Compared to the prior guidelines, this was a major shift. The new guidelines substantially changed which people and how many were recommended statins. They changed the purpose of prescribing statins, away from lowering cholesterol levels to decreasing cardiovascular events.3, 6, 7 They changed how providers are supposed to think about prescribing statins, from a biological perspective of lowering cholesterol to a data-driven perspective of treating patients based on risk. They also changed the recommended process for deciding who should receive statins. In the new guidelines, providers should prescribe statins if a patient has ASCVD (representing a history of having had a heart attack or stroke), a diabetes diagnosis, or an elevated calculated 10-year risk of developing ASCVD (over 7.5% in the guidelines by the American College of Cardiology and American Heart Association, over 12% in the Veterans Affairs/Department of Defense guidelines). Calculating the 10-year risk requires data entry and trust in a risk prediction calculation, neither of which is standard in current medical practice.
Providers’ adoption of the guidelines into practice has been limited.8, 9 Any one of these changes in perspective could create barriers that would prevent providers from effectively following the new guidelines. Developing ways to recognize, address, and correct the gaps to implementation of the new guidelines is necessary to improve care.
We developed a multi-component guideline implementation intervention based on implementation theory and user-centered design to improve statin prescribing.10, 11 Our intervention included an individualized decision support tool, an educational session, reminder cards that summarized the guidelines, and a provider-level performance measurement and audit and feedback system. We then performed a cluster-randomized quality improvement (QI) trial to learn the effects of the intervention. Our primary aim was to examine the impact of the intervention on guideline-concordant statin prescribing. Our secondary aims were to examine the retention of the intervention’s effects after it was completed and to determine in whom the intervention had the largest effect.
METHODS
Context
We performed a single-site clustered randomized controlled trial of a quality improvement intervention on meeting the 2014 Veterans Affairs/Department of Defense (VA/DoD) guidelines Clinical Practice Guidelines for the Management of Dyslipidemia for Cardiovascular Risk Reduction.
Settings
This was a quality improvement intervention at a medium-sized academic clinic. This work occurred in partnership with the primary care clinic. The primary care clinic is organized into five Patient-Aligned Care Teams, each with up to ten attending physicians.
Study Population
Details of the derivation of the study population are described in the Online Supplementary Appendix A. For the primary analysis, inclusion criteria were patients seen in primary care during the period of analysis who were under 75 and who would be recommended a moderate- or high-strength statin according to the VA/DoD guidelines, but were not on one at the time of the visit according to the electronic health record. We had also planned to analyze patients who were on statins but according to the guidelines should not be. This proved to be too small a sample to examine.
Exclusion criteria included patients over the age of 75 or those who had an International Classification of Diseases-9 or -10 codes for end-stage renal disease, muscle pain, pregnancy, or in vitro fertilization in the 2 years prior to the study visit. All visits that were scheduled to occur after the Friday of the week before the visit were also excluded. (This was because visits that are scheduled soon before an appointment are more likely to be for urgent issues and because of our staffing limitations.)
For purposes of analysis, participant visits were divided into six groups. Each visit was either in the intervention arm or in the control arm. They were also in one of three time periods: a pre-QI period from December 12, 2015, to March 20, 2016; a three-month QI period from March 29, 2016, to June 30, 2016; and a post-QI period from July 1, 2016, until September 30, 2016. In addition, a pilot period, during which the intervention was implemented, took place from March 21, 2016, to March 28, 2016.
For a secondary analysis, we also examined a “negative control.” This analysis included patients who were not recommended statins according to the VA/DoD guidelines. These patients all had 12% or lower 10-year calculated probability of developing ASCVD. This population otherwise met the inclusion and exclusion criteria.
We have placed the details of the cohort construction and definitions in Online Supplementary Appendix A.
Risk Assessment
All patients were divided into six hierarchical, mutually exclusive risk groups, as defined by the VA/DoD guidelines, defined in detail in the Appendix. The groups for whom treatment is recommended were those with a history of ASCVD, diabetes, severe hyperlipidemia, or 10-year ASCVD risk ≥ 12%. The groups for whom treatment is not actively recommended by the guidelines are those with risk 6–12% (for whom shared decision-making is recommended) and those with risk < 6%. The differences between the VA/DoD guidelines and the American College of Cardiology and American Heart Association guidelines are summarized in Online Supplementary Appendix B.
ASCVD was defined by diagnostic or procedural codes, diabetes was defined by medicine use or diagnostic codes, and hyperlipidemia by having a low-density lipoprotein (LDL) cholesterol > 190 mg/dl documented in the VA electronic medical record in the prior 2 years. Ten-year ASCVD risk was calculated from the VA electronic medical record. Blood pressure and cholesterol values were only included if they were measured in the 3 years prior to the study.
When there was missing data, we still attempted to categorize patients. Patients with a history of ASCVD, with diabetes, or with measured LDL > 190 mg/dl are classifiable regardless of other missing data. When patients did not have a recent value for blood pressure or cholesterol, we tested the likelihood that the missing value would change their risk category. To do this, we took extreme measures of blood pressure and cholesterol derived empirically from the 95% range of values seen in our dataset. If a patient was in the same risk category for a high and low value for the missing variable, we would include them in that risk category. For example, a 70-year-old man who smokes will have a 10-year ASCVD risk ≥ 12% regardless of his blood pressure or cholesterol value.
Intervention
The design of the intervention has been described in depth.12 In short, we created a prototype multi-component intervention based on existing interventions. We then conducted qualitative interviews with 15 primary care providers to assess the barriers and facilitators to use of the clinical practice guidelines and tools designed to support their use. This was based on Cabana’s Clinical Practice Guidelines Framework for Improvement, which initially divides adoption into knowledge, acceptance, and behavior. In these interviews, we also solicited feedback used to hone the prototype intervention using Neilsen’s Usability Heuristics. Cabana’s Framework for Improvement helped guide what interventions we should consider and our understanding of the outcomes, whereas Neilsen’s Usability Heuristics helped make the interventions themselves effective and user-centered. The most striking finding of this work was that providers’ knowledge and acceptance of the guidelines were fairly high, but their behavior did not align. This was in large part due to difficulties in following the guidelines. We also found that providers cared greatly about the usability of the tools themselves, which led to many small changes to the intervention.
Ultimately, the intervention had four components: a decision support tool, an educational program, a performance measure, and an audit and feedback system.12 The decision support tool provided feedback that was tailored to the patient’s risk, current treatment, and guideline recommendation. Data for the tool was derived from the VA electronic health record. The decision support tool was a paper prototype, which was relatively easy to implement for this time-limited project. Examples of the decision support tool are provided in Online Supplementary Appendix F.
The educational program was a single 15-min session that described the clinical practice guideline, described the rationale for the changes that the guideline recommended, and included three clinical vignettes designed to exemplify the changes. The session was delivered at a regular providers’ meeting. The performance measure credited physicians for the percentage of patients who are eligible for statins per the VA guidelines who met them. In an attempt to influence treatment of high-value care, the performance measure also weighted patients with a history of heart attack or stroke more highly in assigning quality credit than those whose eligibility depended on having a very high LDL cholesterol (> 190) or high calculated risk (≥ 12%). The audit and feedback form summarized the results of the performance measure. Providers received a personalized audit and feedback at weeks 6 and 10 during the 13-week quality improvement period. Neither the performance measure nor the audit and feedback report was linked to pay-for-performance bonuses.
Trial Design and Randomization
We performed a parallel-design, cluster-randomized, usual care-controlled trial. Randomization was done at the level of primary care team. The five Patient-Aligned Care Teams (PACTs) were randomized with two teams receiving the intervention. Randomization was developed with a research assistant choosing a number that was used as a seed in Stata14.
Analysis
The population for the primary analysis was patients who would be recommended moderate- or high-strength statins by the 2014 VA/DoD statin guidelines, but were not on them at the time of their visit. Our analysis used hierarchical logistic regression with patient visits clustered within providers (n = 43) clustered within clinical teams (n = 5). The unit of analysis was the patient visit, and the dependent variable was “patient on statin, yes/no.” Intervention period (before, during, after) and intervention arm were categorical variables. Our primary outcome was the interaction of intervention period and intervention arm, to see if those who received the intervention were different from those who did not during the intervention, compared to before it. Details of the analysis are provided in Online Supplementary Appendix C.
Secondary analyses included examination of statin prescribing for pre-specified clinical subgroups and statin prescribing in patients who were meeting the recommendations of the prior clinical guidelines (the Advanced Treatment Panel III, or ATP III).6 Regarding the latter analysis, our hypothesis was that patients who were meeting prior guidelines would be less likely to have their care changed to meet the new guidelines. All analyses were based on intention to treat.
Ethical Considerations
Veterans Health Administration (VHA) Handbook 1058_05 provides guidance about authorization of manuscripts that have been developed through nonresearch activities (i.e., without institutional review board approval under the authority of VHA operations).13 All VHA authors of this manuscript attest that the activities that resulted in producing this manuscript were not conducted as part of a research project but as part of the nonresearch evaluation conducted under the authority of the VA’s Quality Enhancement Research Initiative. This evaluation follows the SQUIRE 2.0 reporting guidelines.14
RESULTS
Description
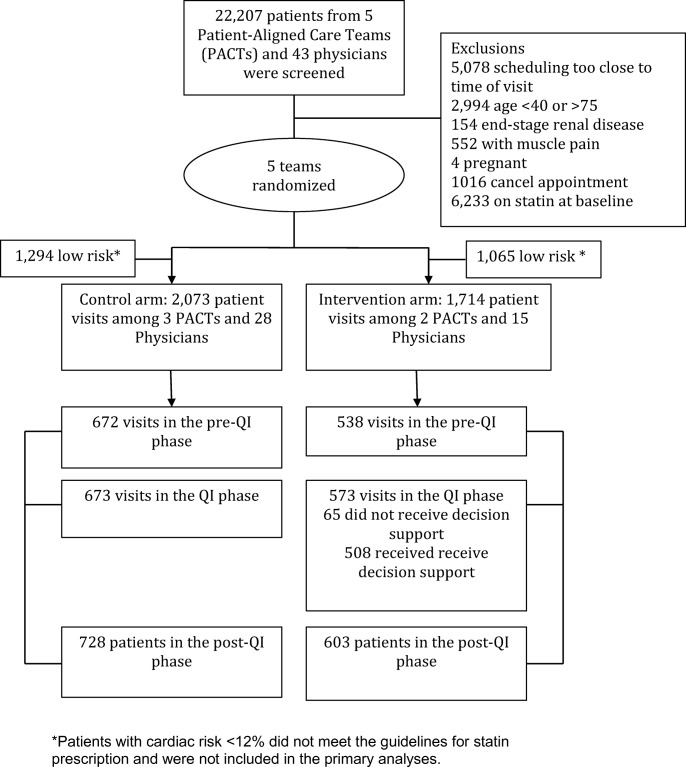
The intervention arm consisted of 15 primary care physicians among 2 PACTs; the control arm had 28 primary care physicians among 3 PACTs (Fig. 1). There were 22,207 patients seen by the participating physicians during the period of the study. Three thousand seven hundred eighty-seven met the inclusion criteria. Of these, 673 were control group patients in the QI phase and 573 were intervention group visits in the QI phase. Of the intervention group visits in the QI phase, 508 received the decision support tool.
Figure 1.
Flow diagram of participation in the QI intervention.
Over 96% of participating patients were men (Table 1). The average age was 65.3 in the control group and 64.2 in the intervention group. In the control group, 10.5% were African-American. In the intervention group, 11.1% were. Average LDL was 112. Twenty-nine percent of participating patients had a history of ASCVD, 21% had diabetes, and 50% had a 10-year risk ≥ 12%.
Table 1.
Description of the Population
| Control (n = 2073) | Intervention (n = 1714) | |
|---|---|---|
| Patient-Aligned Care Teams | 2 | 3 |
| Attending physician providers | 15 | 28 |
| Woman, No. (%) | 67 (3.2) | 62 (3.6) |
| Age (SD) | 65.3 (7.7) | 64.2 (8.5)** |
| African-American, No. (%) | 189 (9.1%) | 158 (9.2%) |
| Tobacco use, No. (%) | 496 (23.9) | 493 (28.8)*** |
| Meeting ATP III guidelines, No. (%) | 1156 (55.8%) | 800 (46.7%)*** |
| LDL, mean (SD), mg/dl | 109 (37) | 116 (41)*** |
| Period | ||
| Pre-QI, No. (%) | 672 (32.4) | 538 (31.4) |
| During QI, No. (%) | 673 (32.5) | 573 (33.4) |
| Post-QI, No. (%) | 728 (35.1) | 603 (35.2) |
| Risk group | ||
| ASCVD, No. (%) | 601 (29.0) | 483 (28.2) |
| Diabetes, No. (%) | 400 (19.3) | 386 (22.5) |
| ≥ 12% 10-year risk, No. (%) | 1051 (50.7) | 821 (47.9) |
| LDL > 190, No. (%) | 21 (1.0%) | 24 (1.4%) |
* p ≤ 0.05
** p ≤ 0.05
*** p ≤ 0.05
In the pre-QI period, patients in the intervention group had lower rate of medication intensification than those in the control group (Table 2). This was not the case in the other time periods.
Table 2.
Statin Initiation for Primary Sample
| Control | Intervention | |
|---|---|---|
| Pre-QI | 110/672 (16%) | 42/538 (8%)*** |
| QI | 95/ 673 (14%) | 102/573 (18%) |
| Post-QI | 95/728 (13%) | 57/603 (9%) |
***p < 0.001
Primary Analyses
Visits in the intervention arm during the QI intervention had an odds ratio of 3.00 (95% CI 1.84–4.89) of prescribing a moderate- or high-strength statin to high-risk patients who were not previously on one after controlling for the provider’s pre-QI statin prescription rate (Fig. 1 and Table 3). After the QI intervention ended, that rate declined to an odds ratio of 1.65 (0.99–2.77) (Fig. 2).
Table 3.
Effect of Being in Intervention Group on Odds of Treatment Correction
| During QI | Post-QI | ||||
|---|---|---|---|---|---|
| Observations in analysis | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| All patients recommended treatment | 3787 | 3.00 (1.84–4.89) | < 0.001 | 1.65 (0.99–2.77) | 0.06 |
| Not meeting ATP III guidelines at baseline | 705 | 4.94 (1.60–15.3) | 0.006 | 3.61 (1.12–11.6) | 0.03 |
| Meeting ATP III guidelines at baseline** | 1956 | 2.34 (1.15–4.77) | 0.02 | 0.89 (0.41–1.96) | 0.78 |
| History of ASCVD | 1984 | 2.58 (0.97–6.9) | 0.06 | 1.80 (0.65–5.0) | 0.26 |
| Diabetes | 786 | 2.48 (0.85–7.2) | 0.10 | 0.94 (0.31–2.8) | 0.91 |
| Calculated risk ≥ 12% | 1872 | 3.73 (1.77–7.4) | < 0.001 | 2.11 (1.0–4.5) | 0.05 |
| Not recommended treatment*** | 2359 | 0.59 (0.18–1.91) | 0.38 | 2.95 (0.85–10.3) | 0.09 |
*Figures of These Results Are Included in Online Supplementary Appendix D
**There are 1126 patients for whom we could not determine if they met ATP III guidelines due to missing data
***These are patients who are not explicitly recommended statins by the VA guidelines, primarily patients whose 10-year cardiac risk is < 12%. They are not in the primary study population and did not receive clinical decision support
Figure 2.
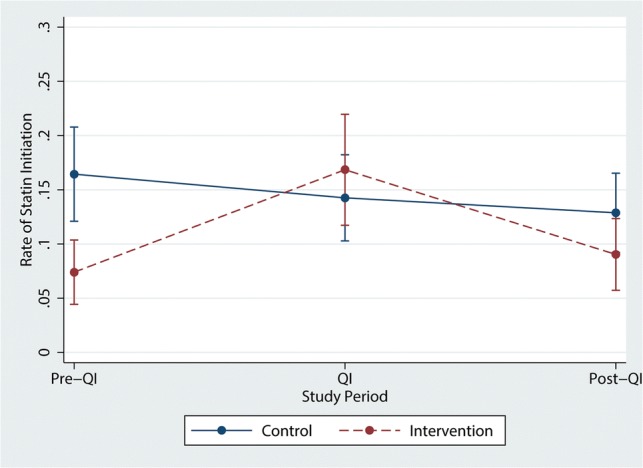
Primary outcomes. Of the people who are recommended moderate- or high-strength statin but not on one at the time of the visit, the percent of patients who are started on that treatment.
Secondary Analyses
There were no clear differences in the effects of the intervention in any risk groups who are recommended treatment. This included no significant difference in rates of intensification in patients who were meeting vs. not meeting the prior cholesterol guidelines (ATP III). Risk eligibility also did not seem to play a role, in that the intervention had the same apparent impact on those with a history of ASCVD vs. those with diabetes vs. those with a calculated high risk.
In a further secondary analysis, in patients who meet none of those criteria and therefore are low ASCVD risk, for whom the guidelines recommend shared decision-making or no statins, and did not receive a decision support reminder, rates of prescribing went down by a nearly significant amount during the intervention (Online Supplementary Appendix D).
We reframe the results in terms of the RE-AIM framework in Online Supplementary Appendix E.15
DISCUSSION
We found that a multi-faceted intervention could help guide the transition to new, risk-based clinical practice guidelines for the use of statin drugs. The intervention increased guideline-appropriate prescribing; the effect substantially declined when the clinical decision support was discontinued. Patients’ risk groupings, such as those who are eligible for statins due to having a history of ASCVD vs. elevated calculated risk, did not seem to alter the influence of the QI intervention.
Our results aligned with Cabana’s conceptual framework, which divides guideline use into knowledge, acceptance, and factors that influence behavior. The impact of the intervention together with the decline in its use once we stopped providing decision support shows that one major limiting step is simply providers’ ability to follow the guidelines. If knowledge or acceptance had been key, the intervention would not have changed their behavior at all. In fact, every provider did provide statins to patients who were eligible by according to the new guidelines, and not the old ones, at some point in the study.
The generalizability of our results is unknown. Our quality improvement project occurred at a single site at a Veterans Affairs clinic; other sites may respond differently. Our population was over 90% men and all US veterans, as is standard at US VA clinics. As a multi-component intervention, we cannot determine which aspect of the intervention had the greatest impact. Limitations in data quality, such as possible errors in smoking status and the assessed race, limit the reliability of our results, though randomization should have minimized the impact of that. Finally, our results are dependent on our finding that in the 3 months before the intervention began, the control group had higher rates of statin prescription than the intervention group. While we appropriately controlled for that difference, since groups were chosen randomly, this is a surprising finding.
Our findings are consistent with those of previous literature. Other multi-component computer-guided cardiovascular risk management programs have found small changes in overall care.16–19 Provision of risk scores has not been shown on their own to consistently change care.20, 21 A well-designed decision aid improved disease understanding, but did not change care choices.20, 22 Decision support tools often have measureable, but not tremendously large, effects.17
Because we have designed the complete multi-component intervention and have developed the decision support tool, the program likely could be scaled up and spread beyond a single site. However, integrating the tool into the EHR would greatly facilitate dissemination and adoption.
We are entering a new clinical era in which risk prediction, guided by big data, will become a key part of clinical practice. The transition to this era, however, will not always be straightforward. Developing clinically relevant, effective risk prediction tools must become a major focus of decision science, but this will only be a first step. Once clinically relevant risk prediction exists, we will need to understand patients’ and providers’ barriers to accurately using these tools and develop interventions that address them.
Electronic Supplementary Material
(PDF 602 kb)
Funding Information
This work was supported through support by VA Quality Enhancement Research (QUERI) Program (Grant No. QUE 15-286) to Drs. Sussman and Lowery and VA Health Services Research and Development (HSR&D) (Grants No. IIR 15-432; CDA 13-021) to Dr. Sussman.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
A version of this work was presented as a plenary talk at the VA Health Services Research and Delivery National Meeting on June 19, 2017.
Change history
1/2/2020
Dr. Sussman's name displayed incorrectly in the html of this paper.
References
- 1.Goff DC, Jr., Cushman WC. Blood-Pressure and Cholesterol Lowering in the HOPE-3 Trial. N Engl J Med. 2016;375(12):1194. [DOI] [PubMed]
- 2.Stone NJ, Turin A, Spitz JA, Valle CW, Kazmi S. Statin therapy across the lifespan: evidence in major age groups. Expert Rev Cardiovasc Ther. 2016;14(3):341–366. doi: 10.1586/14779072.2016.1128825. [DOI] [PubMed] [Google Scholar]
- 3.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. 2010;152(2):69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 4.USPSTF. Final Recommendation Statement. 2014. Last accessed September 24, 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lipid-disorders-in-adults-cholesterol-dyslipidemia-screening.
- 5.Department of Veterans Affairs/Department of Defense. VA/DoD Clinical Practice Guideline for the management of dyslipidemia for cardiovascular risk reduction. 2014:1–112.
- 6.National Cholesterol Education Program Expert Panel. Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed]
- 7.Krumholz HM, Hayward RA. Shifting views on lipid lowering therapy. BMJ. 2010;341:c3531. doi: 10.1136/bmj.c3531. [DOI] [PubMed] [Google Scholar]
- 8.Markovitz Adam A., Hofer Timothy P., Froehlich Whit, Lohman Shannon E., Caverly Tanner J., Sussman Jeremy B., Kerr Eve A. An Examination of Deintensification Recommendations in Clinical Practice Guidelines. JAMA Internal Medicine. 2018;178(3):414. doi: 10.1001/jamainternmed.2017.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pokharel Y, Tang F, Jones PG, et al. Adoption of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guideline in Cardiology Practices Nationwide. JAMA Cardiol. 2017;2(4):361–369. doi: 10.1001/jamacardio.2016.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi A, Arora M, Dai L, Price K, Vizer L, Sears A. Usability of a patient education and motivation tool using heuristic evaluation. J Med Internet Res. 2009;11(4):e47. doi: 10.2196/jmir.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 12.DeJonckheere M, Robinson CH, Evans L, et al. Designing for Clinical Change: Creating an Intervention to Implement New Statin Guidelines in a Primary Care Clinic. JMIR Hum Factors. 2018;5(2):e19. doi: 10.2196/humanfactors.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veterans Health Administration. VHA HANDBOOK 1058.05. Washington, DC 20420. 2011:9.
- 14.Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King DK, Glasgow RE, Leeman-Castillo B. Reaiming RE-AIM: using the model to plan, implement, and evaluate the effects of environmental change approaches to enhancing population health. Am J Public Health. 2010;100(11):2076–2084. doi: 10.2105/AJPH.2009.190959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris DP, Joshi R, Webster RJ, et al. An electronic clinical decision support tool to assist primary care providers in cardiovascular disease risk management: development and mixed methods evaluation. J Med Internet Res. 2009;11(4):e51. doi: 10.2196/jmir.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiris D, Usherwood T, Panaretto K, et al. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: the treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes. 2015;8(1):87–95. doi: 10.1161/CIRCOUTCOMES.114.001235. [DOI] [PubMed] [Google Scholar]
- 18.Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J. 2009;157(3):450–456. doi: 10.1016/j.ahj.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor PJ, Sperl-Hillen JM, Margolis KL, Kottke TE. Strategies to Prioritize Clinical Options in Primary Care. Ann Fam Med. 2017;15(1):10–13. doi: 10.1370/afm.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karmali KN, Persell SD, Perel P, Lloyd-Jones DM, Berendsen MA, Huffman MD. Risk scoring for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;3:CD006887. doi: 10.1002/14651858.CD006887.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekaran NK, Sussman JB, Xu A, Hayward RA. Providing clinicians with a patient’s 10-year cardiovascular risk improves their statin prescribing: a true experiment using clinical vignettes. BMC cardiovascular disorders. 2013;13:90. doi: 10.1186/1471-2261-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80(1):138–140. doi: 10.1016/j.pec.2009.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 602 kb)