Abstract
The mammalian inactive X-chromosome (Xi) is structurally distinct from all other chromosomes and serves as a model for how the 3D genome is organized. The Xi shows weakened topologically associated domains and is instead organized into megadomains and superloops directed by the noncoding loci, Dxz4 and Firre. Their functional significance is presently unclear, though one study suggests that they permit Xi genes to escape silencing. Here, we find that megadomains do not precede Xist expression or Xi gene silencing. Deleting Dxz4 disrupts the sharp megadomain border, whereas deleting Firre weakens intra-megadomain interactions. However, deleting Dxz4 and/or Firre has no impact on Xi silencing and gene escape. Nor does it affect Xi nuclear localization, stability, or H3K27 methylation. Additionally, ectopic integration of Dxz4 and Xist is not sufficient to form megadomains on autosomes. We conclude that Dxz4 and megadomains are dispensable for Xi silencing and escape from X-inactivation.
The mammalian inactive X-chromosome (Xi) is organized into megadomains and superloops directed by the noncoding loci, Dxz4 and Firre. Here the authors provide evidence that megadomains do not precede Xist expression or Xi gene silencing, and suggest that Dxz4, Firre, and megadomains are dispensable for Xi silencing and escape from X-inactivation.
Introduction
A longstanding principle in gene regulation invokes significance of 3D interactions between distant genetic elements. Advances in genomics provide opportunities to probe chromosome architecture and resulted in discovery of three types of long-range interactions. First, “topologically associating domains” (TADs) define continuous regions with extensive cis-contacts1. TADs are usually observed at length scales from 104–106 bp2, depend on cohesins3–5, and are generally bounded by convergent CTCF sites1,6–8. TADs are visible as squares along the diagonal of Hi-C contact maps. Second, “loops” define enhanced contacts between pairs of loci that interact via CTCF. Loops can exist within or between TADs, and are visible as strong “dots” in Hi-C contact maps1. Third, “compartments” transcend TADs and loops and exist as orthogonal structures formed by interactions between chromatin of similar epigenetic states. A-compartments harbor discontinuous chromosomal regions enriched for active genes, whereas B compartments harbor discontinuous regions enriched for repressed genes1,3,4,9–11. A/B compartments are visualized in Hi-C correlation maps by alternating “plaid” patterns of strong and weak interactions. Rapid depletion of CTCF10 or cohesin3 leads to genome-wide loss of TADs and loops, more pronounced A/B compartments (in the case of cohesin depletion) and only modestly affects transcription in the short term3–5,12. While loops are thought to be important for long-range gene regulation, the functional organization of TADs and compartments is presently less well understood.
Recent conformational studies of the inactive X (Xi) provided new insight into 3D chromosomal structure-function relationships13,14. X-chromosome inactivation (XCI) occurs in female cells as part of a dosage compensation mechanism that equalizes dosage of X-linked genes between males and females15–17. Chromosome conformation capture studies have demonstrated that, whereas the active X (Xa) resembles autosomes in having defined TADs, loops, and compartments, the Xi adopts a distinct structure1,18–22. Binding of architectural proteins including CTCF20,23 and cohesins20 are relatively depleted on the Xi, providing a mechanistic explanation for the attenuation of TADs. The Xi also lacks the characteristic separation between A and B compartments. Instead, during XCI, the Xi is partitioned into transitional Xist-rich S1 and Xist-poor S2 compartments, which are later merged into a single compartment by the non-canonical SMC protein, SMCHD124. The merging of S1/S2 structure has physiological consequence, as ablating SMCHD1 precludes this fusion and leads to failure of silencing of >40% of genes on the Xi24. Thus, on the Xi, compartmentalization appears to have an important role in gene silencing.
On the other hand, the significance of domains on the Xi is under debate. Studies have shown that the Xi folds into two “megadomains” separated by the non-coding, tandem repeat “Dxz4”20–22,25. In humans, DXZ4 is heterochromatinized and methylated on the Xa but is euchromatic and unmethylated on the Xi, where it binds CTCF26,27. Murine Dxz4 is not well-conserved at the sequence level, but the syntenic region harbors a repeat array of CTCF sites28. In both mouse and human, Dxz4/DXZ4 resides at the border between the two megadomains of the Xi and binds CTCF and cohesin in an allele-specific manner (Supplementary Fig. 1). Deleting the Dxz4/DXZ4 region in both species results in loss of megadomains and increased frequency of interaction across the border22,25,29. Despite clear disruption of the Xi super-structure, there is presently no agreement regarding functional consequences. One group reported loss of ability of escapees to avoid silencing on the Xi22. Changes in repressive chromatin marks and accessibility have also been reported in mouse22,29. Still others reported minimal effects, or even opposite effects, such as a partial loss of Xi heterochromatin in human cells25. Thus, there exists disagreement as to whether Dxz4 and megadomains enable or oppose silencing.
Additionally, the Xi is characterized by a network of extremely long-range loops termed “superloops”1 and the importance of these structures is also unknown. Superlooping occurs between Xist, DXZ4, and another CTCF-bound tandem repeat called FIRRE30,31 (Supplementary Fig. 1)1,25,27. Far longer than almost all other contacts in mammalian genomes, the loops between Dxz4 and Firre extrude up to 25 Mb of DNA. One study suggests that Firre RNA may direct Xist to the perinucleolar space and influence H3K27me3 deposition on the X32. However, despite the fact that the Firre locus falls at the border between two TADs and contains many CTCF sites, a recent study found that Firre is neither necessary nor sufficient to form borders between TADs, though it is required for superlooping with Dxz433.
Here we combine genetic, epigenomic, and cell biological methods and study the impact of large-scale 3D structures on Xi biology. We observe that megadomains form and TADs weaken several days after the onset of Xist expression. Deletion of Dxz4 disrupts separation between the two megadomains, and deletion of Firre appears to weaken intra-megadomain interactions. However, deletion of Dxz4, Firre or both does not disrupt any feature of XCI. These results suggest the unique tandem repeats of the X-chromosome are necessary for the formation of Xi superstructures but dispensable for XCI itself.
Results
Time course of megadomain formation during XCI
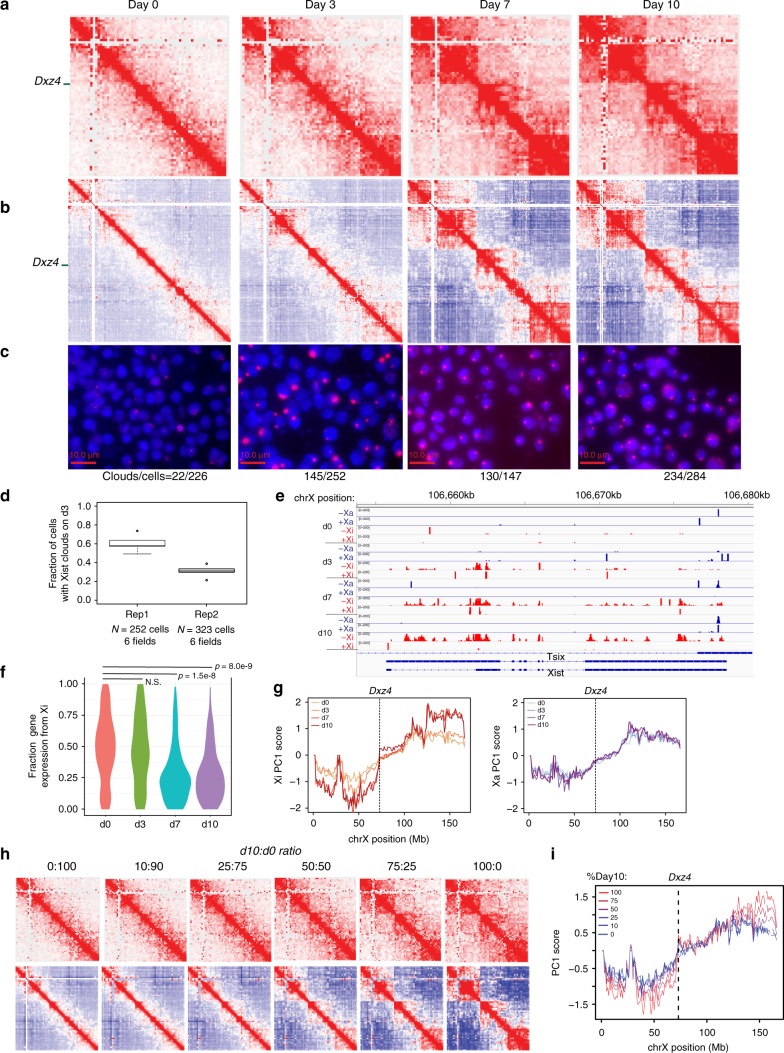
It is presently unknown how the formation of Xi megadomains relates to the timeline of XCI. To assess whether megadomains precede or follow XCI, we performed allele-specific Hi-C in female mouse embryonic stem cells (mESCs), which model different steps of XCI when they are induced to differentiate in culture. We examined timepoints day 0 (before XCI), day 3 (early XCI), day 7 (mid-XCI), and day 10 (late-XCI) in the mESC line, TsixTST/+, and compared the megadomain timeline to the time course of Xist upregulation and Xi silencing in two biological replicates. Allelic analysis was possible in TsixTST/+ in two ways. First, it carries one X-chromosome of M. castaneus (cas) origin and one of M. musculus 129 origin (mus)34,35, the combination of which enabled employment of >600,000 polymorphisms to distinguish alleles. Second, the cell line carries a stop-mutation in the mus Tsix allele34 that ensures the mus X-chromosome is chosen as the Xi in >95% of cells35–37.
For Hi-C analysis, we sequenced to a depth of 25–50 million reads, as megadomains are large (>70 Mb), prominent structures and can be sensitively detected at a resolution of 2.5 megabases (Mb). Allele-specific Hi-C contact maps and corresponding Pearson correlation heatmaps showed that, as expected, megadomains did not appear on the Xa during any timepoint (Supplementary Fig. 2a, b). Focusing in on the Xi, we observed that, in pre-XCI cells (day 0) and in cells undergoing XCI (day 3), Xmus resembled Xcas (the Xa), lacking detectable megadomains (Fig. 1a, b). RNA FISH analysis of these timepoints showed that 30–60% of cells showed robust Xist RNA clouds by day 3 (Fig. 1c, d). RNA-seq analysis also showed robust upregulation of Xist starting on day 3 and continuing throughout differentiation (Fig. 1e, Supplementary Fig. 2c, d). Importantly, Xist was upregulated almost exclusively from Xmus as expected, consistent with the TsixTST allele carried in cis34. [Note: The nonrandom pattern at day 3 agrees with Tsix being a primary determinant of allelic choice38 rather than being a secondary selection mechanism following a stochastic choice process39,40. A small fraction of reads coming from Xcas is likely to be artifactual, as virtually all the Xcas reads fell into one peak near the 5′ end of Xist, rather than distributed across the entire gene (Fig. 1e). This peak fell within a repetitive region of Xist (repeat A) and contained only one SNP (rs225651233)—a 129G ->Cast/EiJ T variant falling within a low complexity 24 bp poly-T tract. Thus, the Xcas reads are likely to be from an improperly-defined SNP.] Despite highly skewed Xist upregulation, allele-specific analysis showed that X-linked gene expression remained unskewed (Fig. 1f), implying that de novo silencing or turnover of preexisting mRNA lagged behind Xist upregulation.
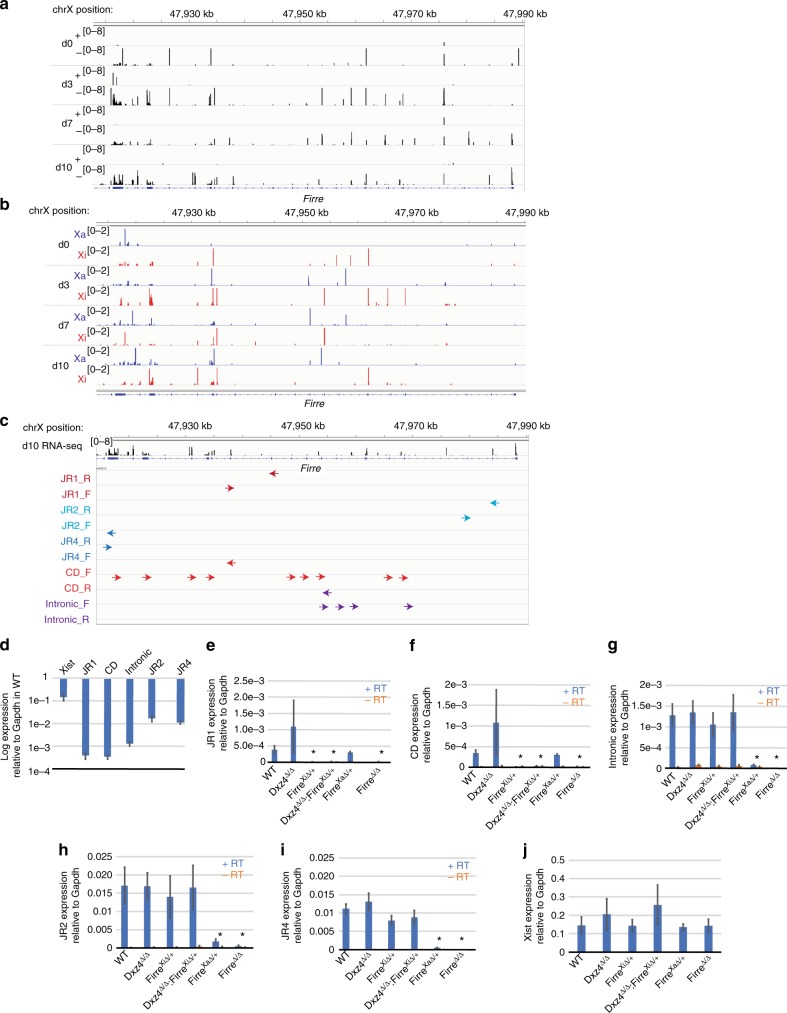
Fig. 1.
Dynamics of megadomain formation during XCI. a KR-normalized Hi-C matrices on future Xi (mus) in female ES cells on days 0, 3, 7, and 10 of differentiation (2.5 Mb resolution). b Pearson correlation of Hi-C matrices on days 0, 3, 7, 10 of differentiation (1 Mb resolution). c Xist RNA FISH on day 0, 3, 7, 10 of differentiation in TsixTST/+ ES cell line. Scale bars: 10 μm. d Fraction of cells showing Xist clouds on day 3 of differentiation in two biological replicates. Error bars represent standard deviation of counts over 6 fields in one imaging session. Box plot parameters: center line = median, lower bound = 25th percentile, upper bound = 75th percentile, whiskers = interquartile range x 1.5. e Allele-specific expression from the Xi (red) or Xa (blue) over Xist in TsixTST/+ during differentiation. f Density plots of the number of X-linked genes with a given level of allelic expression from the Xi on day 0, 3, 7, 10 of differentiation in TsixTST/+. Wilcox p-values comparing the mean allelic expression levels from the Xi between d0 and all other timepoints are indicated (threshold for significance: p < 0.05. p-values above threshold are labeled “not significant” (N.S.)). g 1st principal component of the 1 Mb correlation matrix plotted for all bins on the future Xi (mus, left) and future Xa (cas, right) for Days 0, 3, 7, 10 of differentiation. Dotted lines correspond to the bin containing Dxz4. All Hi-C data in this figure are generated from merging together reads from two biological replicates. h In silico mixing experiment to determine the sensitivity of the Hi-C assay for detecting megadomains. KR-normalized Hi-C matrices at 2.5 Mb resolution (top) and Pearson correlation of Hi-C matrices at 1 Mb (bottom) for the Xi from data sets generated by mixing an indicated ratio of day 0 (d0, megadomain-negative) and day 10 (d10, megadomain-positive) datasets. This mixing experiment uses data from replicate 2 of the timecourse experiments. i 1st principal component of the 1 Mb correlation matrix plotted for all bins on the Xi for datasets within varying ratios of d10:d0 reads
On the other hand, analysis of day 7 cells revealed Xist expression in >80% of cells (Fig. 1c) and robust Xi silencing (Fig. 1f). It was at this timepoint that strong megadomains were first observed (Fig. 1a, b). Analysis of day 10 cells showed similarly strong Xist expression, Xi silencing, and megadomains. To quantify megadomain signals, we computed the Pearson correlation for the Xi contact maps and performed principal component analysis (PCA). On day 7 and day 10, there was a sharp transition in the 1st principal component score (PC1) at Dxz4, indicating changed interaction patterns on each side of Dxz4 at later timepoints but not in days 0 or 3 (Fig. 1g), consistent with appearance of megadomains. By contrast, the PC1 score distribution for the Xa was nearly identical across timepoints and lacked a sharp transition at Dxz4 (Fig.1g).
Our observations suggested that megadomains do not precede Xist spreading and silencing, as they were largely absent in early timepoints (day 0 and day 3). It is however, possible that megadomains were present in a fraction of cells under the detection threshold of our population-based assay. To determine the limits sensitivity, we performed an in silico “mixing” experiment (Fig. 1h, i, Supplementary Fig. 2e, f). We combined megadomain-positive day 10 cells with megadomain-negative day 0 cells in different proportions. The slope of the PC1 score at Dxz4 was directly proportional to the fraction of megadomain-positive (i.e., day 10) cells (Supplementary Fig. 2f), demonstrating that the strength of the Dxz4 border correlated directly with the strength of megadomains. Using a linear fit, we deduced that day 3 megadomains were 5- to 14-fold weaker than on day 10 (Supplementary Fig. 2g). Given the linear fit across all mixing ratios, we furthermore inferred that our Hi-C had sufficient sensitivity to detect megadomains if present in >25% of cells, assuming similar megadomain strengths across time points. Given that Xist spreading had taken place in 30–60% of cells and little silencing had taken place at this time point, megadomains were unlikely to have preceded Xist spreading. The dynamics of megadomain formation were highly reproducible between two biological replicates (Supplementary Fig. 3a). Taken together, these data suggest that megadomains do not precede XCI and appear either concurrently with or (more likely) only after Xist has spread and silenced the Xi.
Time course of TAD attenuation on the Xi
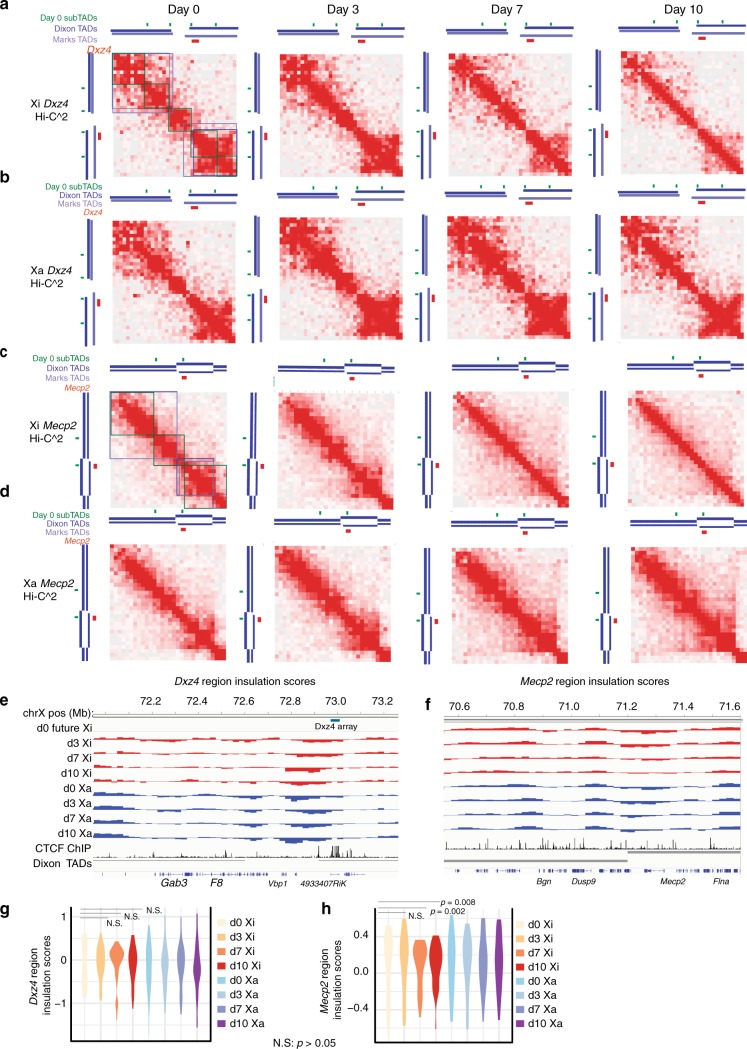
Recent analyses indicate that TADs are not abolished on the Xi but are instead attenuated24,29. Here we investigate the timecourse of TAD attenuation during XCI. To obtain higher resolution allele-specific contact maps, we performed Hi-C^2, a variation of the Hi-C protocol that focuses sequencing on defined regions through hybrid capture6. We investigated ~1.5 Mb regions around (i) Dxz4 to assess the behavior of the megadomain border and (ii) the TAD harboring the disease locus and inactivated gene, Mecp2, to examine how topological domains are weakened during XCI.
Despite megadomains appearing only late during the XCI time course, the Dxz4 region showed a strong boundary during all time points and on both alleles (Fig. 2a, b). Therefore, Dxz4 acts as a border irrespective of XCI status and presence/absence of megadomains. At days 7 and 10, the proximal TAD upstream of Dxz4 expanded on the Xi but not the Xa (Fig. 2a, b). This left only two “boxes” on either side of Dxz4 by day 10, rather than the patchwork of smaller sub-TADs present on the Xa and Xi at earlier timepoints. This finding indicates that Dxz4 insulates interactions at increasingly larger distances when megadomains form. Dxz4 began as a simple TAD border early in differentiation, insulating interactions between loci on either side of Dxz4 but at fairly short distances (<1 Mb). As the megadomain formed, Dxz4 continued to act as border, but insulated interactions between pairs of loci in separate megadomains that were many megabases away from each other. The temporal dynamics of chromatin conformation surrounding Dxz4 were quite similar in two biological replicates (Supplementary Fig. 3b).
Fig. 2.
Dynamics of TADs and sub-TADs during XCI near Mecp2 and Dxz4. a, b Hi-C^2 contact maps around Dxz4 (mm9 coordinates chrX:71,832,976–73,511,687) and on the future Xi (a) or Xa (b) on days 0, 3, 7, 10 of ES differentiation (50 kb resolution). c, d Hi-C^2 contact maps around Mecp2 (mm9 coordinates chrX:70,370,161–71,832,975) and on the future Xi (c) or Xa (d) on Days 0, 3, 7, 10 of differentiation (50 kb resolution). Green bars indicate positions of sub-TAD borders determined from 25 kb day 0 Hi-C^2 matrices (comp matrices use all unique reads); dark blue track shows Dixon et al.2 TAD calls in mESCs, light blue track shows Marks et al.53 TAD calls in mESCs, red bars indicate positions of either Dxz4 or Mecp2. In addition, the regions of the contacts corresponding to sub-TADs (green), Dixon et al. TADs (dark blue), Marks et al. TADs (light blue) have been indicated with boxes on the day 0 Xi contact maps for reference. e, f Insulation scores across the Dxz4 region (e) or Insulation scores across the Mecp2 region (f). Insulation scores on the Xa are blue and insulation scores on the Xi are red. For reference, CTCF ChIP-seq in day 0 F1-2.1 mESCs (black) and TAD calls from Dixon et al. (grey) are shown2. g, h Violin plots showing the distributions of insulation scores across the Dxz4 region (g) and Mecp2 region (h). All data in this figure are generated from merging together reads from two biological replicates. Note: to generate violin plots and evaluate the significance of differences in variance between timepoints we excluded the 6 bins on each edge of the Hi-C^2 region because the regions needed to calculate insulation score fall partly outside the Hi-C^2 region and have far lower read counts than sequences targeted by the capture probes. To evaluate whether differences in the variance of insulation scores is statistically significant between timepoints, we used the pairwise F-test, threshold for significance: p < 0.05. p-values above threshold are labeled “not significant” (N.S.)
Within the region containing Mecp2, Hi-C^2 contact maps showed that Xmus and Xcas behaved similarly on days 0 and 3, in that both were organized into several sub-TADs (Fig. 2c, d). However, once Xist spread over Xmus and the Xi formed as a consequence on days 7 and 10, both TAD and sub-TAD organization become obscured compared to the Xa, where these domains stayed similar to earlier timepoints. In contrast to a previous analysis performed in neural progenitor cells (NPCs)22, we did not observe the persistence of a small domain around Mecp2 in differentiating mESCs. The loss of domain organization in the Mecp2 region was observed in two biological replicates (Supplementary Fig. 3c).
To quantify these changes, we computed insulation scores using standard methods22,41 (Fig. 2e, f). In brief, insulation scores quantify how strongly a given locus acts as a border41–43, and are calculated by running sliding windows across a chromosomal region and measuring the log ratio of reads crossing over a locus to reads neighboring a locus (Supplementary Fig. 3d, Methods). Loci in the interiors of a TAD would be expected to have similar numbers of cross-over interactions and local interactions on each side, leading to insulation scores near zero, whereas loci at borders would register as a local minimum of crossover interactions than local interactions, leading to strong negative insulation scores at domain boundaries. We observed several interesting facets of the insulation score curves on the Xa and Xi at Dxz4 and Mecp2 during differentiation. There was a strong decrease in insulation scores near Dxz4 on both alleles at all timepoints, consistent with Dxz4 acting as a boundary throughout differentiation (Fig. 2e, g). The variance of the insulation scores is a measure of the global strength of insulation, with smaller variance corresponding to weaker insulation44. Near Dxz4, the variance was slightly smaller on the Xi than the Xa for all timepoints. There was no statistically significant difference in variance of insulation scores on the Xi on day 0 compared with the variance insulation scores on the Xi for the later timepoints (pairwise F-test, p-values > 0.05 for all comparisons between day 0 Xi and later Xi timepoints) and this observation held across two biological replicates (Fig. 2g, Supplementary Fig. 3e). Thus, the Dxz4 region is a strong boundary regardless of XCI status, but Dxz4 insulates interactions across larger distances to form megadomains.
The Mecp2 region showed a different pattern of insulation score changes across differentiation. The distribution of insulation scores across the Mecp2 region significantly narrowed on days 7 and 10 on the Xi but not Xa (Fig. 2h). Indeed, across the Mecp2 region, the variance on the day 7 or day 10 Xi was significantly lower than on day 0 (pairwise F-test, day 7 vs day 0 p-value < 0.008443; day 10 vs day 0 p-value < 0.001819). There was no significant difference in the variance on the Xi between day 0 and day 3 (pairwise F-test, p < 0.7369). This clear decrease in the variance on the Xi at day 7 and day 10 relative to day 0 was observed in two biological replicates (Supplementary Fig. 3f). Thus, TAD and sub-TAD structures of the Mecp2-containing TAD region are reduced in strength in the same timeframe that megadomains are gained.
Dxz4 is required but not sufficient for megadomain formation
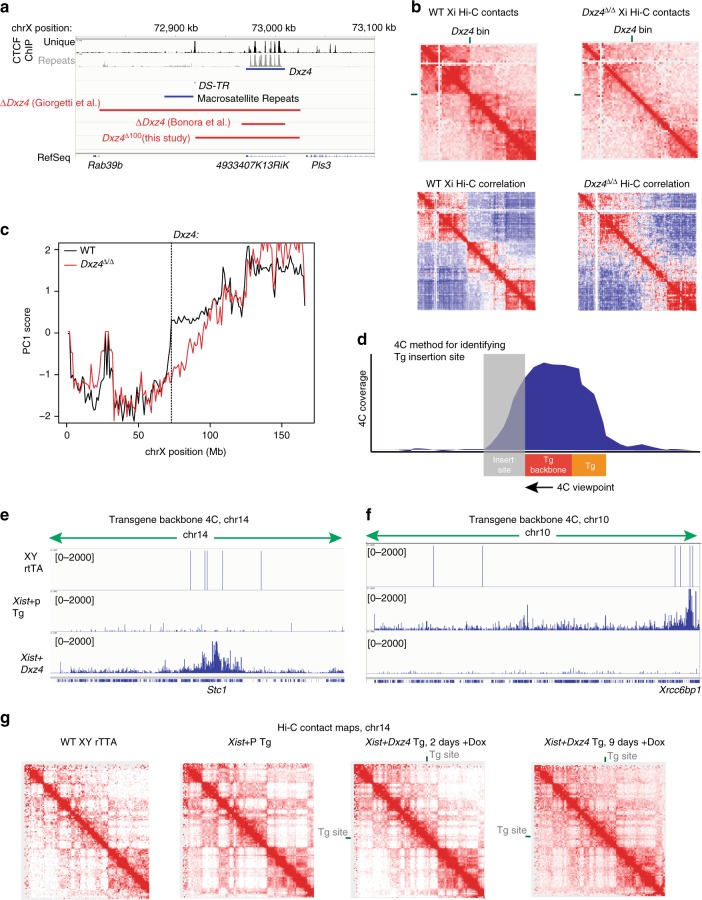
There presently exist several deletions containing Dxz4/DXZ4 created in mouse and human cells22,25,29. One of the mouse deletions22 is a large deletion that contains more than just the noncoding element, Dxz4/DXZ4 (Fig. 3a). We generated a new 100-kb deletion of Dxz4 and its flanking sequences (Dxz4∆100) that left untouched a small cluster of CTCF motifs with very high CTCF coverage and an unusual satellite repeat (Fig. 3a and Supplementary Fig. 4a)28, both of which were deleted in the previous 200-kb Dxz4 deletion29. We validated Dxz4∆100 by Sanger sequencing, DNA FISH with a probe internal to the deleted region, and genomic DNA sequencing (Supplementary Fig. 4, Methods). Importantly, to distinguish Xi from Xa, we performed the deletion analysis in TsixTST/+mESCs.
Fig. 3.
Dxz4 is necessary but not sufficient for megadomain formation. a Schematic of prominent features in the Dxz4 region and deletions generated in this study and previous studies. b Top: Contact maps for the Xi at 2.5 Mb resolution for wild-type (left) and Dxz4∆/∆ (right) cells on d10 of differentiation. Bottom: Pearson correlation matrices at 1 Mb resolution for the wild-type (left) and Dxz4∆/∆ Xi (right). Heatmaps generated by merging reads together from two biological replicates. c 1st principal component of the 1 Mb correlation matrix plotted for wild-type (black) and Dxz4∆/∆ (red) across all bins on the Xi. The dotted line corresponds to the bin containing Dxz4. d Strategy for using 4C to localize the transgene insertion site. e, f 4C interaction profiles using a viewpoint in the backbone of an Xist transgene in either wild-type (top), Xist only Tg (middle) or Xist + Dxz4 Tg (bottom) lines across chr14 (e) or chr10 (f). g Hi-C contact maps for chr14 at 1 Mb resolution in either wild-type (left), Xist only Tg (middle left), Xist + Dxz4 Tg Dox induced for 2 days (middle right) or 9 days (right). The position of the Xist + Dxz4 transgene insertion site at ~69.8 Mb (near Stc1) is indicated by a green bar in the Xist + Dxz4 Tg contact maps
To test the impact of removing Dxz4, we differentiated wild-type and homozygously deleted (Dxz4∆/∆) cells for 10 days and performed Hi-C. Whereas wild-type cells showed strong megadomains, Dxz4∆/∆ cells showed disrupted megadomain structures in Hi-C contact maps (Fig. 3b) and corresponding Pearson correlation maps. Most prominently, the sharp border around Dxz4 was eliminated, though some intra-megadomain interactions remained on either side of the deletion. A disrupted megadomain border was confirmed by loss of the sharp transition in PC1 score around the Dxz4 locus (Fig. 3c). This effect was observed in two biological replicates (Supplementary Fig. 4d). These results are in agreement with prior reports22,25,29 that the 200–300 kb region around Dxz4 is required for megadomain organization. Additionally, our work delineates the required region to a 100-kb domain containing the Dxz4 tandem repeat itself (as opposed to the CTCF motif cluster and the satellite repeats).
Given its necessity for megadomain organization, we asked whether Dxz4 is also sufficient to form a megadomain on an autosome when Xist RNA is expressed in cis. We co-transfected a dox-inducible full-length Xist construct along with a BAC containing Dxz4 into male fibroblasts and used RNA and DNA FISH to identify Xist-inducible clones (Supplementary Fig. 5a) where both Xist and Dxz4 had co-inserted (XPDxz4.4; Supplementary Fig. 5b). To localize the transgene, we adapted the 4C technique45,46 that is ordinarily used to view 3D interactions from a single locus. By placing the 4C viewpoint anchor at the transgene backbone, we could map the transgene through the pattern of cis-interactions on the same chromosome (Fig. 3d), as interaction frequencies are typically highest near the viewpoint. Indeed, in addition to interaction peaks at Xist and Dxz4 as expected (Supplementary Fig. 5c), the only other strong 4C peak in the genome appeared on chr14 near Stc1 (Fig. 3e). This finding contrasts with both a control Xist-only transgene line which showed a peak only on chr10 (Fig. 3f) and the parental rtTA fibroblasts which showed no peaks anywhere in the genome. We confirmed insertion of both Dxz4 and the Xist construct into Stc1 by observing co-localization between Stc1, Xist, and Dxz4 DNA FISH probes (Supplementary Fig. 5d).
To test whether induction of Xist expression could induce megadomain formation at Dxz4 ectopically, we induced Xist and performed Hi-C to determine whether a megadomain formed on transgenic chr14. We induced Xist for 2 days (Supplementary Fig. 5a, n = 207, 88% Xist-positive) because a previous report suggested that induction of Xist from the male X for 2 days was sufficient to at least initiate megadomain formation22. No megadomains formed and the chr14 contact maps looked highly similar to the non-transgenic and Xist-only controls (Fig. 3g). We then extended the timeframe and induced for 9 days (Supplementary Fig. 5a, n = 199, 89% Xist-positive), given that our ES cell time course suggested that several days of Xist upregulation were needed to form megadomains on the Xi. Still, no megadomains formed in these post-XCI cells (Fig. 3g). Furthermore, no obvious local changes were evident near the transgene insertion site (Supplementary Fig. 5e). To assess whether Xist and Dxz4 could do so in cells undergoing de novo XCI, we attempted three times to create the Xist-Dxz4 transgene line in female ES cells, but such a line could not be generated, due to potential lethal consequences of the Xist transgene. These results indicate that Xist and Dxz4 together are not sufficient for megadomain formation in a cell line that had already undergone XCI (fibroblasts). We conclude that Dxz4 and Xist expression are necessary but not sufficient for megadomain formation in post-XCI cells.
Dxz4 and Firre form Xi-specific superloops
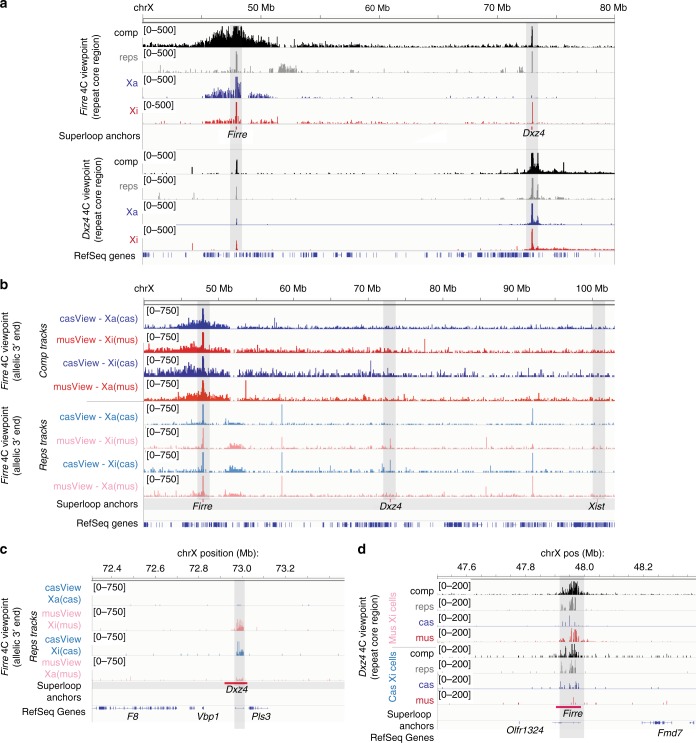
In addition to serving as the border between the megadomains, Dxz4 has been shown to form extremely long (>10 Mb) looping interactions with other loci on the human Xi1,25,27. To further dissect the role of Dxz4 in establishing the large-scale structure of the Xi, we performed 4 C using a viewpoint within the core of the Dxz4 tandem repeats in post-XCI fibroblasts to identify interacting loci that may be important for helping to establish the unique structure of the mouse Xi. Dxz4 generally interacted with the chromosome telomeric to Dxz4 and formed few long-range interactions towards the centromeric side of the chromosome (Fig. 4a, Supplementary Fig. 6a). However, Dxz4 interacted strongly with another non-coding tandem repeat, Firre (Fig. 4a). The two loci formed an extremely strong loop despite the fact that Firre is 25 Mb centromeric to Dxz4. To verify the Dxz4:Firre interaction, we performed a reciprocal 4C using a viewpoint within the core of the Firre tandem repeats and confirmed a strong reciprocal interaction (Fig. 4a). On the Xa, Firre also formed a broad domain of interactions with nearly all sequences within several Mb of itself, as reported for the Firre RNA contact map30. In contrast to this prior study, however, we did not observe any evident interchromosomal contacts from either Xa or Xi allele in fibroblasts.
Fig. 4.
Dxz4, Firre, and Xi-specific superloops. a Reciprocal interaction between Dxz4 and Firre in post-XCI fibroblasts. Top: 4C interaction profiles with the core of the Firre tandem repeat as the viewpoint. Bottom: 4C interaction profiles with the core of the Dxz4 tandem repeat as the viewpoint. Black: all unique reads (comp). Grey: repetitive (non-unique) reads (reps). Blue: Xa (cas)-specific reads. red: Xi (mus)-specific reads. Note: For all tracks and Hi-C datasets, “comp” (“complete”) refers to all unique reads, including non-allelic reads. “reps” refers to all non-unique reads. b 4C from a unique, allele-specific viewpoint at the 3′ end of Firre in cells with either an inactive mus (cas Xa/mus Xi) or an inactive cas (cas Xi/mus Xa). Red: interaction profile for unique (comp) reads on the mus allele. Blue: interaction profile for unique (comp) reads on the cas allele. Pink: interaction profile for non-unique (reps) reads from the mus allele. Light Blue: interaction profile for non-unique (reps) reads from the cas allele. Positions of Firre, Dxz4 and Xist are shaded light grey and indicated with red bars. c 4C interaction profile from a unique, allele-specific viewpoint at the 3’ end of Firre in cells with either a mus Xi (top tracks) or a cas Xi (bottom tracks), zoomed in onto Dxz4. Light blue: interaction profile from the cas Firre viewpoint. Pink: interaction profile from the mus Firre viewpoint. d 4C interaction profile over Firre using the Dxz4 core viewpoint in cells either with a mus Xi (top tracks) or a cas Xi (bottom tracks). Black: all unique (comp) reads. Grey: non-unique (repetitive) reads. Blue: cas reads. Red: mus reads. Note: c and d zoom into Dxz4 and Firre respectively to better highlight the allele-specific superloop interactions. The IgV browser’s windowing function can obscure the relative Xa:Xi interaction strength at large length scales (such as in a), thus these zoomed views allow better comparison of the Xa:Xi ratios
Our analysis revealed that the Dxz4:Firre interaction is primarily detected on the Xi (mus) allele. Indeed, when we repeated this reciprocal 4 C experiment in another hybrid fibroblast line that chose to inactive Xcas, the Dxz4:Firre interaction was detected on Xcas, rather than Xmus. For these experiments, we used both a unique 4 C anchor in the 3′ flanking region of Firre that provides allelic information, as well as an allele-agnostic anchor in the core of Firre repeat. Because Dxz4 and Firre are both highly repetitive, we also examined multiply-aligning reads. The Firre-Dxz4 interaction was only observed on the Xi regardless of whether the Xi was the mus or cas chromosome (Fig. 4b–d). Thus, Firre and Dxz4 formed an Xi-specific superloop conserved between mouse and primate1,25.
Other superloops have been identified on the human Xi using high-resolution Hi-C. FIRRE, DXZ4, XIST, ICCE and X75 are all repetitive loci that bind CTCF on the Xi in human cells, and all form long-range interactions with each other1,25. We examined whether these superloops also occurred in mouse cells. Indeed, in addition to Firre, we observed elevated interaction frequencies between Dxz4 and a region spanning Xist to Ftx (Supplementary Fig. 6a, b) and a region syntenic with human X75 (Supplementary Fig. 6a, c). However, the Xist-Dxz4 and X75-Dxz4 contacts were less prominent than the Firre-Dxz4 contact. Furthermore, we did not observe a Firre-Xist superloop (Fig. 4b), which has been reported on the human Xi1,25. These results suggest that there may only be partial conservation of superloop structures between mouse and human.
Firre is predominantly expressed from the Xa
Previous reports have suggested that Firre escapes X-inactivation30,32,47, and that Firre RNA is necessary for Xist localization and deposition of H3K27me3 on the Xi32. Allele-specific RNA-seq showed Firre reads from both Xa and Xi during differentiation, and expression appeared to be predominantly exonic (Fig. 5a, b). Because Firre is highly repetitive, SNP calls may be not be fully reliable. To confirm allele-specific expression, we used genetic means to examine expression from the Xa and Xi. With allele-specific guide RNAs, we generated an both Xa- and Xi-specific Firre deletions in TsixTST/+ as well as and a homozygous deletion (“Firre∆/∆”) (Supplementary Fig. 7). We then measured Firre expression on day 10 of differentiation using 4 published sets of primers30,32 and one new intronic primer set and deduced the expressed allele(s) by examining differences in expression pattern between the reciprocal heterozygous clones. First, by quantitative RT-PCR of wild-type female ES cells, we inferred that Firre expression was expressed at <10% of Xist RNA overall. The variability between amplicons suggested that there could be multiple isoforms of Firre (Fig. 5d). Second, deleting Firre on the Xi abolished expression of the two lowest expressed amplicons (Fig. 5e, f). By contrast, deleting Firre on the Xa abolished expression of the two most highly expressed exonic amplicons and the intronic amplicon. Together, our results suggest that Firre RNA is expressed from both the Xa and Xi, but the isoforms may be distinct from the two alleles and expression of the Xa-specific isoforms dominates over Xi-specific isoforms.
Fig. 5.
Allele-specific Firre expression in mESCs. a Expression over Firre during differentiation. b Allelic expression over Firre during differentiation, red = Xi reads, blue = Xa reads. c Positions of Firre primers within the Firre locus. d Expression of Xist or Firre amplicons in d10 wild-type cells normalized to Gapdh (logarithmic scale). e–j Expression normalized to Gapdh measured with JR1 (e), CD (f), intronic (g), JR2 (h), JR4 (i) Firre primers or Xist primers (j) in WT, Dxz4∆/∆ FirreXi∆/+, Dxz4∆/∆:FirreXi∆/+, FirreXa∆/+, and Firre∆/∆ cells. Blue bars are + RT, orange bars are -RT. Asterisks (*) indicate a statistically significant (p < 0.05, t-test) difference between normalized expression levels in wild-type and normalized expression levels in the deletion. Error bars show standard error of the mean, three biological replicates
To determine whether Dxz4 influences Firre expression, we also performed RT-PCR in the Dxz4∆/∆ cell line. No changes were evident, indicating that the Dxz4-Firre superloop does not impact transcriptional regulation of Firre (Fig. 5e–i). Finally, to examine whether either repeat locus regulates Xist expression, we performed RT-PCR in cell lines carrying deletions of either Firre or Dxz4, and also generated and tested a double ES cell knockout of Dxz4 and Firre on the Xi carrying the TsixTST allele (Dxz4∆/∆:FirreXi∆/+)(Supplementary Fig. 7, Methods). None of these deletions affected Xist expression (Fig. 5j). Thus, the superloops are irrelevant for expression of Dxz4, Firre, and Xist.
Firre and Dxz4 collaborate to establish bipartite structure
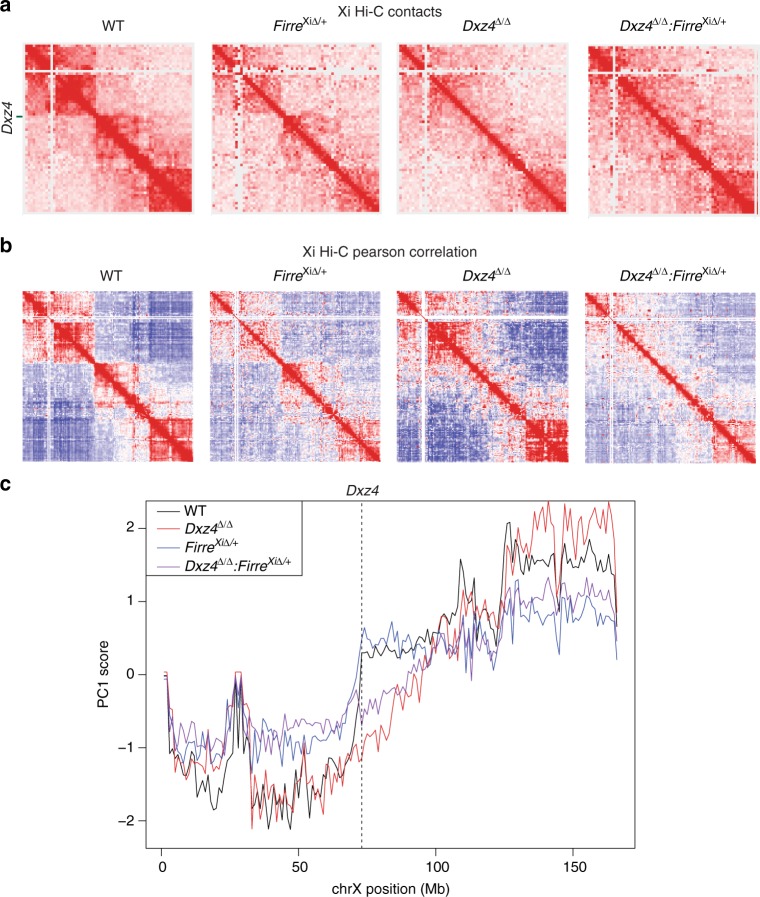
Prior work had not examined whether Xi superloops contribute to the formation of the megadomain boundary at Dxz4. Our Firre deletion lines allowed us to use Hi-C to test whether loss of the other anchor in the Dxz4-Firre superloop perturbed megadomains. To exclude possible trans-effects relating to Firre RNA48 expressed from the Xa and to focus on the role of Firre locus as a superloop anchor, we conducted all analysis in our Xi-specific Firre deletion. We performed Hi-C on day 10 of differentiation in wild-type, FirreXi∆/+ and Dxz4∆/∆:FirreXi∆/+ cells. Despite disruption to the Dxz4-Firre superloop, FirreXi∆/+ cells retained the sharp megadomain boundary on the Xi. Plotting PC1 scores for all bins on the Xi showed a sharp transition in PC1 score at Dxz4 in FirreXi∆/+ similar to that in WT cells (Fig. 6c). Thus, Firre is not required for maintaining the megadomain boundary. However, in two biological replicates, the intra-megadomain interactions in FirreXi∆/+ cells appeared attenuated compared to wild-type (Fig. 6a, b; Supplementary Fig. 8). Consistent with this idea, PC1 scores for the Xi showed high similarity between WT and FirreXi∆/+ cells at the Dxz4 border itself, but differed across the rest of the Xi (Fig. 6c). Thus, Firre may influence the strength of interactions within each megadomain, but is not strictly required for formation of the Dxz4 border and the bipartite mega-structures.
Fig. 6.
The effects of deleting Firre on megadomains. a Contact maps for the Xi at 2.5 Mb resolution for wild-type (left), FirreXi∆/+ (center left), Dxz4∆/∆ (center right; duplicated from Fig. 3) and Dxz4∆/∆:FirreXi∆/+ (right) cells on d10 of differentiation. b Pearson correlation matrices at 1 Mb resolution for wild-type (left), FirreXi∆/+ (center left), Dxz4∆/∆ (center right; duplicated from Fig. 3) and Dxz4∆/∆:FirreXi∆/+ (right). c 1st principal component of the 1 Mb correlation matrix plotted for wild-type (black), Dxz4∆/∆ (red; duplicated from Fig. 3), FirreXi∆/+ (blue) and Dxz4∆/∆:FirreXi∆/+ (purple) across all bins on the Xi. The dotted line corresponds to the bin containing Dxz4. All data in this figure are generated from merging together reads from two biological replicates. Note: The Dxz4∆/∆ contact maps and correlation maps in a and b are the same as in Fig. 3b, and the Dxz4∆/∆ PC1 curve in c is the same as in Fig. 3c. The Dxz4∆/∆ data is included again in this figure to enable comparison across all four genotypes
Given the phenotypes of the Dxz4 and Firre single deletions, we predicted that the double deletion would obliterate all trace of megadomains and their border. Indeed, the Dxz4∆/∆:FirreXi∆/+ cells showed both a loss of the sharp border at Dxz4 and a depletion of intra-megadomain interactions (Fig. 6a–c). Based on the Pearson correlation heatmap and PC1 analysis, the double deletion seemed to reduce the intra-megadomain interactions on either side of Dxz4 more than in the Dxz4∆/∆ single deletion, but the sharp border at Dxz4 was lost in both cases (Fig. 6, Supplementary Fig. 8). Our data suggest that Firre is not required for formation of megadomain border, but may strengthen intra-megadomain interactions. Conversely, Dxz4 is required for formation of the megadomain border but is not absolutely required for intra-megadomain interactions. We conclude that Firre and Dxz4 work together to strengthen the overall bipartitle structure by regulating intra-domain interactions and the megadomain boundary.
Megadomains and superloops are uncoupled from XCI and escape
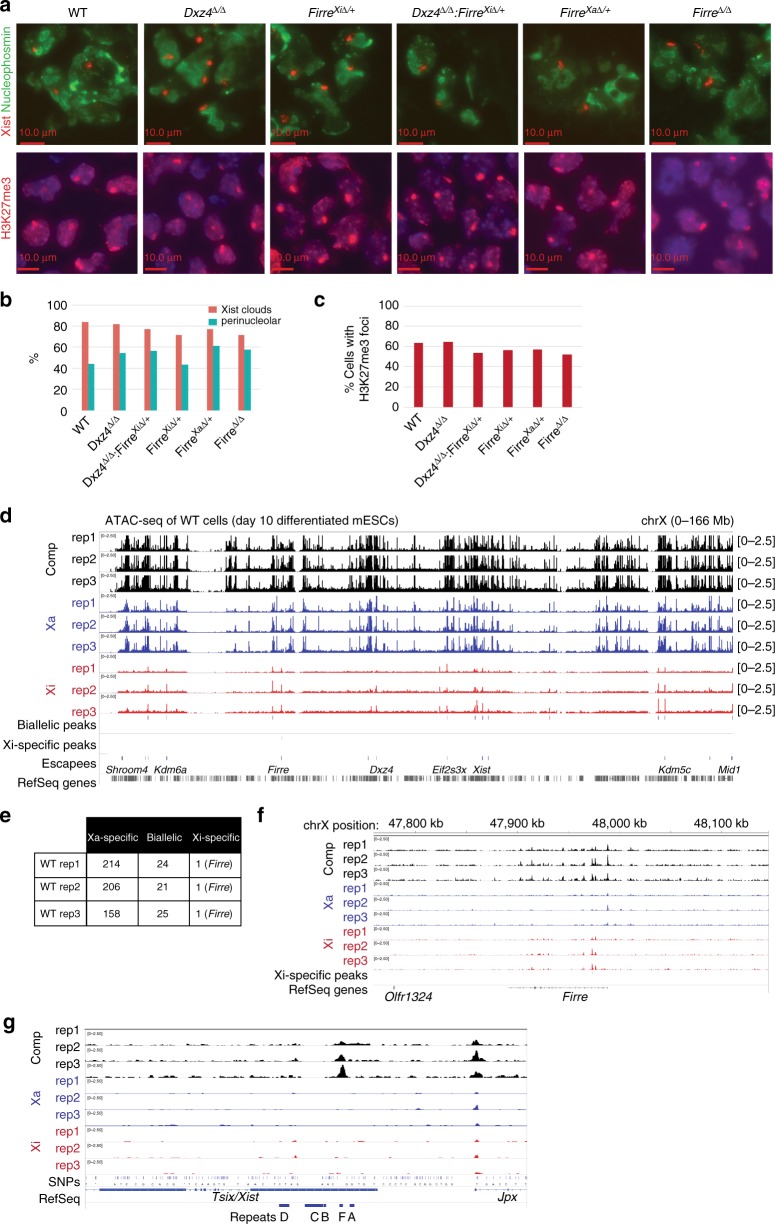
Recent studies have diverged on the effect of deleting the Dxz4 region on XCI, as one study suggested a loss of escape from XCI in mouse NPCs22 and another suggested a partial loss of H3K27me3 on the human fibroblast Xi25. A prior report also suggested that knockdown of Firre RNA disrupts localization of the Xi to the nucleolus and maintenance of H3K27me3 on the Xi32. Here we assessed the effect of deleting Dxz4, Firre, or both on various aspects of XCI. First, we examined effects on the Xist RNA cloud that normally forms over the Xi, but observed no obvious changes in Xist cloud morphology or cloud frequency on day 10 of differentiation in Dxz4∆/∆, FirreXi∆/+, FirreXa∆/+, Dxz4∆/∆:FirreXi∆/+ or Firre∆/∆ (Fig. 7a, b, Supplementary Fig. 9a,b) versus wildtype female cells. There was also no effect on the localization of Xist RNA/Xi to the perinucleolar region49 (Fig.7a, b, Supplementary Fig. 9a, b). We also observed no difference in the enrichment of the H3K27me3 repressive mark on the Xi after 7 days of differentiation in any of the deletions (Fig. 7a, c) or after 10 days in the Dxz4 and Xi-specific Firre deletions (Supplementary Fig. 9a, c). This suggests that neither Firre nor Dxz4 is required for Xist to be expressed, localized, and deposit H3K27me3. To test whether there is a partial loss of H3K27me3 across a macroscopic region of the Xi, as observed in a human DXZ4 deletion25, we produced metaphase spreads in WT and Dxz4∆/∆ cells and performed H3K27ac and H3K27me3 immunofluoresence to visualize the Xi. The Xi stood out as the chromosome with almost no H3K27ac signal and very strong H3K27me3 signal50 (Supplementary Fig. 9d). However, we observed no obvious difference between the H3K27me3 banding pattern on the WT or Dxz4∆/∆ Xi, suggesting no loss of H3K27me3 across a large region of the mouse Xi (Supplementary Fig. 9e).
Fig. 7.
Dxz4 and Firre do not affect Xi localization and accessibility. a Xist RNA FISH (red) combined with nucleophosmin immunofluorescence (green) (top) and H3K27me3 IF (bottom) in wild-type, Dxz4∆/∆ FirreXi∆/+,Dxz4∆/∆:FirreXi∆/+, FirreXa∆/+ and Firre∆/∆ cells. Scale bars: 10 μm. b Fraction of cells with Xist clouds (red) and fraction of Xist clouds in the perinucleolar space (cyan). c Fraction of cells with an H3K27me3 focus. d ATAC-seq coverage across the X (all unique reads, black), Xa (blue) and Xi (red) in wild-type cells, 3 biological replicates. e Allelic status of ATAC peaks in wild-type cells. f ATAC-seq coverage in WT over Firre. g ATAC-seq coverage across Xist. Xist also demonstrates an ATAC peak, consistent with its expression in female cells. The lack of SNPs precluded allele-specific peak-calling
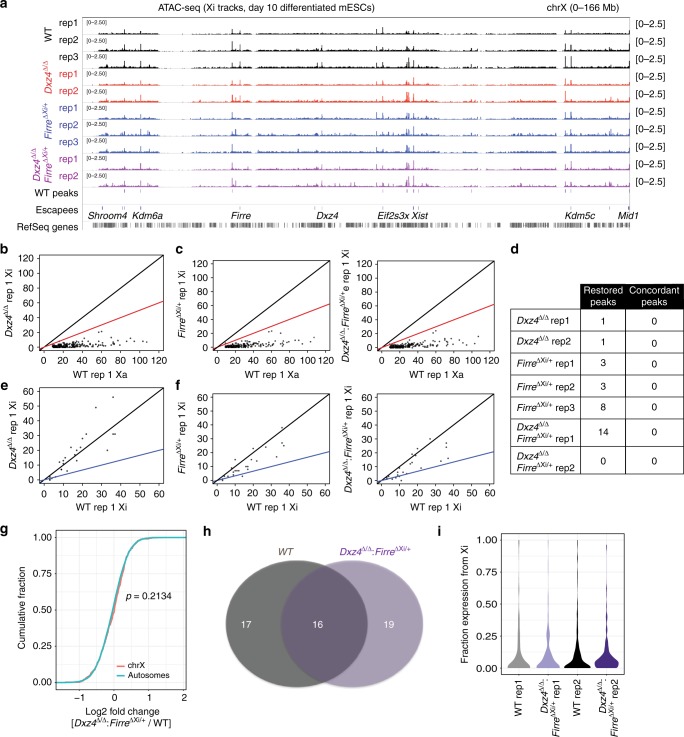
We next used ATAC-seq51 to assay chromatin accessibility on the Xi. In wild-type cells, ATAC signal was heavily skewed towards the Xa, with >85% of all peaks on the X binding specifically to the Xa (Fig. 7d). There were ~20 biallelic sites (e.g., promoters of escapee genes) and only one Xi-specific set of peaks (Fig. 7e, f). Interestingly, the Xi-specific peaks were located in Firre (Fig. 7e, f). While peaks of accessibility were not observed in the allele-specific tracks within Xist, the composite tracks showed a robust peak within Repeat F of Xist (Fig. 7g). The absence of SNPs within this region precluded assignment of the peak to either allele, but it is likely that accessibility comes from the Xi given that Xist is expressed from the Xi. If Dxz4 impairs X-chromosome accessibility as previously proposed for escapee genes22, we would expect biallelic peaks in wild-type to become Xa-specific in the deletion. On the other hand, if Dxz4 or Firre were required to inhibit chromatin accessibility, we would expect many ATAC peaks to appear on the Xi near genes subject to XCI. The overall ATAC-seq patterns were highly similar between wild-type and all mutant genotypes—Dxz4∆/∆, FirreXi∆/+, and Dxz4∆/∆:FirreXi∆/+ (Fig. 8a). We did not observe any “restored” sites on the mutant Xi, when plotting mutant Xi read counts vs. wild-type Xa read counts for peaks that reached at least one half of the wild-type Xa read count (Fig. 8b–d, Supplementary Fig. 10a). We also compared the Xi read counts for biallelic peaks and observed no changes in Dxz4∆/∆ cells (Fig. 8e), FirreXi∆/+ cells, and Dxz4∆/∆: FirreXi∆/+ cells (Fig. 8f, Supplementary Fig. 10b). Thus, we found no decrease in chromatin accessibility at escapee genes, in contrast to a previous study22. Altogether, our results demonstrate that deletion of either Dxz4, Firre, or both has no impact on chromatin accessibility on the Xi.
Fig. 8.
Accessibility and gene silencing on the Xi in the Dxz4 and Firre deletions. a Comparison of Xi ATAC-seq coverage, WT (black), Dxz4∆/∆, FirreXi∆/+ (blue) and Dxz4∆/∆:FirreXi∆/+ (purple). b Comparison between WT Xa and Dxz4∆/∆ Xi ATAC coverage for peaks that are Xa-specific in wild-type. The black line corresponds to a Dxz4∆/∆ Xi:WT Xa ratio of 1:1, the red line corresponds to a ratio of 1:2. c Comparison between WT Xa and FirreXi∆/+ (left) or Dxz4∆/∆:FirreXi∆/+ (right) Xi ATAC coverage for peaks that are Xa-specific in wild-type. The black line correspond to a Dxz4∆/∆ Xi:WT Xa ratio of 1:1, the red lines correspond to a ratio of 1:2. d Number of restored ATAC peaks (Xa-skewed in wild-type but biallelic in deletion) for all deletions. Concordant peaks are peaks reproducibly restored across replicates. e Comparison between WT Xi and Dxz4∆/∆ Xi ATAC coverage for peaks that are Xi-specific in wild-type. The black line corresponds to a Dxz4∆/∆ Xi:WT Xi ratio of 1:1, the blue line corresponds to a ratio of 1:2. f Comparison between WT Xi and FirreXi∆/+ (left) or Dxz4∆/∆:FirreXi∆/+ (right) Xi ATAC coverage for peaks that are Xi-specific in wild-type. The black lines correspond to a Dxz4∆/∆ Xi:WT Xi ratio of 1:1, the blue lines correspond to a ratio of 1:2. g CDF of the fold change in gene expression between WT and Dxz4∆/∆:FirreXi∆/+ for autosomal genes (teal) and X-linked genes with fpm > 1 in all experiments. The Kolmogorov–Smirnov p-value is indicated. h Overlap between escapees in WT and Dxz4∆/∆:FirreXi∆/+. i Density plots of the number of genes with a given level of expression from the Xi in two wild-type and two Dxz4∆/∆:FirreXi∆/+ replicate RNA-seq experiments. Grey and black: wild-type, purple: Dxz4∆/∆:FirreXi∆/+
Finally, we asked whether deleting both Dxz4 and Firre disrupts the pattern of gene silencing or escape on the Xi. We performed allele-specific RNA-seq in wild-type TsixTST/ + and Dxz4∆/∆:FirreXi∆/+ and looked for changes on either a genome-wide or Xi-scale in the mutant relative to wildtype. Surprisingly, no significant differences were detected on a global or Xi-wide scale (Fig. 8g–i), in contrast to previous deletions of Dxz422. Cumulative frequency plots showed balanced X-to-autosomal gene dosages when comparing mutant to wildtype cells (Fig. 8g). The overall number of genes escaping XCI was similar in wild-type and Dxz4∆/∆:FirreXi∆/+, with about half of the escapees being shared between them (Fig. 8h). Examination of allelic contributions to overall X-chromosomal expression showed a predominance of Xa expression in both wild-type and Dxz4∆/∆:FirreXi∆/+, with the pattern being highly similar in two biological replicates (Fig. 8i; p > 0.3 for all pairwise comparisons, Wilcoxon signed-rank test with Bonferroni correction). We conclude that neither Firre nor Dxz4 significantly perturbs Xi silencing and escape. Thus, the unique superloops of the Xi can be uncoupled from XCI biology.
Discussion
An outstanding question in nuclear organization is how higher-order chromatin structure regulates gene expression. Here, using the Xi as a model, we have tested the relevance of two higher order structures—superloops and megadomains—for the biology of mammalian dosage compensation. We find that Dxz4 is required to form the sharp megadomain border and Firre is required for full strength of intra-megadomain interactions. However, abolishing these structures had no impact whatsoever on a range of XCI phenotypes, including (i) chromosome-wide silencing, (ii) escape (iii) subnuclear localization of the Xi, (iv) enrichment of H3K27me3 mark, and (v) chromatin accessibility. By analyzing the timecourse of megadomain formation, we determined that these structures do not obviously precede Xist spreading or XCI. Additionally, our data suggest that loss of insulation and formation of the megadomains do not occur early in XCI, but are observable only concurrently with or after XCI. Irrespectively, Dxz4 and Firre may work together to strengthen megadomains through superloop formation: Deleting Firre weakens intra-megadomain interactions without affecting the strong Dxz4 border, deleting Dxz4 abolishes the sharp megadomain border, and deleting both loci has a more severe effect on the overall bipartite structure than deleting either singly. The significance of attenuated megadomains is unclear, however, given that there was no perturbation to XCI when either Dxz4 or Firre or both were deleted. Our data argue that the superstructures are not necessary for XCI biology, at least in the ex vivo cellular context.
Our findings therefore beg a number of interesting questions. First, what is the purpose of Dxz4, Firre, megadomains, and superloops, and why are they conserved across 80 million years of mammalian radiation? While our observation that Dxz4 is required for megadomains is in agreement with three other studies22,25,29, our results are at odds with the previous proposal that Dxz4 enables genes to escape XCI22. Our study also finds no loss of accessibility on ~35 escapee genes when Dxz4 is deleted on the Xi. The different conclusions may result from differences between our Dxz4∆100 deletion and those of Giorgetti et al.22, use of different cell types (mESC versus NPCs), or clonal variation. Notably, the previous study observed the effects only in one of four NPC clones and the clone showed an unusually high number of escapees—~100 escapees22, a number 2–4 times greater than reported by any other study47,52,53. If this NPC clone were an oddity, comparing it to a Dxz4-deleted cell line of a different NPC background would lead to the impression that the unusual escapees (which ordinarily do not escape XCI) had become silenced.
Our results are in line with other deletions of Dxz4, all of which failed to find an effect on gene silencing22,29. Whatever function Dxz4 might serve, our study indicates that it is necessary but not sufficient for megadomain formation in post-XCI cells, even when Xist is present ectopically together with Dxz4. Because we could not derive the transgenic line in a female ES cell background, we do not formally know whether Dxz4 and Xist together might be sufficient during de novo XCI in an ectopic context. However, given that Dxz4 has no impact on XCI and escape in any measurable way, the sufficiency during the XCI establishment phase seems moot.
Firre was also of interest. What is its function and why do superloops form on the Xi via this CTCF-enriched repeat? Although we failed to find an XCI-related function for Firre after ablating it on the Xi, we found that Firre, Dxz4, Xist, and X75 form a network of superloops on the mammalian Xi, in agreement with a previous study25. Yet, superloops are dispensable for establishing XCI, since both superloop anchors Dxz4 and Firre can be deleted with no impact on XCI establishment. Perhaps Firre RNA has a role in X-inactivation, as suggested from Firre knockdown experiments implicating Firre RNA in maintaining perinucleolar localization of the of the Xi and enrichment of H3K27me352. We leveraged new Xa- and Xi-specific Firre deletions to test which allele expresses Firre RNA and find distinct isoforms associated with the Xa versus Xi. Transcription from the Xa may predominate, though we cannot be certain without knowing all potential isoforms. Deleting Firre on either Xi or Xa does not perturb Xi localization, Xist expression or H3K27me3 deposition on the Xi. Thus, neither the Firre superloop anchor nor any transcript produced by Firre is needed for XCI.
Could megadomains and superloops be default consequences of Xist-mediated attenuation of TADs and compartments? It is important to note that our study follows XCI only in the ex vivo cellular context. It is possible that Dxz4, Firre, megadomains, and superloops have an important role in long-term maintenance of the Xi and that this role would only be revealed by following mice over their lifespan. It is also possible that these Xi megadomains and superloops are incidental structures with no primary impact on gene regulation. While these structures do not disrupt XCI, other macrostructures do. In particular, the recently identified S1/S2 compartments that are revealed by loss of SMCHD1 function have an essential role during de novo Xi silencing24. Why the Xi would be folded in these ways with or without function is unclear. For Dxz4 and Firre superloops, a role in a non-XCI pathway—critical in a whole-organism context and not measurable by our present assays—must also be entertained. Irrespective of function, the megadomains and superloops represent the largest architectural structures identified by Hi-C to date, and both are clearly unique to the Xi. Their evolutionary conservation across 80 million years suggests that the superstructures likely persist for reasons that will become clear with further study.
Methods
Cell lines and growth conditions
The following cell lines were used in these experiments: TsixTST/+ is a female mESC line, derived in the Lee lab and described in Ogawa et al.34 The male XY rtTA fibroblast line was derived in the Lee lab and described in Jeon et al.54 All additional cell lines used are derivatives of one of these cell lines generated in the Lee Lab.
ES cells were grown in regular ES + LIF medium (500 ml DMEM with the addition of 1 ml of β-mercaptoethanol, 6 ml of MEM NEAA, 25 ml of 7.5% NaHCO3, 6 ml of GlutaMAX-1, 15 ml of 1 M HEPES, 90 ml of FBS, 300 μl of LIF, 6 ml of PEN/STREP) on irradiated feeders. To differentiate mESCs and allow them to undergo X-inactivation, mESCs were harvested by trypsinization and quenched in ES medium without LIF. Feeders were removed by adding the cell suspension to tissue culture plates for 45 min at 37 °C. Differentiating embryoid bodies were cultured for 4 days on low-adherence plates in ES medium without LIF. On the 4th day, the embryoid bodies were plated onto gelatinized tissue culture plates and allowed to attach. ES cells for experiments were harvested by extensive trypsinization to detach them from the plates. Unless otherwise noted, all experiments were performed after 10 days of differentiation.
Fibroblasts were grown on un-gelatinized tissue culture plates in MEF media (500 ml DMEM with the addition of 1 ml of β-mercaptoethanol, 6 ml of MEM NEAA, 25 ml of 7.5% NaHCO3, 6 ml of GlutaMAX-1, 15 ml of 1 M HEPES, 60 ml of FBS, 300 μl of LIF, 6 ml of PEN/STREP).
Generation of Dxz4 and Firre deletion cell lines
Oligos encoding gRNAs flanking either Dxz4 or Firre were cloned into wild-type Cas9 + GFP plasmid PX45855 by first linearizing the plasmid with BbsI, purifying the plasmid using the Qiagen PCR Purification Kit, then ligating annealed and phosphorylated oligos into the plasmid using T4 DNA ligase for 1 h at room temperature, then transforming into OneShot Top10 chemically competent E. coli. gRNA plasmid DNA was prepared using the Qiagen Miniprep Kit and gRNA sequences were verified by Sanger Sequencing. To delete Dxz4 or Firre, pairs of gRNAs flanking either Dxz4 or Firre were transfected into mESCs using Lipofectamine LTX. guideRNA sequences are listed in Supplementary Table 1. Briefly, for each transfection, 50 μl of OptiMEM media was added to 2.5 μl LTX reagent and 50 μl OptiMEM was added to 0.5 μl PLUS reagent. The OptiMEM + PLUS mix was added to a mixture of 250 ng of each gRNA, then the OptiMEM + LTX mix was added and incubated at room temperature for 5 min to generate the transfection mixture. Meanwhile, 2 × 105 mESCs were harvested by trypsinization and brought to a volume of 900 μl ES + LIF media. Once the transfection mixture was ready, the mESCs were layered dropwise on top of it and allowed to incubate for 20 min at room temperature. Following incubation, the entire transfection mixture was added to one well of a 12-well dish containing feeders and 1 ml ES + LIF media. The transfected cells were allowed to grow for 16–48 h.
To screen for Cas9-transfected cells, the transfected cells were harvested with trypsin, washed 2× in PBS and re-suspended in 300 μl FACS media (1X Leibowitz’s + 5% FBS) and passed through a cell strainer. The GFP-positive cells were isolated by FACS selection and plated on 10 cm feeder plates (~2000–10,000 GFP + cells/plate). The FACS-sorted GFP positive cells were allowed to grow into large colonies, typically after about 6–8 days of growth. 192 colonies were manually picked and transferred to 96-well plates covered in feeders. Once the 96-well plates were nearly confluent, they were passaged onto 3 new gelatinized plates (no feeders). Freezing media (MEF media + final concentration 10% DMSO) was added to two plates and they were left at −80 °C for storage. The third plate was grown until most wells were fully confluent.
We used a PCR screen to identify Dxz4 or Firre deletion clones. Genomic DNA was prepared from the colonies by incubating them overnight in Laird buffer + proteinase K (50 μl buffer per well) at 55 °C. The genomic DNA was transferred to a new 96-well plate and diluted it 1:10 in H2O, then heated at 95 °C for 10 min to denature it and inactivate the proteinase K. Next, PCR reactions using primers flanking Dxz4 or Firre were prepared in 96-well plates using 20 μl PCR mix + 2 μl denature genomic DNA. 40 cycles of amplication were used, and the PCR reactions were run on 2% agarose gels and visualized by ethidium bromide staining. Deletion clones were identified by PCR reactions that produced a band at the expected size. Deletion clones were thawed onto 12-well plates with feeders, and deletions were verified by Sanger sequencing the PCR product, performing DNA FISH using a fosmid probe entirely within the deleted region, and examining reads over the deleted regions from our genomics experiments.
To generate Xa-specific and homozygous Firre deletions, we employed a restriction assay to determine whether clones carried a deletion on the Xa or Xi (or both). We took advantage of a cas- (Xa-)-specific polymorphism that creates a new TaqI restriction site within the Firre deletion PCR product to screen clones for deletions on particular alleles. We performed PCR amplification as before, but then added 30 μl of 1X Cutsmart buffer + 10 U TaqI (NEB) to each PCR reaction, then incubated the reactions at 65 °C for 45 min before running the reactions on a 2% agarose gel.
Preparation of high molecular weight DNA
Briefly, 500 ml cultures of E. coli containing either Xist + P or RP23-161K4 were grown and spun at 4000 rpm for 15 min. Alkaline lysis was performed by re-supsending in 20 ml Buffer 1, aliquoting the cell suspension into two Oak Ridge polypropylene centrifuge tubes, adding 10 ml of Buffer 2 to each tube and inverting 20 times to mix, then adding 12 ml buffer 3 and inverting 20 times and incubating on ice for 5–10 min. Protein and genomic contaminants were removed by centrifugation at 10,000 rpm in a JA-20 rotor at 4 °C. DNA was precipitated by adding 35 ml isopropanol to 15 ml centrifuged lysate in a 50 ml Falcon tube, incubating 20 min at room temperature, then spinning at 3500 rcf for 20 min at 4 °C. Pellets were resuspended in 500 μl TE + 1%SDS and 15 μl 20 mg/ml Proteinase K was added and the DNA mixture was incubated at 55 °C for 1.5 h to remove protein contaminants. The DNA was phenol:choloroform extracted by adding phenol:cholorform:isoamyl alchohol and shaking by hand for 20 seconds, then the DNA was precipitated with 40 μl 3 M NaOAc and 1 ml isopropanol per 500 μl DNA mixture for 10 min at −20 °C. At this time, the precipitated DNA formed a stringy white mass, the excess liquid was removed from this mass and 1 ml 70% ethanol was added to the DNA. The DNA was centrifuged for 5 min at 16,300×g, the supernatant removed and 1 ml 70% ethanol was added to the pellet and the pellet was spun again for 5 min at 16,300×g. Supernatant was removed and excess ethanol was allowed to evaporate for 5 min, then the DNA pellet was resuspended in 200 μl 10 mM Tris by gentle pipetting with a cut tip.
Generation of a Xist + Dxz4 transgene
To generate an autosomal Xist + Dxz4 transgene, we co-transfected a doxycycline-inducible Xist construct and a BAC containing mouse Dxz4 into male fibroblasts containing rtTA54. First, we prepared DNA from our “Xist + P” construct and the Dxz4-containing BAC RP23-161K4 using a custom high-molecular weight purification protocol. DNA for transfection was only used if it gave the expected digest pattern with either XhoI + BamHI for Xist + P of XhoI for RP23-161K4 and was not excessively smeared. To co-transfect Xist + P and RP23-161K4 into fibroblasts, 2 × 106 fibroblasts were harvested, washed twice in 1× PBS and resuspended in 700 μl 1× PBS. We then added 5 μg Xist + P and 20 ug RP23-161K4 to the cell suspension and electroporated in a 1 mm cuvette at 200 V, 1050 μF using a Bio-RAD Xcell GenePulser electroporation system. Electroporated cells were plated onto three 10 cm dishes in MEF media made with tet-free FBS and grown for one day. The Xist + P construct contains a hygromycin selectable marker, and to select for Xist transgenes, starting one day after transfection, we added 200 μg/ml hygromycin to the media and changed the media every day for 10 days. Once colonies were grown, we manually picked them and transferred them to 96-well plate. We only obtained about 10 hygromycin resistant colonies. Once confluent, we split colonies onto 3 wells of a 24-well plate. One well was kept for maintenance, the other two were used for screening.
To screen for transgenic lines with both inducible Xist and Dxz4 inserted at the same ectopic site, we used the following strategy. We induced each clone with 1000 μg/ml doxycycline overnight and performed Xist RNA FISH to test whether Xist could be induced. We also performed Xist RNA FISH in the same clones without dox induction to ensure Xist expression is inducible. We kept clones that could induce robust Xist RNA FISH clouds. We then used DNA FISH to check whether the Xist + P construct inserted at the same site as the Dxz4-containing BAC. We simultaneously performed DNA FISH using an Xist probe, a probe within the Xist construct backbone, and a fosmid probe against Dxz4. We obtained one clone where all 3 probes co-localize at one spot, indicating co-insertion of Xist and Dxz4 into an autosome. We then used 4 C to localize the candidate insertion site into Stc1 on chr14. We then performed DNA FISH using a fosmid probe overlapping Stc1 combined with a Dxz4 fosmid and a probe overlapping the backbone of the Xist transgenic construct to confirm co-localization of Xist, Dxz4, and Stc1 at one spot.
DNA FISH
BAC or fosmid DNA was prepared using the high molecular weight DNA preparation procedure. Probes were labeled using the Roche Nick Translation kit. 75,000–150,000 cells were cytospun onto slides for 5 min at 1000 rpm. Cells were pre-extracted and fixed by passing the slides through CSK-T for 3 min at 4 °C, CSK for 3 min at 4 °C, 1× PBS + 4% formaldehyde for 10 min at room temperature. RNA was removed by digestion with 0.1 mg/ml RnaseA in 1× PBS for 1 h at 37 degrees. Slides were dehydrated by passage through 70%, 90%, 100% ethanol for 2 min at each concentration, then allowed to dry. Probe was added to hybridization mix (50% formamide, 2× SSC, 10% dextran sulfate, 0.1 mg/ml mouse Cot-1 DNA) and added directly to the slides. Slides were denatured at 92 °C for 10 min on a PCR block, then incubated in a humid chamber at 37 °C overnight. Slides were washed once in 2× SSC, once in 2× SSC + Hoechst 33342 and once in 2× SSC. Mounting media was added and the slides were imaged. The constructs used as FISH probes and their corresponding mm9 coordinates are listed in Supplementary Table 2.
RNA FISH
Slides were prepared for RNA FISH using the same protocol as for DNA FISH but with the RnaseA treatment omitted. Xist RNA FISH was performed using a mixture of Cy3-labeled DNA oligos covering Repeats A, B, and C within Xist. The RNA FISH protocol was the same as the DNA FISH protocol, except that the denaturing step was omitted and the hybridization buffer + probe mixture was heated at 92 °C for 5 min then 37 °C for 5 min and then added directly to the slides. Slides were incubated at 42 °C for 4–8 h and then were washed once in 2× SSC, once in 2× SSC + Hoechst 33342 and once in 2× SSC. Mounting media was added and the slides were imaged.
Immunofluoresence
Overall, 75,000–150,000 cells were cytospun onto slides for 5 min at 1000 rpm. Slides were washed once with 1X PBS, then 1× PBS + 4% formaldehyde was added for 10 min at room temperature, then 1× PBS + 0.5% Triton-X 100 for 10 min at room temperature to remove un-crosslinked proteins. Slides were washed once in 1× PBS, excess buffer was removed from cell spots and 1% BSA in 1× PBS was added for 45 min. Block solution was removed and a 1:200 dilution of H3K27me3 antibody (Active Motif 39155) in 1× PBS + 1% BSA was added for 1 h. Slides were washed 3× in 1× PBS + 0.02% Tween-20. Excess liquid was removed and a 1:2000 dilution of goat-Anti-Rabbit Alexa 555 conjugated antibody (ThermoFisher) was added for 1 h in the dark. Slides were washed once in 1× PBS + 0.02% Tween-20, then twice in 1× PBS and then imaged.
ImmunoFISH
Slides were prepared the same way as for immunofluorescence; with 0.5 U/μl Protector RNase Inhibitor (Sigma) added to the blocking buffer. To visualize the nucleolus, we used a 1:200 dilution of Nucleophosmin antibody (abcam 10530) in blocking solution as the primary antibody. After immunofluorescence, we post-fixed the slides for 10 min in 4% formaldehyde + PBS, and then Xist RNA FISH was performed starting at the dehydration step.
Metaphase immunofluorescence
We added 50 ng/ml Karyomax to the media of day 10 differentiating embryoid bodies for 4 h to arrest cells in metaphase. We harvested the cells via trypsinization, and trypsin was quenched by addition of media. We spun the cells at 1000 rpm for 5 min, aspirated the media, then washed twice in 1× PBS. Cells were then resuspended to a concentration of 5 × 105 cell/ml in 75 mM KCl, and placed at 37 °C for 10 min for swelling. 1 × 105 cells were then cytospun onto a microscope slide at 1000 rpm for 5 min. The cells were fixed in PFA and immunofluorescence was performed as described for interphase cells. We stained H3K27me3 with a 1:200 dilution of Active Motif 39535 and H3K27ac with a 1:200 dilution of Cell Signaling D5E4.
Hi-C library preparation
We used the in situ Hi-C method of Rao et al.1 to prepare all libraries, using 5–10 million cells. Importantly, we sequenced 20–40 million reads per library. This is a lower sequencing depth than many published Hi-Cs, however since the megadomains are large and prominent feature of the organization of the Xi, this depth is appropriate for detecting the megadomains efficiently and economically. We performed a timecourse of Hi-C experiments at 4 timepoints during differentiation (days 0, 3, 7, and 10). To test whether megadomains form in the absence of Dxz4 or Firre, we differentiated cells for 10 days and performed Hi-C in wild-type, Dxz4∆/∆, FirreXi∆/+ or Dxz4∆/∆:FirreXi∆/+. Finally, to test whether megadomains can form on an autosome with Xist and Dxz4 ectopically inserted, we performed Hi-C in the Xist + Dxz4 transgene line after 2 days of induction with 1000 ng/ml dox, as well as an Xist + P only transgene line after 2 days of induction with 1000 ng/ml dox and the parental male XY rtTA line (no induction).
Hi-C analysis
Hi-C alignment to mm9 was performed according to the method of Minajigi and Froberg et al.20 The allele-specific Hi-C reads were filtered for quality and uniqueness with HOMER. Custom scripts were used to convert HOMER tag directories into the format accecpted by Juicebox; contact maps were generated using the Juicer tools ‘pre’ command. All Hi-C contact maps visualized in this study are KR-normalized contact maps generated by Juicebox.
The first principal component of the Hi-C correlation matrix has been used as a quantitative measure of the presence or absence of megadomains56. We used R to generate the Pearson correlation of 1 Mb KR-normalized allele-specific chrX Hi-C matrices, and we plot the first principal component as a function of position along the X-chromosome. Hi-C matrices with a megadomain exhibit a sharp transition in the first principal component score at the bin containing Dxz4.
Hi-C mixing experiment
We mixed together aligned reads from the day 0 and day 10 Hi-C libraries such that 0%, 10%, 25%, 50%, 75%, and 100% of reads were from the day 10 Hi-C. We the generated HOMER tag directories and normalized contact maps in Juicebox as described for the Hi-C experiments. We plotted PC1 scores across the Xi at 1 Mb resolution, and defined the PC1 slope at Dxz4 as the PC1 score @ bin 73 – PC1 score @ bin 71. We performed the analysis for both replicates. Since PC1 scores varied linearly with the “megadomain-positive” fraction (day 10:total ratio), we performed linear regression to infer the strength of megadomains across the population given PC1 score, relative to day 10.
HYbrid Capture Hi-C (Hi-C^2)
HYbrid Capture Hi-C (Hi-C^2) probes were designed and hybridization to in situ Hi-C libraries carried out as described previously6. Probe sets were designed to enrich interactions in two regions of interest: chrX:70,370,161–71,832,975 and chrX:71,832,976–73,511,687 (mm9). Briefly, 120 bp probes were designed around the MboI restriction sites of the regions of interest as previously described6 and custom synthesized pools of single stranded oligodeoxynucleotides ordered from CustomArray, Inc. (Bothell, WA). Probe sequences are included as supplementary files. Single stranded DNA oligos were amplified and biotinylated in a MAXIScript T7 transcription reaction (Ambion). The resulting biotinylated RNA probes were hybridized to 250–300 ng of in situ Hi-C libraries for 24 h at 65C. DNA hybridized to the RNA probes was pulled down by streptavidin beads (Dynabeads MyOne Streptavidin C1, Life Technologies), washed, and eluted as described6. The resulting DNA was desalted using a 1× SPRI cleanup and amplified with Illumina primers for 18 cycles to prepare for sequencing.
Hi-C^2 libraries were sequenced to a depth of 8–15 million 50 bp paired-end reads. Reads were trimmed using cutadapt with the options --adapter = GATCGATC (MboI ligation junction) and --minimum-length = 20. Reads of each pair were individually mapped to the mus and cas reference genomes using novoalign and merged into Hi-C summary files and filtered using HOMER as previously described20. For the chrX:70,370,161–71,832,975 captures, 3–4% of mapped and paired reads fell within the target region (0.05% expected based on size of capture region versus genome) and for the chrX:71,832,976–73,511,687 captures, 1–2% of mapped and paired reads fell within the target region (0.06% expected based on size of capture region versus genome). To avoid computational complexities arising from normalization of sparse, non-enriched regions in the Hi-C contact map, only Hi-C interactions falling within the capture region were analyzed further. For each capture, a custom script was used to pull out the filtered Hi-C interactions falling within the target region from the HOMER tag directories. Hi-C contact maps of the capture regions were then generated from these HOMER tags using the ‘pre’ command of Juicer tools57. The resulting Hi-C contact maps in.hic format were visualized and normalized with the ‘Coverage (Sqrt)’ option in Juicebox58.
Explanation of insulation score analysis
Insulation scoring has become a standard in the Hi-C field for identifying borders and quantifying boundary strength41–43. In brief, insulation scores quantify how strongly a given locus acts as a border41–43, and are calculated by running sliding windows across a chromosomal region and measuring changes in interaction frequencies between successive windows. We show an explanatory diagram in Fig. S3d. The main idea is that the raw insulation score at a particular locus is the ratio of the number of reads that “cross over” that locus (where one end falls to the left and one end falls to the right of the locus) to the number of reads nearby that do not cross over the locus (reads where both ends fall to the right or where both fall to the left of the locus). Once raw insulation scores are calculated for all loci in a region, the insulation score for a given locus is the log-ratio of the raw insulation score at the given locus divided by the mean insulation score for all loci across the region. At loci in the middle of domains, there are a similar number of reads that cross over the locus to the number of reads right beside the locus but that don’t cross over, thus insulation scores in the middle of domains tend to be near zero or perhaps slightly positive. However, at the borders (“insulators”) of domains, there are few reads that cross over the border relative to the reads to the left or to the right in the two domains separated by the border. This means insulation scores are strongly negative at domain borders, and in fact domain borders are defined as the local minima of insulation scores across a region.
Insulation score analysis with Hi-C^2 data
We computed insulation score across the Mecp2 and Dxz4 regions to quantitatively measure changes in domain organization during the timecourse of X-inactivation. To do this, we output the ‘Coverage (Sqrt)’ normalized Hi-C contact maps at 25 kb resolution across either the Mecp2 or Dxz4 regions using Juicer tools ‘dump’ command. We used custom shell and R scripts to convert the densematrix format output from Juicer into the full matrix format accepted by the cworld suite of Hi-C tools (https://github.com/dekkerlab/cworld-dekker). We computed insulation scores across the captured regions using the cworld perl script ‘matrix2insulation.pl’ using the parameters ‘-v --is 125000 --ids 75000 –im sum’. This set of options uses a smaller number of bins to calculate insulation scores, which we found to be optimal for analyzing insulation over small regions with just a few dozen bins. We plotted the distribution of insulation scores across each region and each timepoint. We evaluated changes in insulation across regions by testing whether there was a difference in the variance of insulation scores between timepoints or between the Xa and the Xi using the F-test. This is appropriate as a loss of insulation by definition is a decrease in the variance of insulation across a region44, which can be visualized as a “flatter” insulation score curve. To generate violin plots and calculate F-test p-values, we excluded the 6 bins on the left and right edges of each Hi-C^2 region because the windows used to calculate insulation score at these loci fall partially outside the region covered by Hi-C^2 probes and have far less read coverage than the regions covered by the probes.
4C library preparation and analysis
We previously developed a modified 4C protocol59 to examine chromatin conformation from repetitive viewpoints. Our protocol has several advantages over existing 4C profiles: (1) It sequences the genomic region amplified by the 4C primers, ensuring that on-target priming events can be identified and filtered from numerous off-target priming events. (2) Sequencing the viewpoint allows every read to be assigned to a particular allele if the viewpoint is near a variant, (3) We use a random barcode to identify PCR duplicates, which previously has not been possible in 4C experiments. We performed our modified 4 C using the protocol and analysis pipeline previously described for viewpoints within PAR-TERRA repeats59. Briefly, we digested crosslinked chromatin with restriction enzyme overnight in 320 μl reactions with 50–100 units of 1st restriction enzme, then performed in situ proximity ligation for 4 h at room temperature by adding 90 μl 10× T4 DNA Ligase Buffer (NEB), adjusting the total volume to 900 μl with H2O and adding 20 μl T4 DNA Ligase (Enzymatics). We then purified nuclei by centrifugation at 2500 rpm for 5 min, removed supernatant and added 400 μl 1× TE + 1% SDS and 400 ug Proteinase K to the nuclei and heated overnight at 55 degrees C to reverse crosslinks. We purified DNA using phenol:chloroform extraction followed by EtOH precipitation. We then digested 5–10 ug DNA with secondary restriction enzyme in standard 50 μl reactions and purified the DNA using the QIAGEN PCR Cleanup Kit. Next, we ligated 4 C adaptors onto the secondary restriction sites for 3 h at room temperature, and purified the DNA with SPRI beads. We performed the adaptor ligation using a ~5× molar excess of 4C adaptors relative to the input DNA, in 50 μl 1× T4 DNA Ligase Buffer (NEB) and 2 μl T4 DNA Ligase (Enymatics). We next performed ligation-mediated PCR using one biotinylated primer specific for the 4 C viewpoint and one primer complementary to the adaptors added in the previous step. We then captured the viewpoint-specific PCR products by capturing the biotinylated PCR products with streptavidin pulldown. We performed PCR in 200 μl reactions using Phusion High-Fidelity Master Mix and a primer concentration of 1.5 uM viewpoint-specific primer and 1.5 uM universal primer, and we ran PCR reactions for 12–15 cycles. We performed a final PCR using standard NebNext Illumina multiplex primers and Phusion High-Fidelity Master Mix for an additional 12–15 cycles and purified the PCR reaction with SPRI cleanup.
We performed 4C using several viewpoints within Firre and Dxz4. Some viewpoints were in the core tandem repeats. For these viewpoints, we use the read outside the viewpoint for allelic determination. Others were in unique regions near the tandem repeats; for these we could use known variants to assign every read to the Xa or the Xi. We performed our analysis in two fibroblast lines, one where the mus X is inactive (mus Xi cas Xa), the other where the cas X is inactive (mus Xa cas Xi). We also used 4 C using a viewpoint within the hygromycin resistance marker included in all Xist transgene constructs to localize the insertion sites of the Xist transgenes. 4C viewpoint sequences, as well as the adaptor sequences for ligation-mediated PCR are included in Supplementary Table 3.
Assay for transposase-accessible chromatin
Overall, 50,000 cells were washed in cold PBS and lysed in cold lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% IGEPAL CA-630) containing proteinase inhibitor cocktail (Roche). Nuclei were resuspended in 1× TD Buffer (Illumina FC-121-1030) and 2.5 μl of Tn5 Transposase (Illumina FC-121-1030) were added. Transposition reaction was performed at 37 °C for 30 min, and DNA was purified using a Qiagen MinElute Kit. DNA libraries were amplified for a total of 8 cycles. Libraries were assessed for quality control on the BioAnalyzer 2100 (Aglient) to ensure nucleosomal phasing and complexity. Sequencing was performed on the HiSeq 2500 (Illumina), using 50 bp paired-end reads.
ATAC-seq analysis
Attack seq alignment to mm9 was performed exactly as ChIP-seq alignment was performed in Minajigi and Froberg et al.20 Peaks were called using macs2 with default parameters. Biallelic peaks were identified as peaks with at least 10 alleleic reads in a sample and an Xi:Xa ratio greater than 1/3. Xi-specific peaks were defined as peaks with at least 10 allelic reads and a Xi:Xa ratio less than 1/3. To test whether Xi-specific peaks in wild-type are “restored” (that is: acquire appreciable accessibility on the Xi) in either the Dxz4 or Firre deletion, we plot the wild-type Xa reads on the x-axis and the deletion Xi reads on the y-axis and identify peaks where the deletion Xi/wild-type Xa ratio is greater than ½ (these are peaks where the deletion accessibility level reaches at least half the wild-type accessibility ratio). We also examine the biallelic peaks and plot the wild-type Xi reads on the x-axis and the deletion Xi reads on the y-axis to determine whether the accessibility on the Xi changes for the peaks that are biallelic in wild-type.
RNA-seq library preparation
Total RNA was isolated from 2–5 million trypsinized cells using trizol extraction. polyA + mRNA was isolated using the NEBNext NEBNext® Poly(A) mRNA Magnetic Isolation Module using 5 μg of total RNA as input. Isolated mRNA was reverse-transcribed using Superscript III and actinomycin D to inhibit template switching. Second-strand synthesis was performed using the NEBNext Ultra Directional RNA Second Strand Synthesis Module. Library preparation and NEBNext® ChIP-Seq Library Prep Master Mix Set for Illumina. A USER enzyme treatment was performed following adaptor ligation to specifically degrade the second strand and allow a stranded analysis. Libraries were amplified for 10–15 cycles of PCR using Q5 polymerase and NEBNext multiplex oligos.
RNA-seq analysis
RNA-seq reads were aligned to the cas (Xa) and mus (Xi) genomes allele-specifically using a previously published pipeline20,35,37,60. Following alignment, gene expression levels for each gene were defined using HOMER. Differential expression and fold changes between conditions were calculated using DESeq2. We plotted the cumulative distributions of fold changes for autosomal and X-linked genes and evaluated the significance of any differences between the distributions of the fold changes using the Kolmogorov-Smirnov (KS) test. To examine allele-specific expression from the Xa and the Xi, we summed together allelic reads across both biological replicates and filtered for genes with at least 12 allelic reads in both wild-type and Dxz4∆/∆:FirreXi∆/+and fpm > 0 in all replicates. We also used RNA-seq done in pure hybrid mus or cas fibroblasts to identify and eliminate genes that have incorrect SNP information. We defined escapee genes in a particular condition as genes where at least 10% of allelic reads came from the Xi in either replicate of that condition. We plotted the distribution of expression levels from the Xi (Xi/(Xi + Xa) read counts) for all genes passing our filtered for each replicate. We evaluated the significance in differences of the mean expression level from the Xi using the Wilcoxon-signed rank test with Bonferroni correction for multiple hypothesis testing.
qRT-PCR
Total RNA was isolated from cells using trizol extraction. 500 ng RNA was heated at 70 degrees C for 10 min then cooled to 4 degrees in the presence of 50 ng random primers in 5 μl total volume. The RNA was reverse-transcribed in a 10 μl reaction containing 1X First Strand buffer, 10 mM DTT, 500 uM dNTPs, 6U Protector RNase inhibitor and 100U Superscript III. The reaction was incubated for 5 min at 25 degrees, then 1 h at 50 degrees and 15 min at 85 degrees. Reverse transcription reactions were diluted to 100 μl with water before qPCR. 500 ng RNA was added to 100 μl water as a -RT control. 1 μl template was used per 15 μl qPCR reaction prepared with 1× Taq UniverSYBR Green (BioRad) master mix and 200 nM primers, and reactions were performed in triplicate. All qPCR primers were run using an annealing temperature of 55 degrees. The primers used for qRT-PCR are listed in Supplementary Table 4.
Code availability
The custom analysis pipelines for all genomic analyses are available upon request with no restrictions
Electronic supplementary material
Acknowledgements
We thank Chen-Yu Wang and Eric Aeby for a careful reading of the manuscript. This work was supported by NSF GRFP grants (J.E.F., A.J.K.), a Herchel Smith Fellowship (A.J.K.), an EMBO postdoctoral fellowship (EMBO ALTF 1313-2015) to T.J., and grants from the Howard Hughes Medical Institute and National Institutes of Health (R01-GM090278 and R37-GM58839) to J.T.L.
Author contributions
J.E.F. and J.T.L. designed research, analyzed data, and wrote the manuscript. J.E.F. deleted Dxz4 and Firre, performed all Hi-C, RNA-seq microscopy experiments and all bioinformatics except for 4C analysis. S.F.P. designed, performed, and analyzed 4C experiments. A.J.K. performed the Hi-C^2 experiments. T.J. performed ATAC-seq experiments.
Data availability
All sequencing data have been deposited in the GEO accession GSE116649 All other relevant data supporting the key findings of this study are available within the article and its Supplementary Information files or from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07446-w.
References
- 1.Rao SS, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon J, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao S, et al. Cohesin loss eliminates all loop domains. Cell. 2017;171:305. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzer W, et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature. 2017;551:51–56. doi: 10.1038/551S51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuin J, et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc. Natl Acad. Sci. USA. 2014;111:996–1001. doi: 10.1073/pnas.1317788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanborn AL, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl Acad. Sci. USA. 2015;112:E6456–E6465. doi: 10.1073/pnas.1518552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Ya, Xu Quan, Canzio Daniele, Shou Jia, Li Jinhuan, Gorkin David U., Jung Inkyung, Wu Haiyang, Zhai Yanan, Tang Yuanxiao, Lu Yichao, Wu Yonghu, Jia Zhilian, Li Wei, Zhang Michael Q., Ren Bing, Krainer Adrian R., Maniatis Tom, Wu Qiang. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell. 2015;162(4):900–910. doi: 10.1016/j.cell.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols MH, Corces VG. A CTCF code for 3D genome architecture. Cell. 2015;162:703–705. doi: 10.1016/j.cell.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman-Aiden E, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nora EP, et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell. 2017;169:930–944. doi: 10.1016/j.cell.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowley MJ, et al. Evolutionarily conserved principles predict 3D chromatin organization. Mol. Cell. 2017;67:837–852. doi: 10.1016/j.molcel.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitan V, et al. Cohesin-based chromatin interactions enable regulated gene expression within preexisting architectural compartments. Genome Res. 2013;23:2066–2077. doi: 10.1101/gr.161620.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jegu T, Aeby E, Lee JT. The X chromosome in space. Nat. Rev. Genet. 2017;18:377–389. doi: 10.1038/nrg.2017.17. [DOI] [PubMed] [Google Scholar]
- 14.Pandya-Jones A, Plath K. The “lnc” between 3D chromatin structure and X chromosome inactivation. Semin. Cell. Dev. Biol. 2016;56:35–47. doi: 10.1016/j.semcdb.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disteche CM. Dosage compensation of the sex chromosomes. Annu. Rev. Genet. 2012;46:537–560. doi: 10.1146/annurev-genet-110711-155454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wutz A. Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat. Rev. Genet. 2011;12:542–553. doi: 10.1038/nrg3035. [DOI] [PubMed] [Google Scholar]
- 17.Lee J. Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control. Nat. Rev. Mol. Cell Biol. 2011;12:815–841. doi: 10.1038/nrm3231. [DOI] [PubMed] [Google Scholar]
- 18.Splinter E, et al. The inactive X chromosome adopts a unique three-dimensional conformation that is dependent on Xist RNA. Genes & Dev. 2011;25:1371–1383. doi: 10.1101/gad.633311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nora E, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minajigi A., Froberg J. E., Wei C., Sunwoo H., Kesner B., Colognori D., Lessing D., Payer B., Boukhali M., Haas W., Lee J. T. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science. 2015;349(6245):aab2276–aab2276. doi: 10.1126/science.aab2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng X, et al. Bipartite structure of the inactive mouse X chromosome. Genome Biol. 2015;16:152. doi: 10.1186/s13059-015-0728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorgetti L, et al. Structural organization of the inactive X chromosome in the mouse. Nature. 2016;535:575–579. doi: 10.1038/nature18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese J, et al. Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell. 2012;151:951–963. doi: 10.1016/j.cell.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CY, Jégu T, Chu HP, Oh HJ, Lee JT. SMCHD1 merges chromosome compartments and assists formation of super-structures on the inactive X. Cell. 2018;174:406–421. doi: 10.1016/j.cell.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darrow E, et al. Deletion of DXZ4 on the human inactive X chromosome alters higher-order genome architecture. Proc. Natl Acad. Sci. USA. 2016;113:E4505–E4512. doi: 10.1073/pnas.1609643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chadwick BP. DXZ4 chromatin adopts an opposing conformation to that of the surrounding chromosome and acquires a novel inactive X-specific role involving CTCF and antisense transcripts. Genome Res. 2008;18:1259–1269. doi: 10.1101/gr.075713.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horakova AH, Moseley SC, McLaughlin CR, Tremblay DC, Chadwick BP. The macrosatellite DXZ4 mediates CTCF-dependent long-range intrachromosomal interactions on the human inactive X chromosome. Hum. Mol. Genet. 2012;21:4367–4377. doi: 10.1093/hmg/dds270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horakova Andrea H, Calabrese J Mauro, McLaughlin Christine R, Tremblay Deanna C, Magnuson Terry, Chadwick Brian P. The mouse DXZ4 homolog retains Ctcf binding and proximity to Pls3 despite substantial organizational differences compared to the primate macrosatellite. Genome Biology. 2012;13(8):R70. doi: 10.1186/gb-2012-13-8-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonora G, et al. Orientation-dependent Dxz4 contacts shape the 3D structure of the inactive X chromosome. Nat. Commun. 2018;9:1445. doi: 10.1038/s41467-018-03694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hacisuleyman E, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat. Struct. Mol. Biol. 2014;21:198–206. doi: 10.1038/nsmb.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacisuleyman E, Shukla CJ, Weiner CL, Rinn JL. Function and evolution of local repeats in the Firre locus. Nat. Commun. 2016;7:11021. doi: 10.1038/ncomms11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang F, et al. The lncRNA Firre anchors the inactive X chromosome to the nucleolus by binding CTCF and maintains H3K27me3 methylation. Genome Biol. 2015;16:52. doi: 10.1186/s13059-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barutcu AR, Maass PG, Lewandowski JP, Weiner CL, Rinn JLA. TAD boundary is preserved upon deletion of the CTCF-rich Firre locus. Nat. Commun. 2018;9:1444. doi: 10.1038/s41467-018-03614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa Y, Sun B, Lee J. Intersection of the RNA interference and X-inactivation pathways. Science. 2008;320:1336–1377. doi: 10.1126/science.1157676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinter S, et al. Spreading of X chromosome inactivation via a hierarchy of defined polycomb stations. Genome Res. 2012;22:1864–1876. doi: 10.1101/gr.133751.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon M, et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature. 2013;504:465–469. doi: 10.1038/nature12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kung JT, et al. Locus-specific targeting to the X chromosome revealed by the RNA interactome of CTCF. Mol. Cell. 2015;57:361–375. doi: 10.1016/j.molcel.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Lu N. Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell. 1999;99:47–57. doi: 10.1016/S0092-8674(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 39.Gayen S, Maclary E, Buttigieg E, Hinten M, Kalantry S. A Primary Role for the Tsix lncRNA in Maintaining Random X-Chromosome Inactivation. Cell Rep. 2015;11:1251–1265. doi: 10.1016/j.celrep.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maclary E, et al. Differentiation-dependent requirement of Tsix long non-coding RNA in imprinted X-chromosome inactivation. Nat. Commun. 2014;5:4209. doi: 10.1038/ncomms5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crane E, et al. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature. 2015;523:240–244. doi: 10.1038/nature14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuguchi T, et al. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature. 2014;516:432–435. doi: 10.1038/nature13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lajoie BR, Dekker J, Kaplan N. The Hitchhiker’s guide to Hi-C analysis: practical guidelines. Methods. 2015;72:65–75. doi: 10.1016/j.ymeth.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ke Y, et al. 3D Chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. Cell. 2017;170:367–381.e20. doi: 10.1016/j.cell.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Apostolou E, et al. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013;12:699–712. doi: 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonis M, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) Nat. Genet. 2006;38:1348–1354. doi: 10.1038/ng1896. [DOI] [PubMed] [Google Scholar]
- 47.Berletch JB, et al. Escape from X inactivation varies in mouse tissues. PLoS Genet. 2015;11:e1005079. doi: 10.1371/journal.pgen.1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergmann Jan H., Li Jingjing, Eckersley-Maslin Mélanie A., Rigo Frank, Freier Susan M., Spector David L. Regulation of the ESC transcriptome by nuclear long noncoding RNAs. Genome Research. 2015;25(9):1336–1346. doi: 10.1101/gr.189027.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang LF, Huynh K, Lee J. Perinucleolar targeting of the inactive X during S phase: evidence for a role in the maintenance of silencing. Cell. 2007;129:693–706. doi: 10.1016/j.cell.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 50.Terrenoire E, et al. Immunostaining of modified histones defines high-level features of the human metaphase epigenome. Genome Biol. 2010;11:R110–R110. doi: 10.1186/gb-2010-11-11-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marks H, et al. Dynamics of gene silencing during X inactivation using allele-specific RNA-seq. Genome Biol. 2015;16:149. doi: 10.1186/s13059-015-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeon Y, Lee J. YY1 tethers Xist RNA to the inactive X nucleation center. Cell. 2011;146:119–152. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonora, G. et al. Orientation-dependent Dxz4 contacts shape the 3D structure of the inactive X chromosome. Nat. Commun. 9, 1445 (2018). [DOI] [PMC free article] [PubMed]
- 57.Durand NC, et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 2016;3:95–98. doi: 10.1016/j.cels.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durand NC, et al. Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst. 2016;3:99–101. doi: 10.1016/j.cels.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu HP, et al. PAR-TERRA directs homologous sex chromosome pairing. Nat. Struct. Mol. Biol. 2017;24:620–631. doi: 10.1038/nsmb.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yildirim E, Sadreyev R, Pinter S, Lee J. X-chromosome hyperactivation in mammals via nonlinear relationships between chromatin states and transcription. Nat. Struct. Mol. Biol. 2012;19:56–61. doi: 10.1038/nsmb.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data have been deposited in the GEO accession GSE116649 All other relevant data supporting the key findings of this study are available within the article and its Supplementary Information files or from the corresponding authors upon request.