With the increasing number of prosthetic joints replaced annually worldwide, orthopedic implant-associated infections (OIAI) present a considerable burden. Accurate diagnostics are required to optimize surgical and antimicrobial therapy.
ABSTRACT
With the increasing number of prosthetic joints replaced annually worldwide, orthopedic implant-associated infections (OIAI) present a considerable burden. Accurate diagnostics are required to optimize surgical and antimicrobial therapy. Sonication fluid cultures have been shown in multiple studies to improve the microbiological yield of OIAIs, but uptake of sonication has not been widespread in many routine clinical microbiology laboratories. In this issue, M. Dudareva and colleagues (J Clin Microbiol 56:e00688-18, 2018, https://doi.org/10.1128/JCM.00688-18) describe their unit’s experience with OIAI diagnosis using periprosthetic tissue inoculated into an automated blood culture system and sonication fluid culture.
TEXT
Globally, the prosthetic joint replacement market is expected to grow from $19,144 million to $31,889 million from 2018 to 2024 (1). Proportionately, the number of prosthetic joint infections (PJI), occurring in approximately 2% of joint replacements, are expected to increase. Despite modern microbiological methods, 5% to 34% of PJIs are culture negative (2). Infections after fracture fixation occur in 1% to 30% (depending on whether fractures are closed or open and the degree of contamination associated with the injury). In one study, up to 43% of fixation implant-associated infections were negative by conventional tissue culture techniques (3).
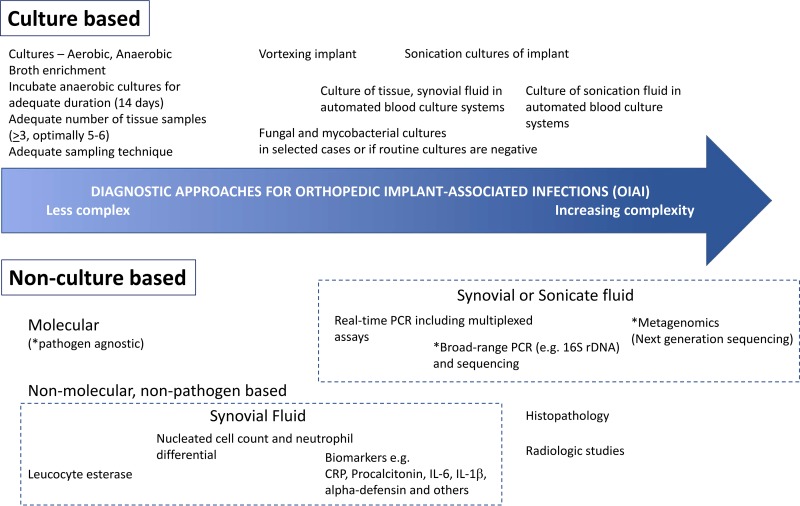
In recent years, a variety of measures and tools have emerged to aid the diagnosis of orthopedic implant-associated infections (OIAI) (Fig. 1). Culture-based techniques remain important, as these facilitate a definitive diagnosis of OIAI and allow phenotypic susceptibility testing, which guides antimicrobial therapy. Because OIAI are associated with biofilm which matures with the chronicity of infection, maneuvers to disrupt bacteria associated with the biofilm, such as sonication, have been found in multiple studies to improve the yield of culture-based diagnostics (4–6). Sonication, although relatively simple, may be labor-intensive (7) and not as accessible to laboratories. In one survey of 262 European orthopedists regarding practices regarding the diagnosis of knee PJI, only 36% utilized sonication (8). Sonication for OIAI generally requires implementation in the local laboratory and is not usually amenable as a “send-out” test to a referral laboratory, given the logistics of transporting a bulky implant and possible degradation of test sensitivity (with delayed processing) and specificity (from contamination with increased handling).
FIG 1.
Tools available for the diagnosis of orthopedic implant-associated infections (OIAIs).
In this issue, Dudareva and colleagues (9) ask the question, are sonication cultures truly superior to an optimized algorithm of tissue sampling and cultures for the diagnosis of OIAI? The authors note that previous studies comparing tissue to sonication cultures have often sampled suboptimal numbers of tissue specimens (<4 to 6), which may have led to diminished sensitivity. Further, most did not utilize automated liquid culture methods which may further increase tissue culture sensitivity (see Table S1 in the supplemental material for reference 9). In a single-center, prospective study performed as a service improvement audit performed over almost 4 years and involving 505 procedures (of which 44% were joint revisions) on 463 patients with suspected OIAI, the authors systemically compared the performance of their optimized tissue culture algorithm with sonication cultures. The tissue culture algorithm comprised of multiple periprosthetic tissue samples (median, 5; interquartile range, 4 to 5) inoculated into Bactec Plus Aerobic/F and Bactec Lytic/10 Anaerobic/F bottles incubated for 10 days or until positivity. Sonication fluid cultures were plated and incubated aerobically (5 days) and anaerobically (10 days), and a cutoff of ≥50 CFU/ml was used for positivity.
The authors found that their tissue culture protocol was superior to sonication by multiple comparisons using various reference standard definitions of infection (clinical, the Infectious Diseases Society of America [IDSA], international consensus, and a composite definition)—regardless of whether OIAI were considered as a whole or in a subgroup analysis of PJI or other orthopedic device-related infections. Using the clinical reference standard for infection (which avoids incorporation bias, as microbiological data from either tissue cultures or sonication are not used as part of the definition of infection), their tissue culture protocol achieved sensitivities of 69% versus 57% for sonication for all devices, 72% versus 62% for PJI, and 64% versus 45% for other OIAI (non-PJI). When tissue culture and sonication were combined, sensitivity (using clinical criteria as the reference standard) increased to 79%. In a subanalysis considering variables which may affect culture yield, for example, time since device implantation, clinical features (e.g., if a sinus or purulence was noted), and recent antibiotic exposure, tissue cultures were still found to be generally superior in sensitivity to sonication cultures. The only subgroup where sonication and tissue culture sensitivity were similar was for OIAI due to less virulent organisms (e.g., coagulase-negative staphylococci and viridans group streptococci), which the authors postulate is in keeping with these organisms predominating in the more indolent OIAI, which are associated with mature biofilm.
While most studies, including the landmark study by Trampuz et al. (5), have found sonication superior to periprosthetic tissue cultures, several studies have found the contrary (10, 11), including the current one (9). Several factors may account for this. First, the threshold for positivity for sonication cultures was higher in the study of Dudareva and colleagues (50 CFU/ml) and others with similar findings (9–11) versus the study by Trampuz et al. (5 CFU/ml, which, compared to a cutoff of 2 CFU/ml, afforded a slightly lower sensitivity but improved specificity) (5). While site-specific differences in sonication technique may influence the cutoffs adopted, the threshold of 5 CFU/ml was also found to have the highest sensitivity (82%) and specificity (99%) in a meta-analysis of 12 studies (6). In the current study, when the threshold for positivity was lowered to 10 CFU/ml, sensitivities were more comparable (77% versus 72%; P = 0.06), although specificity was still significantly higher for the tissue culture group (9). Other studies have also found improved/superior sensitivities for sonication when lower thresholds were used (11).
Second, tissue cultures were inoculated into blood culture bottles, whereas sonication fluid was not in this study. Blood culture bottles may enhance culture yield by neutralizing the effects of antibiotics by dilution and binding to resin, because of culture conditions (shaking/agitation), and also because volume inoculated into bottles are generally larger than that inoculated onto agar plates (12). Several other studies have examined inoculating sonication fluid into blood culture bottles (13–16); however, some of these have not presented the data on the performance of inoculating sonication fluid into blood culture bottles independently from sonication cultures on solid media and other enrichment broths. Two studies which did examined the utility of sonication fluid incubated in blood culture bottles for 5 days; Portillo et al. compared this to periprosthetic tissue cultures (without inoculation into blood culture bottles) (14), and Shen et al. compared this to synovial fluid inoculated into blood culture bottles (13). In the study by Portillo et al., sonication fluid in blood culture bottles achieved a sensitivity of 100%, compared to 87% for conventional sonication cultures and 59% for tissue cultures (number of specimens obtained was unspecified). In the study by Shen et al., sonication fluid in blood culture bottles had a sensitivity of 88%, compared to 64% for synovial fluid in blood culture bottles. To date, no studies have compared head to head the performance of sonicate fluid and tissue cultures inoculated into blood culture bottles cultures.
Third, a higher median number of tissue specimens was obtained in the study by Dudareva et al. than in previous studies. This, in addition to inoculation into blood culture bottles, likely improved the sensitivity of their tissue culture protocol. Using histopathology as a reference standard, older data from Atkins et al. had suggested 5 or 6 specimens to optimize PJI diagnosis (17), while more recently Bémer et al., using the IDSA criteria, found that 4 tissue specimens cultured in pediatric blood culture bottles, chocolate agar, and Schaedler anaerobic broth provided optimal sensitivity for PJI diagnosis (18). In the absence of a reference standard, Peel et al., using Bayesian latent class analysis, found that three periprosthetic tissue specimens inoculated into aerobic and anaerobic blood culture bottles and four tissue specimens using conventional culture techniques offered the greatest accuracy (92% and 91%, respectively) (19).
In a recent study similar to that of Dudareva et al. (9), Yan et al. compared tissue culture in blood culture bottles to sonication fluid cultures and, in contrast to Dudareva et al., found that the two performed similarly (66.4% versus 73.1%; P = 0.07) when using clinical (IDSA) criteria (without microbiological criteria) and also in a separate analysis using Bayesian latent class modeling (86.3% versus 88.7%) (12), where no reference standard is assumed. Besides site-specific differences, diagnostic performance might have varied due to the higher cutoff for sonication culture positivity (50 CFU/ml versus 20 CFU/10 ml, which approximates 2 CFU/ml) and possibly a slightly higher median number of tissue culture samples (5 versus 4) in the study by Dudareva et al. than in the study by Yan et al. (9, 12).
What is to be made of these data in light of what has been previously published? First, multiple tissue samples are required to optimize culture yield, and the available data corroborate IDSA recommendations that at least 3, but optimally 5 or 6, specimens should be obtained. Multiple specimens increase sensitivity, and taken together with data from Peel et al. (19), using up to six specimens optimizes sensitivity (9, 19), but beyond that specificity might suffer (19). Second, tissue samples inoculated into blood culture bottles improve diagnostic yield, and this might approach or be superior to sonication cultures (depending on diagnostic cutoffs used). If resources permit, combining the two would improve the microbiological diagnosis of OIAI (9). However, if resources do not permit (for example, if sonication is not readily available), centers might elect to adopt a tissue culture protocol incorporating the use of blood culture bottles.
Several questions remain, however. Some of these should be the subject of further study, and others relate to practical considerations for implementation. What is the optimal combination of media for tissue cultures for OIAI diagnosis? Are six tissue specimens needed if combined with other culture methods (e.g., culture on solid media and in anaerobic broth and sonication)? One previous study which attempted to address this found that the combination of a single pediatric blood culture bottle along with anaerobic broth and chocolate agar offered the best yield (18), but would a protocol using a single bottle (e.g., pediatric) with anaerobic broth (e.g., thioglycolate or Schaedler) be comparable to one using dual aerobic and anaerobic blood culture bottles? What is the optimal duration of incubation for tissues incubated in blood culture bottles? Time to positivity was not presented in the current study (9), but from other data, incubating aerobic bottles for 7 days and anaerobic bottles for 14 days seems to provide optimal yield, and blind subcultures are unnecessary (20, 21). How would a diagnostic algorithm using sonication fluid inoculated in blood culture bottles compare to tissue cultures inoculated in the same? What are the most appropriate definitions and statistical methodologies when assessing various diagnostic assays for OIAI, and can these be further standardized so that data from different centers can be compared more easily? Practical considerations for implementation include the assessment of laboratory capacity. If six tissue specimens are taken per OIAI, and dual bottles are used, that would translate to 12 blood culture bottles per OIAI specimen, incubated for 7 to 14 days. Laboratories adopting the use of tissue inoculation into culture bottles on automated systems need to consider their OIAI volumes and existing culture capacity and perform the appropriate validation, as blood culture bottles are not approved by the FDA for the culture of tissue.
Despite remaining unanswered questions, the study by Dudareva et al. (9) provides support that an optimized tissue culture algorithm, where periprosthetic tissues are incubated in automated blood culture system, may provide microbiological yield that is at least comparable to that of sonication fluid cultures. A variety of tools now are available to facilitate the diagnosis of OIAI (Fig. 1), including emerging technologies such as novel biomarkers and metagenomic approaches, which may be especially useful in culture-negative OIAI or OIAI due to fastidious organisms. For the majority of OIAI, however, diagnosis may be optimized by first with making the best use of tools “already in the toolbox”—e.g., adopting practices which are straightforward and easily implementable (e.g., holding antibiotics if feasible for at least 2 weeks before sampling, ensuring adequate sampling, and adopting appropriate incubation periods for cultures) and making the best use of technologies already available in one’s laboratory, such as that presented by Dudareva and colleagues (9).
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Energias Market Research. 2018. Global joint replacement market is expected to witness a CAGR of 7.7% during 2018–2024. https://globenewswire.com/news-release/2018/05/15/1502380/0/en/Global-Joint-Replacement-Market-is-expected-to-Witness-a-CAGR-of-7-7-during-2018-2024.html. Accessed 22 September 2018.
- 2.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano MH, Klautau GB, da Silva CB, Nigro S, Avanzi O, Mercadante MT, Salles MJC. 2014. Improved diagnosis of infection associated with osteosynthesis by use of sonication of fracture fixation implants. J Clin Microbiol 52:4176–4182. doi: 10.1128/JCM.02140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portillo ME, Salvadó M, Alier A, Martínez S, Sorli L, Horcajada JP, Puig L. 2014. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect 69:35–41. doi: 10.1016/j.jinf.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Z, Li H, Qin A, Liu G, Liu X, Wu C, Li H, Zhu Z, Qu X, Dai K. 2014. Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J Clin Microbiol 52:1730–1736. doi: 10.1128/JCM.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergidis P, Greenwood-Quaintance KE, Sanchez-Sotelo J, Morrey BF, Steinmann SP, Karau MJ, Osmon DR, Mandrekar JN, Steckelberg JM, Patel R. 2011. Implant sonication for the diagnosis of prosthetic elbow infection. J Shoulder Elb Surg 20:1275–1281. doi: 10.1016/j.jse.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad SS, Becker R, Chen AF, Kohl S. 2016. EKA survey: diagnosis of prosthetic knee joint infection. Knee Surg Sports Traumatol Arthrosc 24:3050–3055. doi: 10.1007/s00167-016-4303-y. [DOI] [PubMed] [Google Scholar]
- 9.Dudareva M, Barrett L, Figtree M, Scarborough M, Watanabe M, Newnham R, Wallis R, Oakley S, Kendrick B, Stubbs D, McNally MA, Bejon P, Atkins BA, Taylor A, Brent AJ. 2018. Sonication versus tissue sampling for diagnosis of prosthetic joint and other orthopedic device-related infections. J Clin Microbiol 56:e00688-18. doi: 10.1128/JCM.00688-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Diek FM, Albers CGM, Van Hooff ML, Meis JF, Goosen JHM. 2017. Low sensitivity of implant sonication when screening for infection in revision surgery. Acta Orthop 88:294–299. doi: 10.1080/17453674.2017.1300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosso MJ, Frangiamore SJ, Yakubek G, Bauer TW, Iannotti JP, Ricchetti ET. 2018. Performance of implant sonication culture for the diagnosis of periprosthetic shoulder infection. J Shoulder Elb Surg 27:211–216. doi: 10.1016/j.jse.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Yan Q, Karau MJ, Greenwood-Quaintance KE, Mandrekar JN, Osmon DR, Abdel MP, Patel R. 2018. Comparison of diagnostic accuracy of periprosthetic tissue culture in blood culture bottles to that of prosthesis sonication fluid culture for diagnosis of prosthetic joint infection (PJI) by use of Bayesian latent class modeling and IDSA PJI criteria for classification. J Clin Microbiol 56:e00319-18. doi: 10.1128/JCM.00319-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen H, Tang J, Wang Q, Jiang Y, Zhang X. 2015. Sonication of explanted prosthesis combined with incubation in BD Bactec bottles for pathogen-based diagnosis of prosthetic joint infection. J Clin Microbiol 53:777–781. doi: 10.1128/JCM.02863-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portillo ME, Salvadó M, Trampuz A, Siverio A, Alier A, Sorli L, Martínez S, Pérez-Prieto D, Horcajada JP, Puig-Verdie L. 2015. Improved diagnosis of orthopedic implant-associated infection by inoculation of sonication fluid into blood culture bottles. J Clin Microbiol 53:1622–1627. doi: 10.1128/JCM.03683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C. 2013. Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop 37:931–936. doi: 10.1007/s00264-013-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hischebeth GTR, Randau TM, Molitor E, Wimmer MD, Hoerauf A, Bekeredjian-Ding I, Gravius S. 2016. Comparison of bacterial growth in sonication fluid cultures with periprosthetic membranes and with cultures of biopsies for diagnosing periprosthetic joint infection. Diagn Microbiol Infect Dis 84:112–115. doi: 10.1016/j.diagmicrobio.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol 36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bémer P, Léger J, Tandé D, Plouzeau C, Valentin AS, Jolivet-Gougeon A, Lemarié C, Kempf M, Héry-Arnaud G, Bret L, Juvin ME, Giraudeau B, Corvec S, Burucoa C. 2016. How many samples and how many culture media to diagnose a prosthetic joint infection: a clinical and microbiological prospective multicenter study. J Clin Microbiol 54:385–391. doi: 10.1128/JCM.02497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peel TN, Spelman T, Dylla BL, Hughes JG, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. 2017. Optimal periprosthetic tissue specimen number for diagnosis of prosthetic joint infection. J Clin Microbiol 55:234–243. doi: 10.1128/JCM.01914-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peel TN, Dylla BL, Hughes JG, Lynch DT, Greenwood-Quaintance KE, Cheng AC. 2016. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. mBio 7:e01776-15. doi: 10.1128/mBio.01776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler ICJW. 2014. Use of an automated blood culture system (BD BACTEC™) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis 14:233. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]