Current guidelines recommend collection of multiple tissue samples for diagnosis of prosthetic joint infections (PJI). Sonication of explanted devices has been proposed as a potentially simpler alternative; however, reported microbiological yield varies.
KEYWORDS: Prosthetic joint infection, accuracy, culture, diagnosis, orthopedic device-related infection, sensitivity, sonication, specificity
ABSTRACT
Current guidelines recommend collection of multiple tissue samples for diagnosis of prosthetic joint infections (PJI). Sonication of explanted devices has been proposed as a potentially simpler alternative; however, reported microbiological yield varies. We evaluated sonication for diagnosis of PJI and other orthopedic device-related infections (DRI) at the Oxford Bone Infection Unit between October 2012 and August 2016. We compared the performance of paired tissue and sonication cultures against a “gold standard” of published clinical and composite clinical and microbiological definitions of infection. We analyzed explanted devices and a median of five tissue specimens from 505 procedures. Among clinically infected cases the sensitivity of tissue and sonication culture was 69% (95% confidence interval, 63 to 75) and 57% (50 to 63), respectively (P < 0.0001). Tissue culture was more sensitive than sonication for both PJI and other DRI, irrespective of the infection definition used. Tissue culture yield was higher for all subgroups except less virulent infections, among which tissue and sonication culture yield were similar. The combined sensitivity of tissue and sonication culture was 76% (70 to 81) and increased with the number of tissue specimens obtained. Tissue culture specificity was 97% (94 to 99), compared with 94% (90 to 97) for sonication (P = 0.052) and 93% (89 to 96) for the two methods combined. Tissue culture is more sensitive and may be more specific than sonication for diagnosis of orthopedic DRI in our setting. Variable methodology and case mix may explain reported differences between centers in the relative yield of tissue and sonication culture. Culture yield was highest for both methods combined.
INTRODUCTION
As more people benefit from arthroplasty surgery, the burden of orthopedic device-related infections (DRI) has grown. Chronic orthopedic infections significantly impact quality of life, and costs of orthopedic implant revision and infection management are projected to rise steeply. An estimated $1.6 billion will be spent in the United States alone over the next 5 years (1, 2).
Bacterial production of organized extracellular matrix (biofilm) presents particular challenges in the diagnosis and management of orthopedic device-related infections and associated osteomyelitis (3, 4). Successful management requires thorough excision of necrotic tissue with or without device removal or exchange, coupled with careful attention to soft tissue and bone reconstruction, accurate microbiological diagnosis, and targeted antimicrobial treatment (4, 5).
Collection of multiple intraoperative deep tissue samples has previously been shown to optimize diagnostic yield and enable assessment of whether cutaneous bacteria of low virulence are likely pathogens or contaminants in individual cases (6). However, a significant proportion of patients with clinical and histopathologic features of orthopedic DRI yield no positive bacterial cultures from operative specimens despite adequate tissue sampling and laboratory processing, and preoperative antibiotic use further decreases culture yield (5, 7).
Sonication of explanted devices may be used to separate adherent bacterial colonies in biofilm and might improve microbiological diagnostic yield (8). Some studies suggest sonication is more sensitive than tissue sample culture, but results vary between centers (see Table S1 in the supplemental material). Furthermore, although culture sensitivity increases with multiple tissue samples (6), few studies have routinely compared sonication with the 4 to 6 tissue samples recommended (6, 9) or used automated liquid culture methods that have been shown to optimize tissue culture sensitivity (10, 11).
We prospectively compared the performance of sonication and tissue sample culture for diagnosis of orthopedic DRI in a large cohort of patients managed at the Oxford Bone Infection Unit.
MATERIALS AND METHODS
Participants and setting.
The Oxford Bone Infection Unit provides specialist multidisciplinary management of complicated bone and joint infections. It provides secondary level care locally and serves as a UK national referral center. Long established local protocols for the diagnosis of prosthetic joint and other orthopedic device-related infections include meticulous tissue sampling for microbiology and histology at the time of device removal (6).
In October 2012 we undertook a service improvement project to implement and locally evaluate sonication for diagnosis of orthopedic DRI. Surgeons were invited to submit explanted orthopedic devices for sonication. Results of both tissue and sonication culture were made available to the clinical team managing the patient, and clinical details were obtained for analysis from the patient notes. The project received institutional approval as a service improvement audit.
Sample processing.
Specimen sampling and processing were performed as previously described (6, 11, 12). Antibiotics were withheld prior to surgery unless the risk of uncontrolled sepsis was considered high. Surgical antibiotic prophylaxis was delayed until after tissue sampling. Multiple tissue samples were obtained for culture and histology, using separate instruments for each sample and avoiding contact with the skin to minimize cross-contamination. Following removal, each device was placed immediately into a sterile, single-use, airtight container (13).
All samples were processed in a class 2 safety cabinet using aseptic technique. Each tissue sample was disrupted by vortexing with sterile glass beads in sterile saline, and equal aliquots of the resulting suspension were inoculated into Bactec Plus Aerobic/F and Bactec Lytic/10 Anaerobic/F bottles (BD Diagnostics, Sparks, MD). Bactec bottles were incubated at 37°C for 10 days or until they flagged positive. Sterile saline was added to the sonication container to cover at least 90% of the device. The container was vortexed vigorously for 30 s, sonicated in an ultrasound bath for 1 min, and vortexed again for 30 s. Aliquots (0.1 ml) of the sonication fluid were inoculated onto blood and chocolate agar; aerobic and anaerobic plates were incubated at 37°C for 5 and 10 days, respectively.
Gram stains were performed on all isolates. Positive Bactec bottles were subcultured onto agar, and isolates were identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker UK Ltd.). Drug susceptibility testing was performed using the BD Phoenix system (BD Diagnostics) or manual EUCAST methods. In keeping with existing guidelines, positive tissue sample culture was defined as isolation of indistinguishable organisms (with identical drug susceptibility profiles) from 2 or more independent tissue specimens (9, 13, 14) and positive sonication culture as ≥50 CFU per milliliter (CFU/ml) (15–23).
Statistical analysis.
Data were analyzed by surgical procedure such that if a patient had more than one procedure during the evaluation period, each procedure would contribute to the analysis. In the absence of a single “gold standard,” we used a range of published definitions of prosthetic joint infection (PJI) (Table S2), including the clinical definition used in previous studies of sonication (presence of a sinus OR visible purulence OR positive histology for infection) (12–14, 24) and combined clinical and microbiological definitions from the Infectious Diseases Society of America (IDSA) (14) and the International Consensus Meeting on Periprosthetic Joint Infection (consensus definition) (9). While the clinical definition excludes microbiological results from the definition, the IDSA and consensus definitions include both clinical features and microbiological parameters in the definition of infection. Since leukocyte counts are not performed routinely on joint fluid in our unit, for the purposes of our analyses we modified the consensus definition of PJI to replace elevated synovial leukocyte and neutrophil counts with visible purulence. Finally, to mitigate incorporation bias in favor of tissue culture in the IDSA and consensus definitions, we compared the performance of both methods against a “composite definition” of PJI requiring either the clinical case definition (12) to be met or a positive culture from either tissue or sonication. We applied both the clinical and the composite case definitions to analyses of other (nonprosthetic-joint) orthopedic DRI.
We calculated the sensitivity and specificity of both culture methods using these definitions, and used McNemar’s χ2 test to compare proportions among paired samples from the same procedure. We calculated the combined sensitivity of sonication and tissue sample culture, first using the independent sonication and tissue sample culture results (independent analysis) and then in a combined analysis including as positive those with identical organisms isolated from both a single tissue specimen and from sonication (analogous to two independent tissue specimens).
To model the effect of the number of specimens taken on the sensitivity of tissue sample culture we used a computer algorithm to randomly sample n specimens from each procedure, excluding procedures from which fewer than n specimens were collected, and calculated the sensitivity based on this sample. For each value of n we repeated this process 100 times to estimate the mean sensitivity for n specimens.
We carried out a blinded case notes review to explore the significance of positive cultures in cases with discordant sonication and tissue sample cultures that did not meet the clinical definition of infection. Case notes were redacted for patient identifiers and the source (tissue or sonication) of isolates. An infectious diseases specialist and an orthopedic specialist in musculoskeletal infections who had not been involved in the patient’s care then reviewed the case notes to judge whether the case was infected. If agreement could not be reached, a third musculoskeletal infection specialist adjudicated the case. We then recalculated the sensitivity and specificity of each culture method against a revised clinical definition of infection incorporating infection assignments of discordant cases from the case notes review.
To explore the effect of other factors on culture sensitivity, we compared the yield of sonication and tissue sample culture among subgroups defined by clinical features, time from device implantation to explantation, antibiotic exposure prior to explantation, isolation of “more virulent” organisms, and mixed infections. For this analysis we defined more virulent organisms a priori as Gram-negative bacilli, Staphylococcus aureus, Staphylococcus lugdunensis, enterococci, beta-hemolytic streptococci, milleri group streptococci, Streptococcus pneumoniae, and Candida species and less virulent organisms as other Gram-positive organisms, including coagulase negative staphylococci, viridans group streptococci, Bacillus species, and mycobacteria. Mixed infection was defined as isolation of more than one pathogen species by either method. Independent associations with a positive culture result from either method were explored using multivariable logistic regression.
Finally, we investigated the effect of lowering the thresholds of positive tissue sample and sonication culture on the performance of each method. We first explored the number of additional cases that would have been identified using the lower sonication threshold of 10 CFU/ml used in some studies (Table S1). Recognizing the polyclonal nature of many infections (25), and in keeping with common clinical practice, we also relaxed the stringent definition of identical tissue culture isolates used in the main analysis to allow up to two differences in drug susceptibility profiles between “indistinguishable isolates” and recalculated the sensitivity of tissue culture using this definition. Using these revised definitions we then carried out a further blinded case notes review of discordant cases that did not meet the clinical definition of infection and recalculated and compared the sensitivity and specificity of each method against the clinical definition of infection after incorporating final infection assignments of discordant cases from this case notes review.
RESULTS
Between 1 October 2012 and 12 August 2016, specimens for sonication were obtained from 528 procedures. We excluded 23 (4%) because <2 tissue specimens were received for culture, leaving 505 procedures on 463 patients in the final analysis (Table 1). Anatomical locations of explanted devices are summarized in Table S3 and Table S4. A median of 5 tissue specimens was obtained per procedure (interquartile range [IQR], 4 to 5).
TABLE 1.
Explanted specimens received for sonication
| Surgical procedure | Specimen type | No. (%) |
|---|---|---|
| Prosthetic joint revision surgery | Entire joint prosthesis (±fixation devices) | 224 (44) |
| Debridement, antibiotics, and implant retention procedure for PJI | Prosthesis components (±fixation devices) | 134 (27) |
| Metalwork removal for infected fracture | Orthopedic fixation devices | 111 (22) |
| Surgical debridement for orthopedic device-related infectionsa | Cement | 26 (5) |
| Bone | 10 (2) | |
| Total | 505 |
Including repeat debridement of prosthetic joint infections and infected fractures.
The median age of patients was 68 years (IQR, 57 to 76). A total of 265 (52%) were male. Date of device implantation was available for 440 (87%), among whom the median time from device implantation to explantation was 28 months (IQR, 8 to 92). A total of 246/505 (49%) met the clinical definition of infection, including 169 PJI. Table 2 shows the number of cases meeting each of the other definitions of infection.
TABLE 2.
Sensitivity of tissue culture and sonication for diagnosis of prosthetic joint and other orthopedic device-related infections
| Reference standard (definition of infection) | Total no. of infected cases |
No. of cases positive by each method |
Sensitivity, % (95% CI) |
P value (tissue vs. sonication) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue or sonication positive |
Tissue | Sonication | |||||||
| Sonication positive |
Sonication negative |
||||||||
| Tissue positivea |
Tissue negative |
Tissue positive |
Tissue negative |
||||||
| PJI | |||||||||
| Clinical | 169 | 96 | 9 | 25 | 39 | 77 (70–83) | 72 (64–78) | 62 (54–69) | 0.006 |
| Consensus | 150 | 99 | 6 | 30 | 15 | 90 (84–94) | 86 (79–91) | 70 (62–77) | <0.001 |
| IDSA | 177 | 100 | 8 | 33 | 36 | 80 (73–85) | 75 (68–81) | 61 (53–68) | <0.001 |
| Composite | 182 | 99 | 14 | 30 | 39 | 79 (72–84) | 71 (64–77) | 62 (55–69) | 0.016 |
| Other orthopedic device-related infection | |||||||||
| Clinical | 77 | 32 | 3 | 17 | 25 | 68 (56–78) | 64 (52–74) | 45 (34–57) | 0.002 |
| Composite | 91 | 34 | 11 | 21 | 25 | 73 (62–81) | 60 (50–71) | 49 (39–60) | 0.077 |
| All device-related infections (PJI and non-PJI) | |||||||||
| Clinical | 246 | 128 | 12 | 42 | 64 | 74 (68–79) | 69 (63–75) | 57 (50–63) | <0.001 |
| Composite | 273 | 133 | 25 | 51 | 64 | 77 (71–81) | 67 (61–73) | 58 (52–64) | 0.003 |
Sonication positive, sonication culture positive (≥50 CFU/ml). Tissue positive, tissue culture positive (i.e., indistinguishable organisms isolated from at least two tissue specimens).
Diagnostic accuracy.
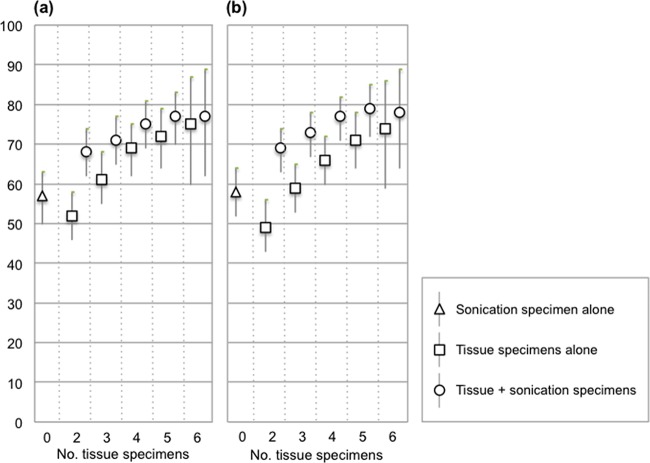
Tissue sample culture was found to be more sensitive than sonication when analyzed independently, with an overall sensitivity against the clinical definition of 69% (63 to 75), compared with 57% (50 to 63) for sonication (P < 0.0001 [Table 2]). In modeling the effect of varying the numbers of tissue specimens obtained, tissue culture sensitivity increased as the number of specimens included in the analysis increased (Fig. 1).
FIG 1.
Sensitivity and 95% confidence intervals for sonication, increasing numbers of tissue samples, and sonication and tissue culture combined for clinical (a) and composite (b) definitions of infection. The effect of tissue sample number was modeled using a computer algorithm to randomly sample required number of specimens from the full set of specimens obtained in each case (see Materials and Methods).
Tissue sample culture was consistently more sensitive among PJI cases, irrespective of the definition of infection used. Despite smaller numbers of non-PJI cases, tissue culture still demonstrated greater sensitivity against the clinical definition of infection, with a trend toward superior sensitivity against the composite endpoint (Table 2).
The combined sensitivity of tissue and sonication culture was higher than for either method alone and increased in line with tissue sample culture as the number of tissue specimens increased (Fig. 1). Combining the independent tissue and sonication culture results, the overall sensitivities were 74% (68 to 79) and 77% (71 to 81) against the clinical and composite definitions of infection, respectively.
In 11 cases that were classed as culture negative by both sonication and tissue culture alone, the same organism was cultured from both sonication and a single tissue specimen, but below the 50-CFU/ml threshold for sonication. In 4 of these cases that grew coagulase-negative staphylococci, drug susceptibility testing was not performed on the sonication isolate so it was not possible to confirm that the tissue and sonication isolates were identical. Inclusion of the remaining 7 cases with identical isolates from tissue and sonication culture as culture positive gave overall sensitivities for tissue and sonication combined of 76% (70 to 81) and 78% (73 to 83) against the clinical and composite definitions of infection, respectively (Table 3).
TABLE 3.
Combined microbiological sensitivity of sonication and tissue culture
| Reference standard definition of infection | Total no. of infected cases |
Independent analysisa
|
Combined analysisb
|
||
|---|---|---|---|---|---|
| No. | Sensitivity, % (95% CI) | No. | Sensitivity, % (95% CI) | ||
| PJI | |||||
| Clinical | 169 | 130 | 77 (70–83) | 133 | 79 (72–85) |
| Consensus | 148 | 135 | 91 (85–95) | 140 | 95 (90–98) |
| IDSA | 179 | 138 | 77 (70–83) | 143 | 80 (73–85) |
| Composite | 184 | 143 | 78 (71–84) | 148 | 80 (74–86) |
| Other orthopedic device-related infection | |||||
| Clinical | 77 | 52 | 68 (56–78) | 53 | 69 (57–79) |
| Composite | 92 | 66 | 72 (61–81) | 67 | 74 (64–83) |
| All device-related infections (PJI and non-PJI) | |||||
| Clinical | 246 | 182 | 74 (68–79) | 186 | 76 (70–81) |
| Composite | 276 | 209 | 76 (70–81) | 216 | 78 (73–83) |
Positive microbiology defined as positive sonication culture or identical isolates from ≥2 tissue specimens (positive tissue culture).
Positive microbiology defined as positive sonication culture or identical isolates from ≥2 specimens of any type (tissue or sonication specimens).
Against the clinical definition of infection, the specificities of tissue and sonication culture were 95% (91 to 97) and 93% (89 to 96), respectively (P = 0.394).
Culture discordant specimens.
Sonication and tissue sample culture results were discordant in 76 cases (Table 2). Of these, 54/76 (71%) met the clinical definition of infection: 27 with a sinus, 29 with visible purulence, and 48 with histological evidence of infection. Clinical and microbiological characteristics of the remaining 22 (29%) cases that did not meet the clinical definition of infection are summarized in Table 4. Following blinded case notes review, 6/9 tissue-positive/sonication-negative cases and 0/13 tissue-negative/sonication-positive cases were judged to be infected (Table 4). After assigning these 6 cases to the clinical infection category and excluding 3 cases classified as “uncertain,” the sensitivities of tissue and sonication culture were 70% (64 to 75) and 56% (49 to 62), respectively (P < 0.001), and 75% (69 to 80) for the two methods combined. Specificities were 97% (94 to 99) for tissue, 94% (90 to 97) for sonication (P = 0.052), and 93% (89 to 96) for the two methods combined. Of 11 tissue-negative/sonication-positive cases not treated and followed up for a median of 3 years, 9 (82%) had a good outcome, 1 patient died of pneumonia, and 1 patient had persistent pain of uncertain etiology.
TABLE 4.
Clinical and microbiological characteristics of cases with discordant tissue and sonication culture results that did not meet the clinical definition of infection
| Case no. | No. of mo since device implantation |
Antibiotics in preceding 14 days |
No. of tissue specimens |
Sonication specimen | Sonication culture (CFU/ml)e |
Tissue culture (no. specimens culture positive for each organism)e |
Treated | Follow-up (mo) | Outcome | Diagnosis following blinded clinical review of case notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Prosthetic joint infection (PJI) cases |
||||||||||
| 9 | 44 | No | 6 | Knee components |
Propionibacterium acnes
(>100), CoNSa (>500) |
Bacillus species (1) | Yes | 85 | Good | Not infected |
| 70 | 5 | Yes | 5 | Hip prosthesis |
Staphylococcus epidermidis (4), Enterococcus faecalis (1) |
Yes | 2 | Died of pneumonia |
Infected (PJI) | |
| 77 | 37 | No | 4 | Elbow components | CoNS (90) | No | 25 | Good | Not infected | |
| 171 | 52 | No | 4 | Hip prosthesis | CoNS (>250) | No | 33 | Good | Not infected | |
| 241 | 13 | No | 5 | Hip components | CoNS (>100) |
Moraxella osloensis (1), Micrococcus luteus (1) |
No | 47 | Good | Not infected (metallosis) |
| 269 | 40 | No | 4 | Knee prosthesis | P. acnes (>100) | No | 44 | Goodb | Not infected (aseptic loosening) | |
| 334 | 0 | No | 5 | Hip components | CoNS-1 (4), CoNS-2 (1) | Yes | 6 | Good | Probably not infected (loosening due to trauma) |
|
| 397 | 124 | No | 4 | Hip components | S. epidermidis (4) | Yes | 19 | Good | Probably not infected (aseptic loosening) |
|
| 432 | 3 | Yes | 5 | Hip prosthesis |
S. epidermidis (3), Bacillus spp. (1) |
Yes | 18 | Good | Infected (PJI) | |
| 491 | 193 | Yes | 4 | Hip prosthesis | S. aureus (4) | Yes | 5 | Good | Infected (PJI) | |
| Other orthopedic device- related (non-PJI) cases |
||||||||||
| 124 | 77 | No | 3 | Bone knee | CoNS (>500) | No | 49 | Good | Not infected (mechanical failure) | |
| 179 | 248 | No | 3 | Metalware spine | CoNS (<50) | CoNS (2), S. epidermidis (1) | No | 23 | Poor | Infected |
| 197 | 8 | No | 5 | Metalware tibia |
S. epidermidis (2), S. aureus (1) |
Yes | 9 | Good | Probably infected (infected nonunion) |
|
| 210 | 17 | No | 5 | Metalware tibia | CoNS (>100) | No | 3 | Died of pneumonia |
Uncertain | |
| 262 | 4 | No | 4 | Metalware | CoNS-1 (>100), CoNS-2 (>100) | No | 45 | Good | Not infected (aseptic nonunion) | |
| 339 | 7 | No | 4 | Metalware femur | P. acnes (>250) | No | 40 | Poor (ongoing pain) |
Uncertain | |
| 368 | 47 | No | 5 | Metalware ankle/foot | CoNSc (<50) | CoNS (2), S. epidermidis-1 (2), S. epidermidis-2 (1) |
Yes | 24 | Good | Probably not infected (aseptic nonunion) |
| 376 | 1 | No | 4 | Metalware ankle/foot | CoNS (>100) | No | 36 | Good | Not infected (aseptic nonunion) | |
| 429 | 3 | No | 3 | Metalware femur | CoNS (>100), Bacillus spp. (>100) | No | 5 | Good | Not infected (mechanical failure; periprosthetic fracture) |
|
| 489 | 9 | No | 4 | Metalware ankle/foot | CoNSd | No | 2 | Good | Not infected | |
| 498 | 1 | Yes | 5 | Metalware ankle/foot | CoNS (>250) | Yes | 6 | Good | Uncertain | |
| 535 | 22 | No | 5 | Metalware ankle/foot |
S. epidermidis-1 (4), S. epidermidis-2 (3), S. epidermidis-3 (1) |
Yes | 17 | Good | Infected (infected nonunion and septic arthritis) |
CoNS, coagulase-negative staphylococci (not further identified).
Later revision for metallosis, but no clinical or microbiological evidence of infection at repeat surgery.
Sonication culture below the 50 CFU/ml threshold for positive culture.
Number of CFU per milliliter not documented.
Organism numerical suffixes denote different isolates of the same species; e.g., “CoNS-1” and “CoNS-2” denote two isolates of coagulase-negative staphylococci that are distinguished by their antimicrobial susceptibility profile.
Subgroup analyses.
Table 5 compares the sensitivities and specificities of tissue and sonication culture stratified by clinical and microbiological characteristics. While smaller numbers limit power in some subgroups, the point estimates suggest that tissue sample culture was more sensitive than sonication in most subgroups. However, no difference in culture yield was observed among cases caused by less virulent organisms. Organism virulence was the only factor independently associated with culture yield in multivariate logistic regression models of tissue and sonication culture (Table 5). The presence of more virulent organisms was strongly associated with positive sample tissue culture (odds ratio [OR], 8.5; 95% confidence interval [CI], 1.7 to 42.9; P = 0.01) but not with positive sonication culture (OR, 0.7; 95% CI, 0.2 to 2.4, P = 0.587).
TABLE 5.
Effect of clinical and microbiological characteristics on tissue and sonication culture yield
| Clinical definition of PJI and other orthopedic DRI clinical subgroup |
No. of patients |
No. sonication positive |
No. sonication negative |
Tissue sensitivity, % (95% CI) |
Sonication sensitivity, % (95% CI) |
P value | ||
|---|---|---|---|---|---|---|---|---|
| Tissue positiveve |
Tissue negative |
Tissue positive |
Tissue negative |
|||||
| Time since device implantation | ||||||||
| <3 mo | 41 | 18 | 2 | 9 | 12 | 66 (49–80) | 49 (33–65) | 0.035 |
| 3–24 mo | 89 | 47 | 5 | 13 | 24 | 67 (57–77) | 58 (47–69) | 0.059 |
| 24 mo | 106 | 59 | 4 | 19 | 24 | 74 (64–82) | 59 (49–69) | 0.002 |
| Clinical features | ||||||||
| Sinus present | 121 | 75 | 5 | 22 | 19 | 80 (72–87) | 66 (57–74) | 0.001 |
| Visible purulence | 139 | 91 | 6 | 23 | 19 | 82 (75–88) | 70 (61–77) | 0.002 |
| No sinus or purulence | 70 | 24 | 3 | 10 | 33 | 49 (36–61) | 39 (27–51) | 0.052 |
| Antibiotic exposure prior to explantationa | ||||||||
| No recent antibiotics | 104 | 56 | 3 | 18 | 27 | 71 (61–80) | 57 (47–66) | 0.001 |
| Antibiotics within 14 days | 109 | 50 | 8 | 19 | 32 | 63 (54–72) | 53 (43–63) | 0.034 |
| Antibiotics within 7 days | 77 | 32 | 5 | 13 | 27 | 58 (47–70) | 48 (37–60) | 0.059 |
| Antibiotics within 3 days | 61 | 27 | 4 | 11 | 19 | 62 (49–74) | 51 (38–64) | 0.071 |
| Surgery while on antibiotics | 49 | 23 | 4 | 9 | 13 | 65 (50–78) | 55 (40–69) | 0.166 |
| Organism virulence | ||||||||
| More virulent organisms | 132 | 93 | 4 | 35 | 0 | 97 (92–99) | 73 (65–81) | <0.001 |
| Less virulent organisms only | 50 | 35 | 8 | 7 | 0 | 84 (71–93) | 86 (73–94) | 1.000 |
Note categories of antibiotic exposure within 14 days are not mutually exclusive.
Mixed infections.
Tissue and/or sonication culture was positive for more than one bacterial species in 47/209 (22%) culture-positive cases. Of these mixed infections, 42 (89%) were positive by tissue sample culture and 18 (38%) by sonication (P < 0.0001 [Table S6]). The two methods shared at least one similar isolate in 35 (74%) cases of mixed infection, including 14 (30%) cases with identical isolates. Sonication identified mixed infection in 9 cases that were tissue culture negative; conversely, mixed infection was identified by tissue sample culture in 4 cases that were negative by sonication culture. In a further 3 cases of mixed infection by tissue sample culture, sonication yielded a completely different organism.
Sensitivity analyses.
Reducing the sonication threshold to 10 CFU/ml would have classified an additional 78 cases as sonication culture positive, of which 41 (53%) met the clinical definition of infection. Of the 37 cases that did not meet the clinical definition of infection, 30 (81%) were not treated with antibiotics, had no evidence of infection during a median of 20 (IQR, 6 to 36) months of follow-up, and were judged on blinded case notes review not to have been infected, 2 (5%) had other clinical evidence of infection, and the infection status of the remaining 5 (14%) who received antibiotic treatment was uncertain.
Relaxing the requirement for defining identical isolates to ≤2 differences in drug susceptibility profile would have classified an additional 21 cases as tissue sample culture positive, of which 18 (86%) met the clinical definition of infection. A further 2 (10%) cases were judged by blinded case notes review to be infected; the remaining case was not treated with antibiotics, had no evidence of infection during 21 months of follow-up and was judged not to have been infected.
Applying these lower thresholds, the sensitivities of tissue and sonication culture against the clinical definition of infection were 77% (71 to 82) and 72% (66 to 77), respectively (P = 0.063). The specificities were 96% (92 to 98) for tissue sample culture and 79% (74 to 84) for sonication (P < 0.0001).
DISCUSSION
This is the largest study to date comparing sonication with standard tissue sample culture for the diagnosis of orthopedic device-related infections. The results suggest that tissue sample culture is more sensitive than sonication for the microbiological diagnosis of both PJI and other orthopedic DRI in our setting.
There is wide variation between centers in the comparative yield of sonication and tissue samples (Table S1). Methodological differences between studies partly explain this heterogeneity; in particular, differences in the number of tissue specimens obtained for culture. Tissue sample culture yield depends critically on specimen number (6, 26), and current recommendations are to collect 4 to 6 specimens (6, 9). Most studies comparing sonication with tissue sample culture did not report the number of tissue specimens obtained (12, 18, 19, 22, 23, 27, 28), and many required a minimum of only 2 tissue specimens (12, 18, 29–31). Use of a suboptimal tissue sampling reference standard may therefore have overestimated the relative yield of sonication in some studies. Meticulous sampling to obtain a median of 5 tissue specimens per case in the current study allows a fairer comparison between tissue and sonication culture, and our analysis reinforces the importance of multiple tissue samples to maximize culture yield.
Differences in laboratory protocols may further affect culture yield. Automated liquid culture as used in this study improves tissue sample culture yield (10, 11) compared with that obtained with more traditional culture media. Variation was noted between studies in the quantitative threshold and culture duration used for sonication. We followed the sonication protocol and threshold for positivity most widely used in previous studies (15–23) but also explored the effect of lowering the sonication threshold (12).
Another reason for heterogeneity between published case series may be differences in the spectrum of cases included. PJI and other orthopedic DRI represent a spectrum of disease, from indolent infections with minimal soft tissue inflammation to more aggressive infections associated with marked soft tissue inflammation, purulence, and sinus formation (Fig. S2) (5, 12, 13).
We tested this hypothesis by comparing the performance of sonication among different clinical subgroups. While tissue sample culture yield was superior overall, sonication was equally sensitive among cases from which only less virulent organisms were isolated. This is consistent with our understanding of the pathophysiology of these more indolent infections, in which biofilm on the prosthesis predominates, with less soft tissue inflammation and lower bacterial density in tissue. At the other end of the spectrum, virulent organisms tend to cause more aggressive soft tissue inflammation with larger numbers of invading bacteria (Fig. S2). Interestingly, although other smaller studies have suggested that sonication may be superior to tissue culture in cases with recent antibiotic exposure and in mixed infections, this was not supported by our results (21, 23). One plausible explanation is that bone and soft tissue from which the tissue samples are taken is not sterilized due to the presence of biofilm and/or collections that persist despite antimicrobial therapy, just as biofilm persists on the surface of prostheses and other devices.
Strengths of this study include prospective inclusion of a large number and range of cases, suggesting that our findings should be generalizable to other settings. Rigorous collection of multiple specimens also ensured a robust tissue sample culture method for comparison. The stringent requirement for indistinguishable tissue culture isolates to have identical drug susceptibility profiles also maximized the specificity of tissue sample culture. We also explored the effect of relaxing this requirement in keeping with routine clinical practice and of reducing the sonication threshold.
In the absence of a perfect reference standard for PJI or other orthopedic DRI, we used a range of published reference definitions. Each of these definitions has limitations. Both the IDSA (13) and consensus (9) definitions, and the Musculoskeletal Infection Society definition (14) on which the later consensus definition is based, suffer from incorporation bias by including tissue culture in the definition. The clinical definition (12) circumvents this problem but does not capture the full spectrum of infection. To overcome these biases, we therefore also included a composite definition of PJI incorporating both tissue and sonication culture in addition to clinical features. While the effect size is slightly smaller using this composite reference standard, tissue culture still appears more sensitive than sonication.
The absence of a reliable clinical reference standard also makes the interpretation of culture specificity difficult, since the clinical significance of culture positive cases that do not meet the clinical definition is unclear. To address this, we conducted a detailed review of all cases with discordant culture results that did not meet the clinical definition of infection. Importantly, to avoid observer bias, reviewers were blinded to the source (tissue or sonication) of culture isolates. The results suggest that sonication may be less specific than tissue culture for clinically relevant infection, particularly when a lower sonication threshold is applied, and that in the absence of clinical or histological evidence of infection, a positive sonication culture may not indicate a need for antibiotic treatment if adequate tissue sampling has been performed and tissue sample cultures are negative. Whether some of these false-positive cases represent true infection that is effectively cured by device removal alone is unclear.
Our study has some limitations. In modeling the incremental yield of additional tissue cultures, we selected available specimens at random. This may not perfectly reflect surgical practice if surgeons are more likely to take samples from the highest yield sites first, based on their macroscopic appearance. This might therefore overestimate the incremental benefit of additional samples.
In the subgroup analyses, the definition of organism virulence is necessarily slightly arbitrary but nevertheless broadly correlates with clinical experience. Data on the time from device implantation also do not completely correlate with early versus late infection, since in many cases it includes a prolonged period from presentation with infection at another hospital, followed by referral and surgery at the Oxford Bone Infection Unit. This may explain why tissue sample culture is more sensitive even among the group >24 months from implantation, as this group does not only include late infections usually associated with less virulent organisms.
In summary, the results from this large prospective study suggest that tissue culture should remain the gold standard for microbiological diagnosis of PJI and other orthopedic DRI. The choice of method in a particular setting may, however, depend on existing infrastructure and available resources. If multiple tissue specimens cannot reliably be obtained, device sonication may provide a simpler though less sensitive alternative to tissue culture. Where rigorous tissue sampling can be established, culture methods should first be optimized (10, 11). Sonication may then have a complementary role in further optimizing microbiological yield.
Supplementary Material
ACKNOWLEDGMENTS
We thank all staff in the Oxford University Hospitals Microbiology laboratory who were involved in processing tissue and sonication specimens.
We declare no competing interests.
This work was supported by Oxford University Hospitals NHS Foundation Trust as a service improvement project for diagnosis of prosthetic joint and other orthopedic device-related infections. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
A.J.B., A.T., B.A.A., M.F., P.B., and R.N. designed and implemented the local evaluation of sonication to improve diagnosis of orthopedic device-related infection in Oxford. M.F., R.W., R.N., and S.O. were responsible for laboratory processing of the specimens. A.J.B., R.W., L.B., M.D., M.F., and M.W. collected and collated the data. A.J.B., A.T., B.A.A., B.K., D.S., M.A.M., M.S., and M.D. conducted the blinded review of discordant cases. A.J.B. performed the analyses and wrote the first draft of the manuscript with input from M.D. All authors contributed to preparation of the final manuscript. A.J.B. had full access to all the data in the study and final responsibility for the decision to submit for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00688-18.
For a commentary on this article, see https://doi.org/10.1128/JCM.01379-18.
REFERENCES
- 1.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27:61–65.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Peel TN, Cheng AC, Lorenzo YP, Kong DCM, Buising KL, Choong PFM. 2013. Factors influencing the cost of prosthetic joint infection treatment. J Hosp Infect 85:213–219. doi: 10.1016/j.jhin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Gristina AG, Oga M, Webb LX, Hobgood CD. 1985. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science 228:990–993. doi: 10.1126/science.4001933. [DOI] [PubMed] [Google Scholar]
- 4.Matthews PC, Berendt AR, McNally MA, Byren I. 2009. Diagnosis and management of prosthetic joint infection. BMJ 338:b1773. doi: 10.1136/bmj.b1773. [DOI] [PubMed] [Google Scholar]
- 5.Trampuz A, Zimmerli W. 2005. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly 135:243–251. [DOI] [PubMed] [Google Scholar]
- 6.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol 36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spangehl MJ, Masri BA, OʼConnell JX, Duncan CP. 1999. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am 81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol 37:3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parvizi J, Gehrke T, Chen AF. 2013. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 95–B:1450–1452. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 10.Hughes HC, Newnham R, Athanasou N, Atkins BL, Bejon P, Bowler ICJ. 2011. Microbiological diagnosis of prosthetic joint infections: a prospective evaluation of four bacterial culture media in the routine laboratory. Clin Microbiol Infect 17:1528–1530. doi: 10.1111/j.1469-0691.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 11.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler ICJW. 2014. Use of an automated blood culture system (BD BACTECTM) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis 14:233. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 13.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. 2011. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandelin GmbH. 2010. Standard operating procedures for the device BactoSonic®, Bandelin GmbH, Berlin; Diagnosis of implant-associated infections with sonication Bandelin GmbH, Berlin, Germany. [Google Scholar]
- 16.Esteban J, Alvarez-Alvarez B, Blanco A, Fernández-Roblas R, Gadea I, Garcia-Cañete J, Sandoval E, Valdazo M. 2013. Prolonged incubation time does not increase sensitivity for the diagnosis of implant-related infection using samples prepared by sonication of the implants. Bone Joint J 95-B:1001–1006. doi: 10.1302/0301-620X.95B7.31174. [DOI] [PubMed] [Google Scholar]
- 17.Puig-Verdié L, Alentorn-Geli E, González-Cuevas A, Sorlí L, Salvadó M, Alier A, Pelfort X, Portillo ME, Horcajada JP. 2013. Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Joint J 95-B:244–249. doi: 10.1302/0301-620X.95B2.30486. [DOI] [PubMed] [Google Scholar]
- 18.Portillo ME, Salvado M, Alier A, Martinez S, Sorli L, Horcajada JP, Puig L. 2014. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect 69:35–41. doi: 10.1016/j.jinf.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Portillo ME, Salvado M, Trampuz A, Siverio A, Alier A, Sorli L, Martinez S, Perez-Prieto D, Horcajada JP, Puig-Verdie L. 2015. Improved diagnosis of orthopedic implant- associated infection by inoculation of sonication fluid into blood culture bottles. J Clin Microbiol 53:1622–1627. doi: 10.1128/JCM.03683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tani S, Lepetsos P, Stylianakis A, Vlamis J, Birbas K, Kaklamanos I. 2017. Superiority of the sonication method against conventional periprosthetic tissue cultures for diagnosis of prosthetic joint infections. Eur J Orthop Surg Traumatol 28:51–57. [DOI] [PubMed] [Google Scholar]
- 21.Van Diek FM, Albers CGM, Van Hooff ML, Meis JF, Goosen JHM. 2017. Low sensitivity of implant sonication when screening for infection in revision surgery. Acta Orthop 88:294–299. doi: 10.1080/17453674.2017.1300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renz N, Feihl S, Cabric S, Trampuz A. 2017. Performance of automated multiplex PCR using sonication fluid for diagnosis of periprosthetic joint infection: a prospective cohort. Infection 45:877–884. doi: 10.1007/s15010-017-1073-5. [DOI] [PubMed] [Google Scholar]
- 23.Rothenberg AC, Wilson AE, Hayes JP, Malley MJ, Klatt BA. 2017. Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res 475:1827–1836. doi: 10.1007/s11999-017-5315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metsemakers W, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, Athanasou NA, Ochsner PE, Kuehl R, Raschke M, Borens O, Xie Z, Velkes S, Hungerer S, Kates SL, Zalavras C, Giannoudis PV, Richards RG, Verhofstad MHJ. 2018. Fracture-related infection: a consensus on definition from an international expert group. Injury 49:505–510. doi: 10.1016/j.injury.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Fife A, Atkins B, Crook D, Berendt AR. 1996. Analysis of clonality of coagulasenegative staphylococcal infections of prosthetic joints using pulsed field gel electrophoresis. Poster. Federation of Infection Societies Meeting, Manchester, UK. J Infect 34:175. [Google Scholar]
- 26.Peel TN, Spelman T, Dylla BL, Hughes JG, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. 2017. Optimal periprosthetic tissue specimen number for diagnosis of prosthetic joint infection. J Clin Microbiol 55:234–243. doi: 10.1128/JCM.01914-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hischebeth GTR, Randau TM, Molitor E, Wimmer MD, Hoerauf A, Bekeredjian-Ding I, Gravius S. 2016. Comparison of bacterial growth in sonication fluid cultures with periprosthetic membranes and with cultures of biopsies for diagnosing periprosthetic joint infection. Diagn Microbiol Infect Dis 84:112–115. doi: 10.1016/j.diagmicrobio.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Portillo ME, Salvadó M, Sorli L, Alier A, Martínez S, Trampuz A, Gómez J, Puig L, Horcajada JP. 2012. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect 65:541–548. doi: 10.1016/j.jinf.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. 2012. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol 50:3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol 47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano MH, Klautau GB, da Silva CB, Nigro S, Avanzi O, Mercadante MT, Salles MJC. 2014. Improved diagnosis of infection associated with osteosynthesis by use of sonication of fracture fixation implants. J Clin Microbiol 52:4176–4182. doi: 10.1128/JCM.02140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.