The rapid identification of blood culture isolates and antimicrobial susceptibility test (AST) results play critical roles for the optimal treatment of patients with bloodstream infections. Whereas others have looked at the time to detection in automated culture systems, we examined the overall time from specimen collection to actionable test results.
KEYWORDS: blood culture, clinical microbiology, laboratory automation, laboratory workflow
ABSTRACT
The rapid identification of blood culture isolates and antimicrobial susceptibility test (AST) results play critical roles for the optimal treatment of patients with bloodstream infections. Whereas others have looked at the time to detection in automated culture systems, we examined the overall time from specimen collection to actionable test results. We examined four points of time, namely, blood specimen collection, Gram stain, organism identification (ID), and AST reports, from electronic data from 13 U.S. hospitals for the 11 most common, clinically significant organisms in septic patients. We compared the differences in turnaround times and the times from when specimens were collected and the results were reported in the 24-h spectrum. From January 2015 to June 2016, 165,593 blood specimens were collected, of which, 9.5% gave positive cultures. No matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry was used during the study period. Across the 10 common bacterial isolates (n = 6,412), the overall median (interquartile range) turnaround times were 0.80 (0.64 to 1.08), 1.81 (1.34 to 2.46), and 2.71 (2.46 to 2.99) days for Gram stain, organism ID, and AST, respectively. For all positive cultures, approximately 25% of the specimens were collected between 6:00 a.m. and 11:59 a.m. In contrast, more of the laboratory reporting times were concentrated between 6:00 a.m. and 11:59 a.m. for Gram stain (43%), organism ID (78%), and AST (82%), respectively (P < 0.001). The overall average turnaround times from specimen collection for Gram stain, organism ID, and AST were approximately 1, 2, and 3 days, respectively. The laboratory results were reported predominantly in the morning hours. Laboratory automation and work flow optimization may play important roles in reducing the microbiology result turnaround time.
INTRODUCTION
The consensus guidelines for managing patients with bloodstream infections specify a collection of blood cultures before the initiation of empirical antibiotic therapy and a de-escalation to the most appropriate therapy as soon as the pathogen identification and antimicrobial susceptibility profile are known (1). The value of blood cultures is emphasized by the observation that empirical antibiotic therapy is frequently inappropriate and associated with increased morbidity and mortality, prolonged hospitalizations, and associated increased hospital costs (2–7). A decrease in the time between the collection of blood cultures and the reportable results is associated with a reduced time to optimal therapy, improved patient outcomes, and decreased hospital costs (8–15). One method to decrease the time to actionable results is to eliminate the needs to subculture positive blood culture bottles and incubate the subculture plates overnight before the identification and susceptibility tests are performed. Variable results have been reported for processing positive blood culture broths directly (16–20). These variable results are a function of the organism identity (e.g., there is better success with Gram-negative rods than with Gram-positive cocci) and the methods used to concentrate the organisms and standardize the test inoculum; however, it is now generally accepted that the direct testing of positive blood culture broths can be performed successfully (21–23). A further reduction in the time to accurate identification of blood culture isolates is obtained by processing positive blood culture broths directly using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (24–27).
Quantitative data regarding current microbiological culture processes and the time needed for clinicians to obtain the results from the specimen collection are scarce. Savinelli et al. (28) reported that the delays in reporting positive blood cultures were significantly impacted by laboratory staffing, and a recent survey across five centers in Italy revealed that the turnaround time for blood culture results was significantly influenced by laboratory workflow practices (28, 29). In this study, we examined blood culture turnaround times (TATs) for three actionable results: Gram-stain, microorganism identification (ID), and antimicrobial susceptibility tests (ASTs).
(The preliminary data were presented in part at the European Congress of Clinical Microbiology and Infectious Diseases 2017 and ASM Microbe 2017.)
MATERIALS AND METHODS
Data.
We used the BD Insights research database (30, 31) of Becton, Dickinson and Company (Franklin Lakes, NJ) to analyze deidentified microbiological data that were electronically captured from 13 acute care hospitals in the United States. The microbiological data were captured as part of automated hospital-wide infections surveillance. The numbers and sizes of the study hospitals were 1 small (<100 beds), 8 medium (100 to 300 beds), and 4 large (>300 beds) hospitals. One was an academic medical center and 12 were nonacademic centers. Six hospitals performed identifications and antibiotic susceptibility tests with Vitek 2 (bioMérieux, Hazelwood, MO), six hospitals used MicroScan (Beckman Coulter, Brea, CA), and the method used by one was unknown at the time of the study. No hospital identified organisms routinely with MALDI mass spectrometry. The study protocol was approved by the New England Institutional Review Board/Human Subjects Research Committee (Wellesley, MA) and conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Definition of TAT.
We examined three blood culture TATs: (i) from blood specimen collection to the first Gram stain report, (ii) from specimen collection to the first organism ID, and (iii) from specimen collection to the first AST. We compared the differences in TATs for the 11 (10 bacterial species and 1 aggregated fungal species) most common, clinically significant organisms in septic patients, based on the literature (32, 33) and by examining the frequency distribution from this study. Among the 10 bacterial species, we combined categories with lower frequencies for further statistical analysis. We also examined the time from when the specimens were collected and the results were reported in the 24-h spectrum.
Statistical analysis.
We conducted descriptive statistical analysis by using the χ2 test to compare the rate differences, with a two-tailed P value of <0.05 designated as a significant difference. The data were analyzed using SAS V9.4 (SAS Institute Inc., Cary, NC).
RESULTS
From 1 January 2015 to 30 June 2016, 165,593 blood specimens were collected, of which 9.5% resulted in positive cultures. The 10 most common and clinically important bacterial isolates (n = 6,412) accounted for 76.5% of the overall isolates (Table 1). Among the bacterial isolates, 61.5% (3,945/6,412) were Gram positive and 38.5% (2,467/6,412) were Gram negative. Approximately 6.2% of the overall isolates (516/8,383) were yeasts and 17.4% (1,455/8,383) were polymicrobial mixtures. They were included in this analysis for comparative purposes. The proportions of bacterial isolates tested for antimicrobial susceptibility ranged from 58.8% (Staphylococcus aureus) to 73.0% (Escherichia coli), with no yeast isolates tested (Table 1).
TABLE 1.
Major organisms and antimicrobial susceptibility test distribution
| Major organism | No. (%) of isolates | Antimicrobial susceptibility testing |
|
|---|---|---|---|
| n | % | ||
| Bacteria | 6,412 (76.5) | 4,166 | 65.0 |
| Gram positive | |||
| Staphylococcus aureus | 2,690 (32.1) | 1,583 | 58.8 |
| Streptococcus agalactiae, S. pneumoniae, S. pyogenes | 688 (8.2) | 420 | 61.0 |
| Enterococcus faecalis, E. faecium | 567 (6.8) | 383 | 67.5 |
| Sum | 3,945 (47.1) | 2,386 | 60.5 |
| Gram negative | |||
| Escherichia coli | 1618 (19.3) | 1,181 | 73.0 |
| Klebsiella pneumoniae | 462 (5.5) | 326 | 70.6 |
| Pseudomonas aeruginosa | 202 (2.4) | 140 | 69.3 |
| Proteus mirabilis | 185 (2.2) | 133 | 71.9 |
| Sum | 2,467 (29.4) | 1,780 | 72.2 |
| Fungi | |||
| Candidaa | 516 (6.2) | 0 | 0.0 |
| Polymicrobial | 1,455 (17.4) | 888 | 61.0 |
| Total | 8,383 (100.0) | 5,054 | 60.3 |
Candida species with the highest frequencies included C. albicans, C. krusei, C. parapsilosis, and C. tropicalis.
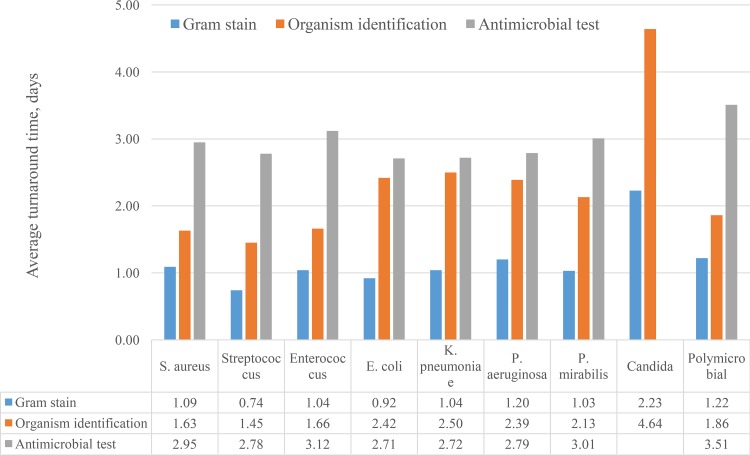
The median (interquartile range) turnaround times for bacterial isolates from specimen collection were 0.80 (0.64 to 1.08), 1.81 (1.34 to 2.46), and 2.71 (2.46 to 2.99) days for Gram stain, organism ID, and AST, respectively (Table 2). The average turnaround times differed by the type of organism for all three measures (all P < 0.001) (Fig. 1). The detection of growth and initial Gram-stain reports were earliest with Streptococcus spp. (average of 0.74 days) and Escherichia coli (average of 0.92 days) and longest with Candida spp. (average of 2.23 days). The Gram stain results for polymicrobial mixtures were intermediate at 1.22 days on average. The average TAT ranged from 1.45 (Streptococcus spp.) to 4.64 (Candida spp.) for ID and from 2.71 (E. coli) to 3.51 (polymicrobial mixtures) days for the AST results (Fig. 1).
TABLE 2.
Laboratory turnaround time for the most common organisms
| Major organism | No. (%) of isolates |
Turnaround time (days) (median [IQRa]) |
||
|---|---|---|---|---|
| Gram stain (n = 8,024) |
Organism identification (n = 8,383) |
Antimicrobial test (n = 5,054) |
||
| Bacteria | 6,412 (76.5) | 0.80 (0.64–1.08) | 1.81 (1.34–2.46) | 2.71 (2.46–2.99) |
| Gram positive | ||||
| Staphylococcus aureus | 2,690 (32.1) | 0.89 (0.72–1.20) | 1.62 (1.00–1.95) | 2.79 (2.55–3.10) |
| Streptococcus agalactiae, S. pneumoniae, S. pyogenes |
688 (8.2) | 0.67 (0.59–0.79) | 1.50 (0.80–1.78) | 2.68 (2.45–2.90) |
| Enterococcus faecalis, E. faecium | 567 (6.8) | 0.82 (0.69–1.07) | 1.62 (0.87–1.99) | 2.90 (2.62–3.20) |
| Gram negative | ||||
| Escherichia coli | 1,618 (19.3) | 0.70 (0.58–0.97) | 2.45 (1.91–2.73) | 2.61 (2.37–2.86) |
| Klebsiella pneumoniae | 462 (5.5) | 0.77 (0.61–1.13) | 2.44 (1.92–2.77) | 2.57 (2.26–2.84) |
| Pseudomonas aeruginosa | 202 (2.4) | 0.82 (0.65–1.18) | 1.96 (1.57–2.61) | 2.67 (2.51–2.88) |
| Proteus mirabilis | 185 (2.2) | 0.95 (0.81–1.37) | 2.14 (1.79–2.81) | 2.83 (2.63–3.00) |
| Fungi | ||||
| Candidab | 516 (6.2) | 1.98 (1.38–2.87) | 3.76 (2.84–5.10) | NAc |
| Polymicrobial | 1,455 (17.4) | 0.99 (0.74–1.48) | 1.69 (1.09–2.34) | 3.10 (2.70–3.89) |
| Overall | 8,383 (100.0) | 0.85 (0.66–1.27) | 1.83 (1.34–2.55) | 2.76 (2.49–3.12) |
IQR, interquartile range.
Candida species with the highest frequencies included C. albicans, C. krusei, C. parapsilosis, and C. tropicalis.
NA, not available.
FIG 1.
Average turnaround time by organism.
Compared to those for smaller hospitals (≤300 beds), larger hospitals (>300 beds) had slightly shorter Gram stain turnaround times for E. coli, K. pneumoniae, and Enterococcus and shorter organism ID times for E. coli and Pseudomonas aeruginosa (all P < 0.05), but this pattern was not consistent across organisms (data not shown).
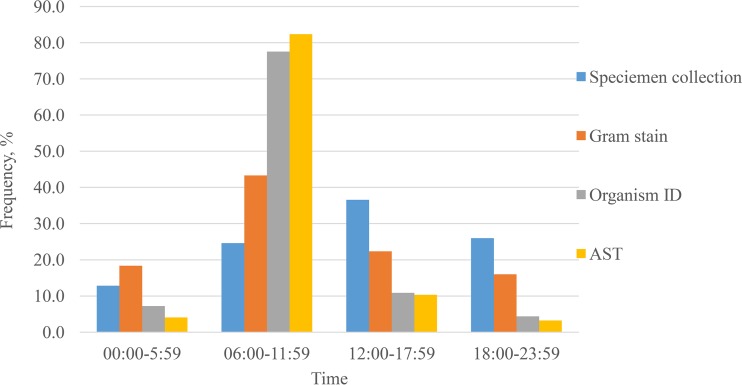
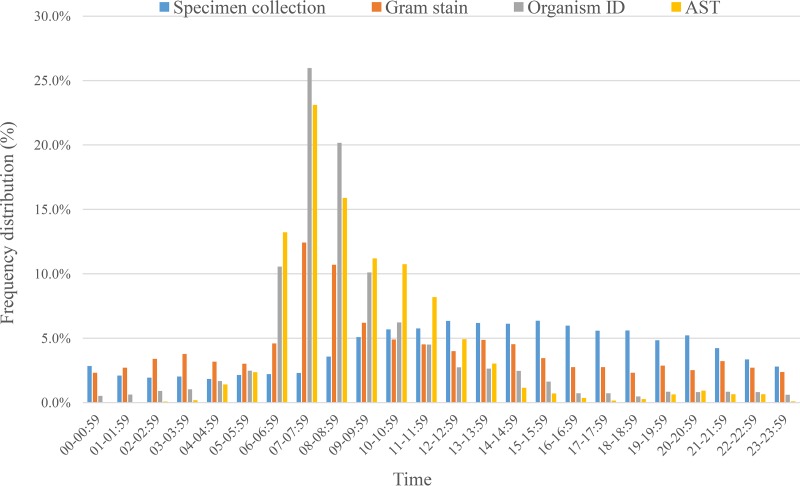
The distributions of blood specimen collection times and culture result reporting times are presented in Fig. 2. A low baseline of blood specimen collections occurred throughout the 24-h period, with approximately 5% of the specimens collected each hour from 09:00 to 22:00. In contrast, the laboratory reporting times were more concentrated between 6:00 a.m. and 11:59 a.m. for Gram stain (43%), organism ID (78%), and AST (82%) results (P < 0.001), with the peak number of all reports between 7:00 and 8:59 a.m. (Fig. 3). When examining by hospital, 10 of the 13 hospitals reported Gram stain results throughout the 24-h period, and three hospitals reported these results only during the day shift (see Table S1 and Fig. S1 in the supplemental material). Organism ID results were reported throughout the 24-h period by only four hospitals (see Table S2 and Fig. S2). No hospital reported AST results throughout the 24-h period (see Table S3 and Fig. S3).
FIG 2.
Distribution of blood culture result reporting times.
FIG 3.
Hourly frequency distributions of blood culture result reporting times.
DISCUSSION
On average, hospital microbiology laboratories in this study took approximately 1 day from the time of specimen collection to obtain Gram stain results, 2 days to identify organisms, and 3 days to report antimicrobial susceptibility results. Previous studies have found that empirical antibiotic therapy is frequently inappropriate, which leads to increased risks for morbidity and mortality, prolonged hospitalizations, and incremental costs (2–8). The recognition of the delays in the availability of microbiological results presents an opportunity for improvement in the care of patients with bloodstream infections. Slow turnaround times for blood culture results were observed despite the availability of rapid identification directly from positive blood culture broths (e.g., MALDI mass spectrometry and spot biochemical tests) and rapid AST results with automated susceptibility platforms such as Vitek and Phoenix. It is interesting that six of the hospitals used the Vitek system for ID and AST, but despite the potential availability of early results, the TATs were similar to those observed for the other six hospitals using MicroScan systems. It is unclear to what extent the 13 hospitals in this study have adopted other advanced technologies or fully implemented the technologies to maximize their benefits.
Although some new technologies may be viewed as expensive, the cost of their adoption may be much lower than the costs associated with delayed appropriate antimicrobial treatment of septic patients. In addition to the adverse impact of inappropriate empirical treatment on patient outcomes, a prolonged use of broad-spectrum antimicrobials is a known risk factor associated with the development and spread of antimicrobial-resistant organisms. Recent studies have shown that antimicrobial infections resulted in a larger net loss for hospitals, exceeding $10,000 per case for multidrug-resistant P. aeruginosa infection (34) or carbapenem-resistant infections (35), compared to that for infection cases with the same pathogens without antimicrobial resistance. Furthermore, the Centers for Medicare and Medicaid has implemented a policy that financially penalizes hospitals that have higher than expected nosocomial infections associated with antibiotic use, such as C. difficile infections (36). All these excess burdens to patients and hospitals call for early identification of organisms and AST status, which enables targeted appropriate antimicrobial therapy to reduce the clinical and financial burdens as well as the antimicrobial resistance risk.
Our finding that organism ID and AST reporting are heavily concentrated in the morning hours indicates that the reporting of the blood culture results is aligned with workflow practices. In theory, the results of Gram stains, ID, and ASTs should have a distribution that parallels the collection time for blood cultures. Although we do not know the exact operational hours of the 13 laboratories, 10 of the 13 hospitals reported Gram stain results throughout the day, evening, and night hours, albeit with a high concentration in the morning hours (Fig. 2 and 3; see also Fig. S1 and Table S1 in the supplemental material). In contrast, ID or AST results were reported infrequently during the 10-h period from 18:00 to 04:00 (Fig. 3), with 9 laboratories reporting almost no ID and all 13 almost no ASTs during this period of time (Fig. 2S and 3S and Table 2S and 3S). Thus, the majority of laboratories apparently had 24-h staffing sufficient for performing and reporting Gram stains but did not appear to use this staffing for reporting ID or AST results. This points to an opportunity to modify laboratory workflow processes by prioritizing the identification of positive blood culture isolates and the reporting of AST results. Although this benefit can be maximized by the adoption of technologies such as MALDI-TOF mass spectrometry for organism identification and rapid AST methodologies, an improved TAT of results could also be realized by modifying workflow practices using the existing laboratory technologies.
Limitations.
Our study hospitals included small, medium, and large hospitals, including one academic teaching hospital and 12 nonteaching hospitals. This might lead to more robust findings than an analysis of a single center, but it did not represent national hospital characteristics. Further investigation on the differences in types of hospitals, such as academic versus nonacademic and rural versus urban, using a larger number of hospitals may shed more light on the microbiology laboratory process in the United States. Given that 12 of 13 sites in our study were confirmed to not use MALDI-TOF mass spectrometry, our study results might more likely represent a conventional testing environment. Future studies should expand the scope of sites to directly assess the impact of the new technology. Although the documentation of current practices with a larger and more diverse data set may be useful, we believe the main value of this study is by challenging microbiologists to look critically at their workflow practices and seek ways to reduce delays in reporting results. The adoption of new rapid technologies and automation can provide opportunities for workflow and reporting improvements, but significant improvements can be made with adjustments in the priorities of processing critically important specimens such as positive blood cultures.
Another limitation of our study is that we were unable to depict comprehensively the technology and staffing and shift policy, such as night and weekend coverage, or determine if each hospital was using an onsite microbiological laboratory or processing in a reference laboratory; hence, we were not able to analyze the underlying contributing factors for the turnaround time delays. However, even without depicting the underlying causes, the TAT results reported in this study might still stimulate the readership to assess their own practices and workflow priorities. In fact, half of the laboratories used a rapid automated platform for ID and AST and still demonstrated significant delays in the time to reported results. This points to the need for integrated process improvement.
Despite these limitations, the consistent findings in this subset of U.S. hospitals are likely to be more broadly applicable. Indeed, laboratory directors may want to review their operational practices and, if they find similar processing delays, look for opportunities to streamline the transfer of the most clinically impactful diagnostic information in a timely manner.
Conclusion.
The average turnaround times from specimen collection for Gram stain, organism ID, and AST in this study were approximately 1, 2, and 3 days, respectively. The laboratory results were reported predominantly in the morning hours. An integrated laboratory workflow optimization and adoption of technological innovations such as MALDI-TOF mass spectrometry, rapid AST platforms, and further laboratory automation will play important roles in reducing the microbiology result turnaround time.
Supplementary Material
ACKNOWLEDGMENTS
We thank Richard Johannes and David Sellers for their clinical insights and Marc Krawitz for his assistance in database management and domain expertise at the early stage of this study.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
All authors are full-time employees of Becton, Dickinson, and Co., Franklin Lakes, NJ, USA. The study was conducted as part of the authors’ routine work. Y.P.T. and P.R.M. conceived of and designed the study and drafted the manuscript; Y.P.T., L.V., G.Y., K.J., V.G., and P.R.M analyzed and interpreted the data and revised and approved the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00500-18.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Diamantis S, Rioux C, Bonnal C, Farfour É, Papy E, Andremont A, Yeni P, Bouvet É, Lucet J-C. 2012. Suitability of initial antibiotic therapy for the treatment of bloodstream infections and the potential role of antibiotic management teams in improving it. Eur J Clin Microbiol Infect Dis 31:1667–1671. doi: 10.1007/s10096-011-1491-8. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Dickstein Y, Raz-Pasteur A. 2016. Antibiotic de-escalation for bloodstream infections and pneumonia: systematic review and meta-analysis. Clin Microbiol Infect 22:960–967. doi: 10.1016/j.cmi.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raman G, Avendano E, Berger S, Menon V. 2015. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect Dis 15:395. doi: 10.1186/s12879-015-1123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH. 2011. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med 39:46–51. doi: 10.1097/CCM.0b013e3181fa41a7. [DOI] [PubMed] [Google Scholar]
- 7.Yokota PK, Marra AR, Martino MD, Victor ES, Durão MS, Edmond MB, dos Santos OF. 2014. Impact of appropriate antimicrobial therapy for patients with severe sepsis and septic shock–a quality improvement study. PLoS One 9:e104475. doi: 10.1371/journal.pone.0104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barenfanger J, Graham DR, Kolluri L, Sangwan G, Lawhorn J, Drake CA, Verhulst SJ, Peterson R, Moja LB, Ertmoed MM, Moja AB, Shevlin DW, Vautrain R, Callahan CD. 2008. Decreased mortality associated with prompt Gram staining of blood cultures. Am J Clin Pathol 130:870–876. doi: 10.1309/AJCPVMDQU2ZJDPBL. [DOI] [PubMed] [Google Scholar]
- 9.Beekmann S, Diekema D, Chapin K, Doern G. 2003. Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. J Clin Microbiol 41:3119–3125. doi: 10.1128/JCM.41.7.3119-3125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French K, Evans J, Tanner H, Gossain S, Hussain A. 2016. The clinical impact of rapid, direct MALDI-ToF identification of bacteria from positive blood cultures. PLoS One 11:e0169332. doi: 10.1371/journal.pone.0169332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockwood AM, Perez KK, Musick WL, Ikwuagwu JO, Attia E, Fasoranti OO, Cernoch PL, Olsen RJ, Musser JM. 2016. Integrating rapid diagnostics and antimicrobial stewardship in two community hospitals improved process measures and antibiotic adjustment time. Infect Control Hosp Epidemiol 37:425–432. doi: 10.1017/ice.2015.313. [DOI] [PubMed] [Google Scholar]
- 12.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, Peterson LE, Musser JM. 2013. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 137:1247–1254. doi: 10.5858/arpa.2012-0651-OA. [DOI] [PubMed] [Google Scholar]
- 13.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM. 2014. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69:216–225. doi: 10.1016/j.jinf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Verroken A, Defourny L, de Waroux OP, Belkhir L, Laterre P-F, Delmée M, Glupczynski Y. 2016. Clinical impact of MALDI-TOF MS identification and rapid susceptibility testing on adequate antimicrobial treatment in sepsis with positive blood cultures. PLoS One 11:e0156299. doi: 10.1371/journal.pone.0156299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlek AL, Bonten MJ, Boel CE. 2012. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589. doi: 10.1371/journal.pone.0032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Cueto M, Ceballos E, Martinez-Martinez L, Perea EJ, Pascual A. 2004. Use of positive blood cultures for direct identification and susceptibility testing with the Vitek 2 system. J Clin Microbiol 42:3734–3738. doi: 10.1128/JCM.42.8.3734-3738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funke G, Funke-Kissling P. 2004. Use of the BD PHOENIX Automated Microbiology System for direct identification and susceptibility testing of Gram-negative rods from positive blood cultures in a three-phase trial. J Clin Microbiol 42:1466–1470. doi: 10.1128/JCM.42.4.1466-1470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gherardi G, Angeletti S, Panitti M, Pompilio A, Di Bonaventura G, Crea F, Avola A, Fico L, Palazzo C, Sapia GF, Visaggio D, Dicuonzo G. 2012. Comparative evaluation of the Vitek-2 Compact and Phoenix systems for rapid identification and antibiotic susceptibility testing directly from blood cultures of Gram-negative and Gram-positive isolates. Diagn Microbiol Infect Dis 72:20–31. doi: 10.1016/j.diagmicrobio.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Lupetti A, Barnini S, Castagna B, Nibbering P, Campa M. 2010. Rapid identification and antimicrobial susceptibility testing of Gram‐positive cocci in blood cultures by direct inoculation into the BD Phoenix system. Clin Microbiol Infect 16:986–991. doi: 10.1111/j.1469-0691.2009.03006.x. [DOI] [PubMed] [Google Scholar]
- 20.Yonetani S, Okazaki M, Araki K, Makino H, Fukugawa Y, Okuyama T, Ohnishi H, Watanabe T. 2012. Direct inoculation method using BacT/ALERT 3D and BD Phoenix System allows rapid and accurate identification and susceptibility testing for both Gram-positive cocci and Gram-negative rods in aerobic blood cultures. Diagn Microbiol Infect Dis 73:129–134. doi: 10.1016/j.diagmicrobio.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Florio W, Barnini S, Morici P, Lupetti A. 2015. Direct inoculation of positive blood cultures using the phoenix system for antimicrobial susceptibility testing of both Gram-positive and Gram-negative bacteria. J Med Microbiol 64:582–585. doi: 10.1099/jmm.0.000053. [DOI] [PubMed] [Google Scholar]
- 22.Lupetti A, Barnini S, Morici P, Ghelardi E, Nibbering P, Campa M. 2013. Saponin promotes rapid identification and antimicrobial susceptibility profiling of Gram-positive and Gram-negative bacteria in blood cultures with the Vitek 2 system. Eur J Clin Microbiol Infect Dis 32:493–502. doi: 10.1007/s10096-012-1762-z. [DOI] [PubMed] [Google Scholar]
- 23.Prod'hom G, Durussel C, Greub G. 2013. A simple blood-culture bacterial pellet preparation for faster accurate direct bacterial identification and antibiotic susceptibility testing with the VITEK 2 system. J Med Microbiol 62:773–777. doi: 10.1099/jmm.0.049361-0. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira L, Sánchez‐Juanes F, Porras ‐Guerra I, García‐García M, García‐Sánchez J, González‐Buitrago J, Muñoz‐Bellido J. 2011. Microorganisms direct identification from blood culture by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Clin Microbiol Infect 17:546–551. doi: 10.1111/j.1469-0691.2010.03257.x. [DOI] [PubMed] [Google Scholar]
- 25.Hazelton B, Thomas LC, Olma T, Kok J, O'Sullivan M, Chen SC-A, Iredell JR. 2014. Rapid and accurate direct antibiotic susceptibility testing of blood culture broths using MALDI Sepsityper combined with the BD Phoenix automated system. J Medical Microbiology 63:1590–1594. doi: 10.1099/jmm.0.075580-0. [DOI] [PubMed] [Google Scholar]
- 26.Kok J, Thomas LC, Olma T, Chen SC, Iredell JR. 2011. Identification of bacteria in blood culture broths using matrix-assisted laser desorption-ionization Sepsityper and time of flight mass spectrometry. PLoS One 6:e23285. doi: 10.1371/journal.pone.0023285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machen A, Drake T, Wang YFW. 2014. Same day identification and full panel antimicrobial susceptibility testing of bacteria from positive blood culture bottles made possible by a combined lysis-filtration method with MALDI-TOF VITEK mass spectrometry and the VITEK2 system. PLoS One 9:e87870. doi: 10.1371/journal.pone.0087870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savinelli T, Parenteau S, Mermel LA. 2004. What happens when automated blood culture instrument detect growth but there are no technologists in the microbiology laboratory? Diagn Microbiol Infect Dis 48:173–174. doi: 10.1016/j.diagmicrobio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Arena F, Argentieri M, Bernaschi P, Fortina G, Kroumova V, Manso E, Montanera PG, Nicoletti P, Pecile P, Rassu M, Rossolini GM, Spanu T, Clerici P, Fontana C. 2016. Real life turnaround time of blood cultures in the clinical microbiology laboratory: results of the first Italian survey, May 2015. Microbiol Med 31. doi: 10.4081/mm.2016.6127. [DOI] [Google Scholar]
- 30.Zilberberg MD, Tabak YP, Sievert DM, Derby KG, Johannes RS, Sun X, McDonald LC. 2011. Using electronic health information to risk-stratify rates of Clostridium difficile infection in U.S. hospitals. Infect Control Hosp Epidemiol 32:649–655. doi: 10.1086/660360. [DOI] [PubMed] [Google Scholar]
- 31.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. 2013. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 34:588–596. doi: 10.1086/670621. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 33.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. 2006. Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med 34:2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 34.Tabak Y, Merchant S, Ye G, Vankeepuram L, Gupta V, Kurtz S, Puzniak L. 2018. 47: incremental clinical and economic burden of multidrug-resistant P. Aeruginosa respiratory infection. Crit Care Med 46:24. doi: 10.1097/01.ccm.0000528102.19156.1a. [DOI] [PubMed] [Google Scholar]
- 35.Tabak Y, Sung A, Johannes R, Ye G, Kurtz S, Vankeepuram L, Gupta V, Mccann E. 2017. Attributable clinical and economic burden of carbapenem non-susceptible Gram-negative infections among patients hospitalized with respiratory infections. Open Forum Infect Dis 4:S147. doi: 10.1093/ofid/ofx163.233. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services 2018. Hospital-acquired condition reduction program. https://www.medicare.gov/hospitalcompare/HAC-reduction-program.html. Accessed 22 March 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.