Abstract
In this article, caspase activation in identifiable reticulospinal neurons of lampreys was inhibited after a complete spinal cord injury using a specific agonist of the GABAA receptor (muscimol). The data presented in this article are quantifications of fluorescent labelling of identifiable descending neurons of larval lampreys after a complete spinal cord injury using fluorochrome-labelled inhibitors of caspases (FLICA) and the corresponding statistical analysis. A single dose of muscimol decreased the intensity of FLICA labelling in giant identifiable reticulospinal neurons following spinal cord injury in lampreys.
Specifications table
| Subject area | Neuroscience |
| More specific subject area | Regenerative biology |
| Type of data | Graph, Figure, Table |
| How data was acquired | Confocal microscope (TCS-SP2; Leica, Wetzlar, Germany) |
| Data format | Analysed data, processed data. |
| Experimental factors | Larval sea lampreys were treated with muscimol after a complete spinal cord injury. Caspase activation was analysed in identifiable descending neurons using fluorochrome-labelled inhibitors of caspases (FLICA). |
| Experimental features | The effect of muscimol on caspase activation after a complete spinal cord injury was analysed using fluorescence microscopy. |
| Data source location | Department of Functional Biology, Faculty of Biology, CIBUS, Universidade de Santiago de Compostela |
| Data accessibility | The data are available within this article. |
| Related research article | Romaus-Sanjurjo et al. [1] |
Value of the data
-
•
Fluorochrome-labelled inhibitors of caspases allow the detection of changes in caspase activation.
-
•
This dataset is of interest for those studying signalling pathways modulating neuronal survival after spinal cord injury in fishes.
-
•
This dataset is of interest for the development of neuroprotectants as a therapy for spinal cord injury.
1. Data
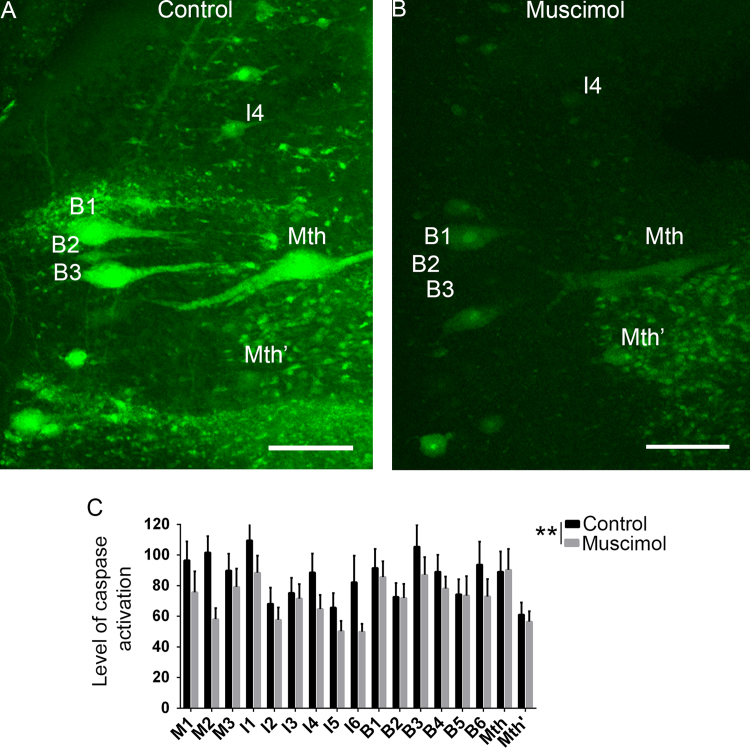
Recently, our group reported that endogenous GABA promotes axonal regeneration of identifiable reticulospinal neurons after a complete spinal cord transection in lampreys [1]. The beneficial effect of GABA appears to be caused by a reduction in the activation of caspases through the activation of GABAB receptors [1]. Other authors also reported that increased GABAergic inhibition trough GABAA receptors is related to a better recovery of function following spinal cord injury in lampreys [2]. Here, we show that the activation of GABAA receptors after a complete spinal cord transection also inhibits caspase activation in identifiable reticulospinal neurons of lampreys. A treatment with a single dose of muscimol, which is a specific agonist of the GABAA receptor, following a complete spinal cord transection reduced the activation of caspases in giant reticulospinal neurons of lampreys as revealed by a decreased fluorescent intensity of FLICA labelling (Fig. 1). Table 1 shows the mean ± S.E.M. of fluorescence intensity of each neuronal type and the number of identifiable neurons used for the statistical analysis. The data from each individual neuron is given in Table S1.
Fig. 1.
Muscimol treatment inhibits caspase activation in identifiable descending neurons. A: Photomicrograph of a whole-mounted brain showing identifiable descending neurons with intense FLICA labelling in control animals. B: Photomicrograph of a whole-mounted brain showing identifiable descending neurons with a reduction in the intensity of FLICA labelling in muscimol treated animals. C: Graph showing a significant change (Mann Whitney U-test, p = 0.0054; asterisks) in the level of caspase activation (intensity of fluorescent FLICA labelling; Y axis) after the muscimol treatment in identifiable descending neurons (X axis). Rostral is up and the ventricle to the left in all photomicrographs. Scale bars: 150 µm.
Table 1.
Table showing the total number of identifiable reticulospinal neurons that were included in the analyses and the mean ± S.E.M. of fluorescence intensity of FLICA labelling of each identifiable neuron.
The data from each individual neuron is given in Table S1.
|
Control |
Muscimol |
|||||
|---|---|---|---|---|---|---|
| Mean | S.E.M. | N | Mean | S.E.M. | N | |
| M1 | 96.53958 | ± 12.3778 | 12 | 75.67378 | ± 13.63257 | 14 |
| M2 | 101.704 | ± 10.53632 | 12 | 58.14621 | ± 7.223279 | 14 |
| M3 | 89.88461 | ± 10.92918 | 13 | 79.14021 | ± 12.03266 | 14 |
| I1 | 109.5599 | ± 15.80085 | 12 | 88.42269 | ± 11.18598 | 16 |
| I2 | 68.21775 | ± 10.45724 | 12 | 57.74719 | ± 7.989043 | 16 |
| I3 | 75.28416 | ± 9.889297 | 13 | 71.58913 | ± 9.475986 | 16 |
| I4 | 88.61723 | ± 12.39366 | 13 | 64.75288 | ± 9.115862 | 16 |
| I5 | 65.69509 | ± 9.503008 | 11 | 50.27394 | ± 6.789322 | 16 |
| I6 | 82.24782 | ± 17.39615 | 11 | 49.91087 | ± 5.125011 | 16 |
| B1 | 91.59154 | ± 12.3856 | 15 | 85.62478 | ± 10.22685 | 18 |
| B2 | 72.6452 | ± 9.034819 | 15 | 71.90128 | ± 9.277125 | 18 |
| B3 | 105.5087 | ± 14.23046 | 15 | 87.06628 | ± 11.55049 | 18 |
| B4 | 89.14333 | ± 10.9955 | 15 | 78.09394 | ± 7.780739 | 18 |
| B5 | 74.33907 | ± 9.773049 | 15 | 73.49995 | ± 12.7075 | 18 |
| B6 | 93.76653 | ± 14.91869 | 15 | 72.91844 | ± 11.33416 | 18 |
| Mth | 89.1006 | ± 13.2872 | 15 | 90.32294 | ± 13.61253 | 18 |
| Mth׳ | 61.15147 | ± 7.887609 | 15 | 56.45844 | ± 6.828169 | 18 |
2. Experimental design, materials, and methods
2.1. Animals
All experiments involving animals were approved by the Bioethics Committee at the University of Santiago de Compostela and the Consellería do Medio Rural e do Mar of the Xunta de Galicia (License reference JLPV/IId; Galicia, Spain) and were performed in accordance to European Union and Spanish guidelines on animal care and experimentation. Animals were deeply anaesthetized with 0.1% MS-222 (Sigma, St. Louis, MO) in lamprey Ringer solution (137 mM NaCl, 2.9 mM KCl, 2.1 mM CaCl2, 2 mM HEPES; pH 7.4) before all experimental procedures and euthanized by decapitation at the end of the experiments.
Mature and developmentally stable larval sea lampreys, Petromyzon marinus L. (n = 17; between 95 and 120 mm in body length, 5–7 years of age), were used in the study. Larval lampreys were collected from the river Ulla (Galicia, Spain), with permission from the Xunta de Galicia) and maintained in aerated fresh water aquaria at 14–20 °C with a bed of river sediment until their use in experimental procedures. Lampreys were randomly distributed between the different experimental groups.
2.2. Spinal cord injury surgical procedures
Complete spinal cord transections were performed as previously described [3]. The rostral spinal cord was exposed from the dorsal midline at the level of the 5th gill by making a longitudinal incision with a scalpel (#11). A complete spinal cord transection was performed with Castroviejo scissors and the spinal cord cut ends were visualized under the stereomicroscope. Animals with a complete spinal cord transection were assigned to either a vehicle treated control group (n = 8) or to muscimol treated group (n = 9). After spinal transections, the animals were returned to fresh water tanks. The animals were allowed to recover in individual fresh water tanks at 19.5 °C. Animals were analysed 2-weeks post-lesion (wpl). The experiment was carried out in 3 different batches of animals.
2.3. Drug treatments
Muscimol was dissolved in distilled water at a concentration of 25 µM, soaked in a small piece of Gelfoam (Pfizer; New York, NY) and placed on top of the site of injury at the time of transection as previously described [4]. Gelfoam soaked in distilled water served as a control.
2.4. Detection of activated caspases in whole-mounted brain preparations
The Image-iT LIVE Green Poly Caspases Detection Kit (Cat. No. I35104, Invitrogen, USA) was used to detect activated caspases in identifiable descending neurons (the M1, M2, M3, I1, I2, I3, I4, I5, I6, B1, B2, B3, B4, B5, B6, Mth and Mth’ neurons) of larval sea lampreys 2 weeks after the complete spinal cord transection. This kit contains 1 vial (component A of the kit) of the lyophilized FLICA reagent (FAM-VAD-FMK). The reagent associates a fluoromethyl ketone (FMK) moiety, which can react covalently with a cysteine, with a caspase-specific aminoacid sequence [valine-alanine-aspartic acid (VAD)]. A carboxyfluorescein group (FAM) is attached as a fluorescent reporter. The FLICA reagent interacts with the enzyme active centre of an activated caspase via the recognition sequence, and then attaches covalently through the FMK moiety. Experiments for the detection of activated caspases in whole-mounted brain preparations using FLICA labelling were done as previously described [5].
2.5. Imaging and quantifications
The quantification of the intensity of FLICA labelling was done as previously described [6]. Briefly, photomicrographs were acquired with a spectral confocal microscope (model TCS-SP2; Leica, Wetzlar, Germany). Images were always acquired under the same microscope conditions for control and treated animals. Quantification of mean fluorescent intensity (mean grey value) of each identifiable neuron was done using the Fiji software [7]. The mean of fluorescence intensity of each type of identifiable descending neuron was used for statistical analyses. Figure plates were generated using Adobe Photoshop CS6 (Adobe Systems).
2.6. Statistical analyses
Statistical analysis was carried out using Prism 6 (GraphPad software, La Jolla, CA). Data are presented as mean ± S.E.M. Normality of the data was determined by the Shapiro-Wilk, D׳Agostino and Pearson omnibus and Kolmogorov–Smirnov normality tests. All data passed all the normality tests. The results of control versus treatment groups were analysed by Mann-Whitney U-test.
Acknowledgments
Grant sponsors: Spanish Ministry of Economy and Competitiveness and the European Regional Development Fund 2007–2013 (Grants number: BFU2014-56300-P and BFU2017-87079-P) to M.C.R. A.B.-I. was supported by a grant from the Xunta de Galicia (Grant number: 2016-PG008) and a grant from the crowdfunding platform Precipita (FECYT; Spanish Ministry of Economy and Competitiveness; grant number 2017-CP081). The authors would like to acknowledge the following individual donors of the crowdfunding campaign in Precipita: Blanca Fernández, Emilio Río, Guillermo Vivar, Pablo Pérez, Jorge Férnandez, Ignacio Valiño, Pago de los Centenarios, Eva Candal, María del Pilar Balsa, Jorge Faraldo, Isabel Rodríguez-Moldes, José Manuel López, Juan José Pita, María E. Cameán, Jesús Torres, José Pumares, Verónica Rodríguez, Sara López, Tania Villares Balsa, Rocío Lizcano, José García, Ana M. Cereijo, María Pardo, Nerea Santamaría, Carolina Hernández, Jesús López and María Maneiro. The authors thank the staff of Ximonde Biological Station for providing lampreys used in this study, and the Microscopy Service (University of Santiago de Compostela) and Dr. Mercedes Rivas Cascallar for confocal microscope facilities and help.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.11.003.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.11.003.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Romaus-Sanjurjo D., Ledo-García R., Fernández-López B., Hanslik K., Morgan J.R., Barreiro-Iglesias A., Rodicio M.C. GABA promotes survival and axonal regeneration in identifiable descending neurons after spinal cord injury in larval lampreys. Cell Death Dis. 2018;9:663. doi: 10.1038/s41419-018-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svensson E., Kim O., Parker D. Altered GABA and somatostatin modulation of proprioceptive feedback after spinal cord injury in lamprey. Neuroscience. 2013;3:109–118. doi: 10.1016/j.neuroscience.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Barreiro-Iglesias A., Zhang G., Selzer M.E., Shifman M.I. Complete spinal cord injury and brain dissection protocol for subsequent wholemount in situ hybridization in larval sea lamprey. J. Vis. Exp. 2014;92:e51494. doi: 10.3791/51494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobrido-Camean D., Robledo D., Sanchez L., Rodicio M.C., Barreiro-Iglesias A. Serotonin inhibits axonal regeneration of identifiable descending neurons after a complete spinal cord injury in lampreys. bioRxiv. 2018:335844. doi: 10.1242/dmm.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreiro-Iglesias A., A, Shifman M.I. Use of fluorochrome-labeled inhibitors of caspases to detect neuronal apoptosis in the whole-mounted lamprey brain after spinal cord injury. Enzym. Res. 2012;2012:835731. doi: 10.1155/2012/835731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreiro-Iglesias A., Sobrido-Cameán D., Shifman M.I. Retrograde activation of the extrinsic apoptotic pathway in spinal-projecting neurons after a complete spinal cord injury in lampreys. Biomed. Res. Int. 2017;2017:5953674. doi: 10.1155/2017/5953674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material