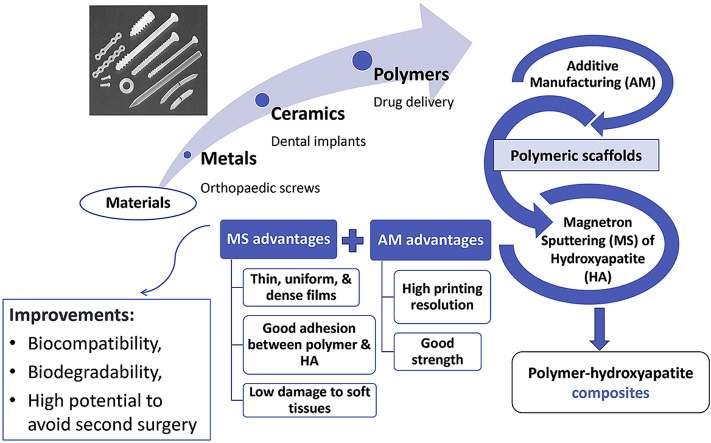
Abstract
The application of various materials in biomedical procedures has recently experienced rapid growth. One area that is currently receiving significant attention from the scientific community is the treatment of a number of different types of bone-related diseases and disorders by using biodegradable polymer-ceramic composites. Biomaterials, the most common materials used to repair or replace damaged parts of the human body, can be categorized into three major groups: metals, ceramics, and polymers. Composites can be manufactured by combining two or more materials to achieve enhanced biocompatibility and biomechanical properties for specific applications. Biomaterials must display suitable properties for their applications, about strength, durability, and biological influence. Metals and their alloys such as titanium, stainless steel, and cobalt-based alloys have been widely investigated for implant-device applications because of their excellent mechanical properties. However, these materials may also manifest biological issues such as toxicity, poor tissue adhesion and stress shielding effect due to their high elastic modulus. To mitigate these issues, hydroxyapatite (HA) coatings have been used on metals because their chemical composition is similar to that of bone and teeth. Recently, a wide range of synthetic polymers such as poly (l-lactic acid) and poly (l-lactide-co-glycolide) have been studied for different biomedical applications, owing to their promising biocompatibility and biodegradability. This article gives an overview of synthetic polymer-ceramic composites with a particular emphasis on calcium phosphate group and their potential applications in tissue engineering. It is hoped that synthetic polymer-ceramic composites such as PLLA/HA and PCL/HA will provide advantages such as eliminating the stress shielding effect and the consequent need for revision surgery.
Keywords: Synthetic polymers, Hydroxyapatite, Magnetron sputtering, 3D printing
Graphical abstract
Highlights
-
•
Polymer-ceramic composites and their biomedical applications.
-
•
Mechanical properties and biocompatibility of polymer-ceramic composites.
-
•
Manufacturing techniques for polymer-ceramic composites.
-
•
Magnetron sputtering in the manufacturing of polymer-hydroxyapatite composites.
1. Introduction
Many clinical applications such as treatments for orthopedic disorders are topics of interest in the field of tissue engineering. Owing to its importance and applications in human life, this area of research has attracted extensive attention from researchers and the field is thus expected to remain active indefinitely.
Bone-related disorders are becoming one of the main worldwide clinical health issues, in particular for the elderly. To repair and treat broken hard tissues, various types of biomaterials and devices have been widely applied in damaged parts of skeletal systems. Biomaterials can be divided into inorganics (metals and ceramics) and organics (polymers). Single-class materials may not be able to satisfy all of the requirements for a given implant application. Therefore, through the combination of two or more classes, composites with multiscale structures and the desired properties for specific applications are achievable. Metals and alloys such as stainless steels, cobalt-chromium (Co—Cr) alloys, and titanium (Ti) and its alloys play an important role as biomaterials for the fixation or replacement of load-bearing bones that have been damaged. Owing to their favorable properties such as high strength, high ductility, and high fracture toughness, more than 70% of implant devices are made from metallic biomaterials [1]. Although metallic materials possess high mechanical strength, there is still an issue with their biomechanical compatibility due to their higher elastic modulus compared to that of natural bone.
There are many other materials such as polymer-ceramic composites that could also be investigated. In the following sections, each of these classes is discussed, including their advantages and disadvantages.
1.1. Ceramics as biomaterials
As some of the main components of human bone tissue, ceramic-based materials such as calcium phosphate, tricalcium phosphate, tetra-calcium phosphate, alumina, silica, and zirconia have been studied for medical applications such as implant devices [2,3]. Because of their biocompatibility and their similar structure to that of natural bone, ceramics are a favorable group of biomaterials.
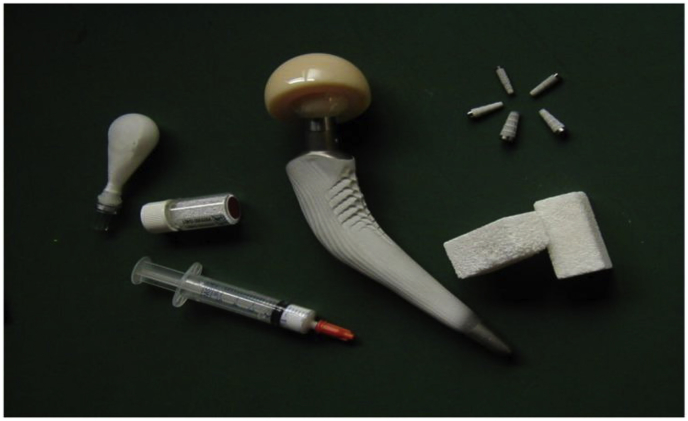
Ceramic nanobiomaterials, especially, calcium phosphate-based materials have been extensively studied for a broad range of orthopedic and dental applications, including β-tricalcium phosphate (β-TCP) and hydroxyapatite (HAp) [4,5]. Calcium phosphates play an important role in the human body and their degradation and bioactivity depend on the Ca/P ratio. These biomaterials can be used for various biomedical applications in the form of nanoparticles, cement, and coating [6]. The current biomedical applications of calcium phosphate-based materials include bone reconstruction [7], coating of orthopedic implants [8,9], dental applications [10], and drug delivery [11,12]. Fig. 1 shows the various biomedical devices made of calcium orthophosphates bioceramics.
Fig. 1.
Calcium phosphate-based biomaterials for bone graft applications (Adopted from Ref. [13]).
Due to the biological functions and possibility to control the mechanical properties of calcium phosphates, this family of ceramics have attracted much attention for different biomedical applications. Currently, great effort has been made to develop calcium phosphate-polymer composites, in particular, HAp and TCP.
1.1.1. Hydroxyapatite
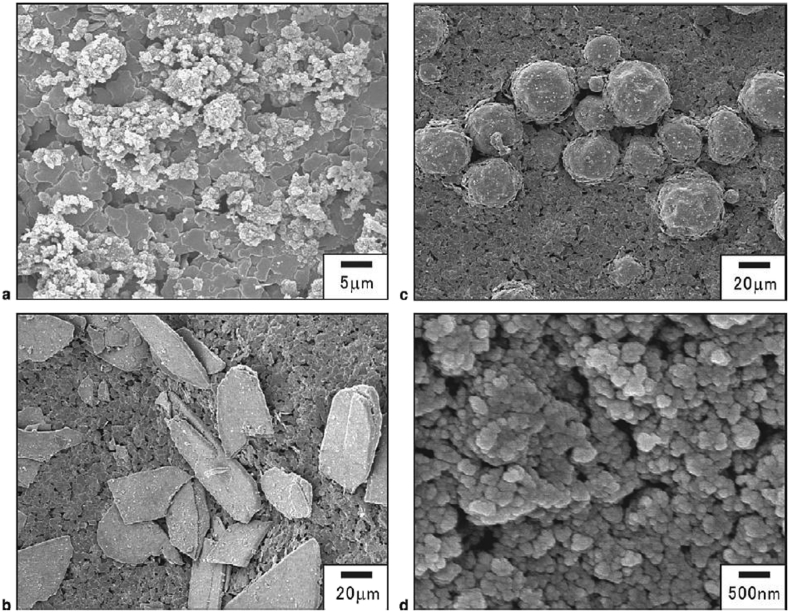
HA with the chemical formula of Ca10(PO4)6(OH)2 as a typical example of calcium phosphates family has been widely used as a bone substitute [[14], [15], [16], [17]]. HAp ceramics with a molar ratio of 1.67 Ca/P are bioactive ceramics that are used as a coating on the surface of various biomaterials [18,19]. However, owing to their brittle nature, they are not suitable to be used in the bulk form or for load-bearing applications [20,21]. To overcome this issue, HAp can be used as thin film coatings on other groups of materials for bone-graft material applications [22,23]. According to previous works, the modification of materials with HAp ceramic coatings can improve the bioactivity, osteoconductivity, osteoinductivity, and resorbability of composite biomaterials [7,24,25] Meanwhile, HAp can also be used in its fine-powder forms as a filler, for example in tablets and capsules, to make the medicines easier to measure [26]. Fig. 2 illustrates the various sizes and shapes of HA particles.
Fig. 2.
SEM micrographs of HA particles with different sizes and shapes: a) microscale, b) plate, c) spherical, d) nanoscale (Adapted from Ref. [27]).
Extensive attempts have been made to achieve a suitable coating on the surface of different materials such as metals and polymers or to incorporate a new phase in matrix materials by considering their biocompatibility and bioactivity. Polymer matrix composite (PMC) is a material consisting of a polymer (resin) matrix combined with a fibrous reinforcing dispersed phase. In this regard, HA can be applied as an appropriate choice of coating or filler for the following reasons:
-
1.
It will enhance the biocompatibility of the substrate biomaterials (or matrix biomaterials) owing to its similarity in structure and composition to those of natural bone and teeth [28,29];
-
2.
The incorporation of HA particles into a polymer matrix can improve the degradation rate of the composites, which is attributed to the percentage of HA particles and enhancement of their bioactivity of them [30,31]; and
-
3.
HA can be considered as a promising bone-graft material for creating strong chemical bonding between the implant and host bone tissues.
In conclusion, owing to its similar chemical structure to that of natural bone, along with its bioactivity, osteoconductivity, and osteoinductivity, HA has been successfully applied in biodegradable polymer-based composites and metallic biomaterials [32,33].
1.1.2. Tricalcium phosphate
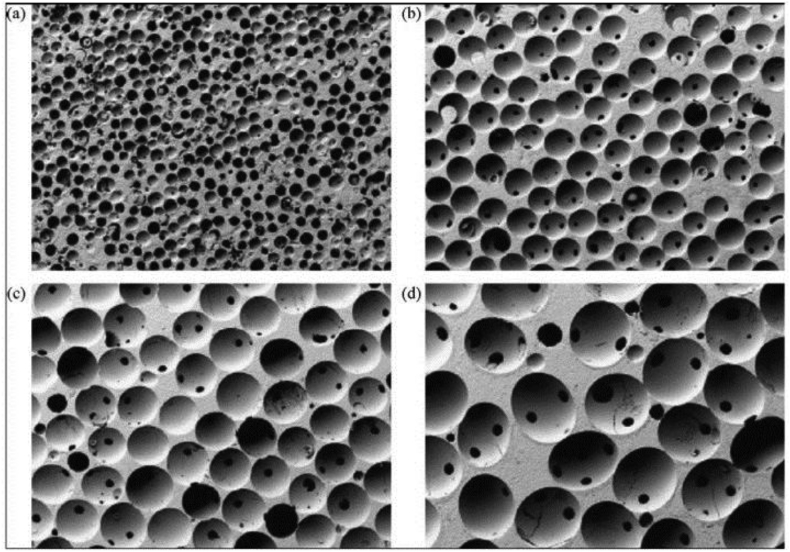
Tricalcium phosphate (TCP) with three polymorphs including α-TCP, β-TCP, and α′-TCP is another well-known bioceramics for bone repair applications. Β-TCP is receiving increasing attention due to its excellent biocompatibility, bioactivity, and bioresorbability [[34], [35], [36], [37]]. Fig. 3 shows porous β-TCP ceramics which polymethylmethacrylate balls are used to obtain various pore sizes.
Fig. 3.
Porous β-TCP with different pore sizes: (a) 100–200 μm, (b) 300–400 μm, (c) 500–600 μm, and (d) 700–800 μm (Adopted from Ref. [13]).
The mechanical strength of HA is higher than β-TCP [10]. However, β-TCP is more resorbable compared to HA which leads to faster growth of new bone surrounding the implanted scaffolds [38,39]. Other types of bioactive ceramics and their applications are summarized in Table 1.
Table 1.
Biomedical applications of various ceramics.
| Ceramics | Applications | Ref. |
|---|---|---|
| Calcium sulphate and carbonate | Bone defects filler, orthopedics and dentistry | [40,41] |
| Alumina ceramics | Dentistry, arthroplasty, antimicrobial activities | [[42], [43], [44]] |
| Zirconia ceramics | Dentistry, HA stabilizer, metallic implants coating | [45] |
| Bioactive glass ceramics | Replacing a vertebral body, material coatings, orthopedic applications | [46] |
| Silicate bioactive glasses | Bone repairing devices, drug delivery, modifier for synthetic and natural polymers | [[47], [48], [49], [50], [51], [52], [53]] |
1.2. Polymers as biomaterials
In recent years, a wide range of polymers have been extensively investigated for bone-tissue engineering applications [54,55]. Biodegradable polymeric materials can be categorized into natural and synthetic polymers. Naturally derived polymers such as collagen (as a biological protein [56]) and gelatin (derived from collagen [57]) have shown problems such as instability, incompatible characteristics, immunogenicity, and poor biodegradability. However, owing to the modifiable properties of most synthetic polymers [58] such as poly(lactic-co-glycolic acid) (PLGA) and polyurethanes (PURs), they offer excellent applicability [59,60]. These biodegradable polymers can be used for wound management, orthopedic devices, dental applications, cardiovascular applications, drug delivery, and tissue engineering. Both natural and synthetic polymers play essential roles in modern medicine [[61], [62], [63], [64]].
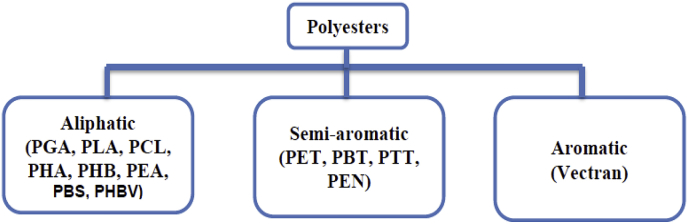
Among synthetic polymers, polyesters have attracted more attention than other types of polymers. This group can be categorized into three important classes, as shown in Fig. 4 and the abbreviations for polyesters are listed in Table 2.
Fig. 4.
Three main categories of polyesters (Adapted from Ref. [65]).
Table 2.
Abbreviations for polyesters.
| Polyester | Abbreviation |
|---|---|
| Polyglycolide or Polyglycolic acid | PGA |
| Polylactic acid | PLA |
| Polycaprolactone | PCL |
| Polyhydroxyalkanoate | PHA |
| Polyhydroxybutyrate | PHB |
| Polyethylene adipate | PEA |
| Polybutylene succinate | PBS |
| Poly (3-hydroxybutyrate-co-3-hydroxy valerate) | PHBV |
| Polyethylene terephthalate | PET |
| Polybutylene terephthalate | PBT |
| Polytrimethylene terephthalate | PTT |
| Polyethylene naphthalate | PEN |
Synthetic polymers such as the polylactic acid (PLA), poly-l-lactic acid (PLLA), PLGA, polyether ether ketone (PEEK), and polymethyl methacrylate (PMMA) are the most frequently employed polymers for medical applications owing to their favorable properties [59,66,67].
PLA can be considered as one of the best options for many biomedical applications, owing to its biocompatibility with host tissue without the need for a second surgery, along with its ease of manufacturing, hydrophobic nature, and biodegradability [[68], [69], [70], [71], [72]]. PLA is a biodegradable thermoplastic polymer that is derived from renewable resources such as corn starch and sugar cane. It is available in various types, including PLLA, poly-d-lactic acid (PDLA), and poly-dl-lactic acid (PDLLA), which can be used for different purposes such as in fabricating screws, pins, rods, and plates. PLA is a semi-crystalline polymer with a slow rate of crystallisation [73,74].
Applications of polymers in soft tissues (e.g., skin, muscle, tendons, and ligaments) and hard tissues (e.g., bone, cartilage, and tendon) depend on the functions of the damaged parts in the human body [[75], [76], [77]].
Table 3 lists the physical and mechanical properties of conventional polymers that have been studied in recent years.
Table 3.
Physical and mechanical properties of commonly used polymers (Adapted from Ref. [78]).
| Polymers | Density, g/cm3 | Tensile strength, MPa | Tensile modulus, GPa | Glass transition temperature, °C | Melting temperature, °C |
|---|---|---|---|---|---|
| PLA | 1.21–1.25 | 21–60 | 0.35–3.5 | 45–60 | 150–162 |
| PLLA | 1.24–1.30 | 15.5–150 | 2.7–4.14 | 55–65 | 170–200 |
| PGA | 1.50–1.71 | 60–99.7 | 6.0–7.0 | 35–45 | 220–233 |
| PCL | 1.11–1.14 | 20.7–42 | 0.21–0.44 | (−60)–(−65) | 58–65 |
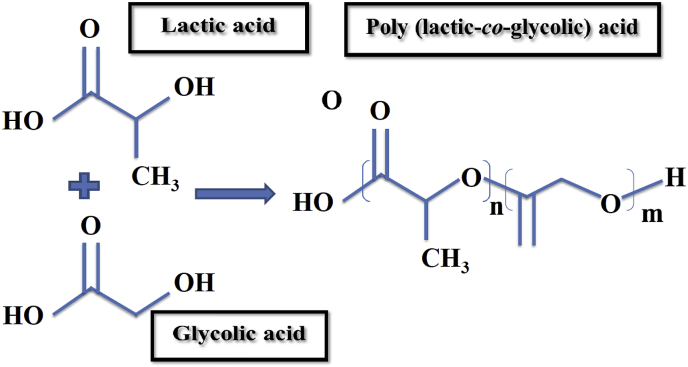
Another synthetic biopolymer which has been the focus of much attention is PLGA. PLGA is a biodegradable polymer that shows great potential to be used for these purposes owing to its safety, desirable mechanical properties, great cell adhesion, and controllable degradation rate [79]. PLGA is known as a random ring-opening copolymerization of PLA and PGA. In this regard, through the variation of the percentage of these two polymers, the degradation rate of PLGA products is controllable. Therefore, PLGA is preferred compared to PGA and could be used in various biomedical applications such as sutures and cancer drug delivery systems [80,81]. When the implants are made from PLGA, the rate of bone healing and growing has demonstrated a great acceleration [82]. Fig. 5 shows the chemical structure of PLGA and its monomers.
Fig. 5.
Chemical structure of PLGA and its monomers (n and m demonstrate the number of repetition of each unit).
1.3. Polymer/ceramic composite biomaterials
Polymer-ceramic composites such as PLLA/HA can be an appropriate choice for non-load-bearing applications that require a high rate of degradation [8].
According to previous work [83], the addition of HA particles to polymeric composites increases the glass transition temperature of the polymers without any changes in the crystallinity and melting temperature.
PLA is one of the most well-researched and commonly used polymers for biodegradable medical applications [[84], [85], [86]]. It was granted approval by the United States Food and Drug Administration (FDA) for medical applications in 1996 [87,88]. Monomers of this polymer such as PLLA, PDLA, and PDLLA are non-toxic [85]. PLA exhibits high mechanical strength but is brittle with a glass transition temperature of approximately 55 °C [89,90]. Various attempts have been made to improve the processability and flexibility of these polymers. For example, the use of plasticisers and polymer blending are the most frequently used methods for enhancing the flexibility of polymers [91,92].
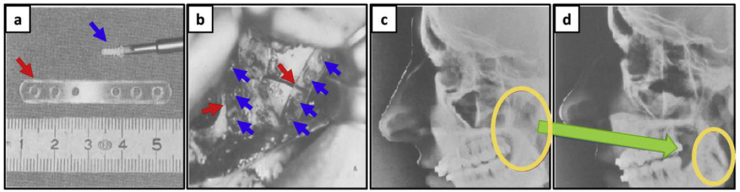
Owing to the favorable features of PLA such as its biodegradability, biocompatibility, and ease of processing, this polymer offers a wide range of applications for medical science [[93], [94], [95]]. Fig. 6 shows the result of using PLA plates to fix a jaw fracture without the need for further support. According to this research, by improving the mechanical properties of PLA through the control of the copolymer's ratio (L/D), PLA plates could be used to fix the damaged parts without the need for additional surgery or supports (in this study the D/L ratio was 85/15).
Fig. 6.
(a) Screws and plate made of PLA, (b) upper jaw with the plates and screws in situ, (c) and (d) lateral cephalogram, with the screws and plate, taken immediately postoperatively and six weeks postoperatively, respectively. (Adapted from Refs. [96,97]).
In general, the degradation products of PLA in the human body can cause inflammatory reactions; thus, ceramics have been investigated about incorporation into PLA-based composites to improve their biocompatibility.
PLLA-based composites have been extensively studied as biodegradable materials for biomedical applications such as in bone-fracture fixations; sutures, interference screws, and meniscus repair [98,99]. On the one hand, HA-PLLA composites can solve some problems that have been posed by metallic implants such as stress-shielding and the need for a second surgery [100,101]. On the other hand, further investigation is required to improve the weak bonding between HA and the PLLA surface [32].
PLGA is another favorable synthetic polymer which has a good biodegradability and biocompatibility [102,103]; however, this polymer suffers from poor mechanical properties and bioactivity and may not provide strong osseointegration [103]. To address these issues, using ceramics, in particular, HA as a coating on PLGA is recommended [104,105]. PLGA-based composites have also been frequently studied for biomedical purposes [106]. Porous PLGA-HA composites have been manufactured through different methods as listed in Table 8. Fig. 7 shows the porous scaffolds of neat PLGA and PLGA/nano-HAP scaffolds after removing the excess powder of HA particles which are prepared through selective laser sintering (SLS) [107]. Table 4 lists the mechanical properties of pure PLGA and PLGA/nano-HAP scaffolds with 0–20 wt % HAP particles.
Table 8.
Different manufacturing methods of conventional synthetic polymer-hydroxyapatite composites.
| Polymer-HA composites | Manufacturing techniques of polymer-ceramic composites | Year |
|---|---|---|
| PLA/HA | Extrusion and injection molding | 2018 [186] |
| Chitosan/HA | Chemical co-precipitation | 2018 [187] |
| PEEK/HA | Electrophoretic deposition (EPD) and suspension | 2018 [188] |
| PEEK/Ti/HAp | Sputtering | 2017 [189] |
| PLGA/HA | Solvent casting and injection molding | 2017 [190] |
| PLA/HA | Fused deposition melting (FDM) | 2017 [131] |
| PCL/HA | Co-extrusion | 2017 [191] |
| PLA/HA | Extrusion and injection molding | 2017 [192] |
| PLLA/nHAP & PLLA/g-HAP | Precipitation | 2016 [193] |
| PLA/HA | 3D printing | 2016 [194] |
| PEEK/HAp | Post-deposition heat treatment | 2016 [195] |
| PLA/HA | Fused deposition melting (FDM) | 2016 [123] |
| PLLA/HA | Thermally induced phase separation (TIPS) | 2016 [196] |
| PLLA/HA | Thermally induced phase separation (TIPS) | 2015 [197] |
| PLGA/HA | Injection molding | 2015 [198] |
| PLA/HA | Extrusion process | 2014 [199] |
| Chitosan-PLGA/HA | Freeze drying | 2014 [200] |
| PLGA/HA | Solution mixing | 2013 [201] |
| PLGA/HA | Selective laser sintering (SLS) | 2013 [107] |
| PCL/HA | Freezing of emulsions | 2013 [202] |
| PLA/HA | Electrospinning | 2013 [203] |
| PLA/nHA | Air jet spinning | 2013 [204] |
| PLLA/HA & PLGA/HA | Solvent casting | 2013 [205] |
| PLA/HA | Stereolithography (SLA) | 2013 [206] |
| PLGA/HA | Co-solution | 2012 [207] |
| PLLA/nHA | Laser melt electrospinning | 2012 [208] |
| PLGA/HA/collagen | Supercritical fluid extractor | 2011 [209] |
| PLGA/HA | Electrospinning | 2011 [210] |
| PMMA/HA | Pulsed laser deposition and magnetron sputtering | 2010 [211] |
| PLLA/HAp | Melt extrusion | 2010 [212] |
| PLLA/HA | Phase inversion | 2010 [213] |
| PLLA/HA | Freeze extraction | 2010 [214] |
| PCL/PLA/HA | Electrospinning | 2010 [215] |
| PCL/HA | Selective laser sintering (SLS) | 2010 [216] |
| Carbonated hydroxyapatite/PLLA | Selective laser sintering (SLS) | 2010 [217] |
| PLLA/nHA | Hot pressing | 2009 [218] |
| PLLA/HA & PLLA/collagen/HA | Electrospinning | 2009 [219] |
| PLLA/HA | A two-step immersing replication method | 2008 [220] |
| PCL/HA | Polymer impregnating | 2008 [221] |
| PLA/HA | Electrospinning | 2008 [222] |
| PCL/HA | Fused deposition melting (FDM) | 2007 [223] |
| PLGA/HA | Gas foaming and particulate leaching (GF/PL) | 2006 [224] |
| PLA/HA | Hot pressing | 2006 [225] |
| PLA/HA | Solvent casting | 2005 [226] |
| PCL/HA | Selective laser sintering (SLS) | 2005 [227] |
| PLLA/HA & PCL/HA | Selective laser sintering (SLS) | 2005 [228] |
| PLLA/HA | Solvent casting | 2004 [229] |
Fig. 7.
SEM images of: (a) neat PLGA (top view), (b) neat PLGA (front view), (c) PLGA/HA composites (top view), and (d) PLGA/HA composites (front view) manufactured by SLS (Adapted from Ref. [107]).
Table 4.
Mechanical properties of pure PLGA and PLGA/nano-HAP scaffolds (Adopted from Ref. [107]).
| Scaffold | Compressive strength, MPa | Modulus, MPa |
|---|---|---|
| PLGA | 1.82 | 22.75 |
| PLGA/nano-HAP | 3.28 | 32.81 |
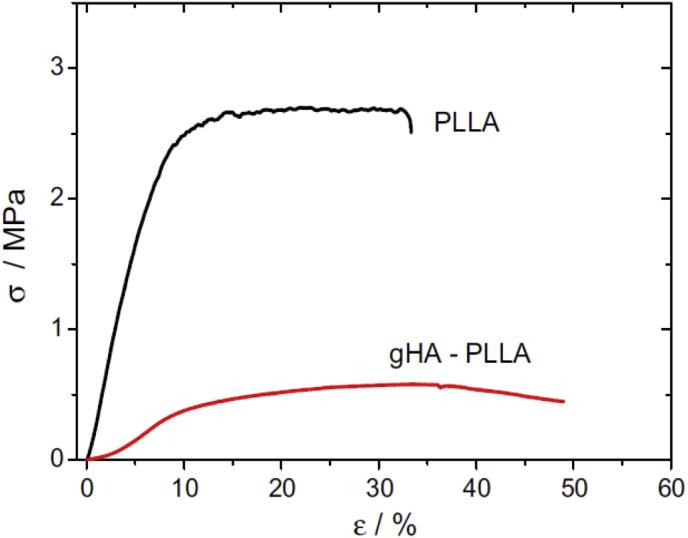
The results of previous investigations have shown that the size and shape of HA particles (micro- or nanoscale; spherical or plate-shaped) have effects on the fracture behaviour of the composites [[108], [109], [110], [111], [112], [113]]. For instance, the highest critical energy release rate with the most extensive surface roughness is related to the micro-HA-PLLA, whereas these nano-HA-PLLA composites have a brittle fracture surface owing to the nanoscale interactions between PLLA fibrils and primary HA particles [113]. Table 5 lists the physical properties of PLLA and gHA-PLLA composite. Fig. 8 shows the effects of HA on the stress-strain behaviour of the composite. In this study, pure PLLA presented a maximum stress of around 2.8 ± 1.2 MPa and a strain at break of 38 ± 7%. However, the microcomposite samples showed a five-fold decrease in maximum stress down to 0.57 ± 0.2 MPa and an increase in the strain at break up to 41 ± 8%.
Table 5.
Physical properties of PLLA and gHA-PLLA (Adapted from Ref. [114]).
| Sample | Average fiber diameter, nm | Porosity, % |
|---|---|---|
| PLLA | 510 ± 150 | 79 ± 3 |
| gHA-PLLA | 440 ± 170 | 88 ± 5 |
Fig. 8.
The stress-strain behaviour for pure PLLA and gHA-PLLA composite (Adapted from Ref. [114]).
2. Manufacturing techniques for polymer-ceramic composite biomaterials
The choice of proper fabrication methods to manufacture composites is one of the most challenging issues in medical science to achieve the desired implants. Traditional fabrication techniques including electrospinning, gas foaming, solvent casting and particulate leaching, phase separation, and melt mixing have been widely used to fabricate scaffolds [83,[115], [116], [117], [118], [119]]. Apart from the advantages of all these manufacturing methods, one of their major drawbacks in the manufacturing of porous structures is the inability of conventional methods to completely control the architecture of scaffolds, such as pore size and interconnections [120]. Further and even more importantly, the use of solvents in some of these methods can affect the biocompatibility of scaffolds [121].
2.1. Additive manufacturing
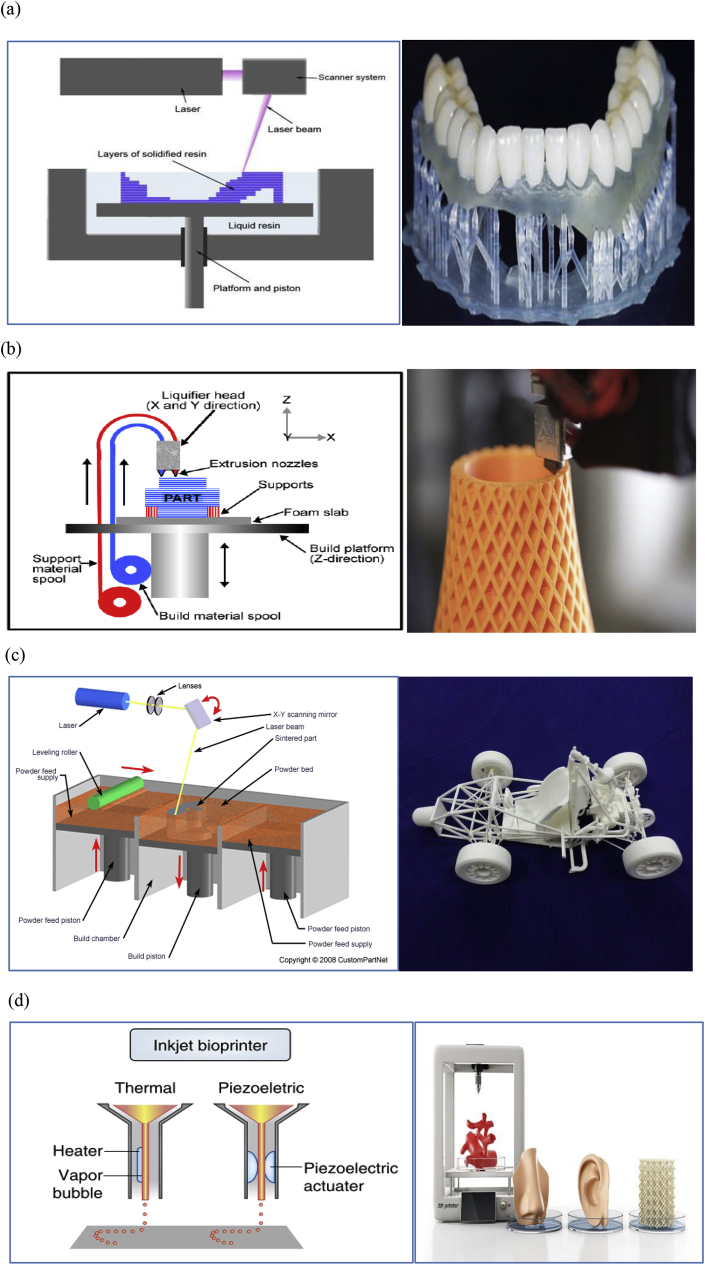
Additive manufacturing is a new and modern technique that shows great potential to offer complete control of architectural details such as pore size, which significantly affects the properties of scaffolds. This technique is receiving much attention, in particular for 3D-printed biopolymers because of the possibility of designing specific and complex structures [[122], [123], [124], [125], [126]]. Owing to the 3D architecture and geometry of native tissue environments, it is necessary to design and manufacture scaffolds to mimic the structure of natural bone. There are different variations of 3D printing, including stereolithography (SLA) [127], fused deposition modelling (FDM) [128], selective laser sintering (SLS) [129], and bioprinting [130]. Fig. 11 shows diagrams for these variations as they are applied in manufacturing different materials.
Fig. 11.
Diagrams of: (a) SLA, (b) FDM, (c) SLS, (d) inkjet bioprinting and their models (Adapted from Refs. [[132], [133], [134], [135], [136], [137], [138], [139]]).
2.1.1. Stereolithography (SLA)
SLA is a manufacturing procedure which takes a short time to complete and allows the creation of functional products within a day. In this method, by applying an electron beam or UV light, a chain reaction will start. Monomers are UV-active and will convert this to polymer chains. A 3D model is built layer by layer on a mobile platform. A laser then touches the container, solidifying the parts required to achieve the creation of the SLA prototype (Fig. 11(a)).
2.1.2. Fused deposition modelling (FDM)
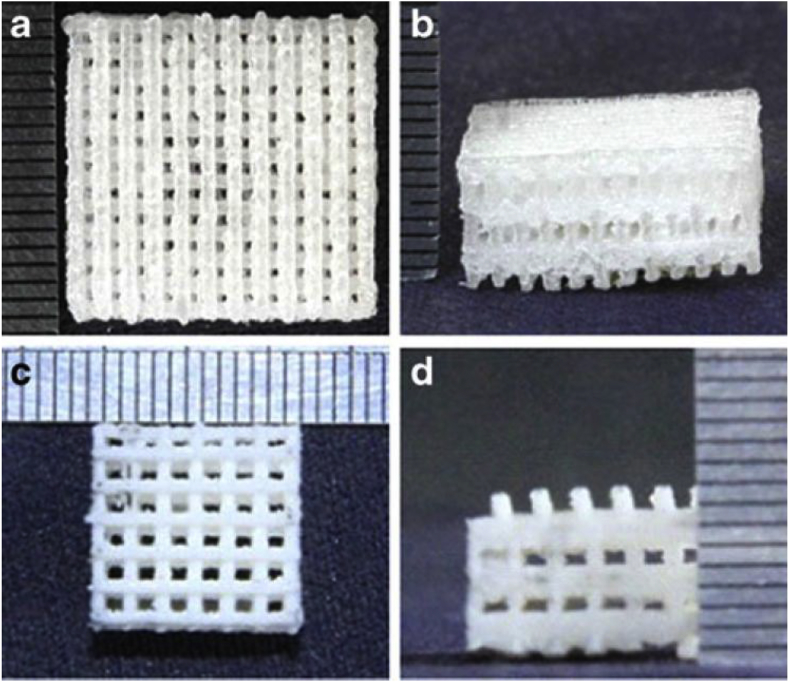
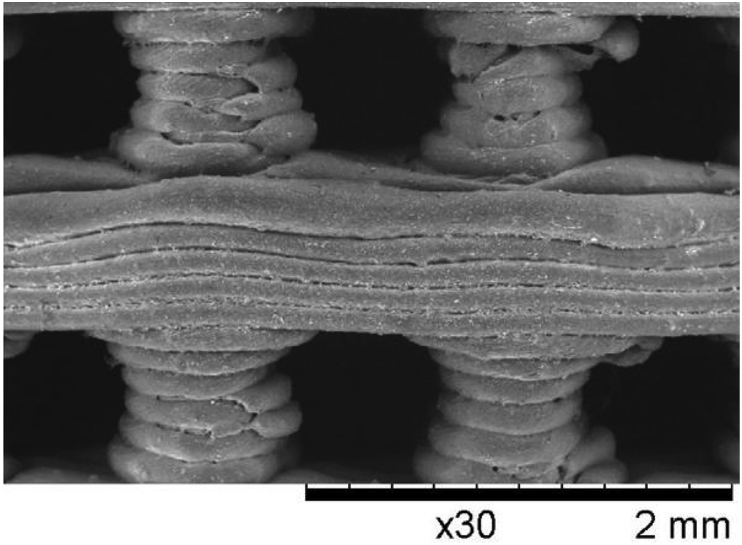
FDM as an additive manufacturing technique is frequently applied to different materials, particularly polymers. As seen in Fig. 11(b), built and support materials are extruded, and the parts form layer by layer. Fig. 9 shows the structure of 3D printed PLA scaffolds via FDM.
Fig. 9.
PLA scaffolds manufactured by FDM (Adapted from Ref. [123]).
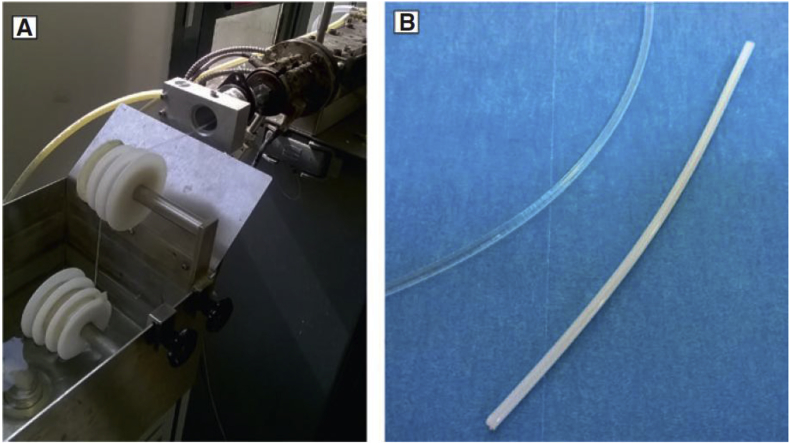
One of the challengeable issues in this method is its difficulty in the direct production of polymer-ceramic composites. Other complementary manufacturing techniques such as melt extrusion are needed to obtain a suitable filament for FDM [131]. Fig. 10 shows manufacturing of PLA/HA filament through melt extrusion.
Fig. 10.
a) Extrusion process of PLA/HA composites, and b) PLA and PLA/HA filament (white one) (Adopted from Ref. [131]).
2.1.3. Selective laser sintering (SLS)
The basic concept of SLS is similar to that of SLA. It uses a moving laser beam to trace and selectively sinter powdered polymer and/or metal composite materials into successive cross-sections of a 3D part. As in all rapid-prototyping processes, the parts are built upon a platform that adjusts in height equal to the thickness of the layer being created. The additional powder is deposited on top of each solidified layer and sintered. This powder is rolled onto the platform from a bin before forming the layer. The powder is maintained at an elevated temperature so that it fuses easily on exposure to the laser (Fig. 11(c)).
2.1.4. Bioprinting
In bioprinting, thermal inkjet bioprinters electrically heat the print head to produce air-pressure pulses that force droplets from the nozzle, whereas acoustic bioprinters use pulses formed by piezoelectric or ultrasound pressure (Fig. 11(d)).
Depending on the materials and applications, any of these methods can be employed. Table 6 summarizes the most commonly used materials with the advantages and disadvantages of each approach.
Table 6.
Advantages and disadvantages of various types of 3D printing.
| Common materials | Technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| PCL, PPF, PDLLA | SLA | High printing resolution | Material limitation, cytotoxicity, high cost | [127,[140], [141], [142], [143], [144]] |
| PCL, TCP, ABS | FDM | Low cost, good strength, multi-material capability | Anisotropy, nozzle clogging | [128,144,145] |
| PCL, PEEK, PLGA | SLS | Good strength, easy removal of support powder | High cost, powdery Surface | [129,144,146,147] |
| Low-viscosity materials (<10 cP) | Inkjet bioprinting | Low cost, multimaterial capability, easy removal of support powder | Clogging of binder jet, binder contamination | [130,144,148] |
| PCL, HA, Hydrogels | Extrusion bioprinting | High printing resolution, soft materials capability | Low mechanical strength, slow | [130,144,[148], [149], [150]] |
Of all the 3D printing techniques, FDM is used more frequently, owing to its lack of need for solvents. One of the most significant drawbacks of FDM is that the type of material employed with this method must be in filament form. The exclusive availability of this technique for thermoplastics is another disadvantage. However, owing to the high speed and low cost of FDM, it is the most commonly used printing technique to fabricate polymer composites.
Another concern regarding 3D printing is how to remove undesirable particles or powders that can be formed in unprinted volumes [151].
In recent studies, researchers applied 3D printing to the fabrication of PLA composites to achieve a uniform structure [126,[152], [153], [154]]. Because the chemical formulae and properties of synthetic HA are similar to those of the main inorganic constituent of bones and teeth, it can be considered the best surface coating. HA has a porous structure with excellent biocompatibility, and bioactivity, and so is used as a coating on the surface of PLA [155,156].
2.2. Surface coating of polymers
There is a wide range of methods, as summarized in Table 7, to produce a thin film on the surface of polymer materials, including plasma spraying [[157], [158], [159]], magnetron sputtering [29,[160], [161], [162], [163], [164], [165], [166], [167], [168]], biomimetic crystallisation techniques [169,170], electrophoretic deposition [171,172], and sol-gel techniques [173,174].
Table 7.
Advantages and disadvantages of various coating techniques.
| Method | Advantages | Disadvantages |
|---|---|---|
| Plasma spraying | Strength bonding, preferable dissolution behaviour | The complex process due to the number of interacting parameters, difficulty in the internal coating of bores, high-cost |
| Magnetron sputtering | Thin, uniform, and dense films, easy to scale up, low damage to soft tissues, high rate of adhesion, high rate of deposition, suitable for a wide range of materials | Nonhomogeneous ion current distribution across the target surface, high-cost |
| Sol-gel | Excellent control of product purity and composition, ability to deposit films and coatings on different surfaces, low temperatures | Easy to crack in the drying process, the high cost of raw materials |
2.2.1. Plasma spraying
The plasma coating process as one of the surface coating methods is an established technology to produce coating films. This method is applied to different polymeric materials as a coating in bone tissue engineering [175,176]. In this technique, the processing temperature can reach 16,000 K. The gas flowing such as He, H2, N2, or mixtures of them is ionised. Particles in powder form are injected by impact into the plasma plume to melt and sprayed onto the substrate to produce a coating. The composition of the used gas and the percentage of it, energy input, and the distance between the substrate and coating can be considered as important factors in this process [177].
2.2.2. Magnetron sputtering
Compared to other methods of physical vapour deposition, magnetron sputtering is widely used in many industries to produce high quality, dense, uniform, and thin films which can be applied for a wide range of materials. Moreover, a high rate of deposition is achievable. Compared to other deposition techniques, magnetron sputtering has the low level of damage to soft substrates, in particular for polymers.
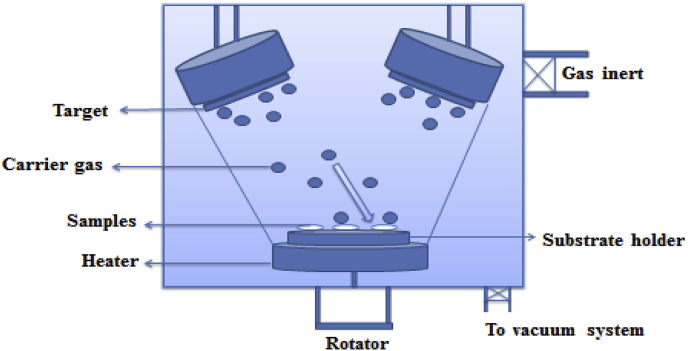
There are different variations of magnetron sputtering, such as pulsed direct current (DC) and radio frequency (RF). The highly flexible method of RF magnetron sputtering is widely used owing to its modifiable conditions such as controllable crystallinity [165]. In this regard, the effects of different processing parameters such as power, the pressure of the used gas (usually argon), and the target substrate and its morphology should be given consideration. The type of gas used, pressure, power, the distance between target and substrate, substrate bias potential and the properties of the target have been investigated as sputtering parameters. These factors affect the morphology, uniformity, and the features of the coating [[178], [179], [180], [181], [182], [183], [184]]. In recent studies to control the uniformity of film coatings, the substrate rotation, movement, and sample position have been experimentally investigated [185]. Inert gases such as argon or helium are used to bombard a target. The ejected atoms from the target will deposit on all surfaces and make a thin film. To cool the target water can be applied. Fig. 12 shows the schematic of the magnetron sputtering method.
Fig. 12.
Schematic of a sputtering technique.
2.2.3. Sol–gel techniques
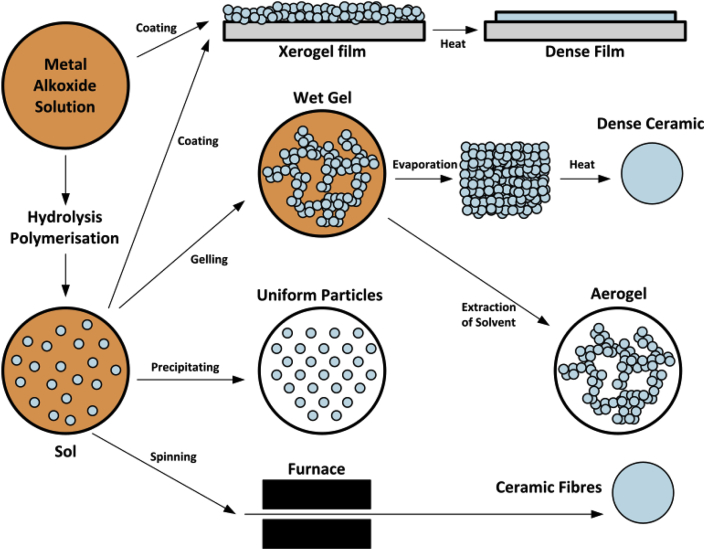
Due to the low synthetic temperature of this method, it is known as the most straightforward technique to produce sub-micron dimensions of HA. The sol-gel process can be considered as aqueous (if water is applied as the reaction medium) and nonaqueous (if organic solvents are used as the reaction medium). This chemical procedure has several steps. First, a sol which is a stable colloidal solution is formed by hydrolysis and partial condensation of precursors such as an inorganic salt or a metal alkoxide. Then it will go towards the formation of a gel-like phase. A new phase has both a liquid and solid phase which their morphologies can be placed between discrete particles and continuous polymer networks. The drying process is accompanied by a significant amount of shrinkage and densification due to the removal of the remaining liquid or solvent. Solid products are known as xerogel and aerogel, respectively. To enhance mechanical properties and structural stability thermal treatment is typically applied. Fig. 13 shows the schematic of a sol-gel method.
Fig. 13.
Schematic of the sol-gel technology (Adapted from Ref. [166]).
The different manufacturing methods of the most common synthetic polymer-hydroxyapatite composites that have been studied in recent years are summarized in Table 8.
As a new generation of materials, biodegradable polymers offer excellent biomedical applications ranging from wound management to drug delivery [230]. Table 9 summarized the biomedical applications of synthetic polymer-ceramic composites with the greatest history of usage.
Table 9.
Biomedical applications of common polymer-ceramic composites.
| Polymer-ceramic composites | Applications | Techniques | Ref. |
|---|---|---|---|
| PCL/ceramic | Bone tissue engineering, hard tissue engineering, bone graft substitutes, neural tissue engineering, tissue generation, wound healing, bone fracture fixation devices, hard tissue repair | Solvent casting particulate leaching, solvent casting, melt-electrospinning, 3D printing, melt extrusion, sol-gel | [47,[231], [232], [233], [234], [235], [236], [237]] |
| PLA/ceramic | Sutures, repair of fractures of the orbital floor, implants, drug-eluting stents, drug delivery | 3D printing, extrusion and injection molding, electrospinning, solvent casting | [123,225,230,[238], [239], [240]] |
| PLLA/ceramic | Bone tissue engineering, various medical applications, interference screws, suture anchors | freeze-extraction, melt extrusion, particulate leaching | [[241], [242], [243], [244]] |
| PLGA/ceramic | Bone tissue engineering, hard & soft tissue engineering, meniscus repair, liver disease | Solvent casting particulate leaching, solvent casting, gas foaming, bone neoplasia and tumours, 3D printing | [52,102,[245], [246], [247], [248], [249], [250]] |
| PDLLA/ceramic | Hard & soft tissue engineering, regeneration of hard-soft tissue defects | Solvent casting particulate leaching, solvent casting | [248,[251], [252], [253]] |
3. Summary and future research directions
According to previous studies, various parameters should be considered as requirements for the achievement of successful implants and scaffolds.
-
1.
The first and foremost requirement is the choice of appropriate materials with good mechanical properties. Biomaterials should have good biocompatibility, bioresorbability, a controllable rate of biodegradation, excellent mechanical strength, and bioactivity.
-
2.
The next requirement is to design scaffolds with suitable structures. Bone-tissue engineering has used various types of structures and architectures to construct medical implants and devices. The appropriate design of scaffold structures has a significant impact on their mechanical properties. Based on previous studies, it is worth emphasising that successful scaffold structures are highly dependent on the porosity, pore size, and shape. 3D porous scaffolds allow fast cell growth and attachments, and high transportation rates of nutrients and waste.
-
3.
Furthermore, these structures provide a large surface area for bone growth. Therefore, highly porous scaffolds in 3D forms play a vital role in meeting these aims. The recommended porosity for scaffolds is approximately 90%.
-
4.
Another essential requirement that should be studied in more detail concerns the adhesion between coatings and substrates. Various efforts have been made to enhance adhesive strength. For example, hydrogel groups of HA can improve the adhesive strength by creating strong chemical bonds between HAp nanoparticles and the PLLA and PLGA matrix. Even more importantly, composites that contain HA demonstrate high mechanical strength compared to pure ones.
-
5.
An appropriate degradation behaviour is another critical requirement for biodegradable materials. The addition of nanofillers could decelerate the degradation rate of a biodegradable composite.
As pointed out above, owing to their unique and beneficial properties, polymeric materials are being used as a new generation of materials for medical applications. Although various types of biomaterials have been extensively studied for biomedical purposes, many other materials could be investigated. In this regard, the physiochemical properties of this new generation of biomaterials, their surface morphology, and their potential applications in tissue engineering should be studied in detail. It is expected that, by employing polymers, the observed problems could at least be reduced.
On the one hand, according to the studies cited above, traditional manufacturing methods may not be able to produce biomaterials that mimic the natural structure of bone. On the other hand, modern techniques face certain limitations regarding raw materials and outcomes. For instance, currently only a limited range of materials such as thermoplastics can be used in 3D-printing devices, and these materials cannot meet all the requirements. Furthermore, owing to the various shapes of scaffolds, as mentioned above, creating strong interfacial bonding is difficult.
To achieve long-lasting implant devices with acceptable biocompatibility, biodegradability, and bioactivity, a variety of materials and manufacturing techniques have been studied in medical science. However, there is still a vital need to develop a range of improved biomaterials and manufacturing approaches.
Conflicts of interest
None.
Acknowledgements
The authors acknowledge the financial support for this research by the Australian Research Council (ARC) through the Discovery Project (DP170102557). YL is also supported by an ARC Future Fellowship (FT160100252).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Niinomi M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A. 2002;33(3):477–486. [Google Scholar]
- 2.Cao W., Hench L.L. Bioactive materials. Ceram. Int. 1996;22(6):493–507. [Google Scholar]
- 3.Dorozhkin S.V., Epple M. 2002. Biological and Medical Significance of Calcium Phosphates, Weinheim; pp. 3130–3146. [DOI] [PubMed] [Google Scholar]
- 4.Denissen H.W., Groot K.d. Immediate dental root implants from synthetic dense calcium hydroxylapatite. J. Prosthet. Dent. 1979;42(5):551–556. doi: 10.1016/0022-3913(79)90253-1. [DOI] [PubMed] [Google Scholar]
- 5.Brown L.C.C.W.E. A new calcium phosphate, water-setting cement. Cem. Res. Prog. 1986:351–379. [Google Scholar]
- 6.Bose S., Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8(4):1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legeros R.Z., Legeros J.P. Woodhead Publishing; 2008. 16 - Hydroxyapatite A2 - Kokubo, Tadashi, Bioceramics and Their Clinical Applications; pp. 367–394. [Google Scholar]
- 8.Xiao-Ming L., Rong-Rong C., Lian-Wen S., Katerina E.A., Yubo F., Qing-Ling F., Fuz-Hai C., Fumio W. 3D-Printed biopolymers for tissue engineering application. Int. J. Polym. Sci. 2014;2014(2):137–149. [Google Scholar]
- 9.Catledge S.A., Fries M.D., Vohra Y.K., Lacefield W.R., Lemons J.E., Woodard S., Venugopalan R. Nanostructured ceramics for biomedical implants. J. Nanosci. Nanotechnol. 2002;2(3–4):293–312. doi: 10.1166/jnn.2002.116. [DOI] [PubMed] [Google Scholar]
- 10.Legeros R.Z. Calcium phosphate materials in restorative dentistry: a review. Adv. Dent. Res. 1988;2(1):164–180. doi: 10.1177/08959374880020011101. [DOI] [PubMed] [Google Scholar]
- 11.Paul W., Sharma C.P. Ceramic drug delivery: a perspective. J. Biomater. Appl. 2003;17(4):253–264. doi: 10.1177/0885328203017004001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Zhang M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 2002;62(3):378–386. doi: 10.1002/jbm.10312. [DOI] [PubMed] [Google Scholar]
- 13.Dorozhkin S.V. Calcium orthophosphates as bioceramics: state of the art. J. Funct. Biomater. 2010;1(1):22–107. doi: 10.3390/jfb1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuadrado T.R., Abraham T.A. Propiedades mecánicas de Biomateriales. In: Sastre R., De Aza S., San Román J., editors. Cooperación Iberoamericana, CYTED, Biomateriales para lasalud. 2004. pp. 151–171. [Google Scholar]
- 15.Ginebra M.P., Driessens F.C., Planell J.A. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials. 2004;25(17):3453–3462. doi: 10.1016/j.biomaterials.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Hench L.L. Major elements in the human body. J. Am. Ceram. Soc. 1998;81:1705. [Google Scholar]
- 17.Shojai M., Khorasani M., Khoshdargi E., Jamshidi A. Research on hydroxyapatite based composite materials. Acta Biomater. 2013;9:7591–7621. doi: 10.1016/j.actbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 18.A H., A H., Y M. Sequential histological Examination and morphometric analysis of osteogenesis in the pores of porous hydroxyapatite with attachment of vascularization periosteum. J. Japan Cranio-Max-Fac. Surg. 2005;21(4):259–270. [Google Scholar]
- 19.Ono I., Tateshita T., Satou M., Sasaki T., Matsumoto M., Kodama N. Treatment of large complex cranial bone defects by using hydroxyapatite ceramic implants. Plast. Reconstr. Surg. 1999;104(2):339–349. doi: 10.1097/00006534-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hench L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991;74(7):1487–1510. [Google Scholar]
- 21.de Groot K. Clinical applications of calcium phosphate biomaterials: a review. Ceram. Int. 1993;19(5):363–366. [Google Scholar]
- 22.Ektessabi A.M., Hamdi M. Surf. Coating. Technol. 2002;10:153. [Google Scholar]
- 23.Suchanek W., Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 2011;13(1):94–117. [Google Scholar]
- 24.Mandracci P., Mussano F., Rivolo P., Carossa S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings. 2016;6(1) [Google Scholar]
- 25.Zhang G.B., Myers E.D., Wallace G.G., Brandt M., Choong F.P. Bioactive coatings for orthopaedic implants—recent trends in development of implant coatings. Int. J. Mol. Sci. 2014;15(7) doi: 10.3390/ijms150711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loghem J.V., Yutskovskaya Y.A., Philip Werschler W.M. Calcium hydroxylapatite: over a decade of clinical experience. J. Clin. Aesthetic Dermatol. 2015;8(1):38–49. [PMC free article] [PubMed] [Google Scholar]
- 27.Todo M., Park S.D., Arakawa K., Takenoshita Y. Relationship between microstructure and fracture behavior of bioabsorbable HA/PLLA composites. Compos A. Appl. Sci. Manuf. 2006;37(12):2221–2225. [Google Scholar]
- 28.Surmenev R.A., Surmeneva M.A., Ivanova A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis – a review. Acta Biomater. 2014;10(2):557–579. doi: 10.1016/j.actbio.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Surmeneva M.A., Chaikina M.V., Zaikovskiy V.I., Pichugin V.F., Buck V., Prymak O., Epple M., Surmenev R.A. The structure of an RF-magnetron sputter-deposited silicate-containing hydroxyapatite-based coating investigated by high-resolution techniques. Surf. Coating. Technol. 2012;218:39–46. [Google Scholar]
- 30.Ang K.C., Leong K.F., Chua C.K., Chandrasekaran M. Compressive properties and degradability of poly(ε‐caprolatone)/hydroxyapatite composites under accelerated hydrolytic degradation. J. Biomed. Mater. Res. A. 2007;80(3):655–660. doi: 10.1002/jbm.a.30996. [DOI] [PubMed] [Google Scholar]
- 31.Liuyun J., Chengdong X., Lixin J., Lijuan X. Degradation behavior of hydroxyapatite/poly(lactic-co-glycolic) acid nanocomposite in simulated body fluid. Mater. Res. Bull. 2013;48(10):4186–4190. [Google Scholar]
- 32.Hong Z., Zhang P., He C., Qiu X., Liu A., Chen L., Chen X., Jing X. Nano-composite of poly( l-lactide) and surface grafted hydroxyapatite: mechanical properties and biocompatibility. Biomaterials. 2005;26(32):6296–6304. doi: 10.1016/j.biomaterials.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Verheyen C., Klein C., Deblieckhogervorst J., Wolke J., Vanblitterswijn C., Degroot K. Evaluation of hydroxylapatite poly(L-lactide) composites - physicochemical properties. J. Mater. Sci. Mater. Med. 1993;4(1):58–65. [Google Scholar]
- 34.Mina A., Castaño A., Caicedo J.C., Caicedo H.H., Aguilar Y. Determination of physical properties for β-TCP + chitosan biomaterial obtained on metallic 316L substrates. Mater. Chem. Phys. 2015;160:296–307. [Google Scholar]
- 35.Rakovsky A., Gotman I., Rabkin E., Gutmanas E.Y. beta-TCP-polylactide composite scaffolds with high strength and enhanced permeability prepared by a modified salt leaching method. J. Mech. Behav. Biomed. Mater. 2014;32:89–98. doi: 10.1016/j.jmbbm.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Zheng L., Yang F., Shen H., Hu X., Mochizuki C., Sato M., Wang S., Zhang Y. The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials. 2011;32(29):7053–7059. doi: 10.1016/j.biomaterials.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Rai B., Oest M.E., Dupont K.M., Ho K.H., Teoh S.H., Guldberg R.E. Combination of platelet-rich plasma with polycaprolactone-tricalcium phosphate scaffolds for segmental bone defect repair. J. Biomed. Mater. Res. A. 2007;81(4):888–899. doi: 10.1002/jbm.a.31142. [DOI] [PubMed] [Google Scholar]
- 38.LeGeros R.Z., Lin S., Rohanizadeh R., Mijares D., LeGeros J.P. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003;14(3):201–209. doi: 10.1023/a:1022872421333. [DOI] [PubMed] [Google Scholar]
- 39.Daculsi G., LeGeros R.Z., Nery E., Lynch K., Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989;23(8):883–894. doi: 10.1002/jbm.820230806. [DOI] [PubMed] [Google Scholar]
- 40.Thomas M.V., Puleo D.A. Calcium sulfate: properties and clinical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2008;88B(2):597–610. doi: 10.1002/jbm.b.31269. [DOI] [PubMed] [Google Scholar]
- 41.Sali S. 2016. Natural Calcium Carbonate for Biomedical Applications. arXiv.org. [Google Scholar]
- 42.Pröbster L., Diehl J. Slip-casting alumina ceramics for crown and bridge restorations. Quintessence Int. 1992;23(1):25–31. [PubMed] [Google Scholar]
- 43.Medvedovski E. Alumina–mullite ceramics for structural applications. Ceram. Int. 2006;32(4):369–375. [Google Scholar]
- 44.Denry I., Kelly J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008;24(3):299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Manicone P.F., Rossi Iommetti P., Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J. Dent. 2007;35(11):819–826. doi: 10.1016/j.jdent.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12(2):155–163. doi: 10.1016/0142-9612(91)90194-f. [DOI] [PubMed] [Google Scholar]
- 47.Dziadek M., Pawlik J., Menaszek E., Stodolak-Zych E., Cholewa-Kowalska K. Effect of the preparation methods on architecture, crystallinity, hydrolytic degradation, bioactivity, and biocompatibility of PCL/bioglass composite scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(8):1580–1593. doi: 10.1002/jbm.b.33350. [DOI] [PubMed] [Google Scholar]
- 48.Vargas G.E., Haro Durand L.A., Cadena V., Romero M., Mesones R.V., Mackovic M., Spallek S., Spiecker E., Boccaccini A.R., Gorustovich A.A. Effect of nano-sized bioactive glass particles on the angiogenic properties of collagen based composites. J. Mater. Sci. Mater. Med. 2013;24(5):1261–1269. doi: 10.1007/s10856-013-4892-7. [DOI] [PubMed] [Google Scholar]
- 49.Tamjid E., Bagheri R., Vossoughi M., Simchi A. Effect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone composites. Mater. Sci. Eng. C. 2011;31(7):1526–1533. [Google Scholar]
- 50.Fabbri P., Cannillo V., Sola A., Dorigato A., Chiellini F. Highly porous polycaprolactone-45S5 Bioglass® scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010;70(13):1869–1878. [Google Scholar]
- 51.Maquet V., Boccaccini A.R., Pravata L., Notingher I., Jerome R. Porous poly(alpha-hydroxyacid)/Bioglass composite scaffolds for bone tissue engineering. I: preparation and in vitro characterisation. Biomaterials. 2004;25(18):4185–4194. doi: 10.1016/j.biomaterials.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 52.Boccaccini A.R., Maquet V. Bioresorbable and bioactive polymer/Bioglass® composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003;63(16):2417–2429. [Google Scholar]
- 53.Blaker J.J., Gough J.E., Maquet V., Notingher I., Boccaccini A.R. In vitro evaluation of novel bioactive composites based on Bioglass-filled polylactide foams for bone tissue engineering scaffolds. J. Biomed. Mater. Res. A. 2003;67(4):1401–1411. doi: 10.1002/jbm.a.20055. [DOI] [PubMed] [Google Scholar]
- 54.Athanasiou K.A., Niederauer G.G., Agrawal C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 55.Prestwich G., Matthew H. Hybrid, composite, and complex biomaterials. Ann. NY Acad. Sci. 2002;961:106–108. doi: 10.1111/j.1749-6632.2002.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 56.Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221(1):1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 57.Young S., Wong M., Tabata Y., Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Contr. Release. 2005;109(1):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 58.Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 59.Nair L.S., Laurencin C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32(8):762–798. [Google Scholar]
- 60.Puppi D., Chiellini F., Piras A.M., Chiellini E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010;35(4):403–440. [Google Scholar]
- 61.López-Noriega A., Quinlan E., Celikkin N., O’brien F.J. Incorporation of polymeric microparticles into collagen-hydroxyapatite scaffolds for the delivery of a pro-osteogenic peptide for bone tissue engineering. Apl. Mater. 2015;3(1) [Google Scholar]
- 62.Przekora A., Palka K., Ginalska G. Biomedical potential of chitosan/HA and chitosan/β-1,3-glucan/HA biomaterials as scaffolds for bone regeneration–A comparative study. Mater. Sci. Eng. C. 2016;58:891. doi: 10.1016/j.msec.2015.09.046. Materials for biological applications. [DOI] [PubMed] [Google Scholar]
- 63.Qin J., Zhong Z., Ma J. Biomimetic synthesis of hybrid hydroxyapatite nanoparticles using nanogel template for controlled release of bovine serum albumin. Mater. Sci. Eng. C. 2016;62:377. doi: 10.1016/j.msec.2016.01.088. Materials for biological applications. [DOI] [PubMed] [Google Scholar]
- 64.Sultana N., Mokhtar M., Hassan M.I., Jin R.M., Roozbahani F., Khan T.H. 2014. Chitosan-based Nanocomposite Scaffolds for Tissue Engineering Applications, Materials and Manufacturing Processes. [Google Scholar]
- 65.Niemela T. Effect of beta-tricalcium phosphate addition on the in vitro degradation of self-reinforced poly-L,D-lactide. Polym. Degrad. Stabil. 2005;89(3):492–500. [Google Scholar]
- 66.Eppley B.L. Biomechanical testing of alloplastic PMMA cranioplasty materials. J. Craniofac. Surg. 2005;16(1):140–143. doi: 10.1097/00001665-200501000-00028. [DOI] [PubMed] [Google Scholar]
- 67.Lampin M., Warocquier‐Clérout R., Legris C., Degrange M., Sigot‐Luizard M.F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 1997;36(1):99–108. doi: 10.1002/(sici)1097-4636(199707)36:1<99::aid-jbm12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 68.Ambrose C., Clanton T. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann. Biomed. Eng. 2004;32(1):171–177. doi: 10.1023/b:abme.0000007802.59936.fc. [DOI] [PubMed] [Google Scholar]
- 69.Baro M., Sánchez E., Delgado A., Perera A., Évora C. In vitro–in vivo characterization of gentamicin bone implants. J. Contr. Release. 2002;83(3):353–364. doi: 10.1016/s0168-3659(02)00179-7. [DOI] [PubMed] [Google Scholar]
- 70.Li R., Yao D. Preparation of single poly(lactic acid) composites. J. Appl. Polym. Sci. 2008;107(5):2909–2916. [Google Scholar]
- 71.Narayanan G., Vernekar V.N., Kuyinu E.L., Laurencin C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016;107:247–276. doi: 10.1016/j.addr.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah S.R., Tatara A.M., Souza R.N., Mikos A.G., Kasper F.K. Evolving strategies for preventing biofilm on implantable materials. Mater. Today. 2013;16(5):177–182. [Google Scholar]
- 73.Lasprilla A.J., Martinez G.A., Lunelli B.H., Jardini A.L., Filho R.M. Poly-lactic acid synthesis for application in biomedical devices - a review. Biotechnol. Adv. 2012;30(1):321–328. doi: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Migliaresi C., De Lollis A., Fambri L., Cohn D. The effect of thermal history on the crystallinity of different molecular weight PLLA biodegradable polymers. Clin. Mater. 1991;8(1):111–118. [Google Scholar]
- 75.Crecelius C. Soft tissue trauma. Atlas Oral Maxillofac. Surg. Clin. 2013;21(1):49–60. doi: 10.1016/j.cxom.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 76.Currey J.D. The design of mineralised hard tissues for their mechanical functions. J. Exp. Biol. 1999;202(23):3285–3294. doi: 10.1242/jeb.202.23.3285. [DOI] [PubMed] [Google Scholar]
- 77.Holzapfel G.A. vol. 3. 2001. Biomechanics of soft tissue; pp. 1049–1063. (The Handbook of Materials Behavior Models). [Google Scholar]
- 78.Van de Velde K., Kiekens P. Biopolymers: overview of several properties and consequences on their applications. Polym. Test. 2002;21(4):433–442. [Google Scholar]
- 79.Ulery B.D., Nair L., Laurencin C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011:832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haghighat F., Ravandi S.A.H. Mechanical properties and in vitro degradation of PLGA suture manufactured via electrospinning. Fibers Polym. 2014;15(1):71–77. [Google Scholar]
- 81.Sadat Tabatabaei Mirakabad F., Nejati-Koshki K., Akbarzadeh A., Yamchi M.R., Milani M., Zarghami N., Zeighamian V., Rahimzadeh A., Alimohammadi S., Hanifehpour Y., Joo S.W. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. APJCP. 2014;15(2):517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- 82.Kleinschmidt J.C., Marden L.J., Kent D., Quigley N., Hollinger J.O. A multiphase system bone implant for regenerating the calvaria. Plast. Reconstr. Surg. 1993;91(4):581–588. doi: 10.1097/00006534-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez G., Albano C., Palacios J. 2012. PLLA-HA Composites: Synthesis and Characterization; pp. 241–243. [Google Scholar]
- 84.Drumright R., Gruber P.R., Henton D.E. 2000. Polylactic Acid Technology. [Google Scholar]
- 85.Lopes M.S., Jardini A.L., Filho R.M. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012;42(Supplement C):1402–1413. [Google Scholar]
- 86.Flores R.L., Liss H., Raffaelli S., Humayun A., Khouri K.S., Coelho P.G., Witek L. The technique for 3D printing patient-specific models for auricular reconstruction. J. Cranio-Maxillofacial Surg. 2017;45(6):937–943. doi: 10.1016/j.jcms.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 87.Dorozhkin S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009;44(9):2343–2387. [Google Scholar]
- 88.Mano J.F., Sousa R.A., Boesel L.F., Neves N.M., Reis R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos. Sci. Technol. 2004;64(6):789–817. [Google Scholar]
- 89.Henton D.E., Gruber P., Lunt J., Randall J. 2005. Natural Fibers, Biopolymers, and Biocomposites, Boca Raton; pp. 527–577. [Google Scholar]
- 90.Ray S.S., Okamoto M. Biodegradable polylactide and its nanocomposites: opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003;24(14):815–840. [Google Scholar]
- 91.Pillin I., Montrelay N., Bourmaud A., Grohens Y. Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid) Polym. Degrad. Stabil. 2008;93(2):321–328. [Google Scholar]
- 92.Younes H., Cohn D. Phase separation in poly(ethylene glycol)/poly(lactic acid) blends. Eur. Polym. J. 1988;24(8):765–773. [Google Scholar]
- 93.Maharana T., Mohanty B., Negi Y.S. Melt–solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009;34(1):99–124. [Google Scholar]
- 94.Tsuji H., Miyauchi S. Poly(l-lactide): VI Effects of crystallinity on enzymatic hydrolysis of poly(l-lactide) without free amorphous region. Polym. Degrad. Stabil. 2001;71(3):415–424. [Google Scholar]
- 95.Yul Lim J., Kim S.-H., Lim S., Ha Kim Y. 2003. Improvement of Flexural Strengths of Poly(L‐lactic Acid) by Solid‐State Extrusion, 2. Extrusion through Rectangular Die. [Google Scholar]
- 96.Haers P.E., Suuronen R., Lindqvist C., Sailer H. Biodegradable polylactide plates and screws in orthognathic surgery: technical note. J. Cranio-Maxillo-Fac. Surg. Official Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 1998;26(2):87–91. doi: 10.1016/s1010-5182(98)80045-0. [DOI] [PubMed] [Google Scholar]
- 97.Hamad K., Kaseem M., Yang H., Deri F., Ko Y. Properties and medical applications of polylactic acid: a review. Express Polym. Lett. 2015;9(5) [Google Scholar]
- 98.Bostman O.M. Absorbable implants for the fixation of fractures. J. Bone Joint Surg. Am. 1991;73(1):148–153. [PubMed] [Google Scholar]
- 99.Leenslag J.W., Pennings A.J., Bos R.R.M., Rozema F.R., Boering G. Resorbable materials of poly(l-lactide). VI. Plates and screws for internal fracture fixation. Biomaterials. 1987;8(1):70–73. doi: 10.1016/0142-9612(87)90034-2. [DOI] [PubMed] [Google Scholar]
- 100.Akeson W.H., Woo S.L.Y., Rutherford L., Coutts R.D., Gonsalves M., Amiel D. The effects of rigidity of internal fixation plates on long bone remodeling: a bio mechanical and quantitative histological study. Acta Orthop. Scand. 1976;47(3):241–249. doi: 10.3109/17453677608991984. [DOI] [PubMed] [Google Scholar]
- 101.Hanafusa S., Matsusue Y., Yasunaga T., Yamamuro T., Oka M., Shikinami Y., Ikada Y. Biodegradable plate fixation of rabbit femoral shaft osteotomies. A comparative study. Clin. Orthop. Relat. Res. 1995;(315):262–271. [PubMed] [Google Scholar]
- 102.Lin H.R., Kuo C.J., Yang C.Y., Shaw S.Y., Wu Y.J. Preparation of macroporous biodegradable PLGA scaffolds for cell attachment with the use of mixed salts as porogen additives. J. Biomed. Mater. Res. 2002;63(3):271–279. doi: 10.1002/jbm.10183. [DOI] [PubMed] [Google Scholar]
- 103.Singh L., Kumar V., Ratner B.D. Generation of porous microcellular 85/15 poly (dl-lactide-co-glycolide) foams for biomedical applications. Biomaterials. 2004;25(13):2611–2617. doi: 10.1016/j.biomaterials.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 104.Boccaccini A.R., Blaker J.J., Maquet V., Day R.M., Jérôme R. Preparation and characterisation of poly(lactide-co-glycolide) (PLGA) and PLGA/Bioglass® composite tubular foam scaffolds for tissue engineering applications. Mater. Sci. Eng. C. 2005;25(1):23–31. [Google Scholar]
- 105.Torres F.G., Nazhat S.N., Sheikh Md Fadzullah S.H., Maquet V., Boccaccini A.R. Mechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle-filled composites for tissue engineering scaffolds. Compos. Sci. Technol. 2007;67(6):1139–1147. [Google Scholar]
- 106.Zhang P., Hong Z., Yu T., Chen X., Jing X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide- co-glycolide) and hydroxyapatite surface-grafted with poly( l-lactide) Biomaterials. 2009;30(1):58–70. doi: 10.1016/j.biomaterials.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 107.Shuai C., Yang B., Peng S., Li Z. Development of composite porous scaffolds based on poly(lactide-co-glycolide)/nano-hydroxyapatite via selective laser sintering. Int. J. Adv. Manuf. Technol. 2013;69(1):51–57. [Google Scholar]
- 108.Hargis M.J., Grady B.P. Effect of sample size on isothermal crystallization measurements performed in a differential scanning calorimeter: a method to determine avrami parameters without sample thickness effects. Thermochim. Acta. 2006;443(2):147–158. [Google Scholar]
- 109.Kuo M.C., Tsai C.M., Huang J.C., Chen M. PEEK composites reinforced by nano-sized SiO2 and Al2O3 particulates. Mater. Chem. Phys. 2005;90(1):185–195. [Google Scholar]
- 110.Lai Y.H., Kuo M.C., Huang J.C., Chen M. On the PEEK composites reinforced by surface-modified nano-silica. Mater. Sci. Eng. A. 2007;458(1):158–169. [Google Scholar]
- 111.Lam T.D., Hoang T.V., Quang D.T., Kim J.S. Effect of nanosized and surface-modified precipitated calcium carbonate on properties of CaCO3/polypropylene nanocomposites. Mater. Sci. Eng. A. 2009;501(1):87–93. [Google Scholar]
- 112.Lin P.-L., Fang H.-W., Tseng T., Lee W.-H. Effects of hydroxyapatite dosage on mechanical and biological behaviors of polylactic acid composite materials. Mater. Lett. 2007;61(14):3009–3013. [Google Scholar]
- 113.Todo M., Park S.D., Arakawa K., Takenoshita Y. Relationship between microstructure and fracture behavior of bioabsorbable HA/PLLA composites. Composites Part A. 2006;37(12):2221–2225. [Google Scholar]
- 114.Santos D., Silva D.M., Gomes P.S., Fernandes M.H., Santos J.D., Sencadas V. Multifunctional PLLA-ceramic fiber membranes for bone regeneration applications. J. Colloid Interface Sci. 2017;504:101–110. doi: 10.1016/j.jcis.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 115.Guan L., Davies J.E. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J. Biomed. Mater. Res. A. 2004;71(3):480–487. doi: 10.1002/jbm.a.30173. [DOI] [PubMed] [Google Scholar]
- 116.Hutmacher D.W. Scaffold design and fabrication technologies for engineering tissues — state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001;12(1):107–124. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 117.Jung Y., Kim S., Kim S.-S., You H.J., Kim B.S., Kim S., Kim Y.H. 2005. Tissue Engineered Bone Formation with Polymer/ceramic Composites by Press-and-baking Method. [Google Scholar]
- 118.Zongliang W., Yu W., Yoshihiro I., Peibiao Z., Xuesi C. A comparative study on the in vivo degradation of poly(L-lactide) based composite implants for bone fracture fixation. Sci. Rep. 2016;6(1) doi: 10.1038/srep20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X., Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 120.Peltola S.M., Melchels F.P.W., Grijpma D.W., Kellomki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008;40(4):268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 121.Sachlos E., Czernuszka J.T. Making Tissue Engineering Scaffolds Work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell. Mater. 2003;5:29–40. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 122.Jakus A.E., Shah R.N. Multi and mixed 3D‐printing of graphene‐hydroxyapatite hybrid materials for complex tissue engineering. J. Biomed. Mater. Res. A. 2017;105(1):274–283. doi: 10.1002/jbm.a.35684. [DOI] [PubMed] [Google Scholar]
- 123.Senatov F.S., Niaza K.V., Zadorozhnyy M.Y., Maksimkin A.V., Kaloshkin S.D., Estrin Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016;57:139–148. doi: 10.1016/j.jmbbm.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 124.Trachtenberg J.E., Placone J.K., Smith B.T., Fisher J.P., Mikos A.G. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J. Biomater. Sci. Polym. Ed. 2017;28(6):532–554. doi: 10.1080/09205063.2017.1286184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu G.-H., Hsu S.-h. Review: polymeric-based 3D printing for tissue engineering. J. Med. Biol. Eng. 2015;35(3):285–292. doi: 10.1007/s40846-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chiulan I., Frone A.N., Brandabur C., Panaitescu D.M. Recent advances in 3D printing of aliphatic polyesters. Bioengineering (Basel, Switzerland) 2017;5(1) doi: 10.3390/bioengineering5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Melchels F.P.W., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 128.Zein I., Hutmacher D.W., Tan K.C., Teoh S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23(4):1169–1185. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 129.Kumar S. Selective laser sintering: a qualitative and objective approach. J. Miner. Met. Mater. Soc. (TMS) 2003;55(10):43–47. [Google Scholar]
- 130.Seol Y.-J., Kang H.-W., Lee S.J., Atala A., Yoo J.J. Bioprinting technology and its applications. Eur. J. Cardio. Thorac. Surg. 2014;46(3):342–348. doi: 10.1093/ejcts/ezu148. [DOI] [PubMed] [Google Scholar]
- 131.Esposito Corcione C., Gervaso F., Scalera F., Montagna F., Maiullaro T., Sannino A., Maffezzoli A. 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J. Polym. Eng. 2017:741. [Google Scholar]
- 132.Vyas D., Udyawar D. A review on current state of art of bioprinting. In: Kumar L.J., Pandey P.M., Wimpenny D.I., editors. 3D Printing and Additive Manufacturing Technologies. Springer Singapore; Singapore: 2019. pp. 195–201. [Google Scholar]
- 133.Sidambe T.A. Biocompatibility of advanced manufactured titanium implants—a review. Materials. 2014;7(12) doi: 10.3390/ma7128168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.3D Bio-printing Approaches for Tissue Engineering Applications, By Liam Critchley. https://www.azom.com/article.aspx?ArticleID=13810.
- 135.3D Rapid Prototyping Selective Laser Sintering SLS Services. https://www.indiamart.com/proddetail/3d-rapid-prototyping-selective-laser-sintering-sls-services-19899095048.html.
- 136.Selective Laser Sintering. https://www.custompartnet.com/wu/selective-laser-sintering.
- 137.Stereolithography: The Future of Dental Fabrication. http://decisionsindentistry.com/article/stereolithography-future-dental-fabrication/.
- 138.Stereolithography (SLA). https://www.tth.com/3d-printing/sla-prototyping/.
- 139.Fused Deposition Modeling (FDM).
- 140.Liu C., Xia Z., Czernuszka J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007;85(7 A):1051–1064. [Google Scholar]
- 141.Melchels F.P.W., Feijen J., Grijpma D.W. A poly( d, l-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials. 2009;30(23):3801–3809. doi: 10.1016/j.biomaterials.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 142.Sean V.M., Anthony A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 143.Sears N., Seshadri D., Dhavalikar P., Cosgriff-Hernandez E. Mary Ann Liebert, Inc.; New Rochelle: 2016. A Review of Three-dimensional Printing in Tissue Engineering; pp. 298–310. [DOI] [PubMed] [Google Scholar]
- 144.Wang X., Jiang M., Zhou Z., Gou J., Hui D. 3D printing of polymer matrix composites: a review and prospective. Compos. B. 2017;110:442–458. [Google Scholar]
- 145.Mohamed O., Masood S., Bhowmik J. Optimization of fused deposition modeling process parameters: a review of current research and future prospects. Adv. Manuf. 2015;3(1):42–53. [Google Scholar]
- 146.Kruth J., Wang X., Laoui T., Froyen L. Lasers and materials in selective laser sintering. Assemb. Autom. 2003;23(4) [Google Scholar]
- 147.Mazzoli A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2012;51(3):1–12. doi: 10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 148.Bajaj P., Schweller R.M., Khademhosseini A., West J.L., Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Binder K.W., Allen A.J., Yoo J.J., Atala A. Drop-on-demand inkjet bioprinting: A primer. Gene Ther. Regul. 2011;06(01):33–49. [Google Scholar]
- 150.Yang S., Leong K.-F., Du Z., Chua C.-K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 151.Butscher A., Bohner M., Doebelin N., Hofmann S., Müller R. New depowdering-friendly designs for three-dimensional printing of calcium phosphate bone substitutes. Acta Biomater. 2013;9(11):9149–9158. doi: 10.1016/j.actbio.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 152.Matsuzaki R., Ueda M., Namiki M., Jeong T.-K., Asahara H., Horiguchi K., Nakamura T., Todoroki A., Hirano Y. Three-dimensional printing of continuous-fiber composites by in-nozzle impregnation. Sci. Rep. 2016;6:23058. doi: 10.1038/srep23058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bin Z., Baekhoon S., VuDat N., Doyoung B. 3D printing of high-resolution PLA-based structures by hybrid electrohydrodynamic and fused deposition modeling techniques. J. Micromech. Microeng. 2016;26(2) [Google Scholar]
- 154.Shaffer S., Yang K., Vargas J., Di Prima M.A., Voit W. On reducing anisotropy in 3D printed polymers via ionizing radiation. Polymer. 2014;55(23):5969–5979. [Google Scholar]
- 155.van Dijk K., Schaeken H.G., Marée C.H.M., Verhoeven J., Wolke J.C.G., Habraken F.H.P.M., Jansen J.A. Influence of Ar pressure on r.f. magnetron-sputtered Ca5(PO4)3OH layers. Surf. Coating. Technol. 1995;76–77:206–210. [Google Scholar]
- 156.Van Dijk K., Schaeken H.G., Wolke J.C.G., Marée C.H.M., Habraken F.H.P.M., Verhoeven J., Jansen J.A. Influence of discharge power level on the properties of hydroxyapatite films deposited on Ti6A14V with RF magnetron sputtering. J. Biomed. Mater. Res. 1995;29(2):269–276. doi: 10.1002/jbm.820290218. [DOI] [PubMed] [Google Scholar]
- 157.Han Y., Xu K., Montay G., Fu T., Lu J. Evaluation of nanostructured carbonated hydroxyapatite coatings formed by a hybrid process of plasma spraying and hydrothermal synthesis. J. Biomed. Mater. Res. 2002;60(4):511–516. doi: 10.1002/jbm.10097. [DOI] [PubMed] [Google Scholar]
- 158.Heimann R.B. Thermal spraying of biomaterials. Surf. Coating. Technol. 2006;201(5):2012–2019. [Google Scholar]
- 159.Yang Y., Kim K.-H., Ong J.L. A review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials. 2005;26(3):327–337. doi: 10.1016/j.biomaterials.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 160.W -Aa N., Thaveedeetrakul A., Virote B. 2012. Effect of Sputtering Power on in Vitro Bioactivity of Zirconia Thin Films Obtained by DC Unbalanced Magnetron Sputtering. [Google Scholar]
- 161.Dinu M., Emil Kiss A., Parau A.C., Braic V., Vitelaru C., Braic M., Pana I., Balaceanu M., Vladescu A. 2013. Influence of Thermal Treatment on the Roughness, Corrosion Resistance and Wettability of Hydroxyapatite Films Deposited by RF Magnetron Sputtering. [Google Scholar]
- 162.López E.O., Mello A., Sendão H., Costa L.T., Rossi A.L., Ospina R.O., Borghi F.F., Silva Filho J.G., Rossi A.M. Growth of crystalline hydroxyapatite thin films at room temperature by tuning the energy of the RF-magnetron sputtering plasma. ACS Appl. Mater. Interfaces. 2013;5(19):9435. doi: 10.1021/am4020007. [DOI] [PubMed] [Google Scholar]
- 163.Pichugin V.F., Surmenev R.A., Shesterikov E.V., Ryabtseva M.A., Eshenko E.V., Tverdokhlebov S.I., Prymak O., Epple M. The preparation of calcium phosphate coatings on titanium and nickel–titanium by rf-magnetron-sputtered deposition: composition, structure and micromechanical properties. Surf. Coating. Technol. 2008;202(16):3913–3920. [Google Scholar]
- 164.Shin J., Lee K., Koh J., Son H., Kim H., Lim H.P., Yun K., Oh G., Lee S., Oh H., Lee K., Hwang G., Park S.W. Hydroxyapatite coatings on nanotubular titanium dioxide thin films prepared by radio frequency magnetron sputtering. J. Nanosci. Nanotechnol. 2013;13(8):5807–5810. doi: 10.1166/jnn.2013.7064. [DOI] [PubMed] [Google Scholar]
- 165.Surmenev R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coating. Technol. 2011;206:2035–2056. [Google Scholar]
- 166.Surmenev R.A., Surmeneva M.A., Evdokimov K.E., Pichugin V.F., Peitsch T., Epple M. The influence of the deposition parameters on the properties of an rf-magnetron-deposited nanostructured calcium phosphate coating and a possible growth mechanism. Surf. Coating. Technol. 2011;205(12):3600–3606. [Google Scholar]
- 167.Takayanagi S., Yanagitani T., Matsukawa M. Unusual growth of polycrystalline oxide film induced by negative ion bombardment in the capacitively coupled plasma deposition. Appl. Phys. Lett. 2012;101(23) [Google Scholar]
- 168.Takuya S., Kazuhiko O., Akira W. The effect of implant surfaces sputter-coated with hydroxyapatite target. J. Hard Tissue Biol. 2013;22(1):67–77. [Google Scholar]
- 169.Crouzier T., Ren K., Nicolas C., Roy C., Picart C. Layer‐by‐layer films as a biomimetic reservoir for rhBMP‐2 delivery: controlled differentiation of myoblasts to osteoblasts. Small. 2009;5(5):598–608. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- 170.Matthias H., Christian H. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010;5(8):565. doi: 10.1038/nnano.2010.83. [DOI] [PubMed] [Google Scholar]
- 171.Boccaccini A.R., Keim S., Ma R., Li Y., Zhitomirsky I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface. 2010;7(5):581–613. doi: 10.1098/rsif.2010.0156.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen F., Lam W.M., Lin C.J., Qiu G.X., Wu Z.H., Luk K.D.K., Lu W.W. Biocompatibility of electrophoretical deposition of nanostructured hydroxyapatite coating on roughen titanium surface: in vitro evaluation using mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2007;82(1):183–191. doi: 10.1002/jbm.b.30720. [DOI] [PubMed] [Google Scholar]
- 173.Gross K., Chai C., Kannangara G., Ben-Nissan B., Hanley L. Thin hydroxyapatite coatings via sol–gel synthesis. Official Journal of the European Society for Biomaterials. 1998;9(12):839–843. doi: 10.1023/a:1008948228880. [DOI] [PubMed] [Google Scholar]
- 174.Layrolle P., Ito A., Tateishi T. Sol‐gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. J. Am. Ceram. Soc. 1998;81(6):1421–1428. [Google Scholar]
- 175.Joshy K.S., Snigdha S., Thomas S. Chapter 16 - plasma modified polymeric materials for scaffolding of bone tissue engineering. In: Thomas S., Mozetič M., Cvelbar U., Špatenka P., M P.K., editors. Non-Thermal Plasma Technology for Polymeric Materials. Elsevier; 2019. pp. 439–458. [Google Scholar]
- 176.Petrovicova E., Schadler L.S. Thermal spraying of polymers. Int. Mater. Rev. 2002;47(4):169–190. [Google Scholar]
- 177.Anon P.s.p. 2010. Plasma spraying process, (2010)http://www.zircotec.com/page/plasma-spray_processing/39 Available at: (accessed April 2011) [Google Scholar]
- 178.AS D., YuV N., YuA F. Vliânie naprâženiâ sme͉eniâ na podložke na teksturu, magnitnye svojstva i morfologiû poverhnosti plenok nikelâ. Geteromagnitnaâ Mikroèlektronika. 2012;13(25) [Google Scholar]
- 179.AS D., YuV N., YuA F. Vliânie naprâženiâ sme͉eniâ na strukturu, morfologiû i magnitnye svojstva plenok nikelâ, polučennyh magnetronnym raspyleniem na postoânnom toke. Nanoinženeriâ. 2013;2(24) [Google Scholar]
- 180.Asanithi P., Chaiyakun S., Limsuwan P. Growth of silver nanoparticles by DC magnetron sputtering. J. Nanomater. 2012;2012 Article Number: 79. [Google Scholar]
- 181.Slavcheva E., Ganske G., Schnakenberg U. Sputtered Pd as hydrogen storage for a chip-integrated microenergy system. Sci. World J. 2014;2014 doi: 10.1155/2014/146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Slavcheva E., Ganske G., Topalov G., Mokwa W., Schnakenberg U. Effect of sputtering parameters on surface morphology and catalytic efficiency of thin platinum films. Appl. Surf. Sci. 2009;255(13):6479–6486. [Google Scholar]
- 183.Slavcheva E.P. Magnetron sputtered iridium oxide as anode catalyst for PEM hydrogen generation. Macedonian J. Chem. Chem. Eng. 2011;30(1):45–54. [Google Scholar]
- 184.YuV N., AS D., YuA F. Formirovanie teksturirovannyh plenok ferromagnitnyh 3-d metallov s različnoj kristallografičeskoj orientaciej i mikrostrukturnym stroeniem metodom magnetronnogo raspyleniâ na postoânnom toke. Nelinejnyj Mir. 2013;2(5) [Google Scholar]
- 185.Ivanova A.A., Surmeneva M.A., Surmenev R.A., Depla D. Influence of deposition conditions on the composition, texture and microstructure of RF-magnetron sputter-deposited hydroxyapatite thin films. Thin Solid Films. 2015;591:368–374. [Google Scholar]
- 186.Akindoyo J.O., Beg M.D.H., Ghazali S., Heim H.P., Feldmann M. Impact modified PLA-hydroxyapatite composites – thermo-mechanical properties. Compos A. Appl. Sci. Manuf. 2018;107:326–333. [Google Scholar]
- 187.Zhang Y., Dong K., Wang F., Wang H., Wang J., Jiang Z., Diao S. Three dimensional macroporous hydroxyapatite/chitosan foam-supported polymer micelles for enhanced oral delivery of poorly soluble drugs. Colloids Surfaces B Biointerfaces. 2018;170:497–504. doi: 10.1016/j.colsurfb.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 188.Baştan F.E., Atiq Ur Rehman M., Avcu Y.Y., Avcu E., Üstel F., Boccaccini A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surfaces B Biointerfaces. 2018;169:176–182. doi: 10.1016/j.colsurfb.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 189.Ozeki K., Masuzawa T., Aoki H. Fabrication of hydroxyapatite thin films on polyetheretherketone substrates using a sputtering technique. Mater. Sci. Eng. C. 2017;72:576–582. doi: 10.1016/j.msec.2016.11.111. [DOI] [PubMed] [Google Scholar]
- 190.Naik A., Shepherd D.V., Shepherd J.H., Best S.M., Cameron R.E. The effect of the type of HA on the degradation of PLGA/HA composites. Mater. Sci. Eng. C. 2017;70:824–831. doi: 10.1016/j.msec.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 191.Jing X., Mi H.-Y., Turng L.-S. Comparison between PCL/hydroxyapatite (HA) and PCL/halloysite nanotube (HNT) composite scaffolds prepared by co-extrusion and gas foaming. Mater. Sci. Eng. C. 2017;72:53–61. doi: 10.1016/j.msec.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 192.Akindoyo J.O., Beg M.D.H., Ghazali S., Heim H.P., Feldmann M. Effects of surface modification on dispersion, mechanical, thermal and dynamic mechanical properties of injection molded PLA-hydroxyapatite composites. Compos A. Appl. Sci. Manuf. 2017;103:96–105. [Google Scholar]
- 193.Wang Z., Wang Y., Ito Y., Zhang P., Chen X. A comparative study on the in vivo degradation of poly(L-lactide) based composite implants for bone fracture fixation. Sci. Rep. 2016;6:20770. doi: 10.1038/srep20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Niaza K.V., Senatov F.S., Kaloshkin S.D., Maksimkin A.V., Chukov D.I. 3D-printed scaffolds based on PLA/HA nanocomposites for trabecular bone reconstruction. J. Phys. Conf. 2016;741(1) 012068. [Google Scholar]
- 195.Durham J.W., Rabiei A. Deposition, heat treatment and characterization of two layer bioactive coatings on cylindrical PEEK. Surf. Coating. Technol. 2016;301:106–113. doi: 10.1016/j.surfcoat.2015.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.G. Ghersi, F.C. Pavia, G. Conoscenti, G.A. Mannella, S. Greco, S. Rigogliuso, V.L. Carrubba, V. Brucato, PLLA scaffold via TIPS for bone tissue engineering, Chem. Eng. Trans. 49 301-306.
- 197.Nishida Y., Domura R., Sakai R., Okamoto M., Arakawa S., Ishiki R., Salick M.R., Turng L.-S. Fabrication of PLLA/HA composite scaffolds modified by DNA. Polymer. 2015;56:73–81. [Google Scholar]
- 198.Naik A., Best S.M., Cameron R.E. The influence of silanisation on the mechanical and degradation behaviour of PLGA/HA composites. Mater. Sci. Eng. C. 2015;48:642–650. doi: 10.1016/j.msec.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 199.Persson M., Lorite G.S., Kokkonen H.E., Cho S.-W., Lehenkari P.P., Skrifvars M., Tuukkanen J. Effect of bioactive extruded PLA/HA composite films on focal adhesion formation of preosteoblastic cells. Colloids Surfaces B Biointerfaces. 2014;121:409–416. doi: 10.1016/j.colsurfb.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 200.Endoğan T., Hasirci V., Hasirci N. 2014. Preparation and Characterization of Chitosan and PLGA-based Scaffolds for Tissue Engineering Applications. [Google Scholar]
- 201.Liuyun J., Chengdong X., Lixin J., Lijuan X. Effect of HA with different grain size range on the crystallization behaviors and mechanical property of HA/PLGA composite. Thermochim. Acta. 2013;565:52–57. [Google Scholar]
- 202.Kim M.-J., Koh Y.-H. Synthesis of aligned porous poly(ε-caprolactone) (PCL)/hydroxyapatite (HA) composite microspheres. Mater. Sci. Eng. C. 2013;33(4):2266–2272. doi: 10.1016/j.msec.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 203.Kutikov A.B., Song J. An amphiphilic degradable polymer/hydroxyapatite composite with enhanced handling characteristics promotes osteogenic gene expression in bone marrow stromal cells. Acta Biomater. 2013;9(9):8354–8364. doi: 10.1016/j.actbio.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abdal-hay A., Sheikh F.A., Lim J.K. Air jet spinning of hydroxyapatite/poly(lactic acid) hybrid nanocomposite membrane mats for bone tissue engineering. Colloids Surfaces B Biointerfaces. 2013;102:635–643. doi: 10.1016/j.colsurfb.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 205.Albano C., Gonzalez G., Palacios Gutiérrez J., Karam A., Covis M. 2013. PLLA‐HA Vs. PLGA‐HA Characterization and Comparative Analysis. [Google Scholar]
- 206.Tanodekaew S., Channasanon S., Kaewkong P., Uppanan P., Scaffolds P.L.A.-H.A. 2013. Preparation and Bioactivity. [Google Scholar]
- 207.Liuyun J., Chengdong X., Dongliang C., Lixin J., xiubing P. Effect of n-HA with different surface-modified on the properties of n-HA/PLGA composite. Appl. Surf. Sci. 2012;259:72–78. [Google Scholar]
- 208.Li X., Liu H., Wang J., Li C. Preparation and characterization of PLLA/nHA nonwoven mats via laser melt electrospinning. Mater. Lett. 2012;73:103–106. [Google Scholar]
- 209.Mou Z.-L., Zhao L.-J., Zhang Q.-A., Zhang J., Zhang Z.-Q. Preparation of porous PLGA/HA/collagen scaffolds with supercritical CO2 and application in osteoblast cell culture. J. Supercrit. Fluids. 2011;58(3):398–406. [Google Scholar]
- 210.Lao L., Wang Y., Zhu Y., Zhang Y., Gao C. Poly(lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011;22(8):1873–1884. doi: 10.1007/s10856-011-4374-8. [DOI] [PubMed] [Google Scholar]
- 211.Socol G., Macovei A.M., Miroiu F., Stefan N., Duta L., Dorcioman G., Mihailescu I.N., Petrescu S.M., Stan G.E., Marcov D.A., Chiriac A., Poeata I. Hydroxyapatite thin films synthesized by pulsed laser deposition and magnetron sputtering on PMMA substrates for medical applications. Mater. Sci. Eng., B. 2010;169(1):159–168. [Google Scholar]
- 212.Delabarde C., Plummer C.J.G., Bourban P.E., Månson J.A.E. Solidification behavior of PLLA/nHA nanocomposites. Compos. Sci. Technol. 2010;70(13):1813–1819. [Google Scholar]
- 213.Papenburg B.J., Bolhuis-Versteeg L.A.M., Grijpma D.W., Feijen J., Wessling M., Stamatialis D. A facile method to fabricate poly(l-lactide) nano-fibrous morphologies by phase inversion. Acta Biomater. 2010;6(7):2477–2483. doi: 10.1016/j.actbio.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 214.Deplaine H., Ribelles J.L.G., Ferrer G.G. Effect of the content of hydroxyapatite nanoparticles on the properties and bioactivity of poly(l-lactide) – hybrid membranes. Compos. Sci. Technol. 2010;70(13):1805–1812. [Google Scholar]
- 215.Fang R., Zhang E., Xu L., Wei S. 2010. Electrospun PCLJPLAIHA Based Nanofibers as Scaffold for Osteoblast-like Cells. [DOI] [PubMed] [Google Scholar]
- 216.Eosoly S., Brabazon D., Lohfeld S., Looney L. Selective laser sintering of hydroxyapatite/poly-ε-caprolactone scaffolds. Acta Biomater. 2010;6(7):2511–2517. doi: 10.1016/j.actbio.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 217.Duan B., Wang M., Zhou W.Y., Cheung W.L., Li Z.Y., Lu W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010;6(12):4495–4505. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 218.Gay S., Arostegui S., Lemaitre J. Preparation and characterization of dense nanohydroxyapatite/PLLA composites. Mater. Sci. Eng. C. 2009;29(1):172–177. [Google Scholar]
- 219.Prabhakaran M.P., Venugopal J., Ramakrishna S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009;5(8):2884–2893. doi: 10.1016/j.actbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 220.Tian T., Jiang D., Zhang J., Lin Q. Fabrication of bioactive composite by developing PLLA onto the framework of sintered HA scaffold. Mater. Sci. Eng. C. 2008;28(1):51–56. [Google Scholar]
- 221.Zhao J., Guo L.Y., Yang X.B., Weng J. Preparation of bioactive porous HA/PCL composite scaffolds. Appl. Surf. Sci. 2008;255(5):2942–2946. Part 2. [Google Scholar]
- 222.Jeong S.I., Ko E.K., Yum J., Jung C.H., Lee Y.M., Shin H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008;8(4):328–338. doi: 10.1002/mabi.200700107. [DOI] [PubMed] [Google Scholar]
- 223.Shor L., Güçeri S., Wen X., Gandhi M., Sun W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials. 2007;28(35):5291–5297. doi: 10.1016/j.biomaterials.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 224.Kim S.-S., Sun Park M., Jeon O., Yong Choi C., Kim B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(8):1399–1409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 225.Russias J., Saiz E., Nalla R.K., Gryn K., Ritchie R.O., Tomsia A.P. Fabrication and mechanical properties of PLA/HA composites: a study of in vitro degradation. Mater. Sci. Eng. C. 2006;26(8):1289–1295. doi: 10.1016/j.msec.2005.08.004. Biomimetic and supramolecular systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kothapalli C.R., Shaw M.T., Wei M. Biodegradable HA-PLA 3-D porous scaffolds: effect of nano-sized filler content on scaffold properties. Acta Biomater. 2005;1(6):653–662. doi: 10.1016/j.actbio.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 227.Williams J.M., Adewunmi A., Schek R.M., Flanagan C.L., Krebsbach P.H., Feinberg S.E., Hollister S.J., Das S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26(23):4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 228.Tan K.H., Chua C.K., Leong K.F., Cheah C.M., Gui W.S., Tan W.S., Wiria F.E. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Bio Med. Mater. Eng. 2005;15(1–2):113–124. [PubMed] [Google Scholar]
- 229.Chen V.J., Ma P.X. Nano-fibrous poly(l-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 230.Rajendra P.P., Sunil U.T., Suresh U.S., Jalinder T.T., Abraham J.D. Biomedical applications of poly(lactic acid) Recent Pat. Regen. Med. 2014;4(1):40–51. [Google Scholar]
- 231.Dziadek M., Menaszek E., Zagrajczuk B., Pawlik J., Cholewa-Kowalska K. New generation poly(ε-caprolactone)/gel-derived bioactive glass composites for bone tissue engineering: Part I. Material properties. Mater. Sci. Eng. C. 2015;56:9–21. doi: 10.1016/j.msec.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 232.Shin Y.M., Park J.-S., Jeong S.I., An S.-J., Gwon H.-J., Lim Y.-M., Nho Y.-C., Kim C.-Y. Promotion of human mesenchymal stem cell differentiation on bioresorbable polycaprolactone/biphasic calcium phosphate composite scaffolds for bone tissue engineering. Biotechnol. Bioproc. Eng. 2014;19(2):341–349. [Google Scholar]
- 233.Ren J., Blackwood K.A., Doustgani A., Poh P.P., Steck R., Stevens M.M., Woodruff M.A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mater. Res. A. 2013;102(9):3140–3153. doi: 10.1002/jbm.a.34985. [DOI] [PubMed] [Google Scholar]
- 234.Patrina S.P.P., Dietmar W.H., Molly M.S., Maria A.W. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication. 2013;5(4):045005. doi: 10.1088/1758-5082/5/4/045005. [DOI] [PubMed] [Google Scholar]
- 235.Lei B., Shin K.-H., Noh D.-Y., Jo I.-H., Koh Y.-H., Kim H.-E., Kim S.E. Sol–gel derived nanoscale bioactive glass (NBG) particles reinforced poly(ε-caprolactone) composites for bone tissue engineering. Mater. Sci. Eng. C. 2013;33(3):1102–1108. doi: 10.1016/j.msec.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 236.Wei J., Chen F., Shin J.-W., Hong H., Dai C., Su J., Liu C. Preparation and characterization of bioactive mesoporous wollastonite – polycaprolactone composite scaffold. Biomaterials. 2009;30(6):1080–1088. doi: 10.1016/j.biomaterials.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 237.Raucci M., Guarino V., Ambrosio L. 2009. Polymeric Composites, Prepared by Sol-gel Method, with Spatial Gradients of Hydroxyapatite Bioactive Signals. [Google Scholar]
- 238.Cutright D.E., Hunsuck E.E. The repair of fractures of the orbital floor using biodegradable polylactic acid. Oral Surg. Oral Med. Oral Pathol. 1972;33(1):28–34. doi: 10.1016/0030-4220(72)90204-6. [DOI] [PubMed] [Google Scholar]
- 239.Lou C.-W., Yao C.-H., Chen Y.-S., Hsieh T.-C., Lin J.-H., Hsing W.-H. Manufacturing and properties of PLA absorbable surgical suture. Textil. Res. J. 2008;78(11):958–965. [Google Scholar]
- 240.Saini P., Arora M., Kumar M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016;107:47–59. doi: 10.1016/j.addr.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 241.Rakovsky A., Gotman I., Rabkin E., Gutmanas E.Y. β-TCP–polylactide composite scaffolds with high strength and enhanced permeability prepared by a modified salt leaching method. J. Mech. Behav. Biomed. Mater. 2014;32:89–98. doi: 10.1016/j.jmbbm.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 242.Lou T., Wang X., Song G., Gu Z., Yang Z. Fabrication of PLLA/β-TCP nanocomposite scaffolds with hierarchical porosity for bone tissue engineering. Int. J. Biol. Macromol. 2014;69:464–470. doi: 10.1016/j.ijbiomac.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 243.El-Kady A.M., Saad E.A., El-Hady B.M.A., Farag M.M. Synthesis of silicate glass/poly(l-lactide) composite scaffolds by freeze-extraction technique: characterization and in vitro bioactivity evaluation. Ceram. Int. 2010;36(3):995–1009. [Google Scholar]
- 244.Damadzadeh B., Jabari H., Skrifvars M., Airola K., Moritz N., Vallittu P.K. Effect of ceramic filler content on the mechanical and thermal behaviour of poly-l-lactic acid and poly-l-lactic-co-glycolic acid composites for medical applications. J. Mater. Sci. Mater. Med. 2010;21(9):2523–2531. doi: 10.1007/s10856-010-4110-9. [DOI] [PubMed] [Google Scholar]
- 245.Filipowska J., Pawlik J., Cholewa-Kowalska K., Tylko G., Pamula E., Niedzwiedzki L., Szuta M., Laczka M., Osyczka A.M. Incorporation of sol–gel bioactive glass into PLGA improves mechanical properties and bioactivity of composite scaffolds and results in their osteoinductive properties. Biomed. Mater. 2014;9(6):065001. doi: 10.1088/1748-6041/9/6/065001. [DOI] [PubMed] [Google Scholar]
- 246.Pamula E., Kokoszka J., Cholewa-Kowalska K., Laczka M., Kantor L., Niedzwiedzki L., Reilly G.C., Filipowska J., Madej W., Kolodziejczyk M., Tylko G., Osyczka A.M. Degradation, bioactivity, and osteogenic potential of composites made of PLGA and two different sol–gel bioactive glasses. Ann. Biomed. Eng. 2011;39(8):2114–2129. doi: 10.1007/s10439-011-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim S.-S., Ahn K.-M., Park M.S., Lee J.-H., Choi C.Y., Kim B.-S. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J. Biomed. Mater. Res. A. 2006;80A(1):206–215. doi: 10.1002/jbm.a.30836. [DOI] [PubMed] [Google Scholar]
- 248.Maquet V., Boccaccini A.R., Pravata L., Notingher I., Jérôme R. Porous poly(α-hydroxyacid)/Bioglass® composite scaffolds for bone tissue engineering. I: preparation and in vitro characterisation. Biomaterials. 2004;25(18):4185–4194. doi: 10.1016/j.biomaterials.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 249.Amini A.R., Laurencin C.T., Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012;40(5):363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kim S.S., Utsunomiya H., Koski J.A., Wu B.M., Cima M.J., Sohn J., Mukai K., Griffith L.G., Vacanti J.P. Survival and function of hepatocytes on a novel three-dimensional synthetic biodegradable polymer scaffold with an intrinsic network of channels. Ann. Surg. 1998;228(1):8–13. doi: 10.1097/00000658-199807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gerhardt L.-C., Widdows K.L., Erol M.M., Burch C.W., Sanz-Herrera J.A., Ochoa I., Stämpfli R., Roqan I.S., Gabe S., Ansari T., Boccaccini A.R. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials. 2011;32(17):4096–4108. doi: 10.1016/j.biomaterials.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 252.Maquet V., Boccaccini A.R., Pravata L., Notingher I., Jérôme R. Preparation, characterization, and in vitro degradation of bioresorbable and bioactive composites based on Bioglass®-filled polylactide foams. J. Biomed. Mater. Res. A. 2003;66A(2):335–346. doi: 10.1002/jbm.a.10587. [DOI] [PubMed] [Google Scholar]
- 253.Blaker J.J., Gough J.E., Maquet V., Notingher I., Boccaccini A.R. In vitro evaluation of novel bioactive composites based on Bioglass®-filled polylactide foams for bone tissue engineering scaffolds. J. Biomed. Mater. Res. A. 2003;67A(4):1401–1411. doi: 10.1002/jbm.a.20055. [DOI] [PubMed] [Google Scholar]