Abstract
Introduction
Solanezumab treatment was previously shown to significantly increase total (bound + unbound) cerebrospinal fluid (CSF) levels of amyloid β (Aβ)1–40 and Aβ1–42 in patients with mild to moderate Alzheimer's disease dementia yet did not produce meaningful cognitive effects. This analysis assessed solanezumab's central nervous system target engagement by evaluating changes in CSF total and free Aβ isoforms and their relationship with solanezumab exposure.
Methods
CSF Aβ isoform concentrations were measured in patients with mild Alzheimer's disease dementia from a pooled EXPEDITION + EXPEDITION2 population and from EXPEDITION3. CSF solanezumab concentrations were determined from EXPEDITION3.
Results
Solanezumab produced statistically significant increases in CSF total Aβ isoforms versus placebo, which correlated with CSF solanezumab concentration. Inconsistent effects on free Aβ isoforms were observed. Solanezumab penetration into the central nervous system was low.
Discussion
Solanezumab administration engaged the central molecular target, and molar ratio analyses demonstrated that higher exposures may further increase CSF total Aβ concentrations.
Keywords: Solanezumab, Alzheimer's disease, Cerebrospinal fluid, Biomarkers, Pharmacodynamics
Highlights
-
•
Solanezumab increased cerebrospinal fluid levels of total amyloid β isoforms.
-
•
Central pharmacodynamics of solanezumab were inconsistent.
-
•
Penetration of solanezumab into the central nervous system was limited.
-
•
Increased dosing may improve target engagement.
1. Background
The amyloid hypothesis postulates that the production and deposition of amyloid β (Aβ) is an early and necessary event in the pathogenesis of Alzheimer's disease (AD). Implicit in the hypothesis is the possibility that reducing synthesis/deposition or increasing clearance of Aβ might slow clinical progression of the disease [1], [2]. Solanezumab, a humanized monoclonal antibody that binds to the mid-domain of the Aβ peptide, was designed to slow AD progression by increasing clearance of soluble Aβ from the brain [3], [4], [5]. The increased clearance of Aβ from the brain via formation of high-affinity antibody-Aβ peptide complexes in plasma (“peripheral sink”) was proposed as one of the possible mechanisms by which passive immunization may reduce Aβ burden and improve cognitive performance in transgenic mouse models of AD [6].
Measurement of cerebrospinal fluid (CSF) Aβ isoform levels permits quantification of biomarkers known to be altered by amyloid pathology [7]. In a phase 2 study, solanezumab was well tolerated using doses up to 400 mg weekly for 12 weeks and produced dose-dependent increases in plasma and CSF total (solanezumab bound + unbound) Aβ1–40 and Aβ1–42 [3]. These increases were attributed to a small percentage of solanezumab crossing into the central compartment and binding Aβ. Furthermore, in patients with AD dementia treated with solanezumab, a numerically small, nonsignificant but potentially exposure-dependent decrease in CSF-free (unbound to antibody) Aβ1–40 relative to placebo was observed, whereas CSF-free Aβ1–42 was increased in an exposure-dependent manner. These disparate effects on free Aβ1–40 and Aβ1–42 in CSF suggest that the amyloid contained in plaque, largely consisting of Aβ1–42, may have been mobilized due to a shift in equilibrium caused by solanezumab treatment [8].
In the previous EXPEDITION and EXPEDITION2 phase 3 clinical trials, plasma levels of both total Aβ1–40 and Aβ1–42 increased up to 300- to 500-fold after ≤80 weeks of solanezumab treatment, administered at a dose of 400 mg every 4 weeks (Q4W), whereas no increases in Aβ1–40 or Aβ1–42 were seen in subjects assigned placebo [8]. Changes from baseline in CSF total Aβ1–40 and CSF total Aβ1–42 were significantly greater in the solanezumab treatment group than in the placebo group. CSF concentration of free Aβ1–40 decreased significantly more in the solanezumab treatment group than in the placebo group, whereas the change in CSF level of free Aβ1–42 was not statistically different between groups [8]. Solanezumab did not significantly reduce cognitive or functional decline in a pooled analysis of patients ranging from mild to moderate AD dementia [4]; however, in a prespecified, pooled secondary analysis, patients with mild AD dementia treated with solanezumab showed less cognitive (approximately 34%) and functional (approximately 18%) decline than placebo-treated patients [8]. Only a subset of these patients had known amyloid status by either florbetapir positron emission tomography (PET) scans or CSF measurements. Of this subset, approximately 25% of patients with mild AD dementia did not appear to have amyloid pathology and thus should not have been expected to respond to a treatment targeting Aβ [8].
EXPEDITION3 was a multicenter, international, randomized study designed to confirm the secondary efficacy analyses from EXPEDITION and EXPEDITION2 [5]. EXPEDITION3 only enrolled patients with mild AD dementia, Mini–Mental State Examination (MMSE) score of 20 through 26, and florbetapir PET or CSF biomarker evidence of amyloid pathology. These inclusion criteria were expected to produce solanezumab treatment outcomes of at least the same magnitude as or greater than those seen in EXPEDITION and EXPEDITION2. As in EXPEDITION and EXPEDITION2, the dose of solanezumab was 400 mg Q4W. Although the solanezumab cognitive and functional treatment effects in EXPEDITION3 were directionally consistent relative to placebo, the slowing of cognitive decline in patients treated with solanezumab was smaller than that observed in the secondary analyses of the mild AD dementia population in EXPEDITION and EXPEDITION2 [5], [8].
The aims of the current analysis include comparing central nervous system (CNS) target engagement of intravenous solanezumab 400 mg versus placebo, administered Q4W. This comparison was conducted by evaluating changes in CSF total Aβ1–40 and Aβ1–42 and CSF free Aβ1–40 and Aβ1–42 in a pooled population of patients with mild AD dementia from the EXPEDITION + EXPEDITION2 trials and patients from the EXPEDITION3 trial. Furthermore, the relationship between the treatment effect on Aβ isoforms and CSF solanezumab exposure observed in EXPEDITION3 was investigated.
2. Methods
2.1. Trial design and participants
EXPEDITION3 (NCT01900665) was a double-blind, placebo-controlled, phase 3 study of 2129 patients with mild dementia due to AD (MMSE scores 20–26), plus a florbetapir PET scan or CSF result consistent with the presence of amyloid pathology at screening [5]. EXPEDITION (NCT00905372) and EXPEDITION2 (NCT00904683) were similarly designed double-blind, placebo-controlled, phase 3 studies of 2052 patients with mild-moderate AD dementia (MMSE score mild = 20–26, moderate = 16–19) but without objective biomarker-based evidence of amyloid pathology [4]. All EXPEDITION studies were carried out in accordance with the Declaration of Helsinki for experiments involving human research. All study participants provided written informed consent before participation in the studies [4], [5]. Patients with mild AD dementia were pooled from EXPEDITION and EXPEDITION2 to provide a population for comparisons with the mild AD dementia population in EXPEDITION3.
For subjects in the EXPEDITION/EXPEDITION2 trials, patients at participating sites were given the opportunity to enroll in a CSF addendum. In EXPEDITION3, all subjects were required to qualify for the study with either a florbetapir PET scan or CSF results consistent with amyloid pathology (depending on site availability of florbetapir scans and investigator preference). For those patients qualifying with CSF results, a second lumbar puncture was conducted at the end of the double-blind period of the trial. Subjects qualifying using a florbetapir scan were offered the opportunity to participate in a CSF addendum to the trial, if their site chose to participate in the addendum.
In all three trials, all patients at a participating site and electing to receive a lumbar puncture were eligible to participate unless they had allergies to local anesthetics, a medical condition requiring treatment with anticoagulants, or any other contraindication, such as increased intracranial pressure.
2.2. Treatment administration
In all EXPEDITION studies, patients were randomized to 400 mg solanezumab or placebo administered as an intravenous infusion of approximately 70 mL over at least 30 minutes Q4W for 18 months (approximately 80 weeks).
2.3. Pharmacodynamics and pharmacokinetics
CSF was obtained by lumbar puncture at baseline (screening or baseline visit) and at the endpoint visit (80 weeks or early discontinuation visit). CSF total Aβ1–40, total Aβ1–42, free Aβ1–40, and free Aβ1–42 concentrations were determined using validated immunoassays [8] in a centralized laboratory. In the EXPEDITION3 study, CSF solanezumab concentrations were determined using a proprietary validated liquid chromatography–mass spectrometry assay. Although CSF solanezumab concentrations were also obtained in the EXPEDITION and EXPEDITION2 studies, an ELISA assay was used, which did not provide comparable results to the assay used in EXPEDITION3. Because more concentration data were obtained in the EXPEDITION3 study than in the other two studies, and because the difference in assays prevents a direct comparison between all 3 studies, only the solanezumab concentration data from EXPEDITION3 are reported here.
2.4. Statistical analysis
EXPEDITION + EXPEDITION2 and EXPEDITION3 changes in CSF Aβ parameters were examined using an analysis of covariance model containing terms for treatment, baseline CSF Aβ1–40 and Aβ1–42, and age at baseline for patients with both baseline and endpoint measures. CSF Aβ parameters are provided as mean results ± standard error or standard deviation and individual patient baseline to endpoint changes (spaghetti plots).
All statistical analyses were performed by Eli Lilly and Company or a contract research organization with SAS software (SAS Institute Inc.) and R [9] using ggplot2 [10]. All tests were conducted at a two-sided α level of 0.05, unless otherwise specified. Baseline demographic characteristics and CSF parameters of patients included in the CSF data set were summarized for each treatment group. Linear regressions were used to explore the relationship between CSF solanezumab concentration and change in CSF Aβ concentrations. Only data from EXPEDITION3 were used in assessing the relationship between solanezumab concentrations and Aβ isoforms due to a change in the CSF solanezumab assay that occurred before the analysis of EXPEDITION3 samples, preventing a comparison of results from across the three studies. These analyses included both placebo- and solanezumab-treated patients. The Spearman rank correlation coefficient (rs) and associated P value were calculated for each analysis.
To measure the extent of target engagement, a calculation was performed to compare the amount of solanezumab in the CSF at the end of the study with the total amount of Aβ present in the CSF at baseline. Total Aβ concentration was approximated by adding together the molar concentrations of Aβ1–40 and Aβ1–42. The concentration of solanezumab in the CSF (expressed as a molar concentration) was divided by the total Aβ concentration to calculate the molar ratio of solanezumab to baseline Aβ concentrations.
To assess the relationship of CSF parameters with cognition and function, Spearman's rank correlation coefficient (rs) was obtained on change from baseline to week 80 for CSF total tau, CSF phosphorylated tau, and CSF-free and total Aβ1–40 and Aβ1–42 in a subset of patients, with change from baseline to week 80 for Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-Cog) ADAS-Cog11, ADAS-Cog12, ADAS-Cog14, Alzheimer's Disease Cooperative Study–[Instrumental] Activities of Daily Living, MMSE, Functional Activities Questionnaire (FAQ), Neuropsychiatric Inventory, and Clinical Dementia Rating–Sum of Boxes by the treatment group.
3. Results
Of the 2052 patients randomized in EXPEDITION + EXPEDITION2, 1027 patients received solanezumab and 1025 patients received placebo [4], [8]. CSF was collected from 120 and 121 patients with mild AD dementia per treatment group, respectively. A total of 2129 patients were randomized in EXPEDITION3, with 1057 patients receiving solanezumab and 1072 patients receiving placebo [5]. CSF was obtained from 211 and 210 patients per treatment group, respectively.
Patient demographics and baseline CSF measurements are summarized in Table 1. CSF measurements in the analyzed trials were similar across treatment groups.
Table 1.
Patient demographics and baseline CSF measures
| Demographic | EXPEDITION + EXPEDITION2 |
EXPEDITION3 |
||
|---|---|---|---|---|
| Solanezumab |
Placebo |
Solanezumab |
Placebo |
|
| CSF sample (n = 120) | CSF sample (n = 121) | CSF sample (n = 211) | CSF sample (n = 210) | |
| Age, years | 71.7 (8.6) | 72.1 (7.45) | 71.4 (7.89) | 71.9 (7.55) |
| Female, N (%) | 59 (49.2) | 67 (55.4) | 122 (57.8) | 115 (54.8) |
| CSF total Aβ1–40, pg/mL | 10,700 (3460) | 10,800 (4000) | 11,100 (4970) | 10,700 (4320) |
| CSF total Aβ1–42, pg/mL | 741 (339) | 698 (326) | 737 (220) | 747 (226) |
| CSF free Aβ1–40, pg/mL | 6110 (1810) | 6310 (2030) | 5700 (2030) | 5750 (2030) |
| CSF free Aβ1–42, pg/mL | 300 (184) | 289 (158) | 272 (99.3) | 278 (103) |
Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid; N, number of patients; n, number of samples; SD, standard deviation.
NOTE. Patient demographics and baseline CSF are for patients with CSF measures. Unless otherwise noted, demographic data are shown as mean (SD).
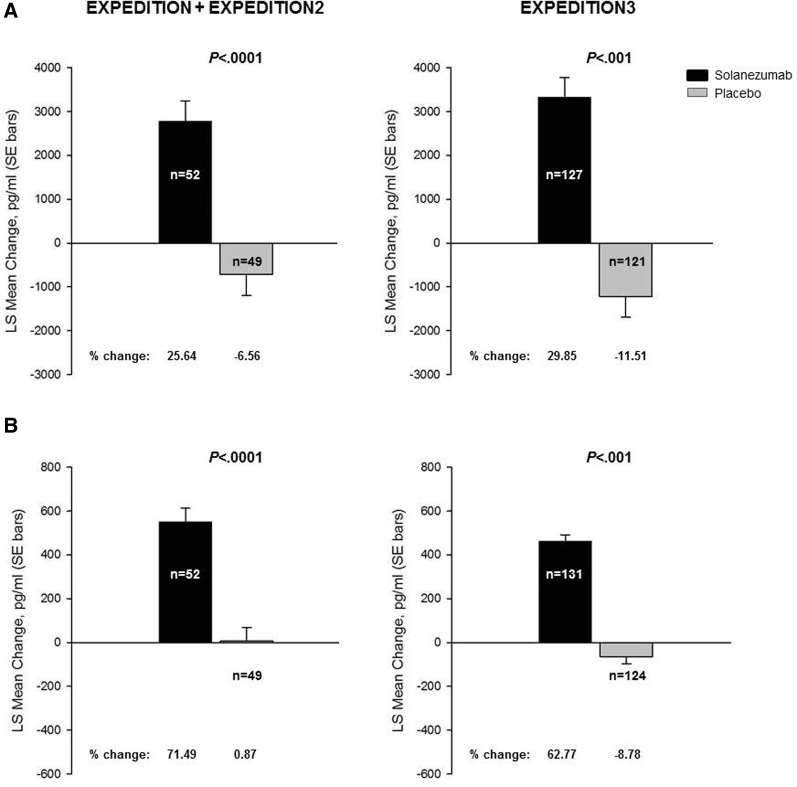
The 400 mg Q4W solanezumab dose regimen studied in all three phase 3 studies (EXPEDITION + EXPEDITION2 and EXPEDITION3) led to statistically significant increases compared with placebo in CSF total Aβ1–40 and total Aβ1–42 (Fig. 1A, B). Overall, the change in total Aβ1–40 was consistent between the studies, showing an increase of up to 29% in solanezumab-treated patients. Likewise, the change in total Aβ1–42 was generally consistent between studies, with an increase of up to 71% in solanezumab-treated patients. In placebo-treated patients, the overall change in either total Aβ1–40 or Aβ1–42 was very modest, with mean changes ranging between 0.9% and −11.5%.
Fig. 1.
Changes from baseline to endpoint in CSF total Aβ1–40 (A) and total Aβ1–42 (B) concentrations (pg/mL) from EXPEDITION + EXPEDITION2 and EXPEDITION3 studies. Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid; LS, least squares; SE, standard error. NOTE: Percent change = LS mean change/baseline mean × 100; n represents the number of patients with CSF samples at endpoint.
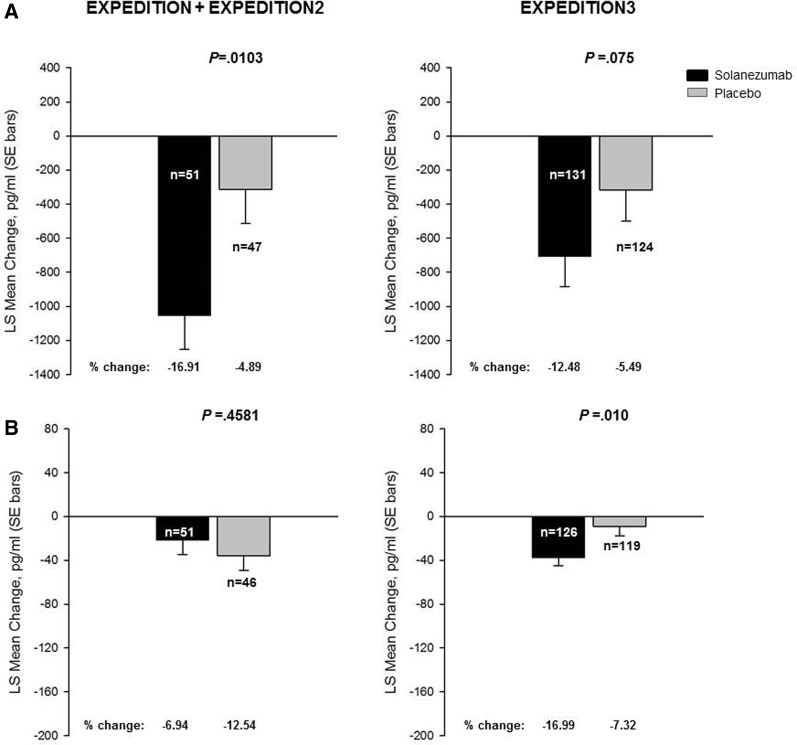
The change from baseline in CSF-free Aβ1–40 was statistically different between the solanezumab- and placebo-treated groups within EXPEDITION + EXPEDITION2 (Fig. 2A); however, in EXPEDITION3, the decrease in CSF-free Aβ1–40 did not achieve statistical significance (P = .075) (Fig. 2A). In contrast, the change from baseline in CSF-free Aβ1–42 was not statistically different between the solanezumab- and placebo-treated groups within EXPEDITION + EXPEDITION2 (Fig. 2B); however, in EXPEDITION3, the decrease in CSF-free Aβ1–42 was statistically significant (P = .010) (Fig. 2B).
Fig. 2.
Changes from baseline to endpoint in CSF-free Aβ1–40 (A) and free Aβ1–42 (B) concentrations (pg/mL) from EXPEDITION + EXPEDITION2 and EXPEDITION3 studies. Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid; LS, least squares; SE, standard error. NOTE: Percent change = LS mean change/baseline mean × 100; n represents the number of patients with CSF samples at endpoint.
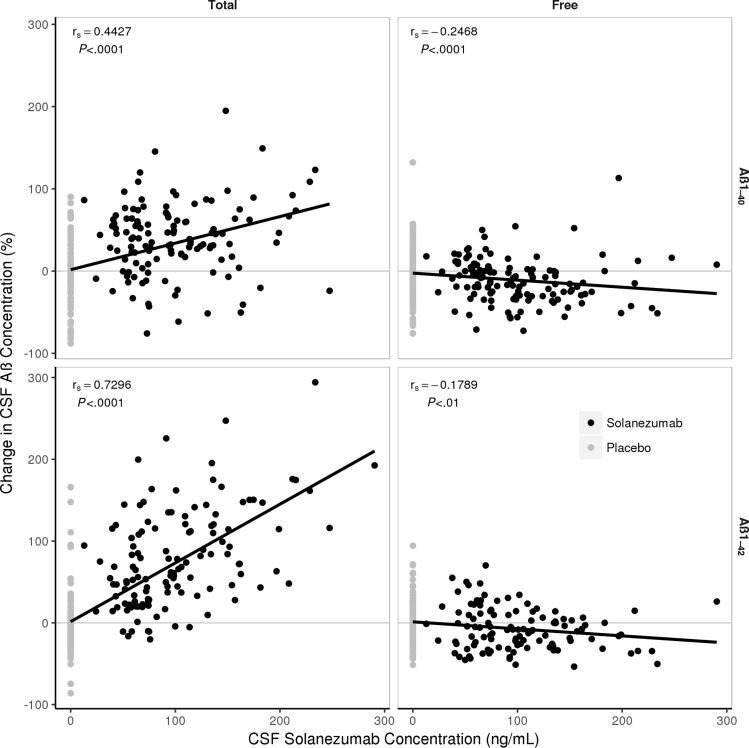
CSF total and free Aβ isoforms were also assessed in comparison to central drug exposure in EXPEDITION3. The mean CSF solanezumab concentration at endpoint was 88.9 ng/mL. CSF total Aβ1–40 and CSF total Aβ1–42 were significantly (P < .0001) correlated with the concentration of solanezumab measured in CSF (Fig. 3), with Spearman's rank correlation coefficient (rs) values of 0.44 and 0.73, respectively. CSF-free Aβ isoforms were correlated to a smaller degree (rs of approximately −0.2) with solanezumab concentration in the CSF, although in both cases the correlation was statistically significant (P < .01). The mean (standard deviation) molar ratio of solanezumab to total Aβ in the CSF was 0.267 (0.153) using the baseline concentration of Aβ.
Fig. 3.
Linear regression analyses of CSF solanezumab (ng/mL) concentrations vs CSF total Aβ1–40 (top left panel), free Aβ1–40 (top right panel), total Aβ1–42 (bottom left panel), and free Aβ1–42 (bottom right panel) (pg/mL) from EXPEDITION3 study. Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid; SD, standard deviation; r, Spearman rank correlation coefficient. NOTE: Mean (SD) concentration of solanezumab at endpoint = 88.9 ng/mL (49.4 ng/mL).
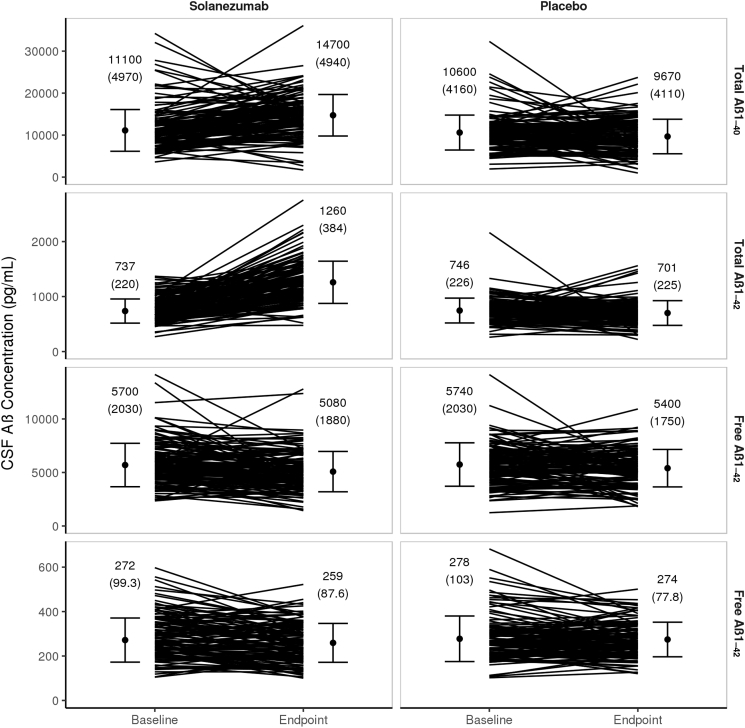
Despite the directionally consistent reduction in CSF-free Aβ isoforms for all trials, the decreases were highly variable from patient to patient in both treatment groups (Fig. 4). In the EXPEDITION3 study, the percent coefficient of variation for change in free Aβ1–40 (P = .075 for change in solanezumab group versus placebo) was approximately 240%, whereas the value for free Aβ1–42 (P = .010 for change in solanezumab group versus placebo) was approximately 200%. Similarly, the individual patient increases in total Aβ isoforms were also greatly variable.
Fig. 4.
Single patient variations from baseline to endpoint of CSF total Aβ1–40 (first row), total Aβ1–42 (second row), free Aβ1–40 (third row), and free Aβ1–42 (fourth row) (pg/mL) from the EXPEDITION3 study. Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid; SD, standard deviation. NOTE: Filled circle = mean (SD) CSF Aβ concentration.
The correlations between the change in free or total Aβ isoforms and various clinical outcomes were investigated for both solanezumab- and placebo-treated patients. Across the 104 (n = 56 in EXPEDITION + EXPEDITION2, n = 48 in EXPEDITION3) comparisons, the correlations were generally small in magnitude, with the absolute value of the Spearman rank correlation coefficient (rs) less than 0.30 in all cases, and only the change in free CSF Aβ1–40 and the change in FAQ in solanezumab-treated patients in EXPEDITION3 meeting nominal statistical significance (P = .050) (Tables S1–S4). Given that no adjustment was made to the P value to account for multiple comparisons, this result is likely spurious.
4. Discussion
Solanezumab administration in EXPEDITION3 did engage the central molecular target, as reflected by statistically significant increases in CSF total Aβ1–40 and CSF total Aβ1–42. This level of target engagement in EXPEDITION3 was comparable to that previously observed in the pooled mild AD dementia population from EXPEDITION + EXPEDITION2. Overall, the reductions in CSF-free Aβ as a marker of solanezumab's central pharmacodynamics in EXPEDITION + EXPEDITION2 and EXPEDITION3 were small, and disparate effects were observed in the CSF-free Aβ isoforms between studies. In EXPEDITION3, the statistically significant decrease in CSF-free Aβ1–42 was greater following solanezumab treatment than placebo, an effect not seen in EXPEDITION + EXPEDITION2. In EXPEDITION + EXPEDITION2 and EXPEDITION3, CSF-free Aβ1–40 decreased more in solanezumab-treated patients, but this difference did not reach significance in EXPEDITION3. Thus, the magnitude of central pharmacodynamics generated by solanezumab as assessed by total Aβ species in CSF was clear and consistent between studies; however, the effects on CSF-free Aβ species were less consistent.
The lack of consistent solanezumab versus placebo treatment effects on CSF-free Aβ1–42 across studies may reflect (1) inherent differences in the enrolled study populations, (2) variation in the size of the populations undergoing lumbar puncture for CSF pharmacodynamic analysis, or (3) difficulties in reliable measurement of the low CSF concentrations of this specific pharmacodynamic marker in patients with mild AD dementia, even before treatment administration. Based on the results of EXPEDITION + EXPEDITION2, solanezumab treatment was thought to only benefit patients with amyloid pathology [8]. Accordingly, to verify amyloid pathology, there was a greater emphasis on CSF collection in EXPEDITION3, leading to almost double the number of subjects with CSF measurements and a greater power to detect treatment effects. Despite the larger power, the high degree of variability in baseline and endpoint free Aβ concentrations suggests that even EXPEDITION3 was not sufficiently powered to reliably detect reductions in free CSF Aβ isoforms. The possibility that dissolution of plaque or shifts in equilibria of other Aβ species, such as Aβ oligomers, might have attenuated the change in free Aβ concentrations exists. However, there were no differences in florbetapir PET imaging in placebo- versus solanezumab-treated patients, suggesting that any dissolution of neuritic amyloid plaques was below the sensitivity of PET imaging. Nonetheless, there were statistically significant (albeit weak) correlations between the change in CSF-free Aβ and CSF solanezumab concentrations, suggesting solanezumab was lowering free Aβ concentrations, as would be expected based on the proposed mechanism of action. The observed exposure-response relationship suggests that higher doses of solanezumab could lead to more substantial reductions in free CSF Aβ isoforms.
Concentrations of solanezumab in the CSF were only about 0.2% of those measured in plasma (Lilly data on file), similar to observations made with other monoclonal antibodies [11], [12]. This low level of blood-brain barrier penetration, while typical for antibodies, drastically limited the amount of solanezumab that was available to bind to Aβ isoforms in the CNS. In EXPEDITION3, the mean molar ratio of solanezumab to baseline Aβ concentrations was relatively low (0.267). This value shows that trough steady-state solanezumab concentrations were lower than Aβ concentrations at baseline, suggesting that the concentration of solanezumab was not adequate to neutralize all the soluble Aβ present in the CSF. Even allowing for solanezumab to bind up to two molecules of Aβ, this analysis indicates that regardless of the binding affinity between solanezumab and Aβ, there were not enough binding sites present to bind all the available Aβ. Thus, it remains unlikely that substantial reductions in free Aβ would be anticipated at the dose used in the EXPEDITION studies. This explanation can be applied to the significantly increased total Aβ concentrations. Turnover of soluble Aβ monomers in the CNS is thought to be relatively rapid (on the order of minutes to hours) [13]. Although the half-life of an antibody (or the antibody-Aβ complex) in the CNS is unknown, it is thought to be much longer, on the order of days. Accordingly, even a small degree of target engagement might be expected to increase total (free + bound) concentrations of Aβ, whereas concentrations of free Aβ might only be slightly decreased. This interpretation of the data is consistent with the changes in Aβ concentrations that were noted at the end of the studies.
A limitation to this analysis is that the solanezumab concentrations were scheduled to be collected at trough (the end of the dosing interval, approximately 28 days post-dose); therefore, the observed solanezumab concentration would be anticipated to be lower than at other times during the dosing interval. While the pharmacokinetics of solanezumab in the CNS have yet to be fully characterized, it seems unlikely that solanezumab concentrations during the dosing interval would be high enough to substantially reduce free Aβ concentrations. Indeed, because this analysis only accounts for the assayed forms of Aβ (Aβ1–40 and Aβ1–42), the actual fraction of Aβ that could be bound to solanezumab at steady state is somewhat lower than might be suggested by the molar ratio. Despite these caveats, the data suggest that higher doses of solanezumab would be required to consistently produce greater reductions in free CSF Aβ isoforms. Future studies would be needed to further support a correlation between greater pharmacodynamic effects and better efficacy.
Given that solanezumab administration in EXPEDITION + EXPEDITION2 and EXPEDITION3 was associated with evidence of central drug disposition, target engagement, relevant target-related pharmacodynamics, and directionally consistent slowing of cognitive and functional decline, an increase in drug dose might be expected to enhance these observed effects. The selection of 400 mg solanezumab Q4W was based at least in part on the peripheral sink hypothesis [6], which suggests that maximizing peripheral target engagement would change the Aβ equilibria and consequently alter amyloid deposition in the central compartment, ultimately slowing disease progression. The phase 3 results indicate that dose selection based on the sink hypothesis may not be optimal and other biomarker studies may provide better predictive value for future dose selection. In the EXPEDITION studies, CNS target engagement was demonstrated and suggested that higher exposures may further increase CSF total Aβ isoform concentrations. A phase 2 study conducted using a weekly dose of 400 mg demonstrated increased CSF total Aβ isoform concentrations, supporting this hypothesis [3]. The possibility also exists that the pathological changes present in the mild dementia stage of the AD clinical continuum may already be so significant that they are not amenable to treatment with a drug targeting soluble Aβ isoforms. Because the most recently reported study population was restricted to patients with mild AD dementia, it may be beneficial to assess earlier stages of disease than those studied in the EXPEDITION trials. A dose of 1600 mg solanezumab Q4W is now being tested in the preclinical AD population in the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) study and in people with autosomal-dominant AD (symptomatic and presymptomatic) in the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU) trial.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Increased clearance of amyloid β (Aβ) from the brain via antibody-Aβ peptide complexes in plasma (“peripheral sink”) was proposed as an important mechanism to reduce Aβ burden and improve cognition in Alzheimer's disease. Solanezumab has previously been shown to significantly increase total plasma and cerebrospinal fluid (CSF) levels of Aβ1–40 and Aβ1–42 yet was inadequate in producing a meaningful cognitive effect.
-
2.
Interpretation: While 400 mg solanezumab treatment Q4W demonstrated target engagement, penetration into the central nervous system was limited as demonstrated by low CSF drug exposure. Therefore, higher exposures may further increase CSF total Aβ isoform concentrations as well as slow cognitive and functional decline.
-
3.
Future directions: Future studies evaluating the pharmacodynamic and cognitive effects of a higher solanezumab dose regimen are warranted.
Acknowledgments
The EXPEDITION studies were fully funded by Eli Lilly and Company. All the authors are current or past employees of Eli Lilly and Company. The authors would like to acknowledge Jayne Talbot of Eli Lilly and Company for analytical methods development, validation, and operational support; Andrea Rossi of Eli Lilly and Company for medical writing assistance; Meghan Greenwood of Syneos Health Clinical for medical writing assistance; and Antonia Baldo of Syneos Health Clinical for editing assistance. The authors also wish to thank the patients and caregivers for their dedicated participation in the EXPEDITION trials.
Footnotes
B.A.W., L.R.F.S., M.G.C., K.H., R.B.D., and J.R. are employees and stockholders of Eli Lilly and Company; K.S., D.R.L., and R.A.D. are former employees and stockholders of Eli Lilly and Company; and E.R.S. is a former employee and stockholder of Eli Lilly and Company, a medical advisor of Cogstate Ltd, the chief medical officer of Acumen Pharmaceuticals Inc, and a consultant of Vaccinex Inc, Cortexyme Inc, and the Alzheimer's Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.trci.2018.10.001.
Supplementary data
References
- 1.Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Karran E., Hardy J. Antiamyloid therapy for Alzheimer's disease—are we on the right road? N Engl J Med. 2014;370:377–378. doi: 10.1056/NEJMe1313943. [DOI] [PubMed] [Google Scholar]
- 3.Farlow M., Arnold S.E., van Dyck C.H., Aisen P.S., Snider B.J., Porsteinsson A.P. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 4.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 5.Honig L.S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378:321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 6.DeMattos R.B., Bales K.R., Cummins D.J., Paul S.M., Holtzman D.M. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 7.Lachno D.R., Evert B.A., Vanderstichele H., Robertson M., Demattos R.B., Konrad R.J. Validation of assays for measurement of amyloid- β peptides in cerebrospinal fluid and plasma specimens from patients with Alzheimer's disease treated with solanezumab. J Alzheimers Dis. 2013;34:897–910. doi: 10.3233/JAD-122317. [DOI] [PubMed] [Google Scholar]
- 8.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 9.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2011. R: A Language and Environment for Statistical Computing.https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing/ Available at: [Google Scholar]
- 10.Wickham H. Springer-Verlag; New York: 2009. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 11.Curtin F., Vidal V., Bernard C., Kromminga A., Lang A.B., Porchet H. Serum pharmacokinetics and cerebrospinal fluid concentration analysis of the new IgG4 monoclonal antibody GNbAC1 to treat multiple sclerosis: A phase 1 study. MAbs. 2016;8:854–860. doi: 10.1080/19420862.2016.1168956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran J.Q., Rana J., Barkhof F., Melamed I., Gevorkyan H., Wattjes M.P. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol Neuroimmunol Neuroinflamm. 2014;1:e18. doi: 10.1212/NXI.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrowolska J.A., Michener M.S., Wu G., Patterson B.W., Chott R., Ovod V. CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition. J Neurosci. 2014;34:8336–8346. doi: 10.1523/JNEUROSCI.0540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.