Abstract
The voltage-dependent anion channels (VDACs) are the workforce of mitochondrial transport and as such are required for cellular metabolism. The elaborate interplay between mitochondria and the apoptotic pathway supports a role for VDACs as a major regulator of cell death. Although VDAC-1 has an established role in apoptosis and cell homeostasis, the role of VDAC-2 has been controversial. In humans, VDAC-2 is best known for its anti-apoptotic properties. In this Viewpoint, we associate the various functional studies on VDAC-2 with structural reports, to decode its unique behavior. The well-structured N-terminus, compact barrel form, differences in the loop regions, specific transmembrane segments and the abundance of thiols in VDAC-2 enable this isoform to perform a different subset of regulatory functions, establish anti-apoptotic features and contribute to gametogenesis. VDAC-2 structural features that demarcate it from VDAC-1 suggest that this particular isoform is better suited for regulating reactive oxygen species, steroidogenesis and mitochondria-associated endoplasmic reticulum membrane regulatory pathways, with ion transport forming a secondary role. A better understanding of the unique structural features of the VDAC family will aid in the design of inhibitors that could alleviate irregularities in VDAC-controlled pathways.
Keywords: apoptosis, cysteines, mitochondrial outer membrane, voltage-dependent anion channel, β-barrel proteins
Introduction
Mitochondria, being the powerhouse of the cell, maintain a continuous bidirectional transport of ATP, ADP, NADH, ions and general metabolite flux through the mitochondrial outer membrane (MOM). The MOM is also responsible for maintaining mitochondrial integrity, which may otherwise lead to cessation of ATP generation, dysregulation of Ca2+ homeostasis, release of cytochrome c, apoptosis and several other irregularities [1,2]. The voltage-dependent anion channel (VDAC), responsible for most of these functions, is also the most abundant protein in the MOM and is hence often regarded as the ‘gatekeeper’ of mitochondria [1,2]. The intricate involvement of mitochondria with the apoptotic pathway, coupled with the presence of VDACs in the MOM, gave rise to the early reports that linked VDACs to apoptosis.
It has been hypothesized that the direct VDAC-mediated pore formation upon homo- or hetero-oligomerization with the Bcl-2 family proteins (Bak/Bax) releases cytochrome c from the mitochondrial intermembrane space, causing apoptosis [2]. VDACs predominantly exist in two channel states – open and closed [3]. Research over the last two decades has revealed that interacting partners such as the Bcl-2 family proteins can influence the channel state: Bcl-xL, for example, promotes the open state, while tBid causes channel closure [1]. Prolonged channel closure can lead to the disruption of metabolite transport, causing mitochondrial swelling and rupture, MOM permeabilization and subsequent cytochrome c release [1]. On this basis, an indirect role for VDACs in apoptosis has been proposed [2]. VDACs were also speculated to be a part of the mitochondrial permeability transition pore (PTP) complex along with adenine nucleotide transporter (ANT) in the inner membrane and cyclophilin D in the mitochondrial matrix [1,4]. The PTP components now include many more proteins, with ANT and VDAC being regarded as modulators rather than active PTP components [4].
There are three isoforms of VDACs in eutherian mammals. VDAC-1, the isoform with highest expression levels in most cell types, has pro-apoptotic properties (reviewed elsewhere [1,2]). The metabolite transport properties of VDAC-1 are also superior to those of VDAC-2 and -3 [3]. Not surprisingly, VDAC-1 has been studied in greater detail [2]. VDAC-2 came into the limelight a few years ago when Cheng et al. demonstrated that it possessed unique characteristics of being embryonically lethal and anti-apoptotic [5]. Further, the large number of cysteines in VDAC-2 and -3 are believed to confer protection from reactive oxygen species (ROS) build-up in the mitochondrial intermembrane space. Cysteine oxidation can either act as a molecular buffer or activate signaling cascades that eventually lead to the production of ROS scavenging enzymes [3].
The recent discovery of distinctive anti-apoptotic features of VDAC-2 piqued interest in this isoform. Although all the three VDAC isoforms have high sequence similarity and some functional redundancy, increasing mechanistic understanding gained for VDAC-2 has identified peculiar features of this isoform. In healthy cells, VDAC-2 primarily acts as an anti-apoptotic protein by preventing Bak oligomerization in the MOM [5]. In addition, VDAC-2 also carries out the primary role of VDACs, namely the transport of metabolites such as ATP/ADP, NAD/NADH, Ca2+ and other small molecules across the MOM [3,6]. This role as a metabolite flux effector is fairly diminished in VDAC-2, as its channel properties are weaker and less defined than VDAC-1 [3], although it still retains the voltage gating property [7]. In addition, VDAC-2 shows diverse conductance states [3,7], weak hexokinase binding [8], and is 10-fold less abundant in MOM than VDAC-1 [3]. Contrastingly, VDAC-2 is crucial for Bak recruitment [9] and apoptosis induction upon viral infection [10] and plays a prominent role in spermatogenesis [1] and oogenesis [11] – features absent in VDAC-1. Additionally, it is expressed in the outer dense fiber of sperm flagellum for regulation of sperm motility (reviewed in [1]). When we consider these facts, in the larger scheme of cellular homeostasis, can a compelling argument for a more important (primary) function of VDAC-2, other than metabolite transport, emerge? Here, we connect the recent VDAC-2 structural studies with functional characterization and attempt to explain the unique facets of VDAC-2 that set it apart from VDAC-1 (Fig. 1).
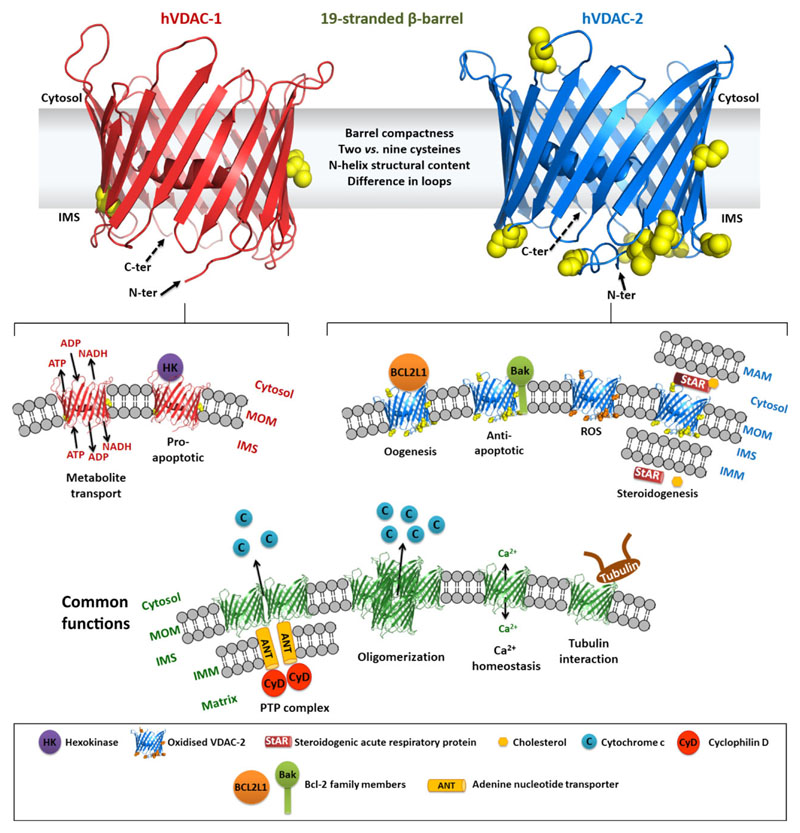
Fig. 1.
Comparison of various cellular functions carried out by VDAC-1 and -2. VDAC-1 and -2 are 19-stranded β-barrel mitochondrial outer membrane proteins with a dynamic N-terminal helix that primarily stays docked in the pore. Both the barrel N (solid arrow, towards the viewer) and C termini (dashed arrow, away from the viewer) face the IMS side of mitochondria, although the flexibility of the N terminus may cause it to translocate to the cytosolic side. Notwithstanding the high sequence similarity between the two isoforms, subtle structural and sequencerelated differences such as cysteine content, barrel compactness, N-terminal extension and loop-specific differences endow them with the ability to execute different functions. VDAC-1 (left) forms the major route for metabolite transport and requires hexokinase binding to suppress its pro-apoptotic nature. In contrast, VDAC-2 (right) displays anti-apoptotic properties/features by actively binding and inhibiting Bcl-2 family members such as BCL2L1 and Bak. Cysteine residues are enriched in loop regions of VDAC-2 that are specifically oriented towards the IMS and confer protection from ROS build-up. Additionally, VDAC-2 also improves the cholesterol fostering ability of StAR protein. By and large, the VDAC family of channels is involved in apoptosis either by regulating the PTP complex or through oligomerization (lower panel). They are also involved in maintaining Ca2+ homeostasis within the cell, and are negatively regulated by interaction with tubulin. N-ter, N terminus; C-ter, C terminus; IMS, intermembrane space; MAM, mitochondria associated endoplasmic reticulum membrane; IMM, inner mitochondrial membrane. The overall barrel geometry of VDAC-1 and -2 is retained in the respective panels.
The newly solved crystal structure of zebrafish VDAC-2 [12] places this protein in the family of unique 19-stranded β-barrel MOM proteins similar to Tom40 [13] with the N-terminal helix docked within the channel pore (Fig. 1). Despite considerable structural similarities of VDAC-1 and -2, closer examination of alterations in electrostatic properties [12], loop orientation, overall barrel scaffold [14] and barrel ellipticity, sequence diversity [12,14] and N-helix [15] identify differences that may be responsible for the unique characteristics of VDAC-2.
The single major difference that is strikingly evident when the isoform protein sequences are compared is the unusual enrichment of cysteines in VDAC-2. In humans, for example, VDAC-1 possesses only two cysteines, while VDAC-2 has nine. The abundance of thiols in VDAC-2 makes it an attractive substrate for neutralizing ROS species through cysteine oxidation (Fig. 1). Our physicochemical studies on recombinant human VDAC-2 suggest that cysteines are retained in the reduced state in the in vitro refolded samples and their presence strengthens barrel–lipid interactions [16], at the expense of diminished channel activity [7]. However, recent in vivo studies also demonstrate that cysteines are dispensable for apoptosis-related functions such as Bak recruitment and tBid-induced cytochrome c release [14]. Nevertheless, the removal or modification of human VDAC-2 cysteines can alter channel function. For example, latest studies show that cysteine succination of VDAC-2 decreases ATP synthesis in mitochondria [17]. This observation with VDAC-2 is also strengthened by recent reports on VDAC-3 (the other cysteine-enriched isoform), which is notorious for its inability to form proper voltage gated channels [3], despite high sequence similarity to the other two isoforms. In VDAC-3 a fully functional voltage-dependent channel could be obtained only when certain thiols (C2, C8 and C122) were retained in the reduced state [18]. Conserved cysteines at corresponding regions in VDAC-2 and -3 allow us to speculate that an analogous change in the thiol status upon oxidative stress can lead to closure of VDAC-2 channels. This can ultimately disrupt metabolite transport, weaken Bak binding, permeabilize the MOM and trigger apoptosis.
The second major difference between the primary protein structures of the two isoforms is the additional 11-residue extension of the N-terminal helix. The extended N terminus adopts a better helical structure in VDAC-2 [15]. This, in turn, is able to enhance the N-helix interaction with the barrel wall and stabilize the overall structure [7]. However, this gain in structure can lead to loss in flexibility of the N-helix and result in differential binding of proteins that interact with the N terminus of VDAC, such as hexokinase and steroidogenic acute respiratory (StAR) protein (Fig. 1). The pro-apoptotic activity of VDAC-1 is kept in check through competitive (constitutive) binding with hexokinase over the Bcl-2 family members [2]. In contrast, weak hexokinase binding shown by VDAC-2 [8] can be a protective mechanism to prevent replacement of pro-apoptotic Bak from this isoform. StAR protein, a mitochondria-associated endoplasmic reticulum membrane (MAM) protein, helps in cholesterol transport into the mitochondria for synthesis of steroid hormones, by specifically interacting with MOM proteins such as VDAC-2, Tom22 and Tom40 [19,20]. StAR, a 37 kDa protein, binds cholesterol near the MOM and is imported into the mitochondrial matrix as an ~ 30 kDa processed cholesterol-bound protein, through a transduceosome complex formed by MOM and inner mitochondrial membrane proteins [19,20]. The transduceosome is formed by Tom40 (translocase of MOM), the translocator protein in complexation with VDAC and the adenine nucleotide transporter of the mitochondrial inner membrane [19,20]. Recently, it was shown that it is the VDAC-2 isoform that specifically interacts with StAR, through residues 221–229, the N-terminal residues 1–12 and C-terminal 20 amino acids [20]. Hence, VDAC-2 is indispensable for StAR import, and thereby steroidogenesis. VDAC-2 facilitates StAR processing and stalls its import into the mitochondrion, because of which StAR gains sufficient time to increase its cholesterol binding [20].
Availability of the zebrafish VDAC-2 crystal structure [12] allowed the identification of important loop-specific differences between the two isoforms. The loops between β-strands 1 and 2 (residues 34–37) differ in their electronegativity, with VDAC-1 possessing an additional glutamate residue in this region. Furthermore, a recent study that used chimeric constructs of VDAC-1 and -2 showed that residues 123–179 of VDAC-2, which comprise strands 7–10, are important for Bak import and tBid-induced apoptosis [14]. Comparing the crystal structures of mouse VDAC-1 and zfVDAC-2 in this region shows that the loops between β-strands 8–9 and 10–11 are oriented differently in VDAC-1 and -2 [14]. This altered orientation allows for the formation of a binding pocket for Bak only in VDAC-2, lending it a Bak-binding anti-apoptotic property. Similar changes in the loop regions of VDAC-2 can also give rise to binding pockets for proteins like glycogen synthase kinase 3β (GSK-3β) and BCL2L1 (a Bcl-2 family member) that bind specifically to VDAC-2. While GSK-3β is a mitochondrial PTP inducer and uses VDAC-2 to translocate to mitochondria under oxidative stress conditions [21], BCL2L1 inhibits autophagy during oogenesis to increase female fecundity [11]. VDAC-2 is also enriched in sperm mitochondria and plays a crucial role in sperm maturation and motility [1].
The N-helix of VDAC-1 barrel translocates into and out of the channel lumen to carry out voltage gating [22], and mediates the apoptosis pathway by binding to cytosolic proteins such as hexokinase, Bcl-2 and Bcl-xL [22,23]. Removal of the VDAC-1 N-helix alters the cylindrical barrel to a collapsed elliptical structure [24] and makes it voltage-independent [22], although conflicting studies exist for the conductance state of such a channel [22,24]. N-helix deleted VDAC-2 also shows voltage-independent behavior [25]; however, electro-physiology measurements suggest that this helix-less isoform does sample the open state more frequently than its Cys-less counterpart [7]. Simulation studies on VDAC isoforms show that the VDAC-2 barrel samples the elliptical conformation less frequently and the overall barrel topology is compact compared with other isoforms [15]. Solid-state NMR studies also reveal that the VDAC-2 barrel carries a far higher degree of conformational heterogeneity [25] than the other isoforms. Taken together, studies now show that the compact yet conformationally flexible VDAC-2 barrel, with a better-structured N-terminal domain, is able to sample diverse conductance states, with varying levels of anion selectivity. Such structural malleability is not seen in VDAC-1 and can form the crux of differential binding of these two isoforms to interacting partners such as hexokinase, StAR, GSK-3β, Bak and other Bcl-2 proteins.
VDAC-3 is the oldest VDAC and the other two isoforms emerged ~ 300–350 million years ago by gene duplication [3]. The abundance of VDAC-1 and -2 over isoform -3 and the coordinated expression levels in a tissue-specific manner suggests that, in the course of evolution, each VDAC isoform accumulated features unique to its function in the cell. VDAC-1 evolved to be metabolically more active and underwent key mutations to enhance its channel activity and maintenance of cellular homeostasis, including the absence of cysteines and N-terminal extension. VDAC-2, on the other hand, gained unique characteristics like Bak binding [5], suppression of ROS [3] and calcium transport in cardiac myocytes [6]. Further, VDAC-2 became vital for gametogenesis [1,11] and preventing embryonic lethality [5], and became an indispensable regulator of cholesterol transport [20] and formation of the PTP complex [21]. Similarly, despite the further limited occurrence of VDAC-3 in the MOM, this isoform also plays an important role in male fertility [1]. With increasing evidence that VDAC-1, -2 and -3 can perform distinct functions, the term ‘paralogs’ might be more suited to describe these proteins, rather than ‘isoforms’.
VDAC-2 abundance levels in the cell, although lower than VDAC-1, are sufficient to power vital cellular activities and dictate cell survival. It is therefore no surprise that VDAC-2 levels are upregulated in certain cancers [1]. Cumulative evidence highlights the importance of VDAC-2 as a regulator of several cellular pathways intricately associated with the mitochondrion and MAM, and possibly executing metabolite flux when VDAC-1 is deficient. Further investigations on the differential nature of this intriguing VDAC isoform point to the specialization that the VDAC-2 sequence underwent during evolution, to achieve a regulatory role in the MOM. We anticipate that future meticulous investigations in this family of MOM barrels will help us pinpoint their interrelated role in apoptosis in greater detail. The uncovering of their unique roles in many other regulatory pathways will help us understand the diverse multifaceted functioning of this channel in the cell.
Acknowledgements
S.R.M. thanks the Council of Scientific and Industrial Research, India, for a senior research fellowship. R.M. is a recipient of the Wellcome/DBT Intermediate Fellowship. This work is supported by the Wellcome Trust/DBT India Alliance award number IA/I/14/1/501305 to R.M.
Abbreviations
- ANT
adenine nucleotide transporter
- GSK-3β
glycogen synthase kinase-3β
- MAM
mitochondria associated endoplasmic reticulum membrane
- MOM
mitochondrial outer membrane
- PTP
permeability transition pore
- ROS
reactive oxygen species
- StAR
steroidogenic acute respiratory protein
- VDAC
voltage-dependent anion channel
Footnotes
Author contributions
S.R.M. and R.M. critically analyzed, discussed and wrote the manuscript.
References
- 1.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 2.Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. The mitochondrial voltagedependent anion channel 1 in tumor cells. Biochim Biophys Acta. 2015;1848:2547–2575. doi: 10.1016/j.bbamem.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Messina A, Reina S, Guarino F, De Pinto V. VDAC isoforms in mammals. Biochim Biophys Acta. 2012;1818:1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Vianello A, Casolo V, Petrussa E, Peresson C, Patui S, Bertolini A, Passamonti S, Braidot E, Zancani M. The mitochondrial permeability transition pore (PTP) - an example of multiple molecular exaptation? Biochim Biophys Acta. 2012;1817:2072–2086. doi: 10.1016/j.bbabio.2012.06.620. [DOI] [PubMed] [Google Scholar]
- 5.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 6.Subedi KP, Kim JC, Kang M, Son MJ, Kim YS, Woo SH. Voltage-dependent anion channel 2 modulates resting Ca(2)+ sparks, but not action potential-induced Ca(2)+ signaling in cardiac myocytes. Cell Calcium. 2011;49:136–143. doi: 10.1016/j.ceca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Maurya SR, Mahalakshmi R. N-helix and cysteines inter-regulate human mitochondrial VDAC-2 function and biochemistry. J Biol Chem. 2015;290:30240–30252. doi: 10.1074/jbc.M115.693978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azoulay-Zohar H, Aflalo C. Binding of rat brain hexokinase to recombinant yeast mitochondria: identification of necessary molecular determinants. J Bioenerg Biomembr. 1999;31:569–579. doi: 10.1023/a:1005469028274. [DOI] [PubMed] [Google Scholar]
- 9.Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin W, Zhang Z, Xu Z, Wang B, Li X, Cao H, Wang Y, Zheng SJ. The association of receptor of activated protein kinase C 1 (RACK1) with infectious bursal disease virus viral protein VP5 and voltagedependent anion channel 2 (VDAC2) inhibits apoptosis and enhances viral replication. J Biol Chem. 2015;290:8500–8510. doi: 10.1074/jbc.M114.585687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan J, Zhang Y, Sheng Y, Fu X, Cheng H, Zhou R. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy. 2015;11:1081–1098. doi: 10.1080/15548627.2015.1040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schredelseker J, Paz A, Lopez CJ, Altenbach C, Leung CS, Drexler MK, Chen JN, Hubbell WL, Abramson J. High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J Biol Chem. 2014;289:12566–12577. doi: 10.1074/jbc.M113.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bay DC, Hafez M, Young MJ, Court DA. Phylogenetic and coevolutionary analysis of the β-barrel protein family comprised of mitochondrial porin (VDAC) and Tom40. Biochim Biophys Acta. 2012;1818:1502–1519. doi: 10.1016/j.bbamem.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Naghdi S, Varnai P, Hajnoczky G. Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis. Proc Natl Acad Sci USA. 2015;112:E5590–E5599. doi: 10.1073/pnas.1510574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guardiani C, Scorciapino MA, Amodeo GF, Grdadolnik J, Pappalardo G, De Pinto V, Ceccarelli M, Casu M. The N-terminal peptides of the three human isoforms of the mitochondrial voltage-dependent anion channel have different helical propensities. Biochemistry. 2015;54:5646–5656. doi: 10.1021/acs.biochem.5b00469. [DOI] [PubMed] [Google Scholar]
- 16.Maurya SR, Mahalakshmi R. Modulation of Human Mitochondrial Voltage-dependent Anion Channel 2 (hVDAC-2) Structural Stability by Cysteine-assisted Barrel-lipid Interactions. J Biol Chem. 2013;288:25584–25592. doi: 10.1074/jbc.M113.493692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piroli GG, Manuel AM, Clapper AC, Walla MD, Baatz JE, Palmiter RD, Quintana A, Frizzell N. Succination is increased on select proteins in the brainstem of the Ndufs4 knockout mouse, a model of Leigh syndrome. Mol Cell Proteomics. 2015 doi: 10.1074/mcp.M115.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki M, Kurabayashi K, Asanuma M, Saito Y, Dodo K, Sodeoka M. VDAC3 gating is activated by suppression of disulfide-bond formation between the N-terminal region and the bottom of the pore. Biochim Biophys Acta. 2015;1848:3188–3196. doi: 10.1016/j.bbamem.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Flis VV, Daum G. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a013235. a013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad M, Kaur J, Pawlak KJ, Bose M, Whittal RM, Bose HS. Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J Biol Chem. 2015;290:2604–2616. doi: 10.1074/jbc.M114.605808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanno M, Kuno A, Ishikawa S, Miki T, Kouzu H, Yano T, Murase H, Tobisawa T, Ogasawara M, Horio Y, et al. Translocation of glycogen synthase kinase-3β (GSK-3β), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2) J Biol Chem. 2014;289:29285–29296. doi: 10.1074/jbc.M114.563924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 23.Arbel N, Ben-Hail D, Shoshan-Barmatz V. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem. 2012;287:23152–23161. doi: 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachariae U, Schneider R, Briones R, Gattin Z, Demers JP, Giller K, Maier E, Zweckstetter M, Griesinger C, Becker S, et al. β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure. 2012;20:1540–1549. doi: 10.1016/j.str.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattin Z, Schneider R, Laukat Y, Giller K, Maier E, Zweckstetter M, Griesinger C, Benz R, Becker S, Lange A. Solid-state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J Biomol NMR. 2015;61:311–320. doi: 10.1007/s10858-014-9876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]