Key Points
Bacterial diversity was restored after FMT with oral frozen capsules, with improvement of diarrhea.
Oral FMT for steroid-refractory acute gGVHD is feasible and could be effective.
Introduction
Acute gut graft-versus-host disease (gGVHD) is the major cause of nonrelapse mortality in allogeneic hematopoietic stem cell transplantation recipients.1-3 Although glucocorticoids are used as the first-line therapy, steroid-refractory gGVHD has a high mortality,2,3 and no satisfactory second-line treatment has been established.4
Recently, several reports showed that impairment of gut microbiota was associated with acute gGVHD.5,6 Moreover, fecal microbiota transplantation (FMT) from a healthy donor could be an effective treatment of refractory acute gGVHD, changing the gut microbiota and restoring bacterial diversity.7,8
We previously reported 4 cases with steroid-resistant acute gGVHD successfully treated with FMT.7 In the previous study, donated fecal suspensions were administered via a nasoduodenal tube. However, insertion and indwelling a nasoduodenal tube would result in discomfort, pain, and a risk of fatal gastrointestinal bleeding because of thrombocytopenia and mechanical mucosal damage.
A case of acute gGVHD refractory to high-dose pulse methylprednisone (mPSL) and rabbit anti-thymocyte globulin (rATG) that was effectively and safely treated with FMT with oral, frozen capsules is presented. To our knowledge, this is the first report of oral FMT for refractory gGVHD.
Case description
A 21-year-old woman with Philadelphia chromosome–positive acute lymphoblastic leukemia underwent allogeneic bone marrow transplantation from a human leukocyte antigen DRB1 1 locus-mismatched unrelated donor in first hematological complete remission. At transplant, polymerase chain reaction for BCR-ABL fusion was positive. The conditioning regimen consisted of etoposide (30 mg/kg), cyclophosphamide (120 mg/kg), and total body irradiation (12 Gy). Cyclosporine A (CyA) and short-term methotrexate were used for GVHD prophylaxis. After engraftment, she was diagnosed as having stage 3 acute gGVHD based on gastrointestinal symptoms and pathological findings 19 days after transplantation. Methylprednisolone therapy (2 mg/kg per day) did not improve the diarrhea, and then high-dose pulse mPSL therapy (1 g/day for 3 days) was started from 32 days after transplantation. The diarrhea improved transiently, and CyA was discontinued 75 days after transplantation to induce graft-versus-leukemia effect. The diarrhea worsened again 91 days after transplantation. Resumption of CyA and the second high-dose-pulse mPSL therapy did not improve the diarrhea, and abdominal pain and bloody feces developed. Although 1 mg/kg rATG was additionally administered on days 99 and 105 after transplantation, the symptoms were exacerbated. FMT for acute gGVHD was planned, and the patient was transferred to our hospital. Although stool volume and frequency partially improved after opioid switching and dose escalation of fentanyl, hemorrhagic characteristics of her stool continued without amelioration.
Methods
Her healthy sister passed the screening examinations for pathogenic microorganisms, and donated feces were prepared as described previously.7 Briefly, sterile saline was added to the 144 g of donated feces and stirred until completely homogenized. The fecal suspension was filtered several times with a metal sieve and clean gauze to remove food-derived debris. After centrifugation at 6000g for 15 minutes, a 16.2-g bacterial pellet was isolated and suspended in saline with glycerol. The concentration of glycerol was adjusted to 10%. After dissolution, 450 µL of bacterial solution were pipetted into #1 hypromellose capsule (DRCaps, Capsugel, Morristown, NJ) and rapidly frozen with liquid nitrogen. Capsules were stored at −80°C. One to 2 hours before administration, the capsules were sealed in #0 capsules and transferred to −20°C.
Fecal 16S ribosomal RNA (rRNA) gene sequencing analyses and operational taxonomic unit clustering analyses were performed as previously reported.7 Bacterial genomic DNA was extracted from feces collected from the patient in the morning of the first FMT day; in the next morning; 1, 2, and 4 weeks after the initiation of the first cycle of FMT; and 1, 2, and 4 weeks after the initiation of the second cycle of FMT. The hypervariable V1-2 region of the 16S rRNA gene was amplified by polymerase chain reaction and sequenced using MiSeq (Illumina, San Diego, CA). We randomly selected 3000 high-quality reads per sample and the reads were sorted and grouped into operational taxonomic units using the UCLUST algorithm (http://www.drive5.com/) with a sequence identity threshold of 97%. The Good coverage index9 of the 3000 reads per sample in this study was 0.98, indicating a high coverage degree that provided sufficient reads for this fecal microbiota analysis. Diversity of the microbiota was estimated by calculating the Shannon index. This study was performed in accordance with the Declaration of Helsinki and approved by the ethics review board of Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital.
Results and discussion
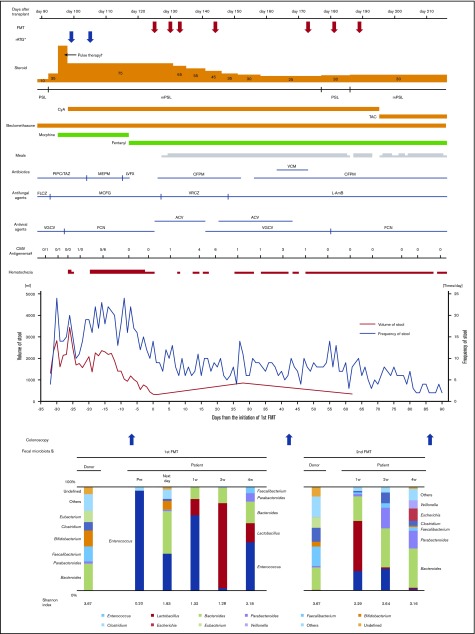
The patient took 15 capsules per day on days 125, 130, 133, and 144 after transplantation. Analysis of 16S rRNA gene sequencing showed that the microbiota composition of the patient before FMT was dominated by Enterococcus, but it became highly diverse the day after initiation of FMT. Digestive symptoms improved soon after initiation of FMT, and mPSL was tapered from 75 to 25 mg/day. Microbiota was transiently dominated by Lactobacillus 2 weeks after the initiation of FMT. However, the proportion of Enterococcus rose again 4 weeks after the first administration of FMT, with a slight exacerbation of diarrhea and hemorrhagic stool.
To further reduce the dose of corticosteroid, the second cycle of FMT was performed with the same protocols. The same donor provided 71 g of feces, and a 15.0-g bacterial pellet was isolated. The patient was administered 15 capsules per day on days 173, 181, and 189 after transplantation. Sequencing analyses showed that the microbiota in the FMT capsules was similar to that of the original fecal suspension of the donor in both cycles of FMT. Although diarrhea and hematochezia remained slightly, the gGVHD improved to stage 1 after the second cycle of FMT (Figure 1), with the improvement of endoscopic findings (supplemental Figure 1). It seemed to be difficult to achieve complete remission of the gastrointestinal symptoms in such short-term period considering the severe mucosal damage before FMT, although degree of hematochezia greatly improved and her abdominal pain mostly disappeared after FMT. In the pathological examination of the colon biopsy at the onset of gGVHD and before FMT, apoptotic body was extensively detected, whereas cytomegalovirus-infected cells were not seen in the immunohistochemical staining. As described in Figure 1, foscarnet and valganciclovir were administered as a preemptive therapy for cytomegalovirus antigenemia, which was detected by plasma C7-horseradish peroxidase assay and C10/11 method. After the first cycle of FMT, when diarrhea and hematochezia largely improved, small numbers of CMV-infected cells were seen in the ascending colon; after the second cycle of FMT, CMV-infected cells were not seen. Thus, we could not completely exclude potentially concomitant CMV colitis, which could be resolved by foscarnet and valganciclovir. However, apoptotic body was extensively detected in colon biopsy specimens before FMT, as well as after the first and second FMT. According to the pathological findings, we concluded that the patient’s gastrointestinal symptoms were primarily the result of gut GVHD, rather than CMV colitis. The diversity of fecal microbiota increased again and persisted after the second cycle of FMT. The Shannon index also increased gradually after FMT. The final microbiota composition was dominated by Bacteroides, Parabacteroides, Clostridium, Faecalibacterium, and Lactobacillus, which was consistent with the previous report.5-8,10 Interestingly, Lactobacillus increased both after the first and second FMT courses, although it was absent in the donor’s fecal microbiota. Lactobacillus was slightly found in the recipient’s fecal microbiota before the first FMT; therefore, it was most likely that the increase of Lactobacillus arose intrinsically, not from donor stool. Antibiotics we administered might play some role for the increase of Lactobacillus, although the exact mechanism remains to be elucidated. It is of interest that our previous study7 and some other studies11,12 also indicated mostly the same phenomenon (eg, increase of Lactobacillus after FMT that was absent in the donor stool). We discontinued antibiotics before the initiation of the first FMT. However, from the second administration of the first cycle of FMT, we performed FMT under the administration of cefepime, as described in Figure 1. Cefepime was empirically administered for transient fever and febrile neutropenia, whose etiology was unknown. We had to continue cefepime for a long period because of the patient’s severe immunosuppression status.
Figure 1.
Clinical course and microbiota dynamics. The fecal sample that was obtained immediately before the initiation of the first FMT on the same day was analyzed as “pre” data. The time points such as “next week,” “1w,” “2w,” and “4w” indicate periods from the initiation of the first or second cycle of FMT. *rATG was administered at 1 mg/kg on days 99 and 105 after transplantation. †Methylprednisone was administered at 1 g/d for 3 days. ‡CMV antigenemia was detected by C10/11 method until day 110 after transplantation in the previous hospital and C7-horseradish peroxidase assay from day 117 after transplantation. §Only the top 10 genus-level organisms were described; organisms not in the top 10 were classified as “others.” ACV, acyclovir; CFPM, cefepime; CMV, cytomegalovirus; FCN, foscarnet; FLCZ, fluconazole; L-AmB, liposomal amphotericin B; LVFX, levofloxacin; MEPM, meropenem; MCFG, micafungin; mPSL, methylprednisone; PIPC/TAZ, piperacillin/tazobactam; PSL, prednisone; TAC, tacrolimus; VCM, vancomycin; VGCV, valganciclovir; VRCZ, voriconazole; w, week.
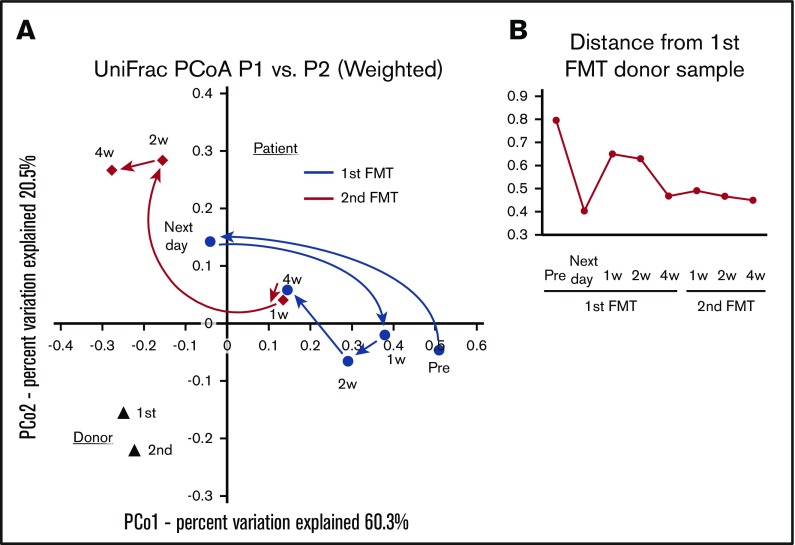
The patient’s fecal microbiota were rather different from the donor fecal microbiota in the UniFrac distance analysis (Figure 2), even after the second cycle of FMT. In our previous study,7 the patient’s fecal microbiota was not necessarily similar to that of donor, whereas the diversity of microbiota increased after the improvement of gastrointestinal symptoms. We speculate that the recovery of the diversity, not persistence of the grafted donor fecal organisms, may be essential for the improvement of gGVHD.
Figure 2.
Weighted UniFrac distance analysis. (A) The fecal microbiota dynamics of the patient and the donor. (B) Distance from the donor fecal microbiota of the first FMT. The fecal sample that was obtained immediately before the initiation of the first FMT on the same day was analyzed as “pre” data. The time points such as “next day,” “1w,” “2w,” and “4w” indicate periods from the initiation of the first or second cycle of FMT.
As to acute GVHD in other organs, acute skin GVHD with exacerbation and remission was found, which was evaluated as stage 2, through almost the entire observation period and there seemed to be no change after FMT. In the meanwhile, liver GVHD was not found. Transient fever, positivity of galactomannan antigen, and recurrence of herpes zoster were found after FMT. The association between these findings and FMT was unclear, and these adverse events might be due to severe immunosuppression induced by corticosteroid and/or rATG.
FMT has been mainly performed by colonoscopy or nasogastric/duodenal tube that exposes the patient to discomfort, pain, and the risk of gastrointestinal bleeding. Our procedure with oral capsules could avoid these disadvantages. An oral FMT procedure was also tried for recurrent Clostridium difficile infection, and a randomized trial showed comparable efficacy and superior safety of oral FMT compared with colonoscopy-delivered procedure.13-15
The present procedure was distinct from the previous reports about FMT7,8 in that the frozen capsules containing only the centrifuged bacterial pellet were given instead of a whole fecal suspension. Although a fecal suspension would contain various metabolites such as short-chain fatty acids,16 the present results indicate the intestinal microbiota itself could ameliorate gGVHD.
In the present case, FMT was performed as third-line treatment after high-dose corticosteroid and rATG. It is challenging to demonstrate the efficacy of FMT, and we could not completely exclude the contribution of other treatments including CyA, tacrolimus, corticosteroid, and rATG. However, the patient’s gastrointestinal symptoms expressly exacerbated after administration of high-dose-pulse mPSL and rATG. This clinical course supported that FMT would be effective for gut GVHD, which was refractory for corticosteroid and rATG. Earlier intervention with FMT might avoid such treatments, which caused severe immunosuppression, and contribute to good outcomes. We conducted FMT with capsules as a compassionate use study in this case, and the protocol including the number of FMT cycle had not been determined before the enrollment. However, according to the previous studies that suggested efficacy and safety of repeated FMT,17,18 we decided to perform the second cycle of FMT with capsules. Further investigation is warranted to determine the optimal protocol.
In this report, the sister was adopted as a fecal donor. However, FMT from a third-party donor could be another potential approach for the management of gGVHD. Indeed, feasibility of prophylactic administration of third party FMT capsules after allo-hematopoietic stem cell transplantation was recently reported.19 Further study that compares the usefulness of prophylactic and therapeutic FMT is warranted.
A case of FMT with capsules for steroid-refractory acute gGVHD was presented. The intestinal bacterial diversity was restored after FMT, with the improvement of diarrhea and colonoscopy findings. Our novel FMT strategy appeared feasible and could be useful for steroid-refractory acute gGVHD.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Hiroaki Maki for giving us the accurate referral document on her clinical course in the previous hospital.
This research was supported by grants from the Japan Agency for Medical Research and Development, Leading Advanced Projects for medical innovation (JP17gm0010003) and Friends of Leukemia Research Fund.
Footnotes
The 16S rRNA gene V1-V2 region sequences analyzed in this study were deposited in DDBJ/GenBank/EMBL (accession number DRA006941).
Authorship
Contribution: K.K., T.T., and K.O. designed the study. S. Kaito, K.Y., S. Kurosawa, and T.T. provided medical treatment to the patient; S. Kaito, K.Y., K.I., T.T., and K.K. collected the sample and clinical data; K.T., W.S., K.K., K.H., and M.H. undertook the sequencing analyses; S. Kaito, T.T., W.S., K.K., and M.H. wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Toya, Hematology Division, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Tokyo, Japan, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan; e-mail: tooya-tky@umin.ac.jp.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?I Identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157(6):732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castilla-Llorente C, Martin PJ, McDonald GB, et al. . Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014;49(7):966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin PJ, Rizzo JD, Wingard JR, et al. . First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenq RR, Ubeda C, Taur Y, et al. . Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holler E, Butzhammer P, Schmid K, et al. . Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakihana K, Fujioka Y, Suda W, et al. . Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spindelboeck W, Schulz E, Uhl B, et al. . Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica. 2017;102(5):e210-e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singleton DR, Furlong MA, Rathbun SL, Whitman WB. Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol. 2001;67(9):4374-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taur Y, Xavier JB, Lipuma L, et al. . Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stripling J, Kumar R, Baddley JW, et al. . Loss of vancomycin-resistant enterococcus fecal dominance in an organ transplant patient with Clostridium difficile colitis after fecal microbiota transplant. Open Forum Infect Dis. 2015;2(2):ofv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Wang C, Tang C, et al. . Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care. 2015;19(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772-1778. [DOI] [PubMed] [Google Scholar]
- 14.Youngster I, Mahabamunuge J, Systrom HK, et al. . Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao D, Roach B, Silva M, et al. . Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318(20):1985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott SJ, Waetzig GH, Rehman A, et al. . Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017;152(4):799-811.e7. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761-767. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Steiner T, Petrof EO, et al. . Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315(2):142-149. [DOI] [PubMed] [Google Scholar]
- 19.DeFilipp Z, Peled JU, Li S, et al. . Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2(7):745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.