Pigs are a “mixing vessel” for influenza A viruses (IAVs) because of their ability to be infected by avian and human IAVs and their propensity to facilitate viral genomic reassortment events. Also, as IAVs may evolve differently in swine and humans, pigs can become a reservoir for old human strains against which the human population has become immunologically naive. Thus, viruses from the novel swine-specific H1N1pdm genogroup may continue to diverge from seasonal H1N1pdm strains and/or from other H1N1pdm viruses infecting pigs and lead to the emergence of viruses that would not be covered by human vaccines and/or swine vaccines based on antigens closely related to the original H1N1pdm virus. This discovery confirms the importance of encouraging swine IAV monitoring because H1N1pdm swine viruses could carry an increased risk to both human and swine health in the future as a whole H1N1pdm virus or gene provider in subsequent reassortant viruses.
KEYWORDS: influenza A virus, antigenic drift, cat, genetic diversity, molecular epidemiology, orthomyxoviridae, pandemic, pig, regional pattern, zoonotic infections
ABSTRACT
The H1N1 influenza virus responsible for the most recent pandemic in 2009 (H1N1pdm) has spread to swine populations worldwide while it replaced the previous seasonal H1N1 virus in humans. In France, surveillance of swine influenza A viruses in pig herds with respiratory outbreaks led to the detection of 44 H1N1pdm strains between 2009 and 2017, regardless of the season, and findings were not correlated with pig density. From these isolates, 17 whole-genome sequences were obtained, as were 6 additional hemagglutinin (HA)/neuraminidase (NA) sequences, in order to perform spatial and temporal analyses of genetic diversity and to compare evolutionary patterns of H1N1pdm in pigs to patterns for human strains. Following mutation accumulation and fixation over time, phylogenetic analyses revealed for the first time the divergence of a swine-specific genogroup within the H1N1pdm lineage. The divergence is thought to have occurred around 2011, although this was demonstrated only through strains isolated in 2015 to 2016 in the southern half of France. To date, these H1N1pdm swine strains have not been related to any increased virulence in swine herds and have not exhibited any antigenic drift compared to seasonal human strains. However, further monitoring is encouraged, as diverging evolutionary patterns in these two species, i.e., swine and humans, may lead to the emergence of viruses with a potentially higher risk to both animal and human health.
IMPORTANCE Pigs are a “mixing vessel” for influenza A viruses (IAVs) because of their ability to be infected by avian and human IAVs and their propensity to facilitate viral genomic reassortment events. Also, as IAVs may evolve differently in swine and humans, pigs can become a reservoir for old human strains against which the human population has become immunologically naive. Thus, viruses from the novel swine-specific H1N1pdm genogroup may continue to diverge from seasonal H1N1pdm strains and/or from other H1N1pdm viruses infecting pigs and lead to the emergence of viruses that would not be covered by human vaccines and/or swine vaccines based on antigens closely related to the original H1N1pdm virus. This discovery confirms the importance of encouraging swine IAV monitoring because H1N1pdm swine viruses could carry an increased risk to both human and swine health in the future as a whole H1N1pdm virus or gene provider in subsequent reassortant viruses.
INTRODUCTION
Influenza A viruses (IAVs) belong to the Orthomyxoviridae family. Their genome consists of eight segments of negative-sense single-stranded RNA, encoding 11 or 12 proteins: the polymerases PB2, PB1 (sometimes PB1-F2), PA, and PA-X; the hemagglutinin (HA); the nucleoprotein (NP); the neuraminidase (NA); the matrix proteins M1 and M2; and the nonstructural proteins NS1 and NS2 (also named NEP) (1). Several viral subtypes are characterized by the two surface glycoproteins, HA and NA. To date, 18 HAs and 11 NAs have been described (2, 3). Although wild aquatic birds are considered the natural reservoir host, IAVs can infect a wide range of domestic birds and mammals, occasionally giving rise to severe outbreaks in animal husbandry and to seasonal epidemics in humans (4). Host switch events are not rare, and some of them have resulted in host-adapted lineages and sometimes in the development of pandemics (5).
In April 2009, a novel genotype of H1N1 virus spread in the human population, causing the first influenza pandemic of the 21st century (6, 7). This pandemic H1N1 virus (H1N1pdm) has a unique genome that combines gene segments originating from swine IAVs (swIAVs), i.e., two segments (encoding M and NA) derived from the “Eurasian avian-like swine H1N1 lineage” (H1avN1) and six other segments (encoding PB2, PB1, PA, HA, NP, and NS) descending from a North American triple-reassortant swIAV (6). This original combination of gene segments suggested that H1N1pdm was most probably generated in swine, even though it was not detected in pigs before it emerged in humans (8–10). Following the pandemic, H1N1pdm replaced the previous H1N1 virus that had been circulating in humans for years, becoming the novel seasonal human H1N1 virus cocirculating with H3N2 and influenza B viruses (11). In 2009, one month after the beginning of the pandemic, the first nonhuman case was reported in a swine herd in Canada (12). It was followed by many outbreaks in pigs worldwide that led to the establishment of a novel enzootic swIAV in almost all pig populations (13–16). In addition, other human-to-animal transmissions were sporadically reported in breeding turkeys, domestic cats, and pet ferrets (17–21).
Following the spread of the H1N1pdm virus to swine, novel reassortant viruses originating from previous enzootic swIAVs of the H1N1, H3N2, or H1N2 subtype and exhibiting one or several H1N1pdm genomic segments were identified worldwide (14, 22, 23). Most of them were not associated with very severe illness in swine, but new gene combinations may lead to the emergence of viruses exhibiting higher pathogenicity and/or increased interspecies transmission potential, as illustrated by cases reported in minks and humans (24–27) as well as by reverse genetic studies and experimental infections in various animal models (28–30). Moreover, once present in pigs, human-derived IAVs continue to evolve, in terms of their antigens, differently than in humans, resulting in lineages that could pose a novel risk to public health because they are no longer similar to contemporary human flu strains (31).
In France, the first H1N1pdm animal case was a domestic cat infection that occurred in November 2009, and outbreaks were then reported in pig herds. In this study, we investigated the genetic and antigenic properties of H1N1pdm isolates obtained from pigs in France from 2010 to 2017. We compared their genetic diversity to those of current H1N1pdm viruses responsible for seasonal influenza in humans in order to evaluate potential switches between hosts and/or divergent evolutionary patterns in both species. While most swine strains were found to be similar to seasonal human H1N1pdm strains at the nucleotide level, we highlight for the first time a new phylogenetic group within the H1N1pdm lineage which comprises only viruses isolated in pigs in France in 2015 to 2016. The mutations introduced at the amino acid level were compared with data from the literature to determine whether they could be assigned to host-specific molecular markers or might be related to any phenotypic or functional virus property in pigs. We also examined their zoonotic risk, searching for markers potentially related to interspecies transmission and evaluating the antigenic drift generated by accumulated mutations through cross-hemagglutination inhibition (HI) assays.
RESULTS
H1N1pdm swine viruses were detected throughout the years of study, and their distribution in France did not correlate with pig density.
From 2009 to 2017, the National Reference Laboratory (NRL) identified 1,016 batches of pigs reared in mainland France that tested positive for the IAV M gene, among which 44 (4.33%) were found positive by reverse transcription-PCRs (RT-PCRs) targeting specifically both the H1pdm and the N1pdm segments of the H1N1pdm virus (Table 1). During this surveillance period, no batch tested positive for only H1pdm or N1pdm genes, indicating that there was no reassortant virus bearing the H1pdm or N1pdm gene together with an NA or HA gene from another IAV lineage, respectively. However, HA and/or NA genes from other enzootic European swIAV lineages were concurrently detected in four H1N1pdm-positive herds, illustrating cocirculation events (data not shown).
TABLE 1.
Annual proportions of H1N1pdm viruses among swine influenza A viruses subtyped in France from 2009 to 2017
| Yr | No. of swIAVs subtyped | No. of H1N1pdm viruses | Proportion of H1N1pdm viruses (%) |
|---|---|---|---|
| 2009 | 15 | 0 | 0 |
| 2010 | 53 | 4 | 7.55 |
| 2011 | 134 | 1 | 0.75 |
| 2012 | 134 | 2 | 1.49 |
| 2013 | 126 | 3 | 2.38 |
| 2014 | 103 | 3 | 2.91 |
| 2015 | 146 | 8 | 5.48 |
| 2016 | 172 | 21 | 12.21 |
| 2017 | 133 | 2 | 1.50 |
| Total | 1,016 | 44 | 4.33 |
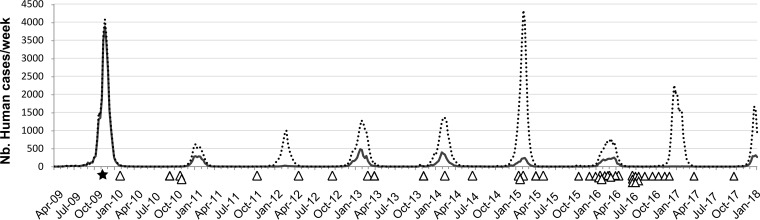
The H1N1pdm swine viruses were detected throughout the years, in contrast to H1N1pdm and other seasonal IAVs in humans, which occurred during winter (Fig. 1). Whereas a drop in the number of H1N1pdm-positive cases was observed in 2017, the annual relative frequency of swine H1N1pdm increased gradually from 2010 to 2016, reaching 12.21% of the swIAVs subtyped in 2016 (Table 1).
FIG 1.
Temporal distribution of swine H1N1pdm in France. The H1N1pdm viruses detected in pigs in France are individually represented by triangles positioned on the time x axis (graduated monthly from April 2009 to January 2018). The first H1N1pdm virus isolated from a mammalian animal in France, i.e., a cat, is represented by a black star. The lines represent the weekly numbers (y axis) of influenza A viruses (dotted line) and H1N1pdm viruses (black full line) detected in humans in France during this period. Human data were extracted from the World Health Organization (WHO)/Global Influenza Surveillance and Response System (GISRS) database (www.who.int/flunet).
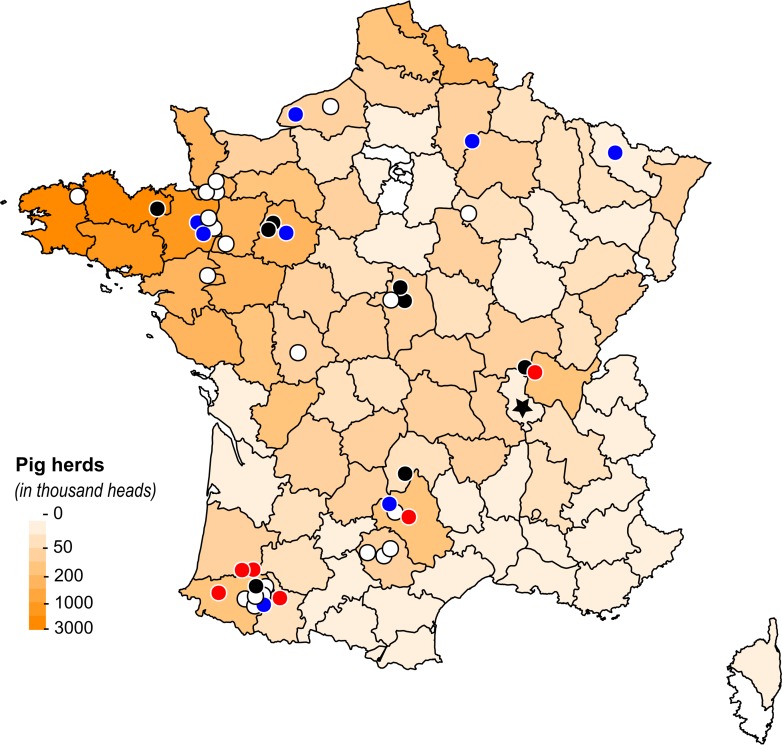
Most of the 44 H1N1pdm swine viruses were detected in distinct pig herds distributed throughout mainland France (Fig. 2). Strains A/swine/Sarthe/0255/2010 (A/Sw/Sarthe/0255/2010) and A/Sw/Sarthe/0262/2010 were isolated in a single herd in animals in two different physiological states and sampled 5 days apart (Table 2). Also, strains A/Sw/France/18-120158/2012 and A/Sw/France/18-120333/2012 were isolated in the same herd but in postweaned piglets sampled 5 months apart. In each French administrative area (department), the number of H1N1pdm viruses detected during the study did not correlate with the number of pigs reared in the area (Pearson’s R = 0.13), in contrast to the total number of swIAVs (Pearson’s R = 0.96) (Fig. 2). Thus, in 2016, for example, only 2 H1N1pdm viruses out of 125 swIAVs were reported in the most western part of Brittany, where nearly 20% of pigs in France are produced, whereas H1N1pdm viruses accounted for 9 out of 10 swIAVs detected in the far southwest of France, where <2% of pigs are bred.
FIG 2.
Distribution of H1N1pdm viruses detected in pigs in France from November 2009 to 2017. The map was colored in an orange gradient according to the size of the pig population in each administrative department, based on data provided by the National Agricultural Census (56). The first mammalian animal H1N1pdm strain isolated in France, i.e., the cat strain, is indicated by a black star. Each circle represents an H1N1pdm virus strain detected in swine. Blue circles are isolates belonging to the seasonal-like (SeL) group, red circles are isolates belonging to the swine divergent (SwD) group, black circles are isolates belonging to neither the SeL group nor the SwD group, and white circles are isolates that were not sequenced. The SeL and SwD genogroups were defined in this study. The map was drawn using R 3.4.0 software (https://CRAN.R-project.org/package=maps).
TABLE 2.
Sample description and GenBank accession numbers of the H1N1pdm sequences obtained in this study
| Strain (H1N1pdm) | Collection date (day/mo/yr) |
Epi form; intensitya | Genogroupb | GenBank accession no. |
Sequencing methodc |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2 | PB1 | PA | HA | NP | NA | M | NS | |||||
| A/Cat/France/0514/2009 | 26/11/2009 | Pneumonia | 3 | CY110768 | CY110769 | HE603922 | FR872140 | HE577050 | FR872139 | FR878009 | HE577051 | Sanger |
| A/swine/Cotes d’Armor/110466/2010 | 03/02/2010 | Recurrent; high | 3 | KC477397 | KC477395 | KC345642 | KC345640 | KC477391 | KC345641 | KC514527 | KC345639 | Sanger |
| A/swine/Sarthe/0255/2010 | 03/11/2010 | ND; high | 7 | HE603920 | HE603924 | CY110772 | CY110773 | HE577053 | HE600191 | HE603923 | HE577052 | NGS1 |
| A/swine/Sarthe/0262/2010 | 08/11/2010 | ND; standard | 7 | KC345635 | HE603925 | KC345637 | FR871195 | KC477393 | CY110914 | KC514526 | KC345636 | NGS1 |
| A/swine/Haute-Loire/0578/2011 | 18/10/2011 | Classical; standard | 7 | KC345645 | KC477394 | KC477392 | CY114522 | KC345643 | CY114523 | KC514528 | KC345644 | NGS1 |
| A/swine/France/18-120158/2012 | 25/04/2012 | None | 7 | KC345621 | KC477389 | KC345620 | KC345616 | KC345618 | KC345617 | KC514524 | KC345619 | NGS1 |
| A/swine/France/18-120333/2012 | 27/09/2012 | Recurrent; standard | 7 | KC345651 | KC477396 | KC345650 | KC345646 | KC345649 | KC345647 | KC514530 | KC345648 | NGS1 |
| A/swine/France/71-130116/2013 | 07/03/2013 | Classical; standard | 7 | KF229767 | KF229768 | KF229769 | KF229766 | KF229770 | KF229771 | KF229772 | KF229773 | NGS2 |
| A/swine/France/57-140136/2014 | 20/02/2014 | Classical; standard | SeL | MH785051 | MH785052 | MH785053 | MH785054 | MH785055 | MH785056 | MH785057 | MH785058 | NGS2 |
| A/swine/France/35-140382/2014 | 24/06/2014 | Recurrent; standard | SeL | MH785035 | MH785036 | MH785037 | MH785038 | MH785039 | MH785040 | MH785041 | MH785042 | NGS2 |
| A/swine/France/35-140384/2014 | 24/06/2014 | Recurrent; standard | SeL | MH785075 | Sanger | |||||||
| A/swine/France/64-150052/2015 | 29/01/2015 | Classical; high | SwD | MH785059 | MH785060 | MH785061 | MH785062 | MH785063 | MH785064 | MH785065 | MH785066 | NGS2 |
| A/swine/France/12-150058/2015 | 22/01/2015 | Recurrent; standard | SwD | KY241117 | KY241118 | Sanger | ||||||
| A/swine/France/64-150091/2015 | 12/02/2015 | Classical; standard | SeL | KY241119 | KY241120 | Sanger | ||||||
| A/swine/France/01-150203/2015d | 11/05/2015 | Classical; high | SwD | MH785027 | MH785028 | MH785029 | MH785030 | MH785031 | MH785032 | MH785033 | MH785034 | NGS2 |
| A/swine/France/65-160089/2016 | 01/02/2016 | Classical; standard | SwD | KY364085 | KY364086 | KY364087 | KY364088 | KY364089 | KY364090 | KY364091 | KY364092 | NGS2 |
| A/swine/France/40-160098/2016 | 29/01/2016 | Recurrent; standard | SwD | KY364101 | KY364102 | KY364103 | KY364104 | KY364105 | KY364106 | KY364107 | KY364108 | NGS2 |
| A/swine/France/40-160120/2016 | 16/02/2016 | Classical; standard | SwD | MH785043 | MH785044 | MH785045 | MH785046 | MH785047 | MH785048 | MH785049 | MH785050 | NGS2 |
| A/swine/France/12-160129/2016 | 07/03/2016 | Classical; standard | SeL | KY364141 | KY364142 | KY364143 | KY364144 | KY364145 | KY364146 | KY364147 | KY364148 | NGS2 |
| A/swine/France/72-160174/2016 | 22/04/2016 | Classical; standard | SeL | KY364165 | KY364166 | KY364167 | KY364168 | KY364169 | KY364170 | KY364171 | KY364172 | NGS2 |
| A/swine/France/35-160233/2016 | 22/06/2016 | Classical; high | SeL | MH785067 | MH785068 | MH785069 | MH785070 | MH785071 | MH785072 | MH785073 | MH785074 | NGS2 |
| A/swine/France/AR2675/2015 | 20/10/2015 | ND | SeL | EPI1080442e | EPI1080443e | Sanger | ||||||
| A/swine/France/AR271/2016 | 19/01/2016 | ND | SeL | EPI1080446e | EPI1080447e | Sanger | ||||||
| A/swine/France/AR9191/2016 | 14/11/2016 | ND | SeL | EPI1080448e | EPI1080449e | Sanger | ||||||
Influenza virus epidemiological form (classical or recurrent) and clinical sign intensity (standard or high) as reported by the veterinarians who collected the samples. ND, not determined.
Genogroup numbers correspond to those defined by Nelson and colleagues (33). Seasonal-like (SeL) and swine divergent (SwD) genogroups were defined in this study.
NGS1/NGS2 sequences were obtained by two different new-generation sequencing pipelines. See Materials and Methods for details.
Strain A/swine/France/01-150203/2015 was isolated from a pig coinfected with viruses from both H1N1pdm and H1avN1 lineages.
GSAID EpiFlu database accession number.
Phylogenetic analyses show that some recent French H1N1pdm strains isolated in pigs diverge from contemporary human strains.
Twenty-three H1N1pdm isolates (dating from 2010 to 2016) were obtained from the 44 H1N1pdm-positive swine batches after biological sample propagation in embryonated eggs or Madin-Darby canine kidney (MDCK) cells, and 17/23 strains were fully sequenced (Table 2). Additionally, sequences of the HA, or both the HA and NA, segments were obtained for the other 6 swine isolates. All H1 genes were confirmed to belong to clade 1A.3.3.2, which comprises H1 genes from H1N1pdm strains, using the “swine H1 clade classification tool” of the Influenza Research Database (IRD) (32).
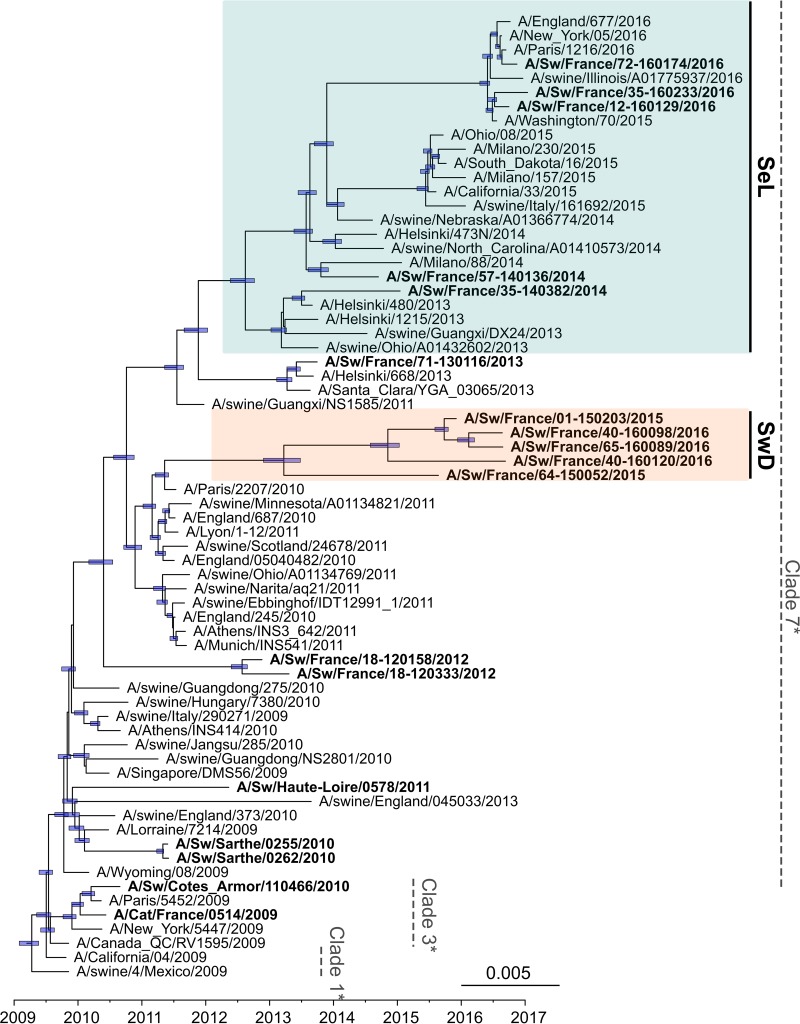
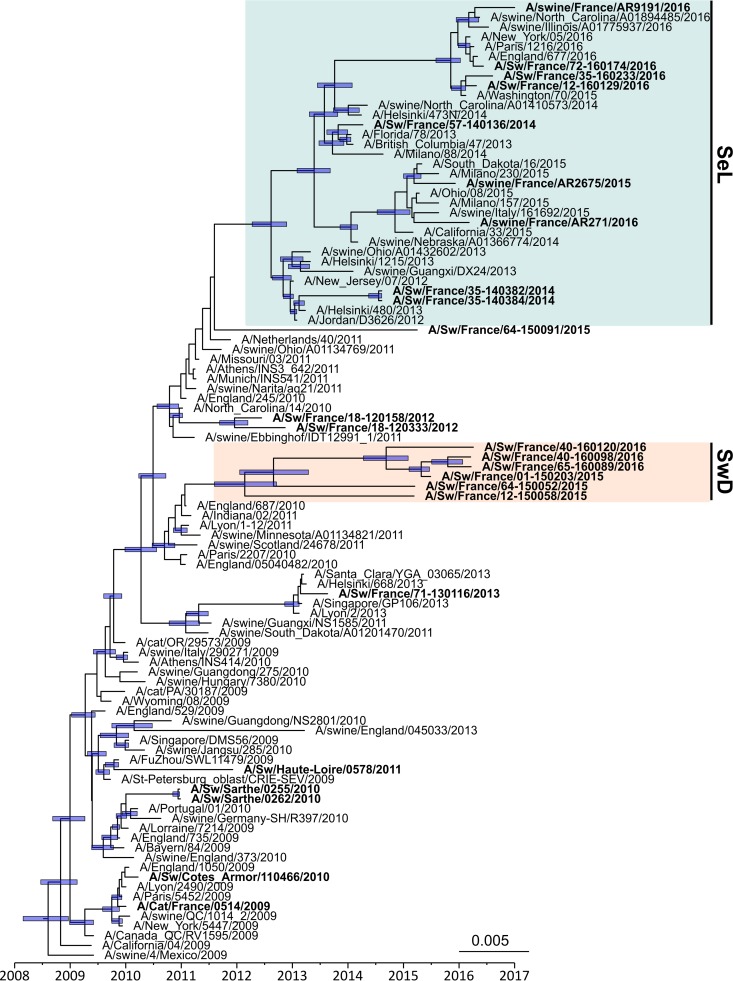
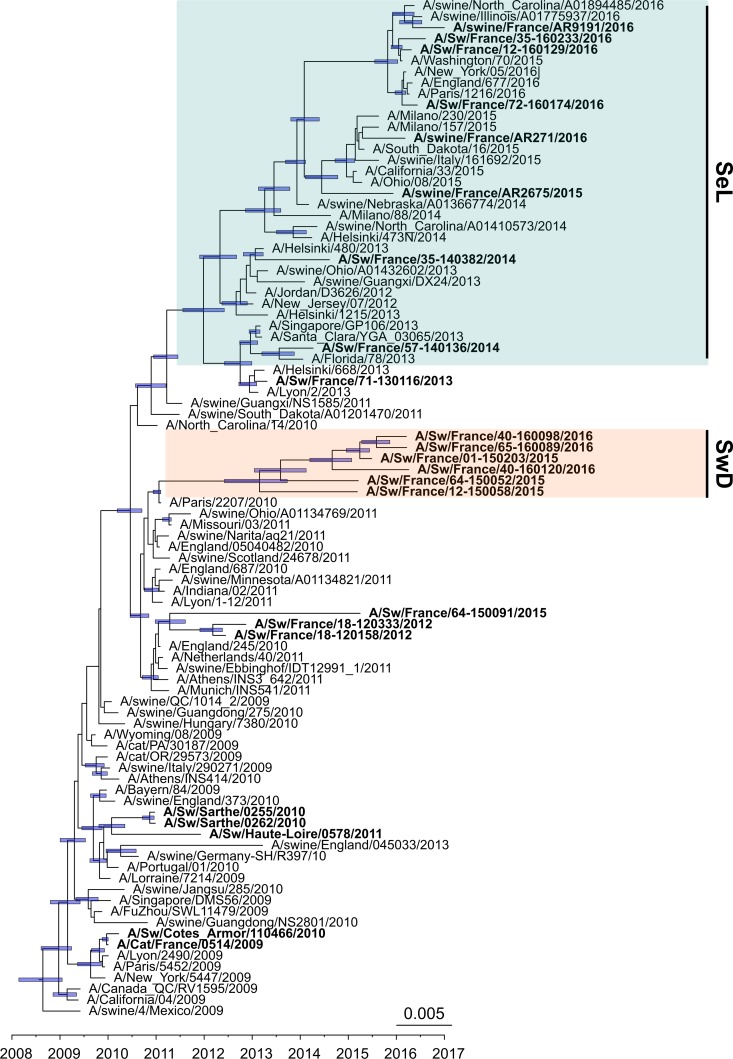
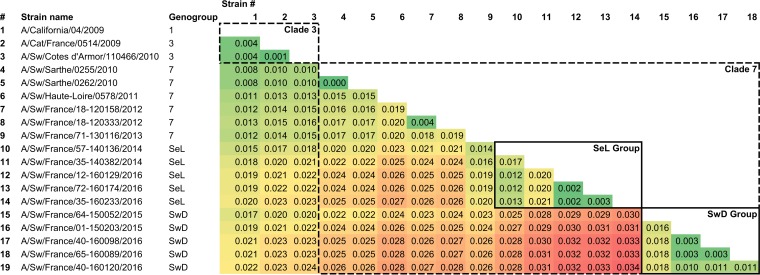
The whole-genome phylogenetic tree (Fig. 3), but also the HA, NA, and M phylogenetic trees (Fig. 4 to 6) as well as the other segment-by-segment phylogenetic trees (data not shown), showed that H1N1pdm viruses isolated from pigs in France from 2010 to 2016 belonged to several genogroups among the H1N1pdm lineage. On the basis of the initial classification proposed by Nelson and colleagues, who described the early genetic diversification of H1N1pdm viruses (33), the first strain isolated in pig in February 2010, i.e., A/Sw/Cotes d’Armor/110466/2010, belonged to so-called “clade 3” together with the strain isolated in cat in November 2009, i.e., A/Cat/France/0514/2009. Subsequently, H1N1pdm strains obtained in pigs from November 2010 to the end of 2013 belonged to, or were derived from, clade 7 and clustered with their contemporary human and swine counterparts isolated worldwide. From 2014 onward, a shift appeared in the French swine H1N1pdm strains, which were divided into two genomic groups (Fig. 3 to 6). In one group, identified as the “seasonal-like” (SeL) group, swine H1N1pdm strains remained close to contemporary human strains (seasonal H1N1), showing 99% identity by BLAST analyses irrespective of the genomic segment. In contrast, in the second group, identified as the “swine divergent” (SwD) group, French swine H1N1pdm strains were classified apart from any other human or swine H1N1pdm strain, based on the sequences available in the different public databases. These strains showed less than 97.5% identity with other H1N1pdm strains in the HA, NA, and NS segments but still between 98% and 99% identity in other segments. Strains from the SeL and SwD groups differed from an average distance of 0.03 substitutions/nucleotide (nt), while the average distances from the reference strain A/California/04/2009 were 0.018 substitutions/nt for SeL strains and 0.02 substitutions/nt for SwD strains (Table 3). Although the SwD group did not comprise any human strains, the most recent common ancestor was shared with strains involved in seasonal epidemics in 2010 to 2011 (Fig. 3). Swine H1N1pdm strains within the SwD group were identified in herds that were located in the South of France, whereas swine strains from the SeL group were detected in herds distributed throughout the country, similarly to H1N1pdm viruses detected in the previous 2009–2013 period (Fig. 2).
FIG 3.
Bayesian inference tree of H1N1pdm virus strains from whole-genome sequences. Strains isolated from pigs (and a cat) in France are indicated in boldface type. Nodes supported by more than 50% of sampled trees are indicated by a blue bar displaying the 95% highest posterior density (HPD) intervals of the node heights. The SeL and SwD genogroups were defined in this study. *, clades 1, 3, and 7 correspond to the classification of Nelson et al. (33).
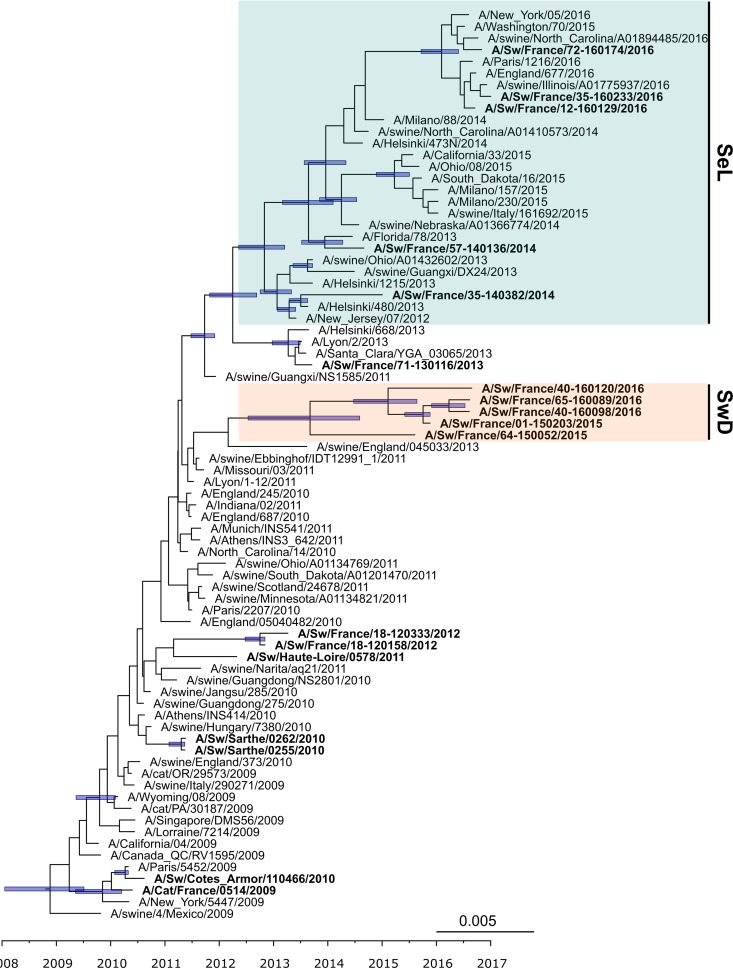
FIG 4.
Bayesian inference tree of the HA segment of H1N1pdm virus strains. Strains isolated from pigs (and a cat) in France are indicated in boldface type. Nodes supported by more than 50% of sampled trees are indicated by a blue bar displaying the 95% HPD intervals of the node heights. The SeL and SwD genogroups were defined in this study.
FIG 5.
Bayesian inference tree of the NA segment of the H1N1pdm virus. Strains from French swine or cat are indicated in boldface type. Nodes supported by more than 50% of sampled trees are indicated by a blue bar displaying the 95% HPD intervals of the node heights. The SeL and SwD genogroups were defined in this study.
FIG 6.
Bayesian inference tree of the M segment of the H1N1pdm virus. Strains from French swine or cat are indicated in boldface type. Nodes supported by more than 50% of sampled trees are indicated by a blue bar displaying the 95% HPD intervals of the node heights. The SeL and SwD genogroups were defined in this study.
TABLE 3.
Pairwise nucleotide distances obtained from whole-genome alignments of A/California/04/2009, A/Cat/France/0514/2009, and swine H1N1pdm strains isolated in Francea
The gradient background color represents the increase in pairwise nucleotide distances, going from the smallest distance, in dark green, to the largest, in dark red. Genogroups, i.e., clades or groups, of affiliations are those defined from phylogenetic analyses.
The molecular clock rate estimated from the whole-genome phylogenetic tree was 3.25 × 10−3 substitutions per site per year (95% highest posterior density [HPD] interval, 3.05 × 10−3 to 3.47 × 10−3). The segment-by-segment molecular clock rate displayed heterogeneous values. It appeared that the highest rates were observed in the three most divergent genes identified in the SwD group, i.e., HA, NA, and NS, with means of 3.98 × 10−3, 4.28 × 10−3, and 4.12 × 10−3 substitutions/site/year, respectively. For the other segments, clock rates varied between 2.67 × 10−3 and 3.06 × 10−3 substitutions/site/year.
Swine H1N1pdm strains belonging to the SwD group accumulated specific amino acid mutations.
The numbers of amino acid mutations observed in swine isolates from France with respect to the reference strain A/California/04/2009 are reported in Table 4. Not surprisingly, the total number of amino acid mutations per viral strain increased over time: strain A/Sw/France/65-160089/2016 contained 75 amino acid (aa) changes, whereas A/Cat/France/0514/2009, the oldest strain isolated in a mammalian animal, exhibited 15 mutations only. Only one exception was observed, with strain A/Sw/France/71-130116/2013, which contained fewer mutations than strains isolated in 2012. In all strains, HA was the protein that showed the highest number of mutations. Nevertheless, proportionally to the protein length, the NS proteins were the ones that accumulated the most mutations since 2015, followed by the HA, NA, and M proteins (Table 4). According to the ratio of the number of nonsynonymous substitutions (dN) to the number of synonymous substitutions (dS), all proteins underwent strong purifying or stabilizing selection. The polymerase proteins (PB1, PB2, and PA) exhibited the highest levels of selection pressure (i.e., lowest dN/dS ratios), while N2 and M2 showed the lowest levels of purifying selection (i.e., highest dN/dS ratios) (Table 4).
TABLE 4.
Amino acid differences, within each influenza A virus protein, between swine and cat H1N1pdm strains isolated in France and reference strain A/California/04/2009
| H1N1pdm strain | % aa differences with A/California/04/2009 in each viral protein (absolute no. of aa differences)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2 (759 aa) | PB1 (757 aa) | PA (716 aa) | PA-X (232 aa) | HA (564 aa) | NP (498 aa) | NA (469 aa) | M1 (252 aa) | M2 (97 aa) | NS1 (219 aa) | NS2 (121 aa) | All (4,684 aa) | |
| A/Sw/France/72-160174/2016 | 1.05 (8) | 0.4 (3) | 0.56 (4) | 1.72 (4) | 3.01 (17) | 0.8 (4) | 3.2 (15) | 1.59 (4) | 2.06 (2) | 3.2 (7) | 4.13 (5) | 1.56 (73) |
| A/Sw/France/12-160129/2016 | 1.05 (8) | 0.4 (3) | 0.70 (5) | 1.72 (4) | 2.84 (16) | 0.8 (4) | 3.2 (15) | 1.59 (4) | 1.03 (1) | 3.2 (7) | 4.13 (5) | 1.54 (72) |
| A/Sw/France/35-160233/2016 | 1.19 (9) | 0.4 (3) | 0.84 (6) | 1.72 (4) | 3.01 (17) | 1 (5) | 2.99 (14) | 1.59 (4) | 1.03 (1) | 3.65 (8) | 4.13 (5) | 1.62 (76) |
| A/Sw/France/40-160098/2016 | 0.92 (7) | 1.32 (10) | 0.98 (7) | 0.86 (2) | 3.72 (21) | 0.4 (2) | 2.56 (12) | 0.4 (1) | 2.06 (2) | 3.2 (7) | 1.65 (2) | 1.56 (73) |
| A/Sw/France/65-160089/2016 | 1.05 (8) | 1.32 (10) | 0.98 (7) | 0.86 (2) | 3.55 (20) | 0.6 (3) | 2.56 (12) | 0.4 (1) | 2.06 (2) | 3.2 (7) | 1.65 (2) | 1.58 (74) |
| A/Sw/France/40-160120/2016 | 0.53 (4) | 1.72 (13) | 0.84 (6) | 0.86 (2) | 3.37 (19) | 0.6 (3) | 2.56 (12) | 0.79 (2) | 4.12 (4) | 4.57 (10) | 2.48 (3) | 1.67 (78) |
| A/Sw/France/01-150203/2015 | 1.19 (9) | 1.19 (9) | 0.98 (7) | 0.86 (2) | 3.72 (21) | 0.4 (2) | 2.56 (12) | 0.4 (1) | 1.03 (1) | 3.2 (7) | 1.65 (2) | 1.56 (73) |
| A/Sw/France/64-150052/2015 | 0.79 (6) | 0.92 (7) | 0.70 (5) | 1.29 (3) | 3.37 (19) | 1.2 (6) | 1.92 (9) | 0.4 (1) | 2.06 (2) | 1.83 (4) | 2.48 (3) | 1.39 (65) |
| A/Sw/France/57-140136/2014 | 1.05 (8) | 0.53 (4) | 0.84 (6) | 1.29 (3) | 2.48 (14) | 0.6 (3) | 1.71 (8) | 1.19 (3) | 3.09 (3) | 2.74 (6) | 1.65 (2) | 1.28 (60) |
| A/Sw/France/35-140382/2014 | 1.05 (8) | 0.79 (6) | 0.70 (5) | 0.43 (1) | 2.30 (13) | 1.2 (6) | 1.92 (9) | 1.19 (3) | 2.06 (2) | 2.74 (6) | 1.65 (2) | 1.30 (61) |
| A/Sw/France/71-130116/2013 | 0.53 (4) | 0.53 (4) | 0.84 (6) | 1.29 (3) | 1.95 (11) | 0.6 (3) | 1.28 (6) | 1.59 (4) | 1.03 (1) | 1.83 (4) | 0.83 (1) | 1.00 (47) |
| A/Sw/France/18-120333/2012 | 0.92 (7) | 0.79 (6) | 1.12 (8) | 0.86 (2) | 3.37 (19) | 0.6 (3) | 1.28 (6) | 1.19 (3) | 3.09 (3) | 1.37 (3) | 0 (0) | 1.28 (60) |
| A/Sw/France/18-120158/2012 | 0.79 (6) | 0.79 (6) | 0.98 (7) | 0.86 (2) | 3.01 (17) | 0.6 (3) | 1.28 (6) | 1.19 (3) | 2.06 (2) | 1.37 (3) | 0 (0) | 1.17 (55) |
| A/Sw/Haute-Loire/0578/2011 | 0.53 (4) | 0.4 (3) | 0.14 (1) | 0 (0) | 2.48 (14) | 0.6 (3) | 1.71 (8) | 0.79 (2) | 0 (0) | 1.37 (3) | 0.83 (1) | 0.83 (39) |
| A/Sw/Sarthe/0255/2010 | 0.4 (3) | 0.13 (1) | 0.56 (4) | 1.29 (3) | 1.60 (9) | 0.4 (2) | 1.71 (8) | 0 (0) | 0 (0) | 0.91 (2) | 0.83 (1) | 0.70 (33) |
| A/Sw/Sarthe/0262/2010 | 0.53 (4) | 0.13 (1) | 0.42 (3) | 1.29 (3) | 1.60 (9) | 0.6 (3) | 1.71 (8) | 0 (0) | 0 (0) | 0.91 (2) | 0.83 (1) | 0.73 (34) |
| A/Sw/Cote d’Armor/110466/2010 | 0 (0) | 0.53 (4) | 0.42 (3) | 0.43 (1) | 1.06 (6) | 0 (0) | 0.43 (2) | 0 (0) | 2.06 (2) | 0.46 (1) | 0 (0) | 0.41 (19) |
| A/Cat/France/0514/2009 | 0 (0) | 0.13 (1) | 0.28 (2) | 0.43 (1) | 1.06 (6) | 0 (0) | 0.21 (1) | 0.4 (1) | 0 (0) | 1.84 (4) | 0 (0) | 0.34 (16) |
| dN/dS ratiob | 0.0779 | 0.0545 | 0.0577 | 0.0948 | 0.1494 | 0.0421 | 0.1876 | 0.1328 | 0.2171 | 0.1859 | 0.3890 | |
The numbers in parentheses are the absolute numbers of amino acid differences in each protein. The two proteins (or three in the case of equality) in which the most mutations were found for each strain are indicated in boldface type.
The dN/dS ratio, the ratio between the number of nonsynonymous substitutions (dN) and the number of synonymous substitutions (dS), was measured from averages of pairwise comparisons of all sequences selected to build the phylogenetic trees with SNAP v2.1.1.
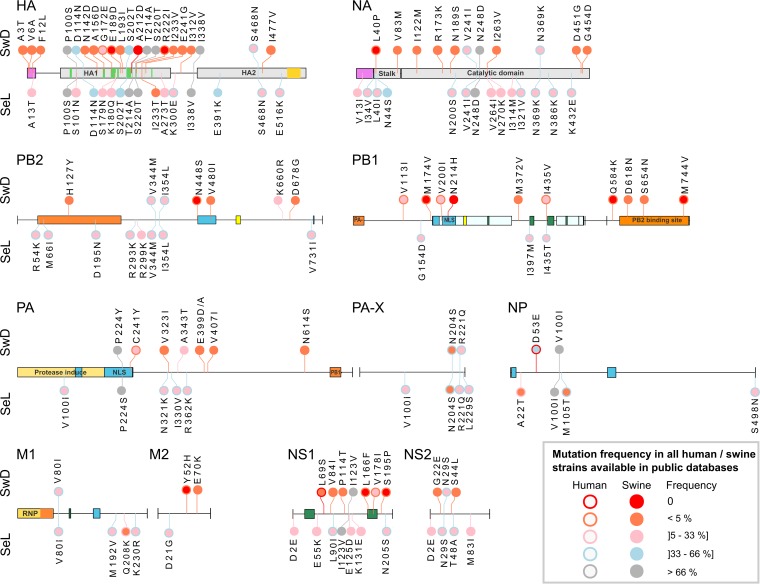
Comparison of swine H1N1pdm viruses isolated in France to A/California/04/2009 revealed that 18 aa changes have been fixed over the years. They were mutations PA-P224S, HA-P100S, HA-T214A, HA-S220T, HA-I338V, NP-V100I, NA-N369K, and NS1-I123V in strains isolated since 2010 and then PA-X-R221Q, HA-D114N, HA-S202T, HA-S468N, and NA-V241I from 2012 and finally PB2-V344M, PB2-I354L, PA-X-N204S, M1-V80I, and NS2-N29S since 2015. Among them, mutations HA-S202T and HA-S220T localized in antigenic sites Sb and Ca1, respectively. Five other mutations, i.e., PB1-G154D, PA-N321K, PA-X-L229S, HA-E391K, and M2-D21G, were observed in all swine H1N1pdm strains isolated since 2012, excluding those belonging to the SwD group.
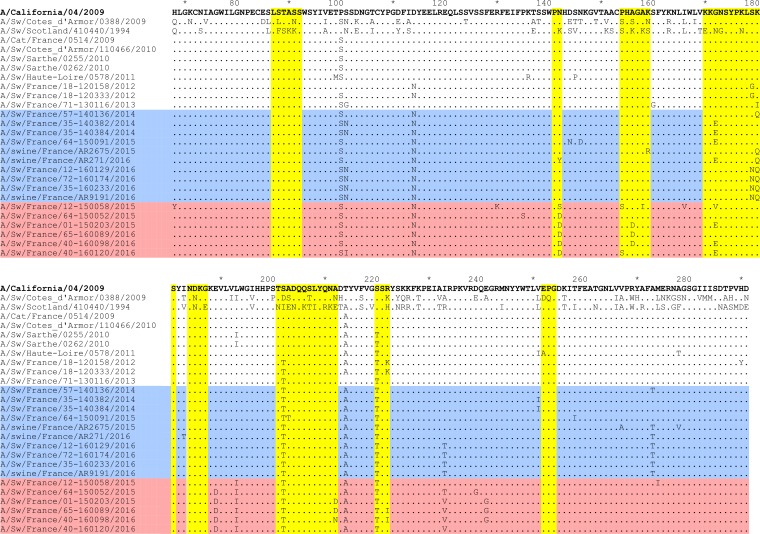
In contrast, strains from the SwD group were characterized by large numbers of amino acid changes that were not inherited from older French swine H1N1pdm strains, going up to 59 new amino acid mutations in one SwD strain. Despite some strain-specific mutations, all H1N1pdm isolates from the SwD group share 12 mutations not observed to date in other swine H1N1pdm isolates and 15 mutations observed in <1% of H1N1pdm haplotypes described in human or swine isolates worldwide (Fig. 7; see also Table SA1 in the supplemental material). Among the mutations shared by all strains within the SwD group, we observed that eight of them affected residues involved in the polymerase protein domains: PB2-V480I, PB1-N213H, and PA-C241Y in the nuclear localization signal (NLS); PB2-H127Y in the PB1-binding site; and PB1-Q584K, D618N, S654N, and M744V around the PB2-binding domain. Three mutations (HA-A3T, -V6A, and -F12L) were localized in the HA signal peptide, while many others concentrated in the globular head of HA, including three mutations in antigenic sites, i.e., N142D and G172E in the Sa site and A156D in the Ca site (Fig. 7). Importantly, HA-G172E was not SwD group specific, as it was encountered in other H1N1pdm strains since 2011, including three recent French strains that were classified in the SeL group. Finally, strains of the SwD group exhibited mutation NP-D53E that was described and suspected to be swine specific since 2011 (34, 35). This mutation, never observed in any human strain, is shared by nearly 80% of swine H1N1pdm strains whose sequences are available in databases, excluding those from the SeL group.
FIG 7.
Distribution and frequency of amino acid mutations in proteins from swine H1N1pdm strains belonging to the SeL and SwD genogroups, respectively. Each bullet represents a mutation in strains from the SeL group (below the line) or the SwD group (above the line), in comparison with the amino acid sequence of reference strain A/California/04/2009 (on the line). The bullet’s color represents the frequency level of a given residue among swine (color inside the bullet) and human (color outside the bullet) H1N1pdm haplotypes available in public databases. Known protein domains or important sites are represented by colored blocks on the reference line: the signal peptide (violet), the antigenic sites (green) inside the HA1 globular head, and the transmembrane domain (yellow) inside the HA2 part for HA; the signal anchor (violet) and the stalk and catalytic domains in NA; two NLSs (cyan) in NP; the PB1-binding site (orange), two NLSs (cyan), and the cap-binding site (yellow) in PB2; the PA-binding site (orange), two nucleotide-cross-linked regions, including two NLSs (cyan), the RNA-binding site (yellow), and four polymerase motifs (in green) (inside and between the regions), three cap-dependent RNase active sites (positions 508, 519, and 522), and the PB2-binding site (orange) in PB1; the protease-induced domain, including two NLSs (cyan), and the PB1-binding site (orange) in PA; the ribonucleoprotein (RNP) (gold), including the membrane-binding site (orange), an RNA-binding site (green), and an NLS (cyan), in M1; and two secondary RNA structures (green) in NS1.
While several mutations observed in strains from the SeL group affected glycosylation sites, such as HA-S179N (4/9 strains), NA-N44S (8/8), and NA-N386K (6/8), there were only 2 strains out of 5 in the SwD group that were affected by changes in glycosylation sites: A/Sw/France/12-150058/2015, which lost one N-glycosylation site due to an HA-T295I mutation, and A/Sw/France/65-160089/2016, which acquired an additional one due to an NA-S35N mutation.
Despite specific amino acid mutations in HA1, swine H1N1pdm strains from the SwD group remain antigenically closely related to A/California/04/2009.
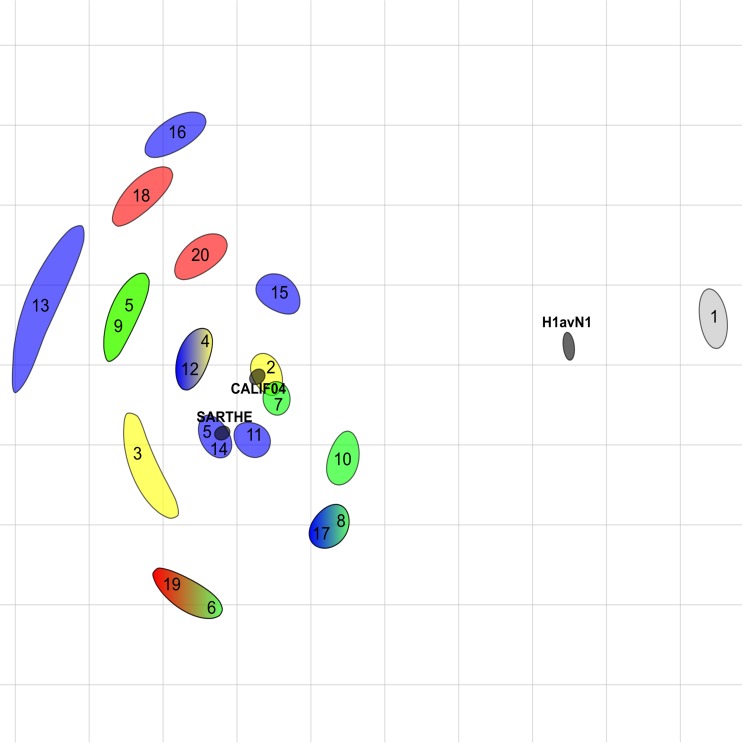
As reported above and as observed from alignments of amino acid sequences, several mutations have been introduced in the major antigenic sites in HA1 since 2010 (Fig. 7 and 8). In order to evaluate the consequences in terms of possible antigenic drift, especially regarding H1N1pdm strains from the SwD group, we compared the reactivities of 17 swine H1N1pdm isolates obtained in France from 2010 to 2016, as well as that of the cat strain obtained in 2009, to porcine hyperimmune sera directed against human or swine reference strains from the H1N1pdm lineage, using hemagglutination inhibition (HI) assays (Table 5). The H1N1pdm isolates were also tested against reference sera containing antibodies to other swIAVs from older European enzootic lineages, i.e., the H1avN1 lineage, the European human-like reassortant H1N2 (H1huN2) lineage, and the European human-like reassortant H3N2 lineage (13). The highest HI titers were obtained with antisera to both H1N1pdm reference strains, i.e., A/California/04/2009 and A/Sw/Sarthe/0255/2010. Stronger reactions were observed with antibodies directed against the swine strain (mean HI titer = 403.2 [range, 80 to 1,280]) than with antibodies directed against the human strain (mean HI titer = 166.3 [range, 80 to 640]). Positive HI titers were also obtained with antibodies directed against the H1avN1 strain, due to cross-reactivity between H1pdm and H1av (36). However, HI titers against the H1avN1 strain were weaker (mean HI titer = 20 [range, <10 to 80]) than those for both H1N1pdm antisera. Similarly to reference strain A/California/04/2009, the cat H1N1pdm and three swine H1N1pdm strains yielded HI titers of 20 with the H1huN2 antiserum, probably due to slight cross-reactivity within the H1 subtype. In contrast, no H1N1pdm strain gave a positive HI titer with the H3N2 antiserum. To better evaluate the antigenic distances between H1N1pdm strains from the different genogroups, HI titers were submitted to antigenic cartography, including all H1N1pdm strains and reference antisera as well as the H1avN1 reference strain and antiserum (Fig. 9). All H1N1pdm strains grouped around the two H1N1pdm reference sera. The viruses most distant from reference antisera to A/California/04/2009 and A/Sw/Sarthe/0255/2010 were A/Sw/France/35-140384/2014 (strain 13) and A/Sw/France/35-160233/2016 (strain 16), both from the SeL group. However, the antigenic distance was only 4 antigenic units (AU) and was not correlated with amino acid changes in HA1 over the years. Strains from the SwD group (strains 18 to 20) did not form a group apart from other strains and were not more distant from reference strains than others.
FIG 8.
Distribution of amino acid mutations in the HA1 domain of swine H1N1pdm strains isolated in France compared to reference strain A/California/04/2009. H1N1pdm strains from the SeL group are highlighted in blue, and those from the SwD group are highlighted in pink. The SeL and SwD genogroups were defined in this study. Strain A/Cat/France/0514/2009 was included as it was the first H1N1pdm strain isolated in a mammalian animal in France. Strains A/Sw/Cotes d’Armor/0388/2009 (H1avN1) and A/Sw/Scotland/410440/1994 (H1huN2) were included as reference strains from other genetic lineages within the swine H1 subtype. The antigenic sites Ca1, Ca2, Cb, Sa, and Sb (57) are marked in yellow.
TABLE 5.
Antigenic relationships between the H1N1pdm strains isolated in pigs in France from 2009 to 2017 and reference strains from H1N1pdm, H1avN1, H1huN2, and H3N2 lineagesa
| Virus strain (identification no.)b | Lineage (clade) | Hemagglutination inhibition titer with sera against: |
||||
|---|---|---|---|---|---|---|
| H1avN1 A/Sw/CA/0388/2009 |
H1N1pdm A/California/04/2009 |
H1N1pdm A/Sw/Sarthe /0255/2010 |
H1huN2 A/Sw/Scotland/410440/1994 |
H3N2 A/Sw/Flandres/1/1998 |
||
| A/Sw/Cotes d’Armor/0388/2009 (1) | H1avN1 | 640 | <10 | <10 | <10 | <10 |
| A/California/04/2009 (2) | H1N1pdm (3) | 40 | 640 | 640 | 20 | <10 |
| A/Cat/France/0514/2009 (3) | H1N1pdm (3) | 10 | 160 | 640 | 10 | <10 |
| A/Sw/Cotes d’Armor/110466/2010 (4) | H1N1pdm (3) | 20 | 320 | 640 | 20 | <10 |
| A/Sw/Sarthe/0255/2010 (5) | H1N1pdm (7) | 10 | 160 | 320 | 10 | <10 |
| A/Sw/Sarthe/0262/2010 (6) | H1N1pdm (7) | 10 | 80 | 320 | 10 | <10 |
| A/Sw/Haute-Loire/0578/2011 (7) | H1N1pdm (7) | 80 | 640 | 1,280 | 20 | <10 |
| A/Sw/France/18-120158/2012 (8) | H1N1pdm (7) | 40 | 160 | 320 | 10 | <10 |
| A/Sw/France/18-120333/2012 (9) | H1N1pdm (7) | 10 | 160 | 320 | 10 | <10 |
| A/Sw/France/71-130116/2013 (10) | H1N1pdm (7) | 80 | 160 | 640 | <10 | 10 |
| A/Sw/France/57-140136/2014 (11) | H1N1pdm (SeL) | 40 | 320 | 1,280 | 20 | <10 |
| A/Sw/France/35-140382/2014 (12) | H1N1pdm (SeL) | 20 | 320 | 640 | 10 | <10 |
| A/Sw/France/35-140384/2014 (13) | H1N1pdm (SeL) | <10 | 80 | 160 | <10 | <10 |
| A/Sw/France/64-150091/2015 (14) | H1N1pdm (SeL) | 20 | 320 | 1,280 | 10 | <10 |
| A/Sw/France/12-160129/2016 (15) | H1N1pdm (SeL) | 20 | 160 | 320 | <10 | <10 |
| A/Sw/France/35-160233/2016 (16) | H1N1pdm (SeL) | 10 | 80 | 80 | 10 | <10 |
| A/Sw/France/72-160174/2016 (17) | H1N1pdm (SeL) | 40 | 160 | 320 | 20 | <10 |
| A/Sw/France/64-150052/2015 (18) | H1N1pdm (SwD) | 10 | 80 | 160 | <10 | <10 |
| A/Sw/France/65-160089/2016 (19) | H1N1pdm (SwD) | 10 | 80 | 320 | 10 | <10 |
| A/Sw/France/40-160098/2016 (20) | H1N1pdm (SwD) | 20 | 160 | 320 | <10 | <10 |
| A/Sw/Scotland/410440/1994 | H1huN2 | 10 | 10 | 20 | 2,560 | 10 |
| A/Sw/Flandres/1/1998 | H3N2 | <10 | <10 | <10 | 10 | 2,560 |
Strain A/Cat/France/0514/2009 was included as the first H1N1pdm strain isolated in a mammalian animal in France. Hemagglutination inhibition titers were measured using antisera produced in SPF pigs. The SeL and SwD genogroups were defined in this study. Homologous titers are indicated in boldface type.
Identification numbers are reported in the antigenic map (Fig. 10).
FIG 9.
Antigenic map of French swine H1N1pdm from HI data. The relative positions of antisera (black shapes) and strains (colored shapes) correspond to the adjusted distance of the HI measurement with the least error calculated by ACMACS. The size of the shape represents the confidence area in the position (0.5-U increase in total error). Black shapes represent antisera directed against strains A/California/04/2009 (CALIF04), A/Sw/Sarthe/0255/2010 (SARTHE), and A/Sw/Cotes d’Armor/0388/2009 (H1avN1). Shapes representing swine virus strains are colored as follows: blue for H1N1pdm strains from the SeL group, red for H1N1pdm strains from the SwD group, green for other H1N1pdm strains from clade 7, yellow for H1N1pdm strains from clade 3, and gray for the H1avN1 reference strain. The SeL and SwD genogroups were defined in this study. Correspondences between numbers and strain names are given in Table 5.
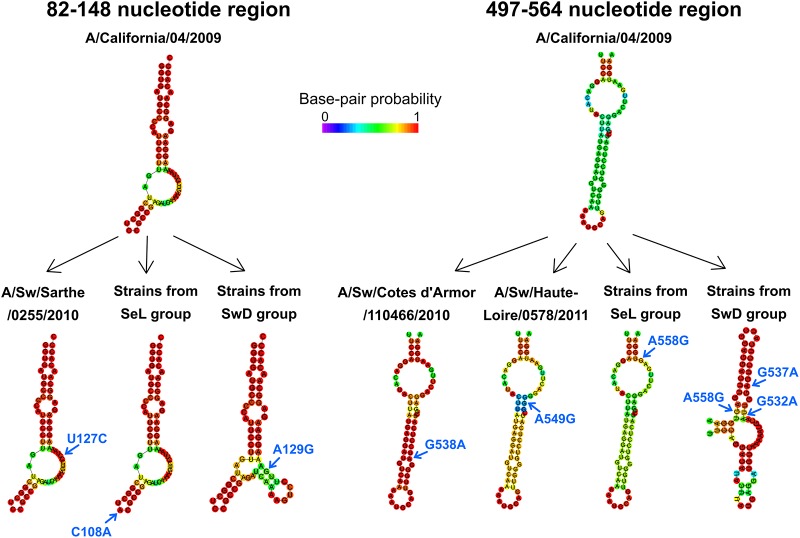
Swine H1N1pdm strains belonging to the SwD group diverge from the H1N1pdm lineage-specific pattern of the predicted RNA secondary structure in the NS segment.
As it was reported previously that the regions spanning positions 82 to 148 and 497 to 564 in the positive-sense RNA of the NS segment would display IAV lineage-specific patterns in predicted RNA secondary structures, we compared the predicted NS secondary RNA structures of swine H1N1pdm strains isolated in France to those of reference strain A/California/04/2009 (37). As previously shown, NS RNA secondary structures of IAVs from A/California/04/2009 fold into stem-loop structures with internal loops and bulges (Fig. 10), resembling those found for swIAVs from North American triple-reassortant and/or classical swine lineages (37). French swine H1N1pdm strains from 2009 to 2011 harbored substitutions in the region spanning positions 82 to 148 or 497 to 564, such as U127C in A/Sw/Sarthe/0255/2010 and A/Sw/Sarthe/0262/2010, G538A in A/Sw/Cotes d’Armor/110466/2010, or A549G in A/Sw/Haute-Loire/0578/2011, but none of these substitutions appear to influence the RNA secondary structures (Fig. 10). Also, the two substitutions C108A and A558G fixed in more-recent strains belonging to the SeL group did not provide any modification of the predicted RNA structure. In contrast, substitution A129G observed in 3/5 strains from the SwD group, as well as substitutions G537A and G532A harbored by all strains from this genogroup, led to a change in predicted structures with the formation of multibranch stem-loops in these two regions (Fig. 10).
FIG 10.
RNA secondary structures predicted in two regions of the NS segment suspected to be lineage-specific regions. Only strains showing nucleotide substitutions compared to reference strain A/California/04/2009 are presented. Strains A/Sw/France/12-160129/2016 and A/Sw/France/01-150203/2015 were used to represent the predicted RNA secondary structures of strains belonging to the SeL and SwD genogroups, respectively. Nucleotide substitutions are indicated by blue arrows.
DISCUSSION
In the present study, we investigated the diversity and evolution of H1N1pdm viruses isolated in pigs in France from 2009 to 2017. Interestingly, this molecular epidemiological survey led to the classification of some recent strains within a new phylogenetic group which appears to be swine specific.
The first case of H1N1pdm infection in a French swine herd was recorded in February 2010 at the time of the pandemic, as in other pig populations elsewhere in Europe and worldwide (12–14, 38–40). Following the pandemic, the H1N1pdm virus adopted a seasonal epidemic pattern in humans (11). However, H1N1pdm outbreaks in pigs were again reported in fall 2010, before the 2010–2011 seasonal epidemic started in humans, leading to the hypothesis that the virus had circulated endemically among swine since its introduction during the pandemic, as demonstrated at the European level (14). Rapid and easy adaptation to the species after human-to-pig transmission was sustained by the swine origin of the H1N1pdm virus itself (41). In addition to intraspecies transmission, it is thought that separate human-to-swine H1N1pdm transmissions also occurred in France almost every year at the time of the seasonal epidemics, as reported at the European level (14). Therefore, both intra- and interspecies transmissions may have increased the annual proportions of H1N1pdm strains detected in French pigs among other swIAVs, gradually from 2010 to 2016 and regardless of the season.
Interestingly, in the northwestern part of France, which is the area with the highest pig density and where 50% of herds were known to be infected with European H1avN1 and H1huN2 swIAVs for years before 2009 (42–44), a small number of H1N1pdm cases were detected among swIAV-infected herds. The preexisting swIAV immunity, cross-reacting with H1N1pdm, most probably reduced the transmission rate and the potential introduction success of the novel H1 antigenic variant in this area (36, 45). This would explain why whole H1N1pdm virus seemed to circulate preferentially in French areas less affected by other swIAV infections. This is also in accordance with the fact that we did not detect any reassortant virus bearing the HA and/or NA gene of the H1N1pdm virus in another swIAV backbone, whereas in several other European countries, such reassortants have become novel enzootic viruses early after the pandemic (13, 14, 46–48). However, novel swIAVs bearing one or more H1N1pdm internal genes in another swIAV backbone were first detected in 2014 and could further contribute to the dissemination of H1N1pdm genes throughout the country (data not shown).
Most H1N1pdm strains isolated in pigs in France from 2010 to 2013 exhibited very high similarity with contemporary seasonal human strains, and current phylogenetic reconstructions were not able to easily distinguish viruses circulating in swine from viruses newly transmitted from humans. However, the nucleotide mutations that accumulated and fixed in swine viruses identified in France, compared to the initial pandemic strain, provided evidence of the divergence of two phylogenetic groups from 2015, which we have called the SeL and SwD groups. The divergence was thought to have occurred around 2011, suggesting that the gap resulted from limited passive surveillance and/or subclinical infections. An active surveillance plan would be necessary to better approach the real distribution of SwD strains in France. However, as these strains were detected in several distant farms and over at least 2 years, it can be hypothesized that they do not constitute a transitory emergence. The SeL group included both swine and human strains isolated in France together with other swine and human strains identified elsewhere worldwide, while the SwD group contained only swine H1N1pdm strains isolated in the southern half of France. At the time of these analyses, no other swine H1N1pdm virus could be classified with these French strains in the SwD group. However, this specificity in France should be further confirmed, as there were only seven swine H1N1pdm strains isolated in other European countries from 2014 to 2017 that are available in public databases.
Strains from the SwD group accumulated a high number of mutations that were rarely or never observed in H1N1pdm viruses previously. They were located in key regions, such as the nuclear localization signals (NLS), cap-binding areas, or regions of interaction between polymerase proteins, mutations that could alter virus replication efficiency and/or virulence. Only one residue, i.e., NP-53E, was previously reported to be related to swine-specific adaptation because of its high frequency in swine isolates and its total absence in human strains (34, 35). For the other 12 mutations never observed in swine H1N1pdm to date, experimental investigations would be needed to evaluate their involvement in host adaptation and virus functional properties. While several mutations occurred in known antigenic sites, cross-HI tests and related antigenic cartography showed that they did not cause antigenic drift in strains from the SwD group, compared to those from the SeL group, those from the 2009–2013 period of time, and A/California/04/2009. However, if strains belonging to the SwD group further persist in the pig population in France, and/or in other pig populations in Europe or elsewhere, we could expect that they will continue to diverge from seasonal H1N1pdm strains circulating in humans and/or from other H1N1pdm viruses infecting pigs. This type of divergent evolution may lead to the emergence of viruses that would no longer be covered by human vaccines and/or vaccines specifically developed for pig protection, based on antigens closely related to the original H1N1pdm virus.
Interestingly, all strains from the SwD group also fixed nucleotide mutations that influenced the two lineage-specific regions of the NS segment RNA secondary structure, sustaining divergence within the H1N1pdm lineage. Although no specific clinical sign and/or increase in the severity of respiratory disease in pigs was associated with infections with SwD group strains, it would be informative to further investigate potential group-specific phenotypic and/or functional characteristics. RNA secondary structure modification could indeed impact the splicing of NS mRNAs and subsequently regulate the expression of NS1 and NS2, two proteins that have important functions in the inhibition of host antiviral gene expression and/or in viral replication (37, 49, 50).
Altogether, these results confirmed the importance of encouraging the surveillance of IAVs in swine and the need to develop further comparative studies on H1N1pdm evolution in both humans and swine, as a novel H1N1pdm swine-specific lineage could present an increased risk to both human and swine health, either as a whole H1N1pdm virus or in further reassortant viruses that will acquire genes from this novel genogroup.
MATERIALS AND METHODS
Biological samples.
Nasal swabs (MW950Sent2mL Virocult; Kitvia, Labarthe-Inard, France) were collected from pigs in France by veterinary practitioners, veterinarians from medical companies, or personnel from the French Agency for Food, Environmental, and Occupational Health and Safety (ANSES), from 2009 to 2017, in the context of diagnostic, passive surveillance or epidemiological investigations in cases of outbreaks of acute respiratory disease. Nasal swabs were mixed vigorously, and the supernatants were stored at −70°C until virological analysis.
H1N1pdm detection and subtyping.
Initial screening for the influenza A virus (IAV) genome was performed by local veterinary laboratories, by the French National Reference Laboratory for Swine Influenza (Ploufragan, France), or by the Friedrich Loeffler Institute (Greifswald-Insel Riems, Germany). It was carried out by a real-time RT-PCR assay targeting the matrix (M) gene, using either the LSI VetMAX swine influenza A/H1N1/2009 kit (Life Technologies, Carlsbad, CA, USA), the Adiavet SIV real-time kit (Bio-X Diagnostics, Rochefort, Belgium), or in-house methods (51, 52). Viral RNA extraction from 200 µl of nasal swab supernatants as well as an amplification step were performed according to the manufacturer’s instructions. RNA extracts that were found to be positive for the IAV M gene were then subjected to real-time RT-PCR assays developed for the specific identification of the HA and NA genes from the H1N1pdm virus in swine samples (51, 52). The LSI VetMAX swine influenza A/H1N1/2009-H1 detection kit (Life Technologies, Carlsbad, CA, USA) and the Adiavet A/H1N1 (2009) real-time kit (Bio-X Diagnostics, Rochefort, Belgium) were used interchangeably to amplify the H1pdm gene, whereas the LSI VetMAX swine influenza A/H1N1/2009-N1 detection kit (Life Technologies, Carlsbad, CA, USA) was used to amplify the N1pdm gene (51). In-house RT-PCR assays specific for the HA or NA genes from other swine IAVs circulating in France and/or in Europe were run in parallel to identify either swine IAVs other than H1N1pdm, reassortant viruses, or virus mixtures (52, 53).
Virus isolation.
Clinical samples positive for the H1pdm and/or N1pdm genes were subjected to virus propagation on Madin-Darby canine kidney (MDCK) cells, according to standard procedures (54). For swine antiserum production and antigenic characterization, reference viruses and/or swine H1N1pdm isolates obtained on MDCK cells were further propagated in 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs. Harvested cell culture supernatants and allantoic fluids were clarified by centrifugation and stored at −70°C until sequencing, pig inoculation, or hemagglutination inhibition (HI) tests.
Sequencing.
Viral RNA was extracted from MDCK cell-propagated H1N1pdm viruses using the NucleoSpin RNA kit (Macherey-Nagel, Düren, Germany). The RNA sequences were obtained by either the Sanger method or next-generation sequencing (NGS) (accession numbers are given in Table 2). For Sanger sequencing, each viral segment was sequenced in both senses after being amplified in a two-step RT-PCR using SuperScript II reverse transcriptase and Platinum Taq high-fidelity DNA polymerase (Invitrogen, Saint-Aubin, France), according to the manufacturer’s instructions. Sequences of forward and reverse primers designed and/or used to amplify each H1N1pdm genomic segment are available upon request. The sequences obtained were manually cleaned and assembled with Vector NTI Advance 11.0 software (Invitrogen, Life Technologies). For NGS sequencing, some viruses (labeled “NGS1” in Table 2) were sequenced at the Sanger Institute, Cambridge, United Kingdom, within European coordinated action ESNIP3 (European Surveillance Network for Influenza in Pigs 3), as previously described (14). Briefly, viral RNA was amplified by using 8-segment RT-PCR, followed by whole-genome sequencing on an FLX Titanium XL genome sequencer (Roche/454 Life Sciences, Branford, CT, USA) or a MiSeq instrument (Illumina, San Diego, CA, USA), using a 150-bp paired-end reagent kit. The reads obtained were de novo assembled using IVA version 0.8.1 and also against a reference sequence using SMALT version 0.7.4. Other viruses (labeled “NGS2” in Table 2) were sequenced on the ANSES (Ploufragan, France) NGS platform. The cDNA libraries were prepared using Ion Total RNA-Seq kit v2 (Life Technologies, Carlsbad, CA, USA) and sequenced on an Ion Proton instrument (Life Technologies). The resulting reads were cleaned and first assembled by mapping on reference genomes using Burrows-Wheeler Aligner software (version 0.7.15-r1140) and in addition were de novo assembled using the SPAdes and MIRA programs (versions v3.10.0 and 4.0.2). The contigs produced by de novo methods were assembled and compared to the alignment on reference genomes to generate a single consensus sequence per viral segment using Vector NTI Advance 11.0 software.
Phylogenetic analyses.
The sequences obtained in this study were compared to sequences retrieved from the Influenza Research Database (IRD) (available at https://www.fludb.org/). IRD sequences were selected from the more similar sequences identified from BLAST searches, taking into account their host species origin, i.e., both human and swine H1N1pdm strains, and their geographical location. Sequences from H1N1pdm strains obtained in cats were also selected, irrespective of their percent identity to French animal strains. For each RNA segment, coding region sequences were aligned in Seaview with the MUSCLE algorithm. The eight alignments were then concatenated in order to build a whole-genome tree and improve the estimation of the dates of divergence between the different clusters. Phylogenetic trees were generated using a Bayesian approach using BEAST v1.8.4. For each individual or concatenated alignment, we performed a maximum likelihood analysis with MEGA 7.0 to select the best-fit substitution model according to the Bayesian information criterion (BIC). The Hasegawa-Kishino-Yano (HKY) model was thus chosen for the phylogeny of the PB2, PB1, NP, M, and NS segments, and the HKY+gamma model was chosen for the other segments (PA, HA, and NA) and for the concatenated tree. Codon positions and collection dates were also incorporated into the BEAST models to improve the phylogenies (55). A Markov chain Monte Carlo (MCMC) run of 10,000,000 generations sampled every 1,000 was performed. The quality of the posterior distribution of each setting was checked through the effective sample size (ESS) with Tracer v1.6. Trees were summarized with TreeAnnotator v1.8.4 and visualized with FigTree v1.4.3.
Amino acid sequence analyses.
DNA sequences were translated into amino acid sequences and compared to the amino acid sequences of the reference strain A/California/04/2009 in Seaview. The frequency of the appearance of each amino acid residue in proteins from H1N1pdm strains isolated in swine was compared to that observed in human strains in order to determine whether some mutations could reflect an adaptation of the virus to the swine host. To do this, amino acid sequences of H1N1pdm virus strains isolated from humans and swine on each continent were downloaded from the IRD using the options “H1N1 subtype,” “all complete segments,” and “2009 pH1N1 sequence only.” Due to the low number of whole genomes from swine H1N1pdm viruses that were available in the IRD, swine H1N1pdm strains registered on the GISAID platform (https://www.gisaid.org/) were added. Protein sequences were analyzed with R 3.4, using the “seqinr” and “plyr” packages. After removal of duplicated sequences representing single strains, identical sequences from several strains were grouped into alleles in order to calculate the frequency of alleles sharing the specified residues.
Antigenic characterization and cartography.
Swine antisera to H1N1pdm and other swIAVs of European H1avN1, H3N2, and H1huN2 lineages, all selected by the French NRL as reference strains (13), were produced in SPF pigs at ANSES facilities. Briefly, 9-week-old SPF pigs were inoculated intranasally with 107 to 108 50% embryo-lethal doses (ELD50) of live virus (in a volume of 4 ml [2 ml per nostril]). Three weeks later, the pigs were inoculated intramuscularly with the same dose of virus diluted (volume/volume) in the adjuvant Montanide ISA206 (Seppic, Givaudan-Lavirotte, France) (total volume of 3 ml). An exception was made for the A/California/04/2009 reference strain, which was inoculated twice intramuscularly with the adjuvant, as it was chemically inactivated. Antisera were collected 2 weeks after the second immunization. Experiments were performed in accordance with the animal welfare experimentation recommendations drawn up by the Direction Départementale de la Protection des Populations des Côtes d’Armor (Departmental Directorate for Protection of the Population) (ANSES registration no. C-22-745-1). They were approved by the French national committee for ethics in animal experimentation (ANSES/ENVA/UPEC) (approval no. 15/02/11-5 and 12/07/16-1) and authorized by the French Ministry for Research. Prior to HI testing, swine antisera were treated with a receptor-destroying enzyme (Sigma-Aldrich, St. Louis, MO, USA) and then heat inactivated at 56°C for 30 min and adsorbed onto 50% chicken red blood cells (RBCs) to remove nonspecific inhibitors of hemagglutination. HI assays were performed by testing reference antisera against H1N1pdm viruses isolated from pigs and a cat in France, according to standard procedures (54). Reference viruses used to generate antisera were also included as controls. Briefly, 4 hemagglutinating units (HAU) of egg-propagated virus were incubated with two 2-fold reference serum dilutions (starting at a dilution of 1:10) and tested against 0.5% chicken RBCs. HI titers were expressed as the reciprocal of the highest dilution inhibiting 4 HAU of virus. Titers of ≥20 were considered positive. Next, the antigenic distances between the different H1N1pdm viruses isolated in mammalian animals in France were mapped, using an antigenic cartography method previously described for human and swine IAVs (31). The antigenic map was built in two dimensions (2D) using ACMACS (University of Cambridge, UK [https://acmacs-web.antigenic-cartography.org/]). Distances for antigenic dimensions are measured in antigenic units (AU), and each unit is equivalent to a 2-fold dilution in HI assay data.
RNA secondary structure prediction.
RNA secondary structures of NS segments were predicted using the RNAfold Web server (http://rna.tbi.univie.ac.at/).
Accession number(s).
The generated nucleotide sequences are available in GenBank. Their accession numbers are reported in Table 2.
Supplementary Material
ACKNOWLEDGMENTS
We thank all farmers and veterinarians who sent biological samples for influenza virus monitoring and subsequent evolution studies. More broadly, we are indebted to members of Résavip, the French national network for surveillance of influenza A viruses in pigs, i.e., the French Ministry of Agriculture (DGAl) and its regional services (SRALs), Coop de France, the Société Nationale des Groupements Techniques Vétérinaires (SNGTV), Groupements de Défense Sanitaire (GDS), the Association Française des Directeurs et Cadres des Laboratoires Publics Vétérinaires d’Analyses (ADILVA), the local veterinary laboratories approved by DGAl to perform swIAV detection, and the National Reference Laboratory for Swine Influenza; to the Plateforme Nationale de Surveillance Épidémiologique en Santé Animale (ESA) for sustaining Résavip operations; to Mérial (Lyon, France); to IDT Biologika GmbH (Dessau-Rosslau, Germany); and to colleagues from the Swine Epidemiological and Welfare Unit, ANSES (Ploufragan, France). We are grateful to colleagues from the SPF Pig Production and Experimentation Unit, ANSES (Ploufragan, France), for antiserum production in SPF pigs. We also thank the French National Influenza Centres located at the Institut Pasteur (Paris, France) and Lyon University (Lyon, France) and the Réseau des Groupes Régionaux d’Observation de la Grippe (GROG network) for sharing human data through public databases.
This study was partially conducted under ANSES/FLI collaboration agreement P610B. It was partially funded by European FP7 project ESNIP3 (no. 259949), by the CoVetLab joint research project 2016 Genetic Characterization of Swine Influenza A Viruses in European Pigs by Next-Generation Sequencing (no. CF0010), and by the European Union’s Horizon 2020 program COMPARE (no. 643476).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00988-18.
REFERENCES
- 1.Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog 9:e1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, NCIRD. 2017. Influenza type A viruses. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm. [Google Scholar]
- 4.Schrauwen EJ, Fouchier RA. 2014. Host adaptation and transmission of influenza A viruses in mammals. Emerg Microbes Infect 3:e9. doi: 10.1038/emi.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taubenberger JK, Kash JC. 2010. Influenza virus evolution, host adaptation and pandemic formation. Cell Host Microbe 7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 7.Girard MP, Tam JS, Assossou OM, Kieny MP. 2010. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 28:4895–4902. doi: 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Babakir-Mina M, Dimonte S, Perno CF, Ciotti M. 2009. Origin of the 2009 Mexico influenza virus: a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch Virol 154:1349–1352. doi: 10.1007/s00705-009-0438-1. [DOI] [PubMed] [Google Scholar]
- 9.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, De Graaf M, Burke DF, Fouchier RAM, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 11.Su YC, Bahl J, Joseph U, Butt KM, Peck HA, Koay ES, Oon LL, Barr IG, Vijaykrishna D, Smith GJ. 2015. Phylodynamics of H1N1/2009 influenza reveals the transition from host adaptation to immune-driven selection. Nat Commun 6:7952. doi: 10.1038/ncomms8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasma T, Joseph T. 2010. Pandemic (H1N1) 2009 infection in swine herds, Manitoba, Canada. Emerg Infect Dis 16:706–708. doi: 10.3201/eid1604.091636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon G, Larsen LE, Durrwald R, Foni E, Harder T, Van Reeth K, Markowska-Daniel I, Reid SM, Dan A, Maldonado J, Huovilainen A, Billinis C, Davidson I, Aguero M, Vila T, Herve S, Breum SO, Chiapponi C, Urbaniak K, Kyriakis CS, Brown IH, Loeffen W. 2014. European surveillance network for influenza in pigs: surveillance programs, diagnostic tools and swine influenza virus subtypes identified in 14 European countries from 2010 to 2013. PLoS One 9:e115815. doi: 10.1371/journal.pone.0115815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson SJ, Langat P, Reid SM, Lam TT, Cotten M, Kelly M, Van Reeth K, Qiu Y, Simon G, Bonin E, Foni E, Chiapponi C, Larsen L, Hjulsager C, Markowska-Daniel I, Urbaniak K, Durrwald R, Schlegel M, Huovilainen A, Davidson I, Dan A, Loeffen W, Edwards S, Bublot M, Vila T, Maldonado J, Valls L, Brown IH, Pybus OG, Kellam P. 2015. Molecular epidemiology and evolution of influenza viruses circulating within European swine between 2009 and 2013. J Virol 89:9920–9931. doi: 10.1128/JVI.00840-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajao DS, Anderson TK, Kitikoon P, Stratton J, Lewis NS, Vincent AL. 2018. Antigenic and genetic evolution of contemporary swine H1 influenza viruses in the United States. Virology 518:45–54. doi: 10.1016/j.virol.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang J, Qiao C, Yang H, Zhang Y, Xin X, Chen H. 2013. Co-circulation of pandemic 2009 H1N1, classical swine H1N1 and avian-like swine H1N1 influenza viruses in pigs in China. Infect Genet Evol 13:331–338. doi: 10.1016/j.meegid.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Mathieu C, Moreno V, Retamal P, Gonzalez A, Rivera A, Fuller J, Jara C, Lecocq C, Rojas M, Garcia A, Vasquez M, Agredo M, Gutierrez C, Escobar H, Fasce R, Mora J, Garcia J, Fernandez J, Ternicier C, Avalos P. 2010. Pandemic (H1N1) 2009 in breeding turkeys, Valparaiso, Chile. Emerg Infect Dis 16:709–711. doi: 10.3201/eid1604.091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sponseller BA, Strait E, Jergens A, Trujillo J, Harmon K, Koster L, Jenkins-Moore M, Killian M, Swenson S, Bender H, Waller K, Miles K, Pearce T, Yoon KJ, Nara P. 2010. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerg Infect Dis 16:534–537. doi: 10.3201/eid1603.091737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohr CV, DeBess EE, Baker RJ, Hiett SL, Hoffman KA, Murdoch VJ, Fischer KA, Mulrooney DM, Selman RL, Hammill-Black WM. 2010. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Vet Pathol 47:378–386. doi: 10.1177/0300985810368393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campagnolo ER, Moll ME, Tuhacek K, Simeone AJ, Miller WS, Waller KO, Simwale O, Rankin JT, Ostroff SM. 2013. Concurrent 2009 pandemic influenza A (H1N1) virus infection in ferrets and in a community in Pennsylvania. Zoonoses Public Health 60:117–124. doi: 10.1111/j.1863-2378.2012.01503.x. [DOI] [PubMed] [Google Scholar]
- 21.Campagnolo ER, Rankin JT, Daverio SA, Hunt EA, Lute JR, Tewari D, Acland HM, Ostrowski SR, Moll ME, Urdaneta VV, Ostroff SM. 2011. Fatal pandemic (H1N1) 2009 influenza A virus infection in a Pennsylvania domestic cat. Zoonoses Public Health 58:500–507. doi: 10.1111/j.1863-2378.2011.01390.x. [DOI] [PubMed] [Google Scholar]
- 22.Ducatez MF, Hause B, Stigger-Rosser E, Darnell D, Corzo C, Juleen K, Simonson R, Brockwell-Staats C, Rubrum A, Wang D, Webb A, Crumpton JC, Lowe J, Gramer M, Webby RJ. 2011. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg Infect Dis 17:1624–1629. doi: 10.3201/1709.110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Ji J, Xie Q, Wang J, Shang H, Chen C, Chen F, Xue C, Cao Y, Ma J, Bi Y. 2011. Isolation and complete genomic characterization of H1N1 subtype swine influenza viruses in southern China through the 2009 pandemic. Virol J 8:129. doi: 10.1186/1743-422X-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremblay D, Allard V, Doyon JF, Bellehumeur C, Spearman JG, Harel J, Gagnon CA. 2011. Emergence of a new swine H3N2 and pandemic (H1N1) 2009 influenza A virus reassortant in two Canadian animal populations, mink and swine. J Clin Microbiol 49:4386–4390. doi: 10.1128/JCM.05676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajao DS, Walia RR, Campbell B, Gauger PC, Janas-Martindale A, Killian ML, Vincent AL. 2017. Reassortment between swine H3N2 and 2009 pandemic H1N1 in the United States resulted in influenza A viruses with diverse genetic constellations with variable virulence in pigs. J Virol 91:e01763-16. doi: 10.1128/JVI.01763-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindstrom S, Garten R, Balish A, Shu B, Emery S, Berman L, Barnes N, Sleeman K, Gubareva L, Villanueva J, Klimov A. 2012. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 18:834–837. doi: 10.3201/eid1805.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowman AS, Sreevatsan S, Killian ML, Page SL, Nelson SW, Nolting JM, Cardona C, Slemons RD. 2012. Molecular evidence for interspecies transmission of H3N2pM/H3N2v influenza A viruses at an Ohio agricultural fair, July 2012. Emerg Microbes Infect 1:e33. doi: 10.1038/emi.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrauwen EJ, Herfst S, Chutinimitkul S, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Kuiken T, Fouchier RA. 2011. Possible increased pathogenicity of pandemic (H1N1) 2009 influenza virus upon reassortment. Emerg Infect Dis 17:200–208. doi: 10.3201/eid1702.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Zhou B, Fan X, Lam TT, Wang J, Chen A, Chen X, Chen H, Webster RG, Webby R, Peiris JS, Smith DK, Guan Y. 2011. Novel reassortment of Eurasian avian-like and pandemic/2009 influenza viruses in swine: infectious potential for humans. J Virol 85:10432–10439. doi: 10.1128/JVI.05352-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Qin K, Wang J, Pu J, Tang Q, Hu Y, Bi Y, Zhao X, Yang H, Shu Y, Liu J. 2011. High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proc Natl Acad Sci U S A 108:4164–4169. doi: 10.1073/pnas.1019109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis NS, Russell CA, Langat P, Anderson TK, Berger K, Bielejec F, Burke DF, Dudas G, Fonville JM, Fouchier RA, Kellam P, Koel BF, Lemey P, Nguyen T, Nuansrichy B, Peiris JM, Saito T, Simon G, Skepner E, Takemae N, Webby RJ, Van Reeth K, Brookes SM, Larsen L, Watson SJ, Brown IH, Vincent AL. 2016. The global antigenic diversity of swine influenza A viruses. Elife 5:e12217. doi: 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson TK, Macken CA, Lewis NS, Scheuermann RH, Van Reeth K, Brown IH, Swenson SL, Simon G, Saito T, Berhane Y, Ciacci-Zanella J, Pereda A, Davis CT, Donis RO, Webby RJ, Vincent AL. 2016. A phylogeny-based global nomenclature system and automated annotation tool for H1 hemagglutinin genes from swine influenza A viruses. mSphere 1:e00275-16. doi: 10.1128/mSphere.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson M, Spiro D, Wentworth D, Beck E, Fan J, Ghedin E, Halpin R, Bera J, Hine E, Proudfoot K, Stockwell T, Lin X, Griesemer S, Kumar S, Bose M, Viboud C, Holmes E, Henrickson K. 2009. The early diversification of influenza A/H1N1pdm. PLoS Curr 1:RRN1126 http://currents.plos.org/influenza/article/the-early-diversification-of-influenza-ah1n1pdm/pdf/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali A, Khatri M, Wang L, Saif YM, Lee C-W. 2012. Identification of swine H1N2/pandemic H1N1 reassortant influenza virus in pigs, United States. Vet Microbiol 158:60–68. doi: 10.1016/j.vetmic.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Liang H, Lam TT, Fan X, Chen X, Zeng Y, Zhou J, Duan L, Tse M, Chan CH, Li L, Leung TY, Yip CH, Cheung CL, Zhou B, Smith DK, Poon LL, Peiris M, Guan Y, Zhu H. 2014. Expansion of genotypic diversity and establishment of 2009 H1N1 pandemic-origin internal genes in pigs in China. J Virol 88:10864–10874. doi: 10.1128/JVI.01327-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyriakis CS, Olsen CW, Carman S, Brown IH, Brookes SM, Doorsselaere JV, Reeth KV. 2010. Serologic cross-reactivity with pandemic (H1N1) 2009 virus in pigs, Europe. Emerg Infect Dis 16:96–99. doi: 10.3201/eid1601.091190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasin AV, Petrova AV, Egorov VV, Plotnikova MA, Klotchenko SA, Karpenko MN, Kiselev OI. 2016. The influenza A virus NS genome segment displays lineage-specific patterns in predicted RNA secondary structure. BMC Res Notes 9:279. doi: 10.1186/s13104-016-2083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofshagen M, Gjerset B, Er C, Tarpai A, Brun E, Dannevig B, Bruheim T, Fostad I, Iversen B, Hungnes O, Lium B. 2009. Pandemic influenza A (H1N1)v: human to pig transmission in Norway? Euro Surveill 14(45):pii=19406 https://www.eurosurveillance.org/content/10.2807/ese.14.45.19406-en. [DOI] [PubMed] [Google Scholar]
- 39.Pereda A, Cappuccio J, Quiroga MA, Baumeister E, Insarralde L, Ibar M, Sanguinetti R, Cannilla ML, Franzese D, Escobar Cabrera OE, Craig MI, Rimondi A, Machuca M, Debenedetti RT, Zenobi C, Barral L, Balzano R, Capalbo S, Risso A, Perfumo CJ. 2010. Pandemic (H1N1) 2009 outbreak on pig farm, Argentina. Emerg Infect Dis 16:304–307. doi: 10.3201/eid1602.091230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreta D, Tantawet S, Na Ayudhya SN, Thontiravong A, Wongphatcharachai M, Lapkuntod J, Bunpapong N, Tuanudom R, Suradhat S, Vimolket L, Poovorawan Y, Thanawongnuwech R, Amonsin A, Kitikoon P. 2010. Pandemic (H1N1) 2009 virus on commercial swine farm, Thailand. Emerg Infect Dis 16:1587–1590. doi: 10.3201/eid1610.100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson MI, Gramer MR, Vincent AL, Holmes EC. 2012. Global transmission of influenza viruses from humans to swine. J Gen Virol 93:2195–2203. doi: 10.1099/vir.0.044974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hervé S, Gorin S, Quéguiner S, Barbier N, Eveno E, Dorenlor V, Eono F, Madec F, Rose N, Simon G. 2011. Estimation de la séroprévalence des virus influenza chez le porc charcutier en France en 2008-2009 Journées Recherche Porcine 43:281–284. http://www.journees-recherche-porcine.com/texte/2011/sante/PS1.pdf. [Google Scholar]
- 43.Kyriakis CS, Brown IH, Foni E, Kuntz-Simon G, Maldonado J, Madec F, Essen SC, Chiapponi C, Van Reeth K. 2011. Virological surveillance and preliminary antigenic characterization of influenza viruses in pigs in five European countries from 2006 to 2008. Zoonoses Public Health 58:93–101. doi: 10.1111/j.1863-2378.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakis CS, Rose N, Foni E, Maldonado J, Loeffen WL, Madec F, Simon G, Van Reeth K. 2013. Influenza A virus infection dynamics in swine farms in Belgium, France, Italy and Spain, 2006-2008. Vet Microbiol 162:543–550. doi: 10.1016/j.vetmic.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Busquets N, Segales J, Cordoba L, Mussa T, Crisci E, Martin-Valls GE, Simon-Grife M, Perez-Simo M, Perez-Maillo M, Nunez JI, Abad FX, Fraile L, Pina S, Majo N, Bensaid A, Domingo M, Montoya M. 2010. Experimental infection with H1N1 European swine influenza virus protects pigs from an infection with the 2009 pandemic H1N1 human influenza virus. Vet Res 41:74. doi: 10.1051/vetres/2010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard WA, Essen SC, Strugnell BW, Russell C, Barass L, Reid SM, Brown IH. 2011. Reassortant pandemic (H1N1) 2009 virus in pigs, United Kingdom. Emerg Infect Dis 17:1049–1052. doi: 10.3201/eid1706.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starick E, Lange E, Grund C, Grosse Beilage E, Dohring S, Maas A, Noe T, Beer M, Harder TC. 2012. Reassortants of pandemic influenza A virus H1N1/2009 and endemic porcine HxN2 viruses emerge in swine populations in Germany. J Gen Virol 93:1658–1663. doi: 10.1099/vir.0.042648-0. [DOI] [PubMed] [Google Scholar]
- 48.Krog JS, Hjulsager CK, Larsen MA, Larsen LE. 2017. Triple-reassortant influenza A virus with H3 of human seasonal origin, NA of swine origin, and internal A(H1N1) pandemic 2009 genes is established in Danish pigs. Influenza Other Respir Viruses 11:298–303. doi: 10.1111/irv.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang X, Zheng M, Wang P, Mok BW-Y, Liu S, Lau S-Y, Chen P, Liu Y-C, Liu H, Chen Y, Song W, Yuen K-Y, Chen H. 2017. An NS-segment exonic splicing enhancer regulates influenza A virus replication in mammalian cells. Nat Commun 8:14751. doi: 10.1038/ncomms14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemeroff ME, Barabino SML, Li Y, Keller W, Krug RM. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol Cell 1:991–1000. doi: 10.1016/S1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- 51.Pol F, Quéguiner S, Gorin S, Deblanc C, Simon G. 2011. Validation of commercial real-time RT-PCR kits for detection of influenza A viruses in porcine samples and differentiation of pandemic (H1N1) 2009 virus in pigs. J Virol Methods 171:241–247. doi: 10.1016/j.jviromet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Henritzi D, Zhao N, Starick E, Simon G, Krog JS, Larsen LE, Reid SM, Brown IH, Chiapponi C, Foni E, Wacheck S, Schmid P, Beer M, Hoffmann B, Harder TC. 2016. Rapid detection and subtyping of European swine influenza viruses in porcine clinical samples by haemagglutinin- and neuraminidase-specific tetra- and triplex real-time RT-PCRs. Influenza Other Respir Viruses 10:504–517. doi: 10.1111/irv.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonin E, Quéguiner S, Woudstra C, Gorin S, Barbier N, Harder TC, Fach P, Hervé S, Simon G. 2018. Molecular subtyping of European swine influenza viruses and scaling to high-throughput analysis. Virol J 15:7. doi: 10.1186/s12985-018-0920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.OIE. 2015. Chapter 2.8.7, Influenza A virus of swine In Manual of diagnostic tests and vaccines for terrestrial animals 2018, 8th ed, vol 1 OIE, Paris, France: http://www.oie.int/en/standard-setting/terrestrial-manual/access-online/. [Google Scholar]
- 55.Shapiro B, Rambaut A, Drummond AJ. 2006. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol Biol Evol 23:7–9. doi: 10.1093/molbev/msj021. [DOI] [PubMed] [Google Scholar]
- 56.Agreste. 2010. Principaux cheptels—nombre d’élevages et cheptel. SSP Ministère de l’Agriculture et de l’Alimentation, Paris, France: http://agreste.agriculture.gouv.fr/enquetes/structure-des-exploitations-964/recensement-agricole-2010/resultats-donnees-chiffrees/. [Google Scholar]
- 57.Sriwilaijaroen N, Suzuki Y. 2012. Molecular basis of the structure and function of H1 hemagglutinin of influenza virus. Proc Jpn Acad Ser B Phys Biol Sci 88:226–249. doi: 10.2183/pjab.88.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.