Abstract
A preferred reporting items for systematic reviews and meta-analyses-compliant meta-analysis was conducted to test the association of metabolic syndrome and its components with the risk of chronic obstructive pulmonary disease (COPD) based on observational studies. Literature retrieval, article selection and data extraction were done by two researchers independently. Total 16 articles (20 independent studies) were analyzed with 3915 COPD patients and 25,790 control participants. Overall analysis indicated that metabolic syndrome was significantly associated with 1.53-fold (95% confidence interval [CI]: 1.23–1.9, P<0.001) increased risk of COPD, with moderate heterogeneity (I2 = 74.3%). Of four metabolic components, hypertension was significantly associated with 1.55-fold (95% CI: 1.14–2.11, P=0.005) increased risk, and averaged levels of systolic blood pressure (weighted mean difference [WMD] = 3.626 mmHg, 95% CI: 1.537–5.714, P<0.001) and glucose (WMD = 2.976 mmol/l, 95% CI: 0.141–5.812; P=0.04) were significantly higher in COPD patients than in control participants, yet that of body mass index (WMD = −1.463 kg/m2, 95% CI: −2.716 to −0.211, P=0.022) were significantly lower. Gender, race, source of control participants, matched status and sample size were identified as accountable factors for significant heterogeneity. Altogether, the presence of metabolic syndrome, especially its component hypertension, was associated with significantly increased risk of COPD.
Keywords: association, chronic obstructive pulmonary disease, metabolic syndrome, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disorder characterized by airflow obstruction. COPD is the leading cause of mortality worldwide, as it afflicts approximately 174.5 million adults [1] and leads to 3.2 million deaths [2] in 2015. The incidence rate of COPD is on the rise, mainly due to ambient air pollution, high cigarette smoking, childhood chronic cough, low awareness and the lack of early detection [3–6]. Hence, pre-identifying individuals at high-risk for COPD may result in improved prevention and early detection. A growing emphasis should be put on clinical epidemiology research and the translation of research findings into advances in screening and clinical care.
It is widely recognized that systemic inflammation plays a central role in the pathogenesis of COPD, yet the exact mechanism remains incompletely understood [7,8]. As a low-grade systemic inflammatory condition, metabolic syndrome has been acknowledged as a common comorbidity of COPD [9]. Some investigators claimed that metabolic syndrome was more frequent in COPD patients than healthy cohorts [10,11]; however, others failed to support this claim [12,13]. A recent systematic review was written by Cebron Lipovec and colleagues who summarized the prevalence of metabolic syndrome in COPD and found that 32% of COPD patients had metabolic syndrome, 2.0 per cent points significantly higher than control participants [14], yet they had not interrogated the contribution of metabolic syndrome to the risk of having COPD. However, literature retrieval failed to reveal any evidence on this risk prediction. To produce more information, we sought to undertake a meta-analysis to test the association of metabolic syndrome with COPD risk by pooling the results of 16 articles.
This meta-analysis of observational studies was conducted according to the guidelines reported in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [15] (PRISMA checklist in Supplementary Table S1).
Methods
Literature retrieval
Three public databases, PubMed, EMBASE and Google/Scholar, were reviewed prior to January 14, 2018 to retrieve eligible articles on the association between metabolic syndrome and COPD from the English-language literature. Terms used for literature retrieval were all MeSH terms, including (‘pulmonary disease, chronic obstructive’ OR ‘chronic obstructive pulmonary disease’) AND (‘metabolic syndrome’) AND (‘prevalence’ OR ‘comorbidity’). Literature search was additionally extended to the reference lists of retrieved articles to avoid possible missing hits.
Inclusion and exclusion criteria
Assessment of each article was based on both inclusion and exclusion criteria. Inclusion criteria that must be met simultaneously incorporated (i) original data analysis, (ii) either cross-sectional or nested case–control study design, (iii) clear diagnoses of metabolic syndrome and COPD, (iv) accessible number of cases with metabolic syndrome between COPD patients and control participants. Meanwhile, exclusion criteria incorporated (i) publication in form of abstracts due to inadequate data for a complete comparison, (ii) case reports or case series, (iii) systematic reviews or meta-analyses, (iv) lack of control participants.
Article selection
Selection process was completed independently by the two researchers (Hualing Yang and Xi Huang), who justified the eligibility of each article through reading title or abstract, and full-text if necessary. Any uncertainty or ambiguity, if exists, was discussed by the two researchers or adjudicated by a third researcher (Zhanxiang Wang), and if necessary original researchers were contacted for confirmation by e-mails. A consensus was reached at the end.
Data extraction
From each eligible article, the following data were extracted: the first author’s name, year of publication, study design, source of control participants, country where participants resided, onset age of COPD diagnosed, diagnostic criteria of COPD and metabolic syndrome, ascertainment of control participants, matched condition between patients and controls, sample size, age, gender, body mass index (BMI), waist circumstance, the percentage of metabolic syndrome in patients and controls, cigarette smoking, pack-year smoking history, alcohol drinking, physical activity, income level, education level, forced expiratory volume in one second (FEV1), FEV1 to forced vital capacity (FVC) ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), glucose, triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), hypertension, diabetes mellitus, dyslipidemia, lipid-lowing therapy, anti-hypertensive treatment and anti-diabetic treatment. If data were provided separately by gender, each was presented and analyzed individually. Data abstraction was independently completed by the two researchers (Hualing Yang and Xi Huang) according to a uniform design table, and data were computerizedly checked for uniformity between the two tables. Any divergence was solved by reviewing original context until a consensus was reached.
Quality assessment
The quality of each eligible study was assessed by the Newcastle–Ottawa Scale (NOS) for case–control studies, and the NOS is an ongoing collaboration between the Universities of Newcastle, Australia and Ottawa, Canada.
Statistical analysis
Risk effect estimates for metabolic syndrome and its categorical components in association with COPD were expressed as odds ratio (OR) and 95% confidence interval (95% CI) under a random-effects model by using the DerSimonian and Laird method [16]. Mean differences in BMI, waist circumstance, FEV1, FEV1 to FVC ratio, SBP, DBP, glucose, triglycerides, TC, HDLC and LDLC between COPD patients and controls were expressed as weighted mean difference (WMD) and 95% CI under a random-effects model.
The I2 statistic was calculated to quantify the magnitude of between-study heterogeneity, and statistical significance was reported if this statistic exceeds 50%. Subgroup analyses by diagnostic criteria of metabolic syndrome, gender, source of control participants, race, country development, matched status and sample size were carried out to see whether these grouping factors can account for between-study heterogeneity. Meta-regression analyses were also conducted to explore other sources of between-study heterogeneity.
Cumulative analysis was conducted to identify the effect of the first published study on the following publications, and to inspect the evolution of cumulated estimates over time. Influential analysis was also conducted to check the effect of any individual studies on the overall estimates.
Begg’s and filled funnel plots (visual appraisal of symmetry) and Egger’s tests at a significance level of 10% were used to assess the likelihood of publication bias. The number of theoretically missing studies with negative results or in small scales can be depicted in the filled funnel plot through the trim-and-fill method.
The STATA/SE version 14.1 (StataCorp LP, College Station, Texas) was used to manage data and figures. Study power was estimated using the PS Power and Sample Size Calculations version 3.0 [17].
Results
Eligible studies
In total, 674 articles were identified from three public databases using predefined key words, only 16 articles met our inclusion and exclusion criteria [10–13,18–29], and the selection process is illustrated in Supplementary Figure S1. Four articles that presented gender-specific data were treated separately [11,22,27,29], and so there were 20 independent studies in the final analysis, including 3915 COPD patients and 25,790 control participants. As for quality assessment, total NOS score ranged from 5 to 8, with a mean value of 6.31. The baseline characteristics of all eligible studies are shown in Table 1.
Table 1. The baseline characteristics of all eligible studies in this meta-analysis.
| Author | Year | Study design | Control source | Country | Matched | COPD diagnosis | MetS diagnosis | Sample size | Mean age (years) | Male gender | Mean BMI (kg/m2) | Mean FEV1 (%) | Mean FEV1 to FVC ratio | MetS | NOS score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |||||||||
| Gupta (ATP III) [10] | 2017 | Cross-sectional | Hospital | India | Yes | GOLD | ATP III | 90 | 45 | 0.689 | 0.578 | 23.29 | 22.59 | NA | NA | NA | NA | 0.156 | 0.000 | 6 | 53.1 | 54.5 |
| Gupta (IDF) [10] | 2017 | Cross-sectional | Hospital | India | Yes | GOLD | IDF | 90 | 45 | 0.689 | 0.578 | 23.29 | 22.59 | NA | NA | NA | NA | 0.333 | 0.000 | 6 | 53.1 | 54.5 |
| Waschki [18] | 2016 | Nested | Hospital | Germany | Yes | GOLD | IDF | 74 | 18 | 0.703 | 0.611 | 26.00 | 25.70 | 55.20 | 116.20 | 51.00 | 78.60 | 0.473 | 0.333 | 6 | 66.0 | 65.9 |
| Munoz-Esquerre [19] | 2016 | Nested | Hospital | Spain | NA | GOLD | JIS (2009) | 17 | 14 | 0.941 | 0.929 | 24.00 | 27.10 | 62.00 | 97.70 | 54.90 | 76.30 | 0.353 | 0.786 | 6 | 63.4 | 58.3 |
| Bozek [20] | 2016 | Cross-sectional | Population | Poland | Yes | GOLD | ICD-10 | 1084 | 1076 | 0.680 | 0.410 | 21.40 | 31.30 | 66.30 | 90.30 | NA | NA | 0.251 | 0.136 | 8 | 66.5 | 68.6 |
| Acharyya (ATP III) [21] | 2016 | Cross-sectional | Population | India | Yes | GOLD | ATP III | 77 | 77 | 0.740 | 0.740 | 23.00 | 24.00 | NA | NA | NA | NA | 0.442 | 0.312 | 7 | 60.0 | 60.0 |
| Acharyya (IDF) [21] | 2016 | Cross-sectional | Population | India | Yes | GOLD | IDF | 77 | 77 | 0.740 | 0.740 | 23.00 | 24.00 | NA | NA | NA | NA | 0.312 | 0.325 | 7 | 60.0 | 60.0 |
| Chung (Male) [22] | 2015 | Nested | Population | Korea | NA | GOLD | ATP III | 760 | 2346 | 1.000 | 1.000 | 23.50 | 24.30 | 77.10 | 96.30 | NA | NA | 0.295 | 0.264 | 6 | 64.5 | 53.2 |
| Chung (Female) [22] | 2015 | Nested | Population | Korea | NA | GOLD | ATP III | 279 | 3731 | 0.000 | 0.000 | 23.30 | 24.10 | 75.70 | 98.50 | NA | NA | 0.380 | 0.320 | 6 | 64.5 | 55.4 |
| Park [23] | 2014 | Cross-sectional | Population | U.S.A. | NA | GOLD | JIS (2009) | 94 | 3661 | 0.447 | 0.511 | 26.98 | 29.30 | 67.00 | 96.00 | 58.00 | 76.00 | 0.575 | 0.536 | 6 | 62.1 | 56.6 |
| Breyer [24] | 2014 | Cross-sectional | Population | Netherlands | NA | GOLD | IDF | 228 | 156 | 0.590 | 0.450 | 26.20 | 27.30 | 52.80 | 120.40 | 40.90 | 78.10 | 0.570 | 0.400 | 6 | 63.7 | 60.1 |
| Ozgen [25] | 2013 | Cross-sectional | Population | Turkey | Yes | GOLD | IDF | 50 | 40 | 0.900 | 0.850 | 27.20 | 27.60 | 46.30 | NA | 53.00 | NA | 0.440 | 0.300 | 8 | 61.3 | 58.4 |
| Hosny [26] | 2013 | Cross-sectional | Hospital | Egypt | Yes | GOLD | ATP III | 50 | 35 | 0.880 | 0.914 | 27.00 | 28.00 | 54.30 | NA | 62.20 | NA | 0.400 | 0.171 | 7 | 57.7 | 55.9 |
| Park (Male) [27] | 2012 | Nested | Population | Korea | NA | GOLD | ATP III | 100 | 437 | 1.000 | 1.000 | 23.30 | 24.10 | NA | NA | NA | NA | 0.330 | 0.222 | 6 | 60.9 | 50.8 |
| Park (Female) [27] | 2012 | Nested | Population | Korea | NA | GOLD | ATP III | 33 | 645 | 0.000 | 0.000 | 24.20 | 24.10 | NA | NA | NA | NA | 0.485 | 0.296 | 6 | 59.2 | 51.4 |
| Akpinar [28] | 2012 | Nested | Hospital | Turkey | Yes | GOLD | ATP III | 91 | 42 | 0.857 | 0.833 | NA | NA | NA | NA | NA | NA | 0.446 | 0.171 | 7 | 63.7 | 62.8 |
| Lam (Male) [29] | 2010 | Nested | Population | China | NA | GOLD | IDF | 128 | 1880 | 1.000 | 1.000 | NA | NA | NA | NA | NA | NA | 0.226 | 0.198 | 6 | 67.1 | 63.5 |
| Lam (Female) [29] | 2010 | Nested | Population | China | NA | GOLD | IDF | 368 | 4982 | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | 0.226 | 0.198 | 6 | 62.7 | 60.7 |
| Funakoshi [12] | 2010 | Cross-sectional | Population | Japan | NA | GOLD | ATP III | 297 | 6544 | 1.000 | 1.000 | 22.70 | 23.70 | 89.00 | 95.80 | 66.10 | 79.50 | 0.168 | 0.258 | 6 | 62.3 | 55.9 |
| Watz [13] | 2009 | Cross-sectional | Hospital | Germany | NA | GOLD | IDF | 57 | 30 | 0.719 | 0.767 | 27.80 | 27.50 | 63.00 | 99.60 | 53.20 | 75.00 | 0.526 | 0.533 | 5 | 63.3 | 62.6 |
| Marquis (Male) [11] | 2005 | Cross-sectional | Hospital | Canada | Yes | ATS (1987) | ATP III | 23 | 20 | 1.000 | 1.000 | 29.00 | 30.00 | NA | NA | NA | NA | 0.609 | 0.200 | 8 | 66.0 | 63.0 |
| Marquis (Female) [11] | 2005 | Cross-sectional | Hospital | Canada | Yes | ATS (1987) | ATP III | 15 | 14 | 0.000 | 0.000 | 27.00 | 29.00 | NA | NA | NA | NA | 0.267 | 0.214 | 8 | 66.0 | 63.0 |
Abbreviations: ATP-III, the Adult Treatment Panel III; ATS (1987), American Thoracic Society in 1987; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IDF, International Diabetes Federation; JIS (2009), a joint interim statement in 2009 (Circulation 2009; 120:1640–1645); MetS, metabolic syndrome; NA, not available; NOS, Newcastle–Ottawa Scale.
Overall analyses – metabolic syndrome and COPD
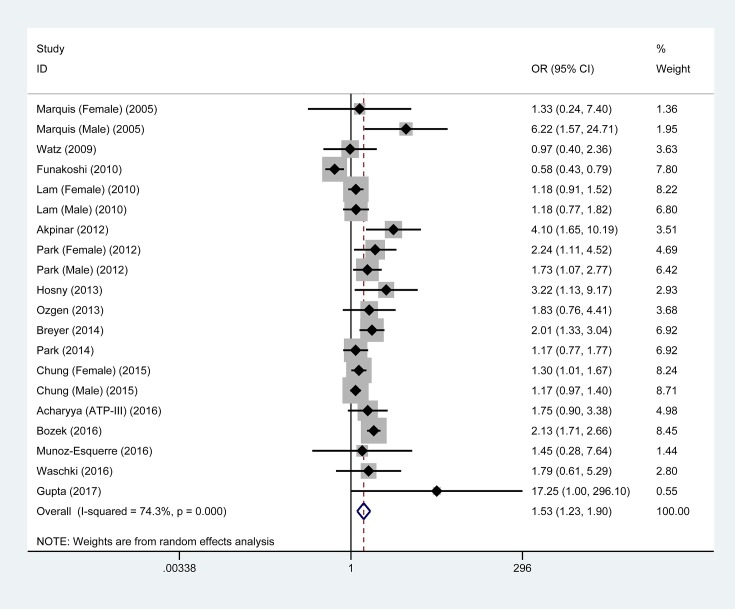
As shown in Figure 1, metabolic syndrome was significantly associated with 1.53-fold (95% CI: 1.23–1.90, P<0.001) increased risk of COPD when pooling the results of 20 eligible studies together. The I2 statistic was 74.3%, indicating moderate between-study heterogeneity. With an estimated prevalence of COPD of 13.5% for adults aged 20–79 years old [30], the power to derive an OR of 1.53 in 3915 COPD patients and 25,790 control participants was 100% using a 2-sided alpha of 5%.
Figure 1. Overall association of metabolic syndrome with COPD risk.
Abbreviations: 95% CI, 95% confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio. OR is denoted by the center of a solid diamond, and the length of solid line cross this diamond denotes its 95% CI. The hollow diamond with a vertical broken line denotes overall risk estimate. The solid vertical line is set at the null value (OR = 1.0).
The results of cumulative and influential analyses are presented in Supplementary Figure S2. In the cumulative analysis, the overall estimation tended to be stabilized since 2012, and significance was reached after 2013. In the influential analysis, no single study was noted to affect the overall estimation remarkably.
Overall analyses – metabolic components and COPD
Table 2 presents the association of metabolic components with COPD using available data from all eligible studies. At a categorical scale, hypertension was significantly associated with 1.55-fold increased risk of COPD (95% CI: 1.14–2.11, P=0.005), while the risk conferred by obesity was marginally reduced by 32% (95% CI: 0.46–1.01, P=0.057). No hints of significance were observed for diabetes mellitus, high waist circumstance, high triglycerides and low HDLC (P>0.05). When metabolic components were analyzed continuously, averaged levels of SBP (WMD = 3.626 mmHg, 95% CI: 1.537–5.714, P<0.001) and glucose (WMD = 2.976 mmol/l, P=0.040) were significantly higher in COPD patients than in controls, yet that of BMI (WMD = −1.463 kg/m2, 95% CI: −2.716 to −0.211, P=0.022) were significantly lower. There was no significance for the other continuous variables (P>0.05).
Table 2. Effect estimates of metabolic components in association with COPD.
| Metabolic components | Number | EE | 95% CI | P | I2 | PQ-test | Egger’s P |
|---|---|---|---|---|---|---|---|
| Categorical scale (EE = OR) | |||||||
| Hypertension | 11 | 1.55 | 1.14–2.11 | 0.005 | 64.4% | 0.002 | 0.539 |
| Diabetes | 12 | 1.1 | 0.93–1.32 | 0.273 | 13.5% | 0.312 | 0.01 |
| Obesity | 7 | 0.68 | 0.46–1.01 | 0.057 | 81.6% | <0.001 | 0.243 |
| High WC | 9 | 1.19 | 0.78–1.79 | 0.42 | 73.0% | <0.001 | 0.006 |
| High triglycerides | 10 | 1.28 | 0.9–1.81 | 0.169 | 66.2% | 0.002 | 0.063 |
| Low HDLC | 10 | 1.09 | 0.82–1.45 | 0.536 | 26.8% | 0.197 | 0.568 |
| Continuous scale (EE = WMD) | |||||||
| BMI | 17 | –1.463 | –2.716 to –0.211 | 0.022 | 98.2% | <0.001 | 0.728 |
| WC | 16 | 0.247 | –0.666 to 1.16 | 0.596 | 72.3% | <0.001 | 0.101 |
| SBP | 15 | 3.626 | 1.537 to 5.714 | 0.001 | 81.7% | <0.001 | 0.786 |
| DBP | 15 | –0.708 | –1.842 to 0.426 | 0.221 | 80.9% | <0.001 | 0.574 |
| Glucose | 15 | 2.976 | 0.141 to 5.812 | 0.04 | 81.8% | <0.001 | 0.025 |
| Triglycerides | 17 | –4.827 | –10.685 to 1.031 | 0.106 | 68.0% | <0.001 | 0.706 |
| TC | 5 | –2.385 | –10.346 to 5.701 | 0.563 | 86.4% | <0.001 | 0.809 |
| HDLC | 17 | 0.234 | –0.825 to 1.293 | 0.665 | 63.2% | <0.001 | 0.248 |
| LDLC | 7 | –3.609 | –12.038 to 4.82 | 0.401 | 87.6% | <0.001 | 0.912 |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EE, effect estimate; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumstance; WMD, weighted mean difference. The term ‘Number’ in the first row referred to the number of eligible studies.
Pulmonary functional testing
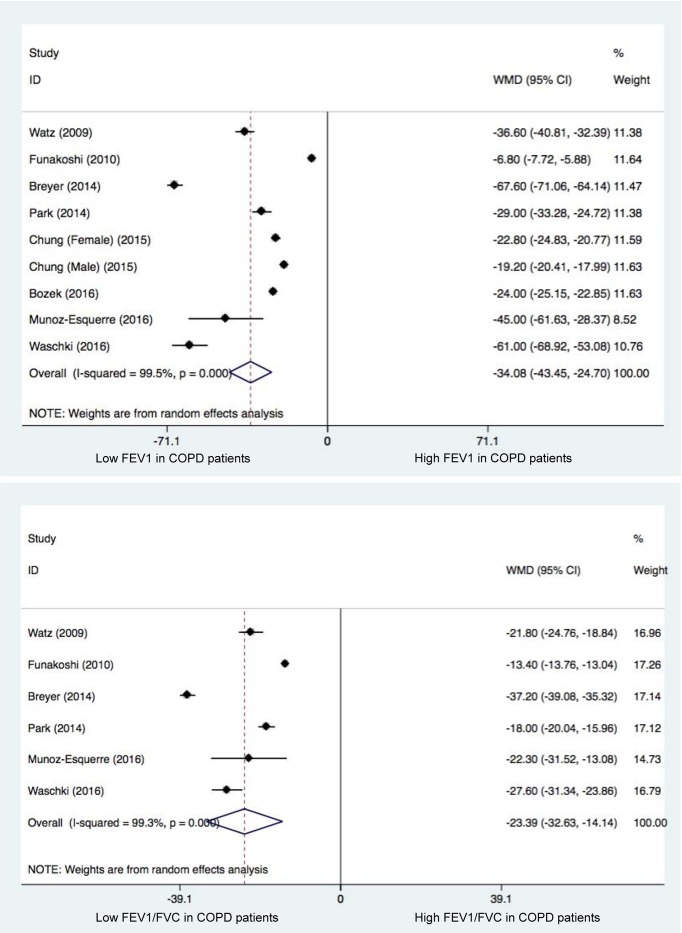
Two indices of pulmonary functional testing, FEV1 and FEV1 to FVC ratio, were summarized and compared between COPD patients and controls (Figure 2). As expected, the two indices were remarkably lower in COPD patients than in control participants (P<0.001).
Figure 2. Changes of FEV1 (the upper panel) and FEV1 to FEV ratio (the lower panel) between COPD patients and control participants.
Abbreviations: 95% CI, 95% confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; WMD, weighted mean difference. WMD is denoted by the center of a solid diamond, and the length of solid line cross this diamond denotes its 95% CI. The hollow diamond with a vertical broken line denotes overall risk estimate. The solid vertical line is set at the null value (WMD = 0.0).
Stratified analyses – metabolic syndrome and COPD
Stratified effect estimates of metabolic syndrome for COPD risk are presented in Table 3. By COPD diagnosis, metabolic syndrome was significantly associated with COPD in studies adopting the GOLD criteria (OR = 1.48, 95% CI: 1.20–1.84, P<0.001), but not in studies adopting the American Thoracic Society (ATS) criteria in 1987. By the diagnostic criteria of metabolic syndrome, most studies adopted the criteria developed by ATP-III (Adult Treatment Panel III) and IDF (International Diabetes Federation), and the association of metabolic syndrome with COPD was statistically significant using the two major criteria (OR = 1.72 and 1.39, 95% CI: 1.24–2.4 and 1.03–1.88, P=0.001 and 0.032, respectively). By gender, the risk prediction of metabolic syndrome for COPD was only significant in females (OR = 1.28, 95% CI: 1.08–1.53, P=0.004). By study design, significance was attained in studies of both cross-sectional and nested designs (OR = 1.67 and 1.35, 95% CI: 1.09–2.58 and 1.14–1.6, P=0.02 and <0.001, respectively). By source of controls, effect estimate was significant for both hospital-based and population-based sources, especially in hospital-based controls (OR = 2.43, 95% CI: 1.41–4.18, P=0.001). By race, the risk of COPD was significant in Caucasian and Middle-Eastern populations (OR = 2.05 and 2.83, 95% CI: 1.7–2.46 and 1.65–4.86, both P<0.001). By development, the association of metabolic syndrome with COPD was significant in both developed and developing countries (OR = 1.44 and 1.76, 95% CI: 1.09–1.9 and 1.19–2.6, P=0.01 and 0.004, respectively). By matched status, studies with matched patients and controls reported a significant association of metabolic syndrome with COPD (OR = 2.21, 95% CI: 1.83–2.68, P<0.001). Finally, by sample size, the risk estimate was 2.16 (95% CI: 1.47–3.16) in small studies (total sample size <300) and 1.34 (95% CI: 1.05–1.71) in large studies (total sample size ≥300) (P<0.001 and P=0.02, respectively).
Table 3. Stratified effect estimates of metabolic syndrome for COPD risk.
| Stratified groups | Number | OR | 95% CI | P | I2 | PQ-test |
|---|---|---|---|---|---|---|
| COPD diagnosis | ||||||
| GOLD | 18 | 1.48 | 1.20–1.84 | <0.001 | 75.4% | <0.001 |
| ATS (1987) | 2 | 3.14 | 0.70–14.09 | 0.134 | 46.9% | 0.170 |
| By MetS | ||||||
| ATP-III | 12 | 1.72 | 1.24–2.4 | 0.001 | 83.6% | <0.001 |
| IDF | 8 | 1.39 | 1.03–1.88 | 0.032 | 49.1% | 0.056 |
| JIS (2009) | 2 | 1.18 | 0.79–1.77 | 0.41 | 0.0% | 0.802 |
| By gender | ||||||
| Males | 5 | 1.22 | 0.78–1.91 | 0.384 | 84.8% | <0.001 |
| Females | 4 | 1.28 | 1.08–1.53 | 0.004 | 0.0% | 0.414 |
| By study design | ||||||
| Cross-sectional | 11 | 1.67 | 1.09–2.58 | 0.02 | 83.2% | <0.001 |
| Nested | 9 | 1.35 | 1.14–1.6 | <0.001 | 34.5% | 0.142 |
| By source of controls | ||||||
| Hospital | 8 | 2.43 | 1.41–4.18 | 0.001 | 33.7% | 0.159 |
| Population | 12 | 1.38 | 1.1–1.73 | 0.006 | 80.6% | <0.001 |
| By race | ||||||
| Asian | 9 | 1.24 | 0.97–1.59 | 0.085 | 74.2% | <0.001 |
| Caucasian | 7 | 2.05 | 1.7–2.46 | <0.001 | 0.0% | 0.445 |
| Middle Eastern | 3 | 2.83 | 1.65–4.86 | <0.001 | 0.0% | 0.440 |
| Mixed | 1 | 1.17 | 0.77–1.77 | 0.46 | NA | |
| By country development | ||||||
| Developed | 13 | 1.44 | 1.09–1.9 | 0.01 | 80.0% | <0.001 |
| Developing | 7 | 1.76 | 1.19–2.6 | 0.004 | 57.5% | 0.028 |
| By matched status | ||||||
| NR | 11 | 1.23 | 1.0–1.52 | 0.051 | 69.7% | <0.001 |
| Yes | 9 | 2.21 | 1.83–2.68 | <0.001 | 0.0% | 0.460 |
| By total sample size | ||||||
| <300 | 10 | 2.16 | 1.47–3.16 | <0.001 | 19.6% | 0.262 |
| ≥300 | 10 | 1.34 | 1.05–1.71 | 0.02 | 83.8% | <0.001 |
Abbreviations: 95% CI, 95% confidence interval; ATP-III, the Adult Treatment Panel III; COPD, chronic obstructive pulmonary disease; IDF, International Diabetes Federation; JIS (2009), a joint interim statement in 2009 (Circulation 2009; 120:1640–1645); MetS, metabolic syndrome; NA, not reported; OR, odds ratio. The term ‘Number’ in the first row referred to the number of eligible studies.
In stratified analyses, heterogeneity was non-significant in studies with the diagnosis of metabolic syndrome using IDF and JIS-2009 (I2 = 49.1% and 0.0%, respectively), involving female gender only (I2 = 0.0%), with nested design (I2 = 34.5%), involving hospital-based controls (I2 = 33.7%), including Caucasian or Middle Eastern populations (both I2 = 0.0%), with matched patients and controls (I2 = 0.0%) and with total sample size less than 300 (I2 = 19.6%).
Meta-regression analyses
Besides stratified analyses, meta-regression analyses were conducted to explore other potential sources of heterogeneity for the association of metabolic syndrome with COPD by modelling age, gender, smoking, pack-year smoking history, drinking, physical activity, income and education. None of these factors exhibited significant contributions to the association between metabolic syndrome and COPD (all P>0.1).
Publication bias
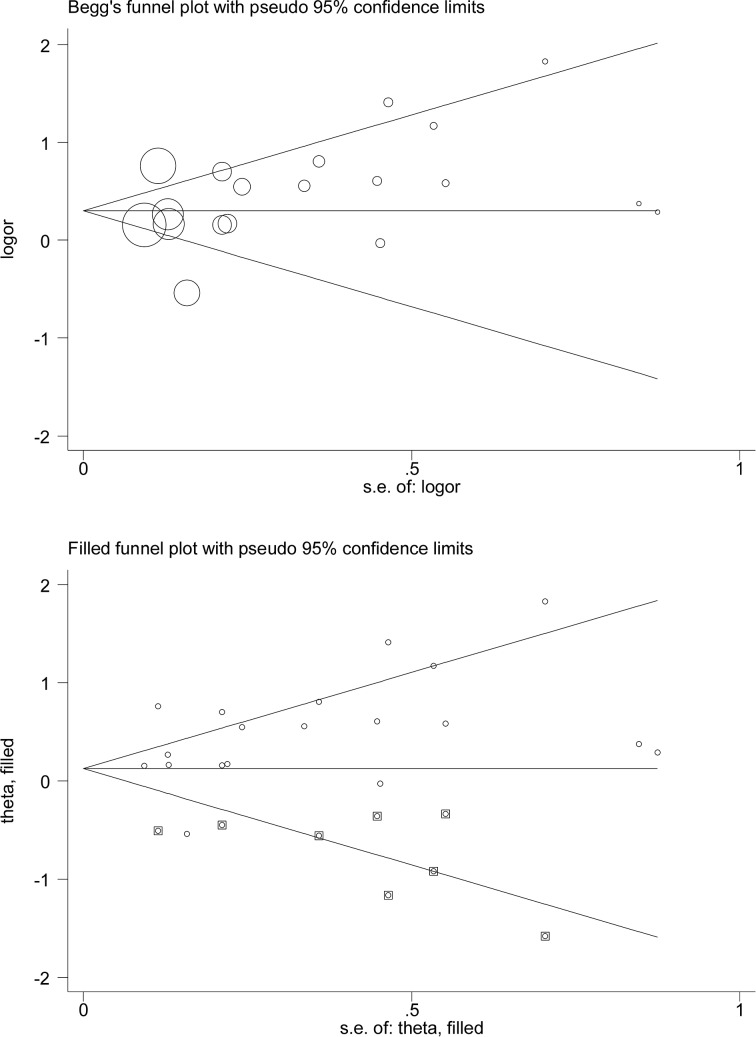
For metabolic syndrome in association with COPD, there was a low probability of publication bias, as reflected by Begg’s and filled funnel plots (Figure 3), although there were an estimated eight missing studies to make the filled funnel plot symmetrical. After considering the impact of missing studies, the association between metabolic syndrome and the risk of COPD was still statistically significant (OR = 1.13, 95% CI: 1.05–1.23, P=0.002).
Figure 3. Begg’s (the upper panel) and filled (the lower panel) funnel plots for the association of metabolic syndrome with COPD risk.
Abbreviations: COPD, chronic obstructive pulmonary disease; logor, the logarithm of odds ratio; S.E., standard error. In Begg’s funnel plot, the symbols denoting the data in the plot are sized proportionally to inverse variance. In filled funnel plot, hollow circles denote the actual studies included in this meta-analysis, and solid squares denote missing studies required to achieve symmetry of funnel plot.
For the association of metabolic components with COPD, the probability of publication bias was significant for diabetes mellitus, high waist circumstance, high triglycerides and glucose at a significance level of 10% (Egger’s P=0.01, 0.006, 0.063 and 0.025, respectively). There was no evidence of publication bias for other metabolic components, either categorical or continuous (Egger’s P>10%).
Discussion
The aim of this meta-analysis is to test the association of metabolic syndrome and its components with COPD risk. Importantly, our data indicate that the comorbidity of metabolic syndrome, especially its component hypertension, was associated with significant risk of experiencing COPD. Moreover, gender, race, source of control participants, matched status and sample size were accountable factors for significant heterogeneity. To our knowledge, this is the first quantitative assessment of metabolic syndrome and COPD in the medical literature.
Metabolic syndrome as a potential risk factor for the development of COPD has been widely evaluated, while no consensus has been attained yet, mainly because of racial diversity of study populations, insufficient power of some individual studies and heterogeneous methodological designs [13,23]. A recent systematic review concluded that the prevalence of metabolic syndrome was significantly higher in COPD patients than controls, and hypertension, abdominal obesity and hyperglycemia constituted the most prevalent components [14]. However, to what magnitude metabolic syndrome contributes to the occurrence of COPD has not yet been clarified in this review [14]. Moreover, it is worth noting that metabolic syndrome is significantly more common in non-emphysematous COPD [31], which is defined by airflow obstruction with a paucity of emphysema on chest CT scan. However, considering that only one [19] of all eligible studies in this meta-analysis provided data on emphysema in COPD patients and control participants, we here focused on COPD irrespective of emphysema phenotype. To yield more information, we, in a larger sample size, found that patients with metabolic syndrome were about 1.5 times more like to develop COPD, and especially its component hypertension played a dominant role in predicting the disease risk. Additionally, we observed that COPD patients tended to have a lower level of BMI, which differed from that reported in this previous review [14]. In support of our findings, a large number of studies have demonstrated that low BMI was not only a high-risk factor for COPD [24,32–35], but also a significant predictor for all-cause mortality in COPD patients [36–38]. There is evidence that low BMI was associated with systemic inflammation that served as one of the possible mechanisms in COPD patients [39]. It is widely recognized that COPD is characterized by low-grade systemic inflammation that affects body composition [40], and systemic inflammation can promote insulin resistance and further contribute to the development of metabolic syndrome [41,42]. In addition, some systemic inflammatory markers such as leukocyte count [43] were observed to be significantly higher in COPD patients with metabolic syndrome than in patients without metabolic syndrome. It is suggested that several factors may contribute to the co-existing COPD and metabolic syndrome, including the presence of physical inactivity and systemic inflammation related to a smoking habit, sedentary lifestyle, airway inflammation and obstruction, adipose tissue and inflammatory marker activation [44]. Based on above lines of evidence, it is reasonable to postulate that the COPD risk conferred by metabolic syndrome might be mediated through an inflammation-related process. An in-depth assessment of this process is beyond the scope of this present study but certainly warrants further investigations.
Another key finding of this meta-analysis is that in stratified analysis, gender, race, source of control participants, matched status and sample size were identified as accountable factors for significant between-study heterogeneity. In particular, we found that the magnitude of COPD risk conferred by the presence of metabolic syndrome was markedly reinforced when analysis was restricted to females, Caucasian populations, studies involving hospital-based controls and studies of matched patients and controls, respectively. The reasons for differed risk magnitude are multifaceted, relating either to different exposures to environmental and lifestyle factors (such as cigarette smoking) or to different genetic backgrounds across races or ethnicities. For example, cigarette smoking is a predisposing factor for the development of both metabolic syndrome and COPD, and predisposition to smoking varies by gender [45,46]. Moreover, although sample size can explain some heterogeneity, the association between metabolic syndrome and COPD remained significant when analysis was restricted to larger studies in this study, suggesting the robustness of our findings. Nevertheless, to derive a more reliable estimate, we agree that some large, soundly designed, prospective studies are required.
Limitations
The first limitation is that the association between metabolic syndrome and COPD does not prove causality. The second limitation revolves around the use of published aggregate data, not individual participant data, limiting further explorations, such as the three-way interaction between smoking, metabolic syndrome and COPD, which could provide clinically relevant insights into the increased risk of COPD among susceptible individuals. The third limitation is that the exclusion of non-English research papers, might lead to exaggerated estimates of observed association. The fourth limitation is that we did not consider the impact of therapies (especially steroids) on the association between metabolic syndrome and COPD due to sparse data. The fifth limitation is that this meta-analysis involved studies in varying definitions regarding both metabolic syndrome and COPD, which might produce a misclassification bias.
Conclusions
Taken together, in a quantitative analysis of 29,705 subjects, our data indicate that the presence of metabolic syndrome, especially its component hypertension, was associated with significantly increased risk of COPD. Moreover, gender, race, source of control participants, matched status and sample size were accountable factors for significant heterogeneity. So for practical reasons, prevention and early detection of COPD patients prone to the development of metabolic syndrome and its sequelae may facilitate intensive surveillance or targeted interventions for high-risk patients, and thereby be of significant clinical importance.
Supporting information
Supporting Figure 1. PRISMA flow chart for article selection in this meta-analysis.
Supplementary Figure 2. Cumulative (the left panel) and influential (the right panel) analyses for overall association of metabolic syndrome with the risk of chronic obstructive pulmonary disease. In the lower panel, 1 denotes the study by Marquis et al in females, 2 denotes denotes the study by Marquis et al in males, 3 denotes the study by Watz et al, 4 denotes the study by Funakoshi et al, 5 denotes the study by Lam et al in females, 6 denotes the study by Lam et al in males, 7 denotes the study by Akpinar et al, 8 denotes the study by Park et al in females in 2012, 9 denotes the study by Park et al in males in 2012, 10 denotes the study by Hosny et al, 11 denotes the study by Ozgen et al, 11 denotes the study by Breyer et al, 12 denotes the study by Park et al in 2014, 13 denotes the study by Chung et al in females, 14 denotes the study by Chung et al in males, 15 denotes the study by Acharyya et al, 16 denotes the study by Bozek et al, 17 denotes the study by Munoz-Esquerre et al, 18 denotes the study by Waschki et al and 19 denotes the study by Gupta et al. Abbreviations: 95% CI, 95% confidence interval.
Supplementary Table 1. The PRISMA checklist.
Abbreviations
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- FEV1
forced expiratory volume in one second
- FVC
FEV1 to forced vital capacity
- HDLC
high-density lipoprotein cholesterol
- LDLC
low-density lipoprotein cholesterol
- NOS
Newcastle–Ottawa Scale
- SBP
systolic blood pressure
- TC
total cholesterol
- WMD
weighted mean difference
Funding
The authors declare that there are no sources of funding to be acknowledged.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
Z.W. designed the study; L.Y. and X.H. completed literature search, article selection and data abstraction; Q.W., H.Y. and D.C. did the data preparation, quality control and data analyses; L.Y., H.Y. and Z.W. wrote the manuscript.
References
- 1.Disease GBD, Injury I and Prevalence C (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortality GBD and Causes of Death C (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S., Zhou Y., Liu S., Chen X., Zou W., Zhao D.. et al. (2017) Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax 72, 788–795 10.1136/thoraxjnl-2016-208910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Zheng X.Y., Chung K.F. and Zhong N.S. (2016) Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet 388, 1939–1951 10.1016/S0140-6736(16)31597-5 [DOI] [PubMed] [Google Scholar]
- 5.Wang C., Xu J., Yang L., Xu Y., Zhang X., Bai C.. et al. (2018) Prevalence and risk factors of chronic obstructive pulmonary disease in China the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 391, 1706–1717 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 6.Fang L., Gao P., Bao H., Tang X., Wang B., Feng Y.. et al. (2018) Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir. Med., 6, 421–430 10.1016/S2213-2600(18)30103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chhabra S.K., Gupta M., Ramaswamy S., Dash D.J., Bansal V. and Deepak K.K. (2015) Cardiac sympathetic dominance and systemic inflammation in COPD. COPD 12, 552–559 10.3109/15412555.2014.974743 [DOI] [PubMed] [Google Scholar]
- 8.Tan D.B.A., Armitage J., Teo T.H., Ong N.E., Shin H. and Moodley Y.P. (2017) Elevated levels of circulating exosome in COPD patients are associated with systemic inflammation. Respir. Med. 132, 261–264 10.1016/j.rmed.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Rubinsztajn R., Przybylowski T., Maskey-Warzechowska M., Paplinska-Goryca M., Nejman-Gryz P., Karwat K.. et al. (2017) Metabolic syndrome as a factor affecting systemic inflammation in patients with chronic obstructive pulmonary disease. Adv. Exp. Med. Biol. 1021, 55–62 10.1007/5584_2017_28 [DOI] [PubMed] [Google Scholar]
- 10.Gupta K.K., Singh J., Gupta P., Patel M.L., Kumar V. and Chaudhary S.C. (2017) Uncovering metabolic syndrome among chronic obstructive pulmonary disease patients in a Tertiary Care Hospital, India. J. Clin. Diagn. Res. 11, OC08–OC11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquis K., Maltais F., Duguay V., Bezeau A.M., LeBlanc P., Jobin J.. et al. (2005) The metabolic syndrome in patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 25, 226–232, discussion 233-224 10.1097/00008483-200507000-00010 [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi Y., Omori H., Mihara S., Marubayashi T. and Katoh T. (2010) Association between airflow obstruction and the metabolic syndrome or its components in Japanese men. Intern. Med. 49, 2093–2099 10.2169/internalmedicine.49.3882 [DOI] [PubMed] [Google Scholar]
- 13.Watz H., Waschki B., Kirsten A., Muller K.C., Kretschmar G., Meyer T.. et al. (2009) The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest 136, 1039–1046 10.1378/chest.09-0393 [DOI] [PubMed] [Google Scholar]
- 14.Cebron Lipovec N., Beijers R.J., van den Borst B., Doehner W., Lainscak M. and Schols A.M. (2016) The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD 13, 399–406 10.3109/15412555.2016.1140732 [DOI] [PubMed] [Google Scholar]
- 15.Ito H., Matsuo K., Hamajima N., Mitsudomi T., Sugiura T., Saito T.. et al. (2004) Gene-environment interactions between the smoking habit and polymorphisms in the DNA repair genes, APE1 Asp148Glu and XRCC1 Arg399Gln, in Japanese lung cancer risk. Carcinogenesis 25, 1395–1401 10.1093/carcin/bgh153 [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Dupont W.D. and Plummer W.D. Jr (1990) Power and sample size calculations. A review and computer program. Control Clin. Trials 11, 116–128 10.1016/0197-2456(90)90005-M [DOI] [PubMed] [Google Scholar]
- 18.Waschki B., Kirsten A.M., Holz O., Meyer T., Lichtinghagen R., Rabe K.F.. et al. (2016) Angiopoietin-like protein 4 and cardiovascular function in COPD. BMJ Open Respir. Res. 3, e000161 10.1136/bmjresp-2016-000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Esquerre M., Lopez-Sanchez M., Escobar I., Huertas D., Penin R., Molina-Molina M.. et al. (2016) Systemic and pulmonary vascular remodelling in chronic obstructive pulmonary disease. PLoS One 11, e0152987 10.1371/journal.pone.0152987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozek A., Rogala B. and Bednarski P. (2016) Asthma, COPD and comorbidities in elderly people. J. Asthma 53, 943–947 10.3109/02770903.2016.1170139 [DOI] [PubMed] [Google Scholar]
- 21.Acharyya A., Shahjahan M.D., Mesbah F.B., Dey S.K. and Ali L. (2016) Association of metabolic syndrome with chronic obstructive pulmonary disease in an Indian population. Lung India 33, 385–390 10.4103/0970-2113.184871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung J.H., Hwang H.J., Han C.H., Son B.S., Kim D.H. and Park M.S. (2015) Association between sarcopenia and metabolic syndrome in chronic obstructive pulmonary disease: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2011. COPD 12, 82–89 10.3109/15412555.2014.908835 [DOI] [PubMed] [Google Scholar]
- 23.Park S.K. and Larson J.L. (2014) Metabolic syndrome and associated factors in people with chronic obstructive pulmonary disease. West J. Nurs. Res. 36, 620–642 10.1177/0193945913512423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breyer M.K., Spruit M.A., Hanson C.K., Franssen F.M., Vanfleteren L.E., Groenen M.T.. et al. (2014) Prevalence of metabolic syndrome in COPD patients and its consequences. PLoS One 9, e98013 10.1371/journal.pone.0098013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozgen Alpaydin A., Konyar Arslan I., Serter S., Sakar Coskun A., Celik P., Taneli F.. et al. (2013) Metabolic syndrome and carotid intima-media thickness in chronic obstructive pulmonary disease. Multidiscip. Respir. Med. 8, 61 10.1186/2049-6958-8-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosny H., Abdel-Hafiz H., Moussa H. and Soliman A. (2013) Metabolic syndrome and systemic inflammation in patients with chronic obstructive pulmonary disease. Egypt J. Chest Dis. Tuberc. 62, 85–89 10.1016/j.ejcdt.2013.02.007 [DOI] [Google Scholar]
- 27.Park B.H., Park M.S., Chang J., Kim S.K., Kang Y.A., Jung J.Y.. et al. (2012) Chronic obstructive pulmonary disease and metabolic syndrome: a nationwide survey in Korea. Int. J. Tuberc. Lung Dis. 16, 694–700 10.5588/ijtld.11.0180 [DOI] [PubMed] [Google Scholar]
- 28.Akpinar E.E., Akpinar S., Ertek S., Sayin E. and Gulhan M. (2012) Systemic inflammation and metabolic syndrome in stable COPD patients. Tuberk Toraks 60, 230–237 10.5578/tt.4018 [DOI] [PubMed] [Google Scholar]
- 29.Lam K.B., Jordan R.E., Jiang C.Q., Thomas G.N., Miller M.R., Zhang W.S.. et al. (2010) Airflow obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur. Respir. J. 35, 317–323 10.1183/09031936.00024709 [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Guzman E. and Mannino D.M. (2014) Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin. Chest Med. 35, 7–16 10.1016/j.ccm.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 31.Hersh C.P., Make B.J., Lynch D.A., Barr R.G., Bowler R.P., Calverley P.M.. et al. (2014) Non-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitus. BMC Pulm. Med. 14, 164 10.1186/1471-2466-14-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Y., Xu J., Yao J., Chen Y., He P., Ouyang Y.. et al. (2015) The analyses of risk factors for COPD in the Li ethnic group in Hainan, People’s Republic of China. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 2593–2600 10.2147/COPD.S86402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Wang D., Liu S., Lu J., Zheng J., Zhong N.. et al. (2013) The association between BMI and COPD: the results of two population-based studies in Guangzhou, China. COPD 10, 567–572 10.3109/15412555.2013.781579 [DOI] [PubMed] [Google Scholar]
- 34.Hunter L.C., Lee R.J., Butcher I., Weir C.J., Fischbacher C.M., McAllister D.. et al. (2016) Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ Open 6, e009121 10.1136/bmjopen-2015-009121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park H., Jung S.Y., Lee K., Bae W.K., Lee K., Han J.S.. et al. (2015) Prevalence of chronic obstructive lung disease in Korea using data from the fifth Korea national health and nutrition examination survey. Korean J. Fam. Med. 36, 128–134 10.4082/kjfm.2015.36.3.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y., Zhang T., Wang Z., Yu F., Xu Q., Guo W.. et al. (2016) Body mass index and mortality in chronic obstructive pulmonary disease: a dose-response meta-analysis. Medicine (Baltimore) 95, e4225 10.1097/MD.0000000000004225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi Y., Hasegawa W., Yasunaga H., Sunohara M., Jo T., Takami K.. et al. (2014) Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 1337–1346 10.2147/COPD.S75175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao C., Wang R., Wang J., Bunjhoo H., Xu Y. and Xiong W. (2012) Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One 7, e43892 10.1371/journal.pone.0043892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higashimoto Y., Yamagata T., Honda N., Satoh R., Sano H., Iwanaga T.. et al. (2011) Clinical and inflammatory factors associated with body mass index in elderly patients with chronic obstructive pulmonary disease. Geriatr. Gerontol. Int. 11, 32–38 10.1111/j.1447-0594.2010.00629.x [DOI] [PubMed] [Google Scholar]
- 40.Spruit M.A., Gosselink R., Troosters T., Kasran A., Van Vliet M. and Decramer M. (2005) Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest 128, 3183–3190 10.1378/chest.128.5.3183 [DOI] [PubMed] [Google Scholar]
- 41.Kern E. (2011) Metabolic syndrome and systemic inflammation in COPD. COPD 8, 395–396 10.3109/15412555.2011.636554 [DOI] [PubMed] [Google Scholar]
- 42.Poulain M., Doucet M., Drapeau V., Fournier G., Tremblay A., Poirier P.. et al. (2008) Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 5, 35–41 10.1177/1479972307087205 [DOI] [PubMed] [Google Scholar]
- 43.Vujic T., Nagorni O., Maric G., Popovic L. and Jankovic J. (2016) Metabolic syndrome in patients with chronic obstructive pulmonary disease: frequency and relationship with systemic inflammation. Hippokratia 20, 110–114 [PMC free article] [PubMed] [Google Scholar]
- 44.Clini E., Crisafulli E., Radaeli A. and Malerba M. (2013) COPD and the metabolic syndrome: an intriguing association. Intern. Emerg. Med. 8, 283–289 10.1007/s11739-011-0700-x [DOI] [PubMed] [Google Scholar]
- 45.Corwin E.J., McCoy C.S., Whetzel C.A., Ceballos R.M. and Klein L.C. (2006) Risk indicators of metabolic syndrome in young adults: a preliminary investigation on the influence of tobacco smoke exposure and gender. Heart Lung 35, 119–129 10.1016/j.hrtlng.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 46.Miller M.R., Jordan R.E. and Adab P. (2011) Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 66, 921–922, author reply 922 10.1136/thx.2010.152348 [DOI] [PubMed] [Google Scholar]