Abstract
The immunological function of patients with malignant tumors may be suppressed during the perioperative period. However, details on the effects of transcutaneous electrical acupoint stimulation (TEAS) on immunological function are relatively lacking. We designed this study to examine the effects of TEAS on the immunological function of patients with non-small cell lung cancer (NSCLC) during the perioperative period. Participants (n = 144) were enrolled and randomly assigned into group TEAS or group sham TEAS. TEAS on bilateral Feishu (BL13), Hegu (L14), and Zusanli (ST36) was performed continuously throughout the procedure. The primary outcome was the quantities of natural killer (NK) cells at 30 minutes before induction (T0), 5 minutes after intubation (T1), at the beginning of the operation (T2), at the beginning of the lobectomy (T3), at the beginning of the lymphadenectomy (T4), and immediately after extubation (T5). The secondary outcomes were the serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) at T0 to T5, the mean arterial pressure (MAP) and heart rate (HR), the intraoperative consumption of propofol and remifentanil, the incidence of hypoxemia, postoperative nausea and vomiting (PONV), and the length of hospital stay. The quantities of NK cells were decreased in group sham TEAS after intubation compared to that in group TEAS, while the quantities of NK cells in group TEAS were similar at T0 to T5. Meanwhile, the quantities of NK cells in group sham TEAS at T1 (P = .012), T2 (P < .001), T3 (P = .027), T4 (P = .045), and T5 (P = .021) were lower than those in group TEAS. In group TEAS, the serum levels of TNF-α were lower at T1 to T5, while the levels of IL-6 were lower at T2 to T5. Furthermore, the intraoperative MAP and HR were more stable, the total propofol and remifentanil consumptions were lower, and the length of hospital stay was shorter than those in group sham TEAS. The application of TEAS can effectively reverse the decrease in NK cells, decrease the serum levels of TNF-α and IL-6, maintain hemodynamic stability during the perioperative period, decrease the consumption of propofol and remifentanil, and shorten the length of the hospital stay.
Keywords: transcutaneous electrical acupoint stimulation, non-small cell lung cancer, video-assisted thoracic surgical lobectomy, perioperative period, immunological function
Introduction
Lung cancer is one of the most common malignancies observed in daily clinical practice and the leading cause of cancer-related death worldwide. Cancer leads to approximately 1 million deaths, for which non-small cell lung cancer (NSCLC) accounts for approximately 80%.1 Patients with lung cancer exhibit numerous immune abnormalities, including cellular immune dysfunction, cytokine alterations, microcirculatory disturbances, and antigen presentation defects,2,3 due to surgical trauma and postoperative pain. Surgical treatment is the main method of therapy for early-stage NSCLC. However, immunological function could be further depressed during the perioperative period, which may predispose patients to septic complications, multiple organ dysfunction, tumor spread or metastases, and mortality during this period.4 Therefore, it is of great importance to find approaches to attenuate perioperative immunological dysfunction. The traditional thoracotomy causes more trauma and can lead to a strong stress reaction and severe fluctuation of hemodynamics intraoperatively. Compared to traditional thoracotomy, video-assisted thoracic surgery (VATS) lobectomy causes less trauma and has better visualization during the procedure, which helps to depress the inflammatory response, protect immune function,5 shorten the length of hospital stay, hasten postoperative rehabilitation, and decrease in-hospital mortality.6 Protection of perioperative immunological function is one of the most important clinical concerns, particularly for patients with malignant tumors.
Transcutaneous electrical acupoint stimulation (TEAS) is a type of acupuncture that combines the effect of transcutaneous electrical nerve stimulation (TENS) and acupoint therapy. According to the basic theory of traditional Chinese medicine, surgical trauma could imbalance the state of the human body and disturb the movement of Qi (vital energy),7 while acupoint stimulation may restore the balance of Qi and facilitate recovery from bodily injury via exerting effects on the central nervous, autonomic nervous, immune, metabolic, and endocrine systems.8,9 Acupuncture could promote the release of cytokines activated by natural killer (NK) cells and enhance nonspecific antitumor immunity.10 The NK cells are natural lymphocytes that play a significant role in antitumor immunity and can express receptors, such as NK group 2D receptors, which recognize tumor cells and pathogen-infected cells via surface ligands.11 Perioperative surgical trauma and other stimuli could increase the release of inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, leading to inflammatory reactions and tissue damage. In addition, high levels of IL-6 not only mediate inflammatory reactions but also promote tumor angiogenesis, tumor cell growth, and metastasis,12 which are detrimental to the prognosis of patients with cancer. TNF-α is one of the earliest released and most sensitive inflammatory factors,13 which can also activate the production of IL-6. Generally, serum levels of TNF-α and IL-6 can reflect the levels of inflammatory reaction and humoral immunity. Research suggests that electroacupuncture could alleviate the levels of inflammatory reaction by downregulating the serum levels of cytokines TNF-α and IL-6.14 Therefore, NK cells, TNF-α, and IL-6 were selected as the immunological factors of interest. The TEAS has been proven to provide perioperative immune system protection by regulating the balance of T lymphocytes and the expression levels of related cytokines and transcription factors,15 and the procedure may be a promising strategy for treating postoperative immunological dysfunction in patients with NSCLC. However, relevant studies regarding these effects are relatively lacking.
We conducted this randomized controlled study primarily to examine the effects of TEAS on the immunological function in patients with NSCLC undergoing VATS lobectomy.
Methods
Patients
Ethical approval was obtained from the Ethical Committee of Human Research of Tangshan People’s Hospital, North China University of Science and Technology (file number: RMYY-YWLL-2017-1110). The study was performed in accordance with the Declaration of Helsinki and the guidelines on good clinical practice. Approval by the regional research ethics committee was obtained. All patients enrolled in the study signed informed consent.
Participants who underwent VATS lobectomy were enrolled in the study. The eligibility criteria were as follows: (1) patients who provided consent; (2) patients aged more than 18 years; (3) patients with an American Society of Anesthesiology physical status classification grade I or II; and (4) patients with sufficient cognitive function and language skills for the study. The exclusion criteria were as follows: (1) patients with a painful condition (eg, chronic migraine, rheumatoid arthritis, cancer pain); (2) patients with a major organ system disease or hypersensitivity to the study medications; (3) patients fitted with a pacemaker; (4) participants using an analgesic drug or had a history of significant abuse of certain drugs; (5) patients who received acupuncture point stimulation or had experience with acupuncture electrodes; (6) patients in who the target acupoint stimulation site was infected, had trauma, or TEAS implementation was not appropriate to carry out for other reasons; (7) patients who had already underwent thoracotomy; and (8) patients who had uncontrolled hypertension, diabetes, history of epilepsy, were pregnant, lactating or had childbearing potential, or were enrolled in other clinical trials.
The perioperative management sequences of TEAS (group TEAS) and sham TEAS (group sham TEAS) were determined, and randomization was stratified according to the time of admission in a block size of 10. Eligible participants were randomly assigned to receive either TEAS or sham TEAS via a central randomization system for clinical research using a 1:1 ratio. The random number list was generated by an independent statistician, and the block size was disclosed to other researchers. An independent, blinded statistician concealed the file of the generated random number table using a password and provided information regarding which group each participant was assigned to the clinical research coordinator. Researchers who assessed outcome measures and those who performed data management and statistical analysis were blinded to each participant’s allocation status. Outcome assessors were not allowed to have any conversations with participants regarding the treatment process. To avoid any bias due to deliberate intervention, each practitioner performed only one of the interventions (TEAS or sham TEAS), and none of the practitioners were allowed to participate in the analysis of outcomes. In addition, patients were treated separately to prevent communication throughout the trial.
Study Design
All patients were admitted 1 day before surgery, and no preoperative medication was administered prior to anesthesia induction. Upon arrival in the operating room, a dedicated intravenous cannula was inserted by a circuit nurse, and an infusion of Ringer’s lactate solution was commenced. The patient’s temperature, heart rate (HR), electrocardiogram (lead II), pulse oximetry, and bispectral index (BIS; Model A 3000, Aspect Medical Systems, Inc, Natick, Massachusetts) were continuously monitored during the procedure. Puncture and catheterization of the radial artery were performed after local anesthesia to monitor continuous arterial blood pressure.
Patients in group TEAS received acupoint electrical stimulation on the bilateral Feishu (BL13, located under the spinous process of the third thoracic vertebrae approximately 5 cm apart), Hegu (L14, between the first and second metacarpal bones on the dorsum of the hand, the midpoint of the radial side of the second metacarpal bone), and Zusanli (ST36, located 5 mm below and lateral to the anterior tubercle of the tibia) 30 minutes before induction of anesthesia. The HANS LH-202 electrical stimulator (Nanjing Ji Sheng Medical Technology Co, Ltd, Nanjing, China.) was used to provide electrical stimulation. After skin disinfection, electrode tabs were placed on the bilateral BL13, L14, and ST36; the output wire was connected to the electrode tabs; and the electrode intensity was adjusted, with the intensity set at the highest level the patients could tolerate. The TEAS was maintained continuously throughout the procedure with a dilatational wave at a frequency of 2/100 Hz.16 The intensity of the current ranged from 5 to 30 mA for TEAS, from 5 to 10mA for the upper limbs, and from 10 to 30 mA for the lower limbs and trunk.17 The presence of de qi sensation was used to establish the efficacy of the acupoint stimulation. For patients in group sham TEAS, electrode tabs were placed on the bilateral BL13, L14, and ST36 similar to patients in group TEAS, but electrical stimulation was not initiated. The electrodes were well protected from detaching during the operation. The TAES intervention was performed by a research nurse who was a qualified member of the research team and then verified by 2 traditional Chinese medicine physicians. Throughout the trial, participants were treated separately to prevent communication.
All patients were provided 100% oxygen for 3 minutes before induction. Anesthesia induction was initiated by experienced anesthesiologists with 0.1 mg/kg midazolam, 4 μg/kg fentanyl, 0.4 mg/kg etomidate, and 0.6 mg/kg rocuronium. A left double-lumen endotracheal tube (Mallinckrodt; Covidien, Manseld, Massachusetts) was used for intubation, and its correct position was confirmed by fiberoptic bronchoscopy. All the patients received propofol and remifentanil, controlled by the same closed-loop automated system, during the induction and maintenance of general anesthesia. The BIS values were maintained between 40 and 50, with the intermittent addition of cis-atracurium, to ensure the progress of the surgery. All anesthetics were discontinued at the end of the surgery. No anti-inflammatory drugs were administered after surgery. All participants were transferred to the postanesthesia care unit (PACU) after surgery and then escorted back to the cardiothoracic surgery ward by the anesthesia nurse after extubation. All surgeries were performed by an experienced, skilled chief surgeon who was blinded to the grouping. Patients were discharged when they met the following criteria: (1) surgical wounds were healing well without infection; (2) pain wound score was less than 3 points (assessed by the Visual Analog Scale); (3) vital signs were stable; and (4) they had no postoperative complications, such as pneumothorax, pleural effusion, and pulmonary infection.
Measurements
The primary outcome was the quantities of NK cells. For all patients, a 5-mL venous blood sample was drawn from the forearm vein with a syringe containing heparin at 30 minutes before induction (T0), 5 minutes after intubation (T1), at the beginning of the operation (T2), at the beginning of the lobectomy (T3), at the beginning of the lymphadenectomy (T4), and immediately after extubation (T5), which was used to count NK cells and detect the serum levels of TNF-a and IL-6. The quantity of NK cells was counted using Attune NxT flow cytometry (Beijing Xinlei Zhongsen Biotech Co Ltd, Beijing, China). The secondary outcomes were the serum levels of TNF-α and IL-6, the mean arterial pressure (MAP) and HR, the interoperative consumption of propofol and remifentanil, the incidence of hypoxemia, postoperative nausea and vomiting (PONV) in the PACU, and the length of hospital stay. The serum levels of TNF-α and IL-6 were detected by enzyme linked immunosorbent assay (Shanghai West Tang Biotechnology Co, Ltd, Shanghai, China). The detection process was completed by the Department of Clinical Laboratory of our hospital in accordance with the manufacturer’s instructions. The laboratory physicians were blinded to the study group assignments.
Sample Size Calculation
The sample size was determined based on the results of the primary outcome of NK cell counts from a pilot study. The power analysis suggested that the number of patients should be 54 in each group, with α = .05 and power (1 − β) = 0.8. Considering a drop-off rate of 20%, the preferred sample size of each group was determined to be 66 patients.
Statistics Analysis
Data were processed using SPSS version 20.0 (SPSS Inc, Chicago, Illinois). Data are presented as the means and standard deviations for continuous variables and as proportions for categorical variables. Normally distributed continuous data (determined by the Kolmogorov-Smirnov method) were compared using Student t test. The χ2 test was used to analyze categorical variables. One-way analysis of variance was used to analyze differences between the baseline values and other time points. Repeated measurements were used to analyze differences in the interaction effects between groups and different time points. A 2-tailed P value of <.05 was considered statistically significant.
Results
Participant Enrollment
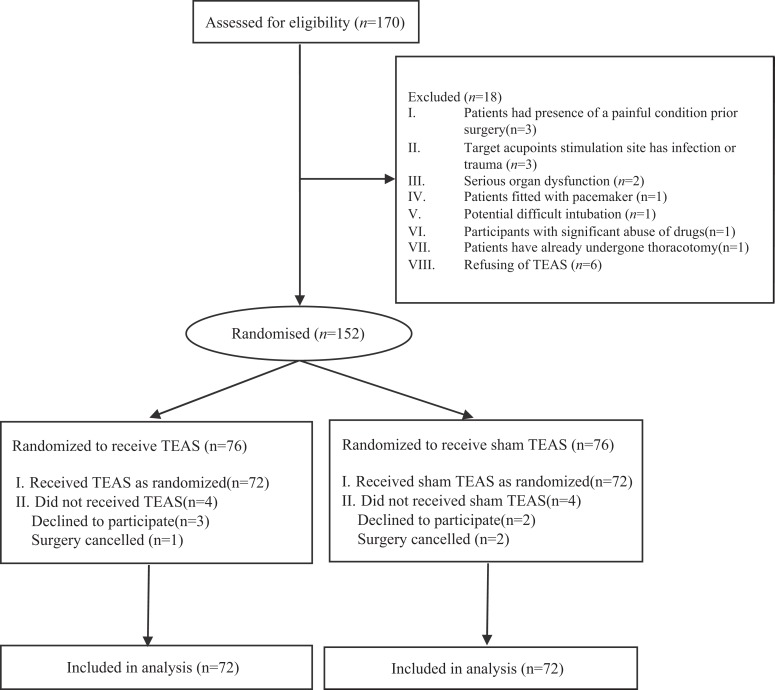
We screened 170 patients selected to undergo VATS lobectomy in our teaching hospital for eligibility, of who 144 were randomly assigned to receive either TEAS (n = 72) or sham TEAS (n = 72). Data from 144 (84.7%) patients were obtained. Twenty-six (15.3%) patients were excluded for meeting the exclusion criteria and other reasons after consent. Three patients had a painful condition prior to surgery, and three patients had target acupoint stimulation site infection or trauma. Two patients had serious organ dysfunction, and one patient was fitted with a pacemaker. One patient had the potential for a difficult intubation, one patient had significant drug abuse, and one patient had already undergone a lobectomy. Six patients refused to receive TEAS. In all, 5 patients declined to participate, 3 cases in group TEAS and 2 cases in group sham TEAS. In total, 3 surgeries were canceled for various reasons, 1 case in group TEAS and 2 cases in group sham TEAS. The flow of randomized patients is shown in Figure 1. The baseline characteristics were similar between the 2 groups (Table 1).
Figure 1.
Flow of participants randomized to receive TEAS or sham TEAS. TEAS indicates transcutaneous electrical acupoint stimulation.
Table 1.
Patient and Surgical Characteristic.a
| Characteristics | Group TEAS, n = 72 | Group Sham TEAS, n = 72 | P Value |
|---|---|---|---|
| Age, years | 64.34 (8.25) | 62.88 (8.37) | .642 |
| Sex | .383 | ||
| Male | 42 | 39 | |
| Female | 30 | 33 | |
| BMI, kg/m2 | 59.42 (8.44) | 58.43 (8.90) | .572 |
| Smoking history | 47 | 44 | .174 |
| ASA physical status | .726 | ||
| I | 38 | 35 | |
| II | 34 | 37 | |
| TNM stage | .477 | ||
| Stage I | 42 | 41 | |
| Stage II | 30 | 31 | |
| Side of surgery | .941 | ||
| Left side | 36 | 38 | |
| Right side | 36 | 34 | |
| Operation time, minutes | 165.26 (16.74) | 168.48 (12.82) | .656 |
| Anesthesia time, minutes | 187.47 (14.26) | 188.58 (13.66) | .824 |
Abbreviations: ASA, American Society of Anesthesiologist; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); TNM, tumor, node and metastasis.
a Values are shown as mean (SD) or number of patients.
Quantities of NK Cells
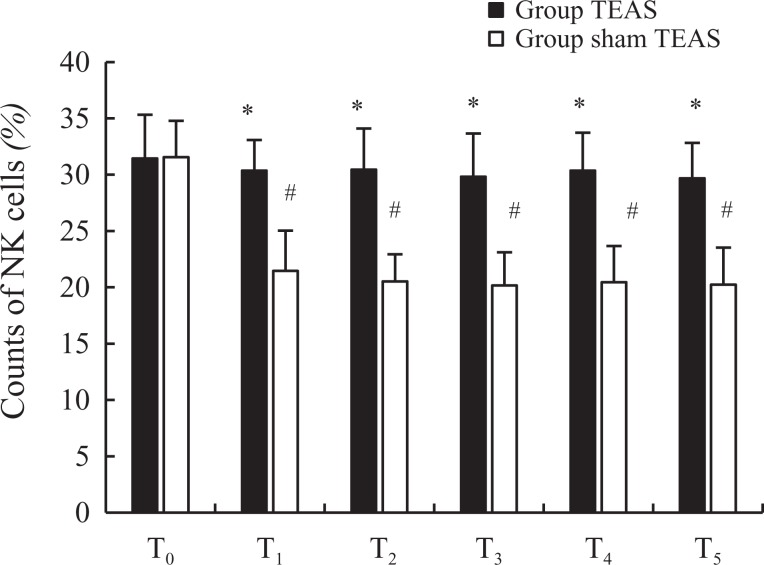
There were no significant differences in the quantities of NK cells at T0 (31.45 [3.87] vs 31.55 [3.24], P = .237) between the 2 groups. Compared to the quantity of NK cells at T0, the counts were decreased in group sham TEAS at T1 to T5 (P < .05), similar to those in group TEAS at T0 to T5 (P > .05). However, the quantities of NK cells in group sham TEAS at T1 (P = .012), T2 (P < .001), T3 (P = .027), T4 (P = .045), and T5 (P = .021) were lower than those in group TEAS (Figure 2).
Figure 2.
Counts of NK cells between 2 groups. NK indicates natural killer. *P < .05 versus group sham transcutaneous electrical acupoint stimulation (TEAS), # P < .05 versus T0. T0, 30 minutes before induction; T1, 5 minutes after intubation; T2, the beginning of the operation; T3, the beginning of the lobectomy; T4, at the beginning of the lymphadenectomy; T5, immediately after extubation.
Serum Levels of TNF-α and IL-6
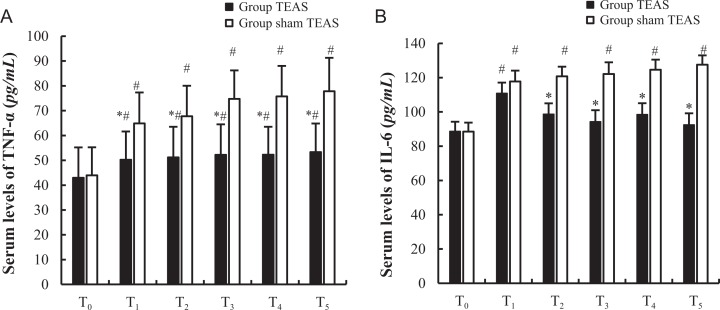
The baseline serum levels of TNF-α and IL-6 at T0 were comparable between the 2 groups (P > .05). The serum levels of TNF-α and IL-6 at T1 to T5 were increased to varying degrees in group sham TEAS. In group sham TEAS, the serum levels of TNF-α increased at T1 to T5, while the levels of IL-6 were elevated only at T1, T2, and T4, compared to the baseline levels at T0 (P < .05). However, in group TEAS, the serum levels of TNF-α at T1 (P = .014), T2 (P = .033), T3 (P < .001), T4 (P = .017), and T5 (P = .016) were lower than those in group sham TEAS, while the serum levels of IL-6 at T2 (P < .001), T3 (P < .025), T4 (P = .008), and T5 (P = .031) were lower than those in group sham TEAS (Figure 3A and B).
Figure 3.
Serum levels of TNF-α and IL-6 between 2 groups. * P < .05 versus group sham transcutaneous electrical acupoint stimulation (TEAS), # P < .05 versus T0. T0, 30 minutes before induction; T1, 5 minutes after intubation; T2, the beginning of the operation; T3, the beginning of the lobectomy; T4, at the beginning of the lymphadenectomy; T5, immediately after extubation.
Changes in MAP and HR
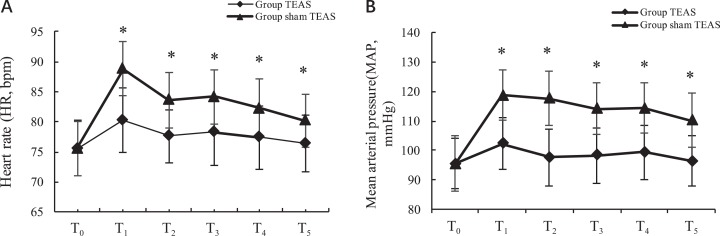
The MAPs and HRs in both groups were similar at T0 (P > .05). Compared to T0, the MAPs and HRs were significantly increased at T1to T5 in group sham TEAS (P < .05). However, in group TEAS, there were no statistically significant differences in the MAPs and HRs between time points (P > .05), and the MAPs and HRs at T1to T5 in group TEAS were lower than those in group sham TEAS (P < .05; Figure 4A and B).
Figure 4.
Heart rate and mean arterial pressure changes between 2 groups. *P < .05 versus group sham transcutaneous electrical acupoint stimulation (TEAS). T0, 30 minutes before induction; T1, 5 minutes after intubation; T2, the beginning of the operation; T3, the beginning of the lobectomy; T4, at the beginning of the lymphadenectomy; T5, immediately after extubation.
Consumption of Propofol and Remifentanil, Incidence of Hypoxemia and PONV in the PACU, and Length of Hospital Stay
Total propofol (P = .002) and remifentanil (P = .028) consumption were lower in group TEAS than that in group sham TEAS. The incidence of neither hypoxemia (P = .872) nor PONV in the PACU (P = .437) differed significantly between the 2 groups. Meanwhile, the length of hospital stay in group TEAS was shorter than that in group sham TEAS (P = .014; Table 2).
Table 2.
Consumption of Propofol and Remifentanil Intraoperative, the Incidence of Hypoxemia, Postoperative PONV in PACU, and the Length of Hospital Stay.a
| Group TEAS, n = 72 | Group Sham TEAS, n = 72 | P Value | |
|---|---|---|---|
| Propofol, mg | 827.74 (118.37) | 1029.26 (126.47) | .002b |
| Remifentanil, μg | 1145.56 (216.67) | 1864.82 (244.34) | .028b |
| Hypoxemia, n (%) | 1 (1.39) | 2 (2.78) | .872 |
| PONV, n (%) | 6 (8.33) | 8 (11.11) | .437 |
| Length of hospital stay, days | 7.42 (1.23) | 9.17 (1.31) | .014b |
Abbreviations: PACU, postanesthesia care unit; PONV, postoperative nausea and vomiting; TEAS, transcutaneous electrical acupoint stimulation.
a Values are presented as mean (SD) or number of patients.
b P < .05 versus group sham TEAS.
Discussion
Unlike other types of lung cancer, the growth and metastasis rates of NSCLC are relatively slow. Therefore, early surgical treatment has a chance to cure the disease. The VATS lobectomy surgical procedure has an efficacy comparable to that of traditional thoracotomy but is less likely to cause further trauma, pain, and perioperative morbidity.18 However, since malignant tumors are transferrable and commonly reoccur, clinical treatments for the disease are particularly difficult. Although surgical resection is the main method of treatment, the proliferation and distant metastasis of residual tumor cells can occur during the perioperative period.19 This phenomenon is primarily related to tumor cell dissemination, perioperative stress response, and the inhibition of cellular immune function.20,21 Perioperative immunosuppression allows tumor cells to escape immune surveillance and promotes tumor metastasis.22 Accordingly, the application of preventive measures is of great significance to protect patients’ immunological function, decrease perioperative immunosuppression, reduce tumor recurrence and metastasis, and improve the long-term prognosis of patients with cancer.
The effects of acupuncture and related techniques on perioperative immunological function have been focused on extensively for the last decade. However, the underlying mechanisms are still unclear. The TEAS adds an electrical stimulation pulse to target acupoints using electrodes placed on the surface instead of acupuncture needles. The TEAS has been found to reduce analgesic consumption and the incidence of nausea and vomiting and to accelerate anesthesia recovery.16,23 Traditional Chinese medicine holds that acupuncture at BL13 can treat respiratory diseases. An animal experiment showed that electroacupuncture at BL13 and Zusanli (ST36) downregulated the expression of orexins and their receptors, reduced lung inflammation, improved rat lung function, and delayed the development of chronic obstructive pulmonary disease.24 Luo et al suggested that the electroacupuncture stimulation of BL13 downregulated the lung index and serum TNF-α levels and upregulated the serum IL-10 levels in mice with viral pneumonia.25 These studies indicated that stimulation at BL13 can improve immunological function and the prognosis for respiratory diseases. Electroacupuncture at LI4 improved perioperative immunological function26,27 and inhibited tumor growth.28 Recently, some experimental and clinical studies have shown that sequential electroacupuncture stimulation applied at ST36 performed well in the treatment of stress-induced immunodeficiency.29,30 Electrical stimulation at ST36 can also activate the immune system and support anticancer treatments.31 Accordingly, BL13, LI4, and ST36 were selected as target acupoints for electrical stimulation in our study.
Studies demonstrated that acupuncture promoted the release of cytokines activated by NK cells and enhanced nonspecific antitumor immunity.10 Additionally, a current study by Wu et al suggested that TAES was able to partially attenuate the postoperative immune depression of patients with lung cancer by regulating the balance of T lymphocytes and the expression levels of related cytokines and transcription factors,15 but they did not discuss the impact of TEAS on NK cells and related immunologic factors, such as TNF-α and IL-6. Actually, NK cells play an all-star role in protecting hosts from carcinogenesis and metastases. The NK cells are essential and critical effector cells of the innate immune system that spontaneously kill transformed and infected cells and represent the first line of the host immune defense against cancer and pathogens. The NK cells recognize and kill cancer cells by 2 major constitutive mechanisms: the secretory/necrotic mechanism and the nonsecretory/apoptotic mechanism. Studies have shown that IL-6 is the key factor in many inflammatory responses, and serum IL-6 concentrations are positively correlated with the degree of tissue injury.32,33
In this study, the quantity of NK cells was decreased slightly in group TEAS after induction. In contrast, the quantity of NK cells decreased significantly after induction in group sham TEAS. As expected, in group sham TEAS, the number of cells declined markedly after induction compared to that in group TEAS. This result suggested that TEAS may reverse the decrease in NK cells during the perioperative period. Cytokines of TNF-α and IL-6 play the core role in the pathogenesis of inflammatory damage.34 Therefore, inhibiting the expression and release of inflammatory cytokines can reduce perioperative inflammatory response and protect the immune system. A review addressed that acupuncture exerted anti-inflammatory actions and inhibited macrophage activation and the production of TNF, IL-6, and other proinflammatory cytokines through central inhibition of the innate immune system.35 Another clinical research showed that TEAS diminished the lower limb ischemia–reperfusion injury and improved pulmonary function by downregulating the levels of inflammatory cytokines, such as TNF-α and IL-6.36 Previous studies proved that increased levels of IL-6 may inhibit the cytotoxic activity of NK cells by decreasing the expression of perforin, granzyme B (a serine protease), and tyrosine phosphatase-2 (SHP-2) but do not decrease NK cell numbers.37,38 As an important inflammatory cytokine, TNF-α directly influences NK cell activation and cytotoxic effects on tumor cells.39 In our study, the serum levels of TNF-α and IL-6 were increased to varying degrees in both the groups. The levels of TNF-α in group TEAS were lower at T1 to T5 than those in group sham TEAS, while the levels of IL-6 were lower at T2 to T5 in group TEAS. These results indicated that TEAS decreased the levels of TNF-α and IL-6 and demonstrated that the intensity of inflammatory reactions in the body was decreased and may also account for the stable NK cell levels. Conversely, the increased number of NK cells may theoretically inhibit the increase in TNF-α and IL-6 levels. However, we did not detect the cytoactivity of the NK cells. Additional studies are needed to investigate the relationship between changes in TNF-α and IL-6 levels and the stability of NK cells under the intervention of TEAS. Simultaneously, the MAPs and HRs in group TEAS were comparable at all 5 time points. Nevertheless, the MAPs and HRs after induction were higher than the baseline values in group sham TEAS, demonstrating that TEAS was beneficial to maintaining hemodynamic stability and reducing the stress response.
In addition, the consumption of propofol and remifentanil in group sham TEAS was much higher than that in group TEAS. The results were in accordance with a previous study by Huang et al.16 However, no significant differences in the incidences of hypoxemia or PONV in the PACU existed between the 2 groups. These observations may be related to the following factors: Researchers enrolled participants according to strict criteria; the respiratory functions of the participants were in a good state; partial lobectomy had little effect on respiratory function; and combined with good intraoperative management, the incidence of hypoxemia was relatively small. Thus, the effect of TEAS on respiratory function could not be accurately evaluated. Second, research suggested that electrical stimulation at Neiguan (PC6) can reduce the incidence of PONV.16 In our study, however, PC6 was not selected as a target acupoint. This may explain why the incidences of PONV were similar between the 2 groups. The length of hospital stay in group TEAS was also shorter than that in group sham TEAS, indicating that TEAS was beneficial to accelerating postoperative recovery and improving prognosis.
Limitations
As both study groups were comprised of participants from the same hospital, our findings might not be generalizable to other hospitals despite that both groups were highly homogeneous. Since we can determine the effectiveness of TEAS by the de qi sensations, the patients were not completely blinded to the interventions, which may have affected the overall results. However, although the patients experienced the sensation of de qi, they did not know the group in which they were placed, since we excluded patients who have received acupuncture point stimulation or had experience with acupuncture electrode. Additionally, all patients were told that TEAS was carried out throughout the surgery. In addition, patients were treated separately to prevent communication throughout the trial and to reduce the bias of blinding as much as possible. The study was supposed to use absolute time, which would improve the objectivity of data collection and the reliability of results. We did not follow the long-term prognosis or tumor-free survival periods of the participants in our study. The long-term assessment of tumor progression after surgery is an important focus, and we recorded only the length of the hospital stay in the current study. In future studies, larger sample-sized, double-blind multicenter randomized controlled trials with a long-term assessment of tumor progression after surgery are warranted to gather more evidence on the detailed effects of TEAS on the immunological function of patients with cancer.
Conclusion
The application of TEAS can effectively reverse the decrease in NK cells, reduce the serum levels of TNF-α and IL-6, and depress the inflammatory reaction during the perioperative period. The TEAS was also beneficial for maintaining hemodynamic stability, reducing the stress response, decreasing the interoperative consumption of propofol and remifentanil, and decreasing the length of the hospital stay.
Acknowledgments
The authors would like to thank the study investigators, study center staff, and all trial participants and their families. The authors thank the professional guidance given by doctors from Department of Traditional Chinese Medicine and Department of Clinical Laboratory of our hospital.
Abbreviations
- BIS
bispectral index
- HR
heart rate
- IL
interleukin
- MAP
mean arterial pressure
- NK
natural killer
- NSCLC
non-small cell lung cancer
- PACU
postanesthesia care unit
- PONV
postoperative nausea and vomiting
- TEAS
transcutaneous electrical acupoint stimulation
- TNF
tumor necrosis factor
- VATS
video-assisted thoracic surgery.
Authors’ Note: Qing Tu and Zhou Yang drafted the manuscript. Qiaofeng Song were responsible for patient recruitment. Yan Wang collected individual data. Jian Zhang performed statistical analyses. Jianhui Gan and Bin Que contributed to study conception. Jianhui Gan reviewed the manuscript and approved final submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jianhui Gan, MD  https://orcid.org/0000-0002-8399-827X
https://orcid.org/0000-0002-8399-827X
References
- 1. Esposito L, Conti D, Ailavajhala R, Khalil N, Giordano A. Lung cancer: are we up to the challenge? Curr Genomics. 2010;11(7):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opin Biol Ther. 2012;12(7):923–937. [DOI] [PubMed] [Google Scholar]
- 3. Micheli DC, Jr, Fernandes PC, Cruvinel JC, Nomelini ID, Murta EF, Tavares-Murta BM. Circulating cytokines and nitric oxide are involved in the inhibition of neutrophil migration in patients with uterine cervical neoplasia. Clin Med Insights Oncol. 2012;6:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bobocea AC, Trandafir B, Bolca C, Cordoş I. Minimally invasive surgery in cancer. Immunological response. Chirurgia (Bucur). 2012;107(2):154–157. [PubMed] [Google Scholar]
- 5. Zhang W, Huang J. Effect of thoracoscopic lobectomy on inflammatory factors and immune function in the patients with non-small cell lung cancer [in Chinese]. Prac J Cancer. 2017;32(2):295–298. [Google Scholar]
- 6. Desai H, Natt B, Kim S, Bime C. Decreased in-hospital mortality afterlobectomy using video-assisted thoracoscopic surgery compared with open thoracotomy. Ann Am Thorac Soc. 2017;14(2):262–266. [DOI] [PubMed] [Google Scholar]
- 7. Chapman CR, Schimek F, Gehrig JD, Gerlach R, Colpitts YH. Effects of nitrous oxide, transcutaneous electrical stimulation, and their combination on brain potentials elicited by painful stimulation. Anesthesiology. 1983;58(3):250–256. [DOI] [PubMed] [Google Scholar]
- 8. Acupuncture in physiotherapy: key concepts and evidence based-practice In: Allen H, Edwards R, eds. 1st ed Oxford, UK: Butterworth-Heinemann; 2004. [Google Scholar]
- 9. Kondo T, Kawamoto M. Acupuncture and moxibustion for stress-related disorders. Biopsychosoc Med. 2014;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnston MF, Ortiz Sánchez E, Vujanovic NL, Li W. Acupuncture may stimulate anticancer immunity via activation of natural killer cells. Evid Based Compl Alternat Med. 2011;2011:481625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrari de Andrade L, Tay RE, Pan D, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359(6383):1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54–74. [DOI] [PubMed] [Google Scholar]
- 13. Kharbanda RK, Peters M, Walton B, et al. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia–reperfusion in humans in vivo. Circulation. 2001;103(12):1624–1630. [DOI] [PubMed] [Google Scholar]
- 14. Tian L, Huang YX, Tian M, Gao W, Chang Q. Downregulation of electroacupuncture at ST36 on TNF-alpha in rats with ulcerative colitis. World J Gastroenterol. 2003;9(5):1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu H, Wang K, Li G, et al. Effects of transcutaneous acupoint electrical stimulation on the imbalance of Th1, Th2, Th17 and Treg cells following thoracotomy of patients with lung cancer. Exp Ther Med. 2016;11(2):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang S, Peng W, Tian X, et al. Effects of transcutaneous electrical acupoint stimulation at different frequencies on perioperative anesthetic dosage, recovery, complications, and prognosis in video-assisted thoracic surgical lobectomy: a randomized, double-blinded, placebo-controlled trial. J Anesth. 2017;31(1):58–65. [DOI] [PubMed] [Google Scholar]
- 17. Qu F, Li R, Sun W, et al. Use of electroacupuncture and transcutaneous electrical acupoint stimulation in reproductive medicine: a group consensus. J Zhejiang Univ Sci B. 2017;18(3):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila). 2007;2(6):261–292. [DOI] [PubMed] [Google Scholar]
- 19. Green JS, Tsui BC. Impact of anesthesia for cancer surgery: continuing professional development. Can J Anaesth. 2013;60(12):1248–1269. [DOI] [PubMed] [Google Scholar]
- 20. Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–1339. [DOI] [PubMed] [Google Scholar]
- 21. Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10(8):972–992. [DOI] [PubMed] [Google Scholar]
- 22. Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):S32–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Xie Y, Zhang Q, et al. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: a prospective, randomized, placebo-controlled trial. Br J Anaesth. 2014;112(6):1075–1082. [DOI] [PubMed] [Google Scholar]
- 24. Zhang XF, Zhu J, Geng WY, et al. Electroacupuncture at Feishu (BL13) and Zusanli (ST36) down-regulates the expression of orexins and their receptors in rats with chronic obstructive pulmonary disease. J Integr Med. 2014;12(5):417–424. [DOI] [PubMed] [Google Scholar]
- 25. Luo W, Wang JY, Liu CL, Huang C. Effect of electroacupuncture stimulation of “Feishu” (BL13) on lung index, serum and lung IL-10 and TNF-alpha levels in mice with viral pneumonia. Zhen Ci Yan Jiu. 2014;39(4):293–297. [PubMed] [Google Scholar]
- 26. Lai M, Wang SM, Wang Y, Tang CL, Kong LW, Xu XY. Effects of electroacupuncture of “Zusanli” (ST36), “Hegu” (LI4) and/or “Sanyinjiao” (SP9) on immunofunction in gastric carcinectomy rats. Zhen Ci Yan Jiu. 2008;33(4):245–249. [PubMed] [Google Scholar]
- 27. Gu CY, Shen LR, Ding YH, et al. Effect of different anesthesia methods on immune function in patients of laparoscopic cholecystectomy in peri-operational period. Zhongguo Zhen Jiu. 2011;31(4):236–240. [PubMed] [Google Scholar]
- 28. Lai M, Wang SM, Zhang WL, et al. Effects of electroacupuncture on tumor growth and immune function in the Walker-256 model rat. Zhongguo Zhen Jiu. 2008;28(8):607–609. [PubMed] [Google Scholar]
- 29. Richardson PH, Vincent CA. Acupuncture for the treatment of pain: a review of evaluative research. Pain. 1986;24(1):15–40. [DOI] [PubMed] [Google Scholar]
- 30. Lu GW. Characteristics of afferent fiber innervation on acupuncture points Zusanli. Am J Physiol. 1983;245(4):R606–R612. [DOI] [PubMed] [Google Scholar]
- 31. Rogers PA, Schoen AM, Limehouse J.Acupuncture for immune-mediated disorders. Literature review and clinical applications. Probl Vet Med. 1992;4(1):162–193. [PubMed] [Google Scholar]
- 32. Gonçalves de Freitas AT, Lemonica L, De Faveri J, Pereira S, Bedoya Henao MD. Preemptive analgesia with acupuncture monitored by c-fos expression in rats. J Acupunct Meridian Stud. 2016;9(1):16–21. [DOI] [PubMed] [Google Scholar]
- 33. Van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280–293. [DOI] [PubMed] [Google Scholar]
- 34. Ni X, Xie Y, Wang Q, et al. Cardioprotective effect of transcutaneous electric acupoint stimulation in the pediatric cardiac patients: a randomized controlled clinical trial. Paediatr Anaesth. 2012;22(8):805–811. [DOI] [PubMed] [Google Scholar]
- 35. Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6(3):251–257. [DOI] [PubMed] [Google Scholar]
- 36. Mo Y, Chen S, Yang L, et al. The effect of transcutaneous electrical acupoint stimulation on inflammatory response in patients undergoing limb ischemia–reperfusion. Mediators Inflamm. 2017;2017:8369737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cifaldi L, Prencipe G, Caiello I, et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67(11):3037–3046. [DOI] [PubMed] [Google Scholar]
- 38. Kang YJ, Jeung IC, Park A, et al. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum Reprod. 2014;29(10):2176–2189. [DOI] [PubMed] [Google Scholar]
- 39. Balasa B, Yun R, Belmar NA, et al. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol Immunother. 2015;64(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]