Abstract
Background:
Precise local radiotherapy for adrenal metastasis can prolong the useful life of patients with oligometastasis. The aim of this retrospective, 2-center study was to establish the safety and effectiveness of real-time tumor-tracking radiotherapy and general stereotactic body radiotherapy in treating patients with adrenal metastatic tumors.
Materials and Methods:
Thirteen lesions in 12 patients were treated with real-time tumor-tracking radiotherapy (48 Gy in 8 fractions over 2 weeks) and 8 lesions in 8 patients were treated with general stereotactic body radiotherapy (40-50 Gy in 5-8 fractions over 2 weeks or 60-70 Gy in 10 fractions over 2 weeks). Overall survival rates, local control rates, and adverse effects were analyzed.
Results:
The actuarial overall survival rates for all patients at 1 and 2 years were 78.5% and 45.8%, respectively, with a median follow-up of 17.5 months, and the actuarial local control rates for all tumors at 1 and 2 years were 91.7% and 53.0%, respectively, with a median follow-up of 9 months. A complete local tumor response was obtained in 3 tumors treated by real-time tumor-tracking radiotherapy (lung adenocarcinomas with diameters of 35, 40, and 60 mm). There was a statistically significant difference in the local control between the groups treated by real-time tumor-tracking radiotherapy (100% at 1 year) and general stereotactic body radiotherapy (50% at 1 year; P < .001). No late adverse reactions at Grade 2 or higher were reported for either treatment group.
Conclusions:
This study showed that although both treatments are safe and effective, the real-time tumor-tracking radiotherapy is more effective than general stereotactic body radiotherapy in local control for adrenal metastasis.
Keywords: adrenal gland, metastasis, stereotactic body radiotherapy, retrospective, survival rates
Introduction
Local therapy for oligometastatic tumors is receiving considerable attention because of its potential to prolong the life of patients with cancer.1 We have previously reported that adrenal metastatic tumors can be successfully treated with real-time tumor-tracking radiotherapy (RTRT), with a high local control (LC) rate and low complication rate.2,3 The RTRT method tracks the adrenal gland movement along the craniocaudal direction during respiration, which can be large and so needs to be taken into consideration during radiotherapy.3,4 Stereotactic body radiotherapy (SBRT) has also been used for treating adrenal metastatic tumors; although initial reports described results that did not agree with the results of LC rates,5,6 an increasing number of studies have confirmed high LC rates for these tumors.6-18 In general, SBRT has minimal acute and chronic adverse effects, but serious gastrointestinal (GI) adverse reactions have been reported in rare instances.19,20 It has been suggested that the prognostic parameters for serious adverse reactions with SBRT are the dose administered to the GI tract and the use of concurrent chemotherapy.19 A systematic review suggested that SBRT is a valid alternative treatment for adrenal metastasis in patients with oligometastasis when surgery is not feasible or when the operative risk is unacceptable.21 However, there have been few reports about the appropriate dosimetric parameters and their relationship to clinical outcomes.
In this study, we retrospectively investigated dosimetric parameters and clinical outcomes related to treatment with RTRT and general SBRT (g-SBRT) for adrenal metastatic tumors at Hokkaido University Hospital and the University of Yamanashi Hospital in Japan.
Materials and Methods
This study included 20 patients with adrenal metastatic tumors treated without the use of concurrent chemotherapy between 2004 and 2017, 15 in the Hokkaido University Hospital and 5 in the University of Yamanashi Hospital. Before receiving treatment, the patients were given a detailed explanation about the procedure and each provided written informed consent. The present retrospective study was approved by the institutional review boards of both institutions (the approval number is 017-0109 from the Hokkaido University Hospital and 961 from the University of Yamanashi Hospital).
The patients were 19 men and 1 woman, with a median age of 66 years (range 48-86 years). Table 1 presents the clinical characteristics. The primary site was the lung in 9 patients (1 small cell cancer, 1 squamous cell cancer, and 7 adenocarcinomas), the liver in 5 patients (all hepatocellular carcinomas), the kidney in 4 patients (all renal cell carcinomas), transitional cell carcinoma of the bladder in 1 patient, and prostate cancer in 1 patient. One of the 20 patients received treatment for both adrenal glands at different times; thus a total of 21 tumors were investigated for LC and adverse reactions. The median tumor size was 40 mm (range, 14-80 mm).
Table 1.
Patient Characteristics.
| RTRT (n = 12) | g-SBRT (n = 8) | |
|---|---|---|
| Sex | ||
| Men | 11 | 8 |
| Women | 1 | 0 |
| Age (years) | ||
| Median (range) | 66 (55-80) | 65 (48-86) |
| ECOG performance status | ||
| 0 | 1 | 3 |
| 1 | 10 | 4 |
| 2 | 1 | 1 |
| Primary tumor site | ||
| Lung (NSCLC) | 7 (6) | 2 (2) |
| Liver | 1 | 4 |
| Kidney | 3 | 1 |
| Prostate | 1 | 0 |
| Bladder | 0 | 1 |
| Lateralitya | ||
| Left | 6 | 3 |
| Right | 7 | 5 |
| Diameter (mm) | ||
| Median (range) | 56 (19-80) | 38 (14-74) |
| Synchronous extra-adrenal lesion | 7 | 7 |
| Symptoms before RT chemotherapy | 1 | 2 |
| Before RT | 7 | 3 |
| After RT | 10 | 5 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; g-SBRT, general stereotactic body radiotherapy; NSCLC, non-small cell lung cancer; RT, radiotherapy; RTRT, real-time tumor-tracking radiotherapy; SD, standard deviation.
aBoth glands were treated in 1 patient.
The treatment method for the RTRT has been described elsewhere.3,22 In the RTRT, treatment planning was based on computed tomography (CT) images with a slice thickness of 2.0 or 2.5 mm recorded while the patients were holding their breath at the end of normal expiration after insertion of a 2-mm gold marker near the tumor. The gross tumor volume (GTV) was delineated as the visible tumor on the CT image by the attending physician. The GTV-to-clinical target volume (CTV) margin, internal margin, and set-up margin were determined to be 3.0, 2.0, and 3.0 mm, respectively, from the preclinical physical evaluation. A summation of these margins is used to determine the planning target volume (PTV). The coordinates of the fiducial gold marker in the same CT images were also delineated and registered. The RTRT system consists of 4 sets of diagnostic fluoroscopic X-ray imaging units and a conventional linear accelerator with multileaf collimators which enable 3-dimensional conformal irradiation. At the start of the treatment, the patient is aligned so that the fiducial marker at the end-of-expiration is at the planned position, corresponding to the center of the gating window. After the start of therapeutic irradiation, the coordinates of the gold marker are monitored every 0.033 seconds by fluoroscopy and the linear accelerator is gated to irradiate the tumor only when the coordinates of the gold marker are within ± 2.0 mm of the planned position.3,22 Patients were not immobilized and were treated while breathing freely. When a baseline shift of the coordinates of the gold marker is detected and it exceeds 2 mm, the patient is realigned by adjusting the couch position so that the marker at the end-of-expiration again is at the center of the gating window.22
In the g-SBRT at Hokkaido University Hospital, free-breathing CT images and 4-dimensional CT (4DCT) images with a slice thickness of 2.5 mm were obtained in all the 3 patients. The 4DCT scan parameters have been described elsewhere.23 The GTV was delineated as the visible tumor on the free-breathing CT images by the attending physician. The internal margin was determined to include the whole tumor position during respiration using the 4DCT images in all 10 respiratory phases. The set-up margin was 5.0 mm according to the preclinical physical evaluation. The GTV-to-CTV margin was set at 0 mm because there must be an overlap between these imaginary margins. Patients were not immobilized and treated while breathing freely. Daily cone beam CT images were obtained before each treatment fraction in all patients.
In the g-SBRT at the University of Yamanashi Hospital, the patients were trained in voluntarily holding the breath during the inspiration phase using a respiratory indicator.24 Treatment planning was based on CT images with a slice thickness of 2.0 mm, which were recorded under voluntary breath-holding during the inspiration phase. A personal internal margin was calculated from 3 CT scans. The GTV was delineated as the visible tumor in the CT image by the attending physician. The GTV-to-CTV margin was set at 0 mm. The PTV was determined as the GTV plus the personal internal margin with an additional margin of 2.0 mm to compensate for intrasession reproducibility and to provide a safety margin. Vacuum cushions were used for immobilization and patients were treated with the breath held voluntarily during the inspiration phase. Before every radiotherapy fraction, the beam isocenter was visually adjusted with in-room CT images of 2-mm thickness taken under voluntary breath-holding to correspond to the planned isocenter.19
The X-ray energies of 6, 9, and 10 MV were used for 14, 1, and 6 tumors, respectively. The dose–volume metrics were based on 3-dimensional radiotherapy planning systems such as Pinnacle and Xio. Superposition, convolution, and Clarkson algorithms were used for 19, 1, and 1 tumors, respectively. Multiportal noncoplanar irradiation or rotational irradiation was used for all the patients. Quality assurance for the RTRT and g-SBRT was performed based on the guidelines for respiratory motion management in radiotherapy published by the Japan conformal external beam radiotherapy group and others in 2013.25
The dose prescription was based on published studies about hypofractionated SBRT. Eight fractionation schedules were used at Hokkaido University Hospital for lung and liver disease. Five or 10 fractionation schedules were used at the University of Yamanashi Hospital for lung diseases including those of the lower lung field. Dose constraints for each fractionation were as determined at either hospital and the target doses were determined accordingly. For comparison, the radiation dose was converted to the biologically effective dose (BED) by using a linear-quadratic model, with BED defined as nd×(1 + d/[α/β]), where n is the number of fractions and d is the dose per fraction, assuming α/β ratios of 10 (BED10) for tumors and 3 (BED3) for organs at risk. At Hokkaido University Hospital, all the 12 patients with 13 tumors treated with RTRT were administered a prescribed dose of 48 Gy in 8 fractions (BED10 = 76.8 Gy) at the isocenter and the 3 patients treated with g-SBRT were administered doses as follows: 48 Gy in 8 fractions at the isocenter (1 patient), 48 Gy in 8 fractions to the 95% volume of PTV (PTVD95; 1 patient), and 40 Gy in 8 fractions (BED10 = 60 Gy) to PTVD95 (1 patient). The dose constraint for the stomach and duodenum was that the dose to 1 cm3 (D1 cc) would be 35 Gy or less, with the BED3 86 Gy.3 At the University of Yamanashi Hospital, for the 5 patients treated with g-SBRT, the dose administration was as follows: 60 Gy in 10 fractions (BED10 = 96 Gy) at the isocenter (1 patient), 70 Gy in 10 fractions (BED10 = 119 Gy) at the isocenter (1 patient), 40 Gy in 5 fractions (BED10 = 72 Gy) to PTVD95 (1 patient), 50 Gy in 5 fractions (BED10 = 100 Gy) to PTVD95 (1 patient), and 42 Gy in 7 fractions (BED10 = 67.2 Gy) to CTVD90 (1 patient).
The local tumor response was assessed in accordance to the Revised Response Evaluation Criteria in Solid Tumors Guideline (version 1.1). A locally controlled disease was defined as the absence of progressive disease. Treatment-related toxicities based on the information in the patients’ medical records were assessed with the Common Terminology Criteria for Adverse Events v4.0, and toxicities of Grade 2 or higher were recorded. The follow-up duration was calculated from the start date of the radiotherapy. The Kaplan-Meier method was used to calculate overall survival (OS) and LC rates. Fisher exact test, Student t test, and the log-rank test were used to compare subgroups. A P value <.05 was considered statistically significant. The statistical analysis was performed using JMP Pro version 12.2 (SAS, Cary, North Carolina).
Results
The metrics for the nominal dosimetric parameters are presented in Tables 2 and 3. Because the dose distributions for RTRT, which uses gating, and for g-SBRT, which does not use gating, cannot be compared directly using these values, the values are shown as nominal doses. The statistical comparison illustrates the differences in nominal doses between the gated and nongated irradiation but not the physical dose deposited at the same location in the moving organs. All the tumors were treated without violation of the dose constraint protocol, except for 1 RTRT patient who had a left adrenal tumor attached to the gastric wall. This patient received 48 Gy in 8 fractions at the isocenter for a 6.5 cm diameter left adrenal tumor with the D1 cc of the stomach of 37.1 Gy, higher than the dose constraint of 35 Gy, while other parameters were within the dose constraints. In 5 patients in the RTRT series, the dose to 1.0 cm3 of the bowel exceeded 35 Gy.
Table 2.
BED10 Parameters for the GTV and PTV.
| RTRT (n = 13) | g-SBRT (n = 7a) | P Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| GTV | |||
| Volume (mL) | 50.5 (42.0) | 48.6 (61.9) | .94 |
| Dmean (Gy) | 73.9 (1.4) | 86.9 (14.9) | .0049 |
| Dmin (Gy) | 47.2 (14.5) | 73.0 (25.1) | .0088 |
| Dmax (Gy) | 80.9 (3.7) | 90.1 (14.7) | .041 |
| D98 (Gy) | 59.1 (9.4) | 89.3 (15.0) | .0013 |
| D50 (Gy) | 75.0 (1.1) | 87.1 (14.5) | .0067 |
| D2 (Gy) | 79.5 (2.6) | 81.1 (16.6) | .029 |
| PTV | |||
| Volume (mL) | 148.6 (87.7) | 118.1 (102.9) | .49 |
| Dmean (Gy) | 69.1 (2.8) | 81.7 (13.7) | .0043 |
| Dmin (Gy) | 29.0 (11.1) | 44.6 (17.8) | .026 |
| Dmax (Gy) | 81.4 (3.8) | 90.2 (14.7) | .027 |
| D98 (Gy) | 46.7 (12.3) | 66.5 (18.6) | .0098 |
| D95 (Gy) | 40.0 (13.4) | 61.0 (19.7) | .011 |
| D50 (Gy) | 72.7 (1.5) | 83.1 (13.3) | .010 |
| D2 (Gy) | 80.0 (2.5) | 89.1 (14.8) | .036 |
a One patient was excluded from this analysis because dosimetric data were not available.
Abbreviations: BED, biologically effective dose; Dmax, maximum dose; Dmean, mean dose; Dmin, minimum dose; Dxx, the dose received by xx % of the GTV or PTV volume; g-SBRT, general stereotactic body radiotherapy; GTV, gross tumor volume; PTV, planning target volume; RTRT, real-time tumor-tracking radiotherapy; SD, standard deviation.
P values in boldface indicate statistical significance (P < 0.05).
Table 3.
BED3 Parameters for Organs at Risk.
| RTRT (n = 13) | g-SBRT (n = 7a) | P Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Stomach | |||
| D1cc (Gy) | 47.2 (22.4) | 32.8 (9.5) | .123 |
| Dmax (Gy) | 65.4 (31.7) | 47.6 (26.9) | .22 |
| Duodenum | |||
| D1cc (Gy) | 47.8 (19.1) | 47.6 (35.6) | .99 |
| Dmax (Gy) | 69.7 (31.8) | 77.1 (45.4) | .67 |
| Bowel | |||
| D1cc (Gy) | 68.8 (43.5) | 47.5 (36.2) | .28 |
| Dmax (Gy) | 77.9 (47.5) | 64.3 (59.3) | .58 |
| Ipsilateral kidney | |||
| Dmean (Gy) | 20.7 (10.4) | 13.5 (10.8) | .16 |
| Liver | |||
| Dmean (Gy) | 8.8 (6.4) | 9.5 (8.6) | .83 |
a One patient was excluded from this analysis because dosimetric data were not available.
Abbreviations: BED, biologically effective dose; Dmax, maximum dose; Dmean, mean dose; D1 cc, the dose to 1 cm3 of the organ at risk; g-SBRT, general stereotactic body radiotherapy; RTRT, real-time tumor-tracking radiotherapy; SD, standard deviation
The local tumor response and adverse reactions are summarized in Table 4. A complete response was obtained in 3 tumors (lung adenocarcinomas with diameters of 35, 40, and 60 mm) out of the 13 tumors treated with RTRT, but in none of the 8 tumors treated with g-SBRT. The differences in overall response rates (complete response plus partial response) did not differ significantly between RTRT and g-SBRT (P = .673).
Table 4.
Clinical Outcomes for the 21 Tumors.
| RTRT (n = 13) | g-SBRT (n = 8) | |
|---|---|---|
| Local tumor response | ||
| CR | 3 | 0 |
| PR | 2 | 4 |
| SD | 4 | 2 |
| PD | 4 | 2 |
| Acute adverse effect | ||
| Grade 2 | 4 | 0 |
| Grade 3-5 | 0 | 0 |
| Late adverse effect | ||
| Grade 2-5 | 0 | 0 |
Abbreviations: CR, complete response; g-SBRT, general stereotactic body radiotherapy; PD, progressive disease; PR, partial response; RTRT, real-time tumor-tracking radiotherapy; SD, stable disease.
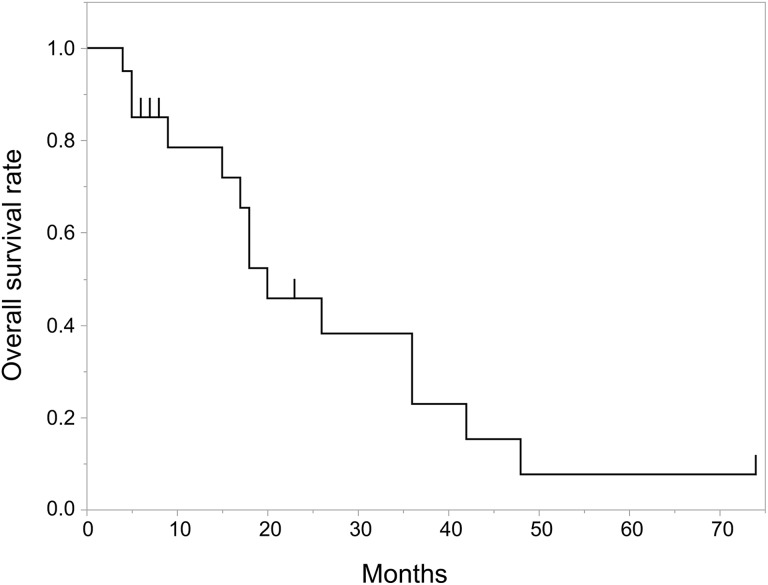
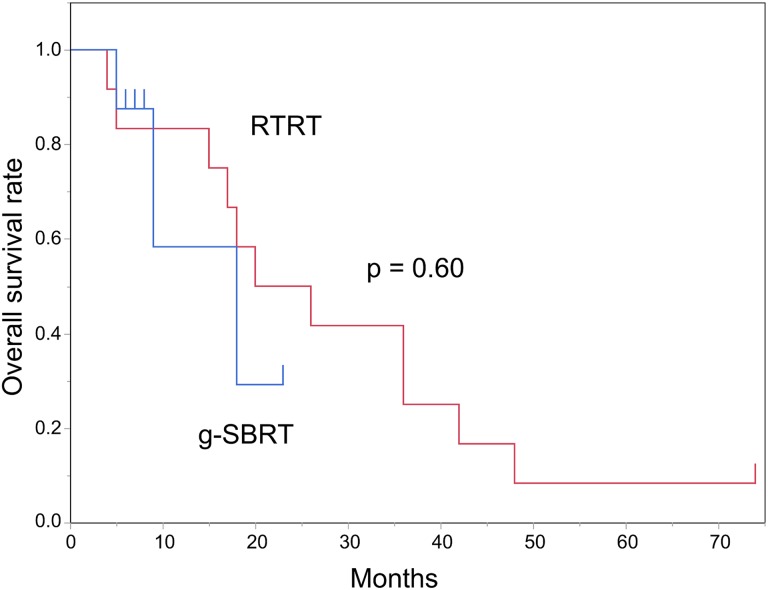
The actuarial OS at 1 and 2 years were 78.5% and 45.8%, with a median follow-up of 17.5 months for all patients (Figure 1). For the 12 patients treated with RTRT, the actuarial OS at 1 and 2 years was 83.3% and 50.0%, with a median follow-up of 23 months. For the 8 patients treated with g-SBRT, the actuarial OS at 1 year was 58.3% with a median follow-up of 7.5 months. There was no statistically significant difference in OS between the RTRT and g-SBRT groups (P = .60; Figure 2). For the 8 patients with non-small cell lung cancer, the actuarial OS at 1 and 2 years was 87.5% and 58.3%, with a median follow-up of 12 months.
Figure 1.
Kaplan-Meier curve of overall survival rates for all patients.
Figure 2.
Overall survival rates following treatment by real-time tumor-tracking radiotherapy (RTRT, blue line) or general stereotactic body radiotherapy (g-SBRT, red line).
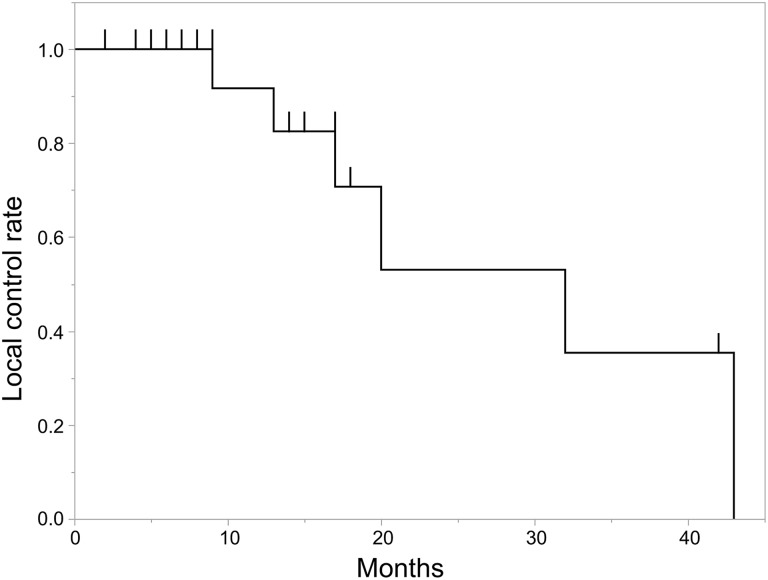
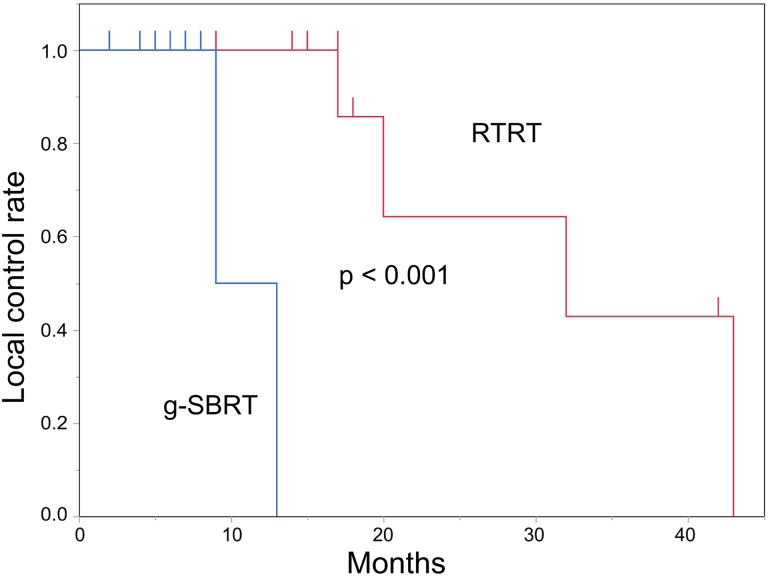
The actuarial LC for all tumors at 1 and 2 years was 91.7% and 53.0%, with a median follow-up of 9 months (Figure 3). For the 12 patients (13 tumors) treated with RTRT, the actuarial LC at 1 and 2 years was 100% and 64.3%, respectively, with a median follow-up of 17 months. For the 8 patients treated with g-SBRT, the actuarial LC at 1-year was 50.0%, with a median follow-up of 6.5 months. There was a statistically significant difference in LC between the 2 treatment groups (P < .001; Figure 4). For the 8 patients with non-small cell lung cancers (9 tumors), the actuarial LC at 1 and 2 years was 100% and 40.0%, respectively, with a median follow-up of 13 months; and for the 4 patients with renal cell carcinomas, the actuarial LC at 1 and 2 years was 100% and 66.7%, respectively, with a median follow-up of 29.5 months.
Figure 3.
Local control rates for all tumors.
Figure 4.
Local control rates for real-time tumor-tracking radiotherapy (RTRT, blue line) and for general stereotactic body radiotherapy (g-SBRT, red line).
Grade 2 acute reactions (appetite loss, nausea, and vomiting) were reported in 4 patients in the RTRT series; no acute reactions at Grade 3 or higher or late adverse reactions at Grade 2 or higher were reported in either treatment group (Table 4). There was no difference in the incidence of adverse reactions between the left and right adrenal glands (P = 1.00).
Discussion
The location of the adrenal glands makes them unsuitable for conventional radiotherapy. Irradiation to a tumor in the left or right adrenal gland using coplanar fields will unavoidably include the GI tract, liver, or kidney. The SBRT technique can deliver high doses to the tumor while reducing the dose to these organs at risk around the adrenal glands. In the present 2-institutional retrospective studies, we reviewed 20 patients treated with RTRT or g-SBRT for adrenal gland metastasis. The LC and OS rates at 1 year were 91.7% and 78.5%, respectively, and no grade 3 or higher adverse events were reported. Several previous studies on SBRT for adrenal gland metastasis are detailed in Table 5. Our results are consistent with previous studies of SBRT for adrenal gland metastasis with LC rates ranging from 60% to 100% and OS rates ranging from 39.7% to 93.3%. The dose–volume metrics and the clinical outcomes observed in this study suggest that both RTRT and g-SBRT are useful in reducing the risk of serious adverse reactions in the treatment of adrenal metastatic tumors.
Table 5.
Studies of stereotactic body radiotherapy (SBRT) for Adrenal Gland Metastasis.
| Study (year) | Number of Patients (Lesions) | Primary Tumor Site | GTV-to-CTV Margin | SBRT Schedule | Prescription | Median Follow-Up | LC | OS |
|---|---|---|---|---|---|---|---|---|
| Holy et al 7 (2011) | 18 (18) | Lung 100% | 2 mm | 15-40 Gy/ 3-12 Fr | 100% isodose line | 21 months | 77% (objective response rate) | 23 months (median) |
| Scorsetti et al 8 (2011) | 34 (36) | Lung 71% | 3 mm | 32 Gy/4 Fr (median) | 95% isodose line | 41 months | 66% (1 year) | 22 months (median) |
| Casamassima et al 6 (2012) | 48 (NR) | Lung 50% | 0 mm | 34.9 Gy /3 Fr (mean) SRS: 23.5 Gy (mean) |
70% isodose line | 16.2 months | 90% (1 year) | 39.7% (1 year) |
| Ahmed et al 9 (2013) | 13 (13) | Lung 46% | 0 mm | 45 Gy/5 Fr (median) | NR | 12.3 months | 100% (crude rate) | 62.9% (1 year) 7.2 month (median) |
| Rudra et al 14 (2013) | 10 (13) | Lung 80% | 0 mm | 36 Gy/3 Fr (median) | 80%-90% isodose line | 14.9 months | 73% (1 year) | 90% (1 year) 17.3 month (median) |
| Celik et al 16 (2017) | 15 (15) | Lung 100% | 0 mm | 42Gy/6 Fr | 65% isodose line | 24 months | 60% (1 year) | 93.3% (1 year) |
| Franzese et al 17 (2017) | 46 (46) | Lung 65.2% | 0 mm | 40 Gy/4 Fr | CTV V98% > 98% | 7.6 months | 65.5% (1 year) | 87.6% (1 year) |
| This study | 20 (21) | Lung 45% | 0 or 3 mm | 48 Gy/8 Fr (median) | Isocenter, PTVD95 | 17.5 months | 91.7% (1 year) | 78.5% (1 year) |
Abbreviations: CTV, clinical target volume; Dxx, xx% of the PTV volume received by the dose; Fr, fractions; GTV, gross tumor volume; LC, local control; NR, data not reported; OS, overall survival; PTV, planning target volume; SBRT, stereotactic body radiotherapy.
As pointed out by Scorsetti et al, there are no consensus or defined guidelines for radiation treatment of adrenal gland metastases,12 the GTV-to-CTV margins, dose prescription, and treatment schedules vary in the different studies (Table 5). Above, we have also reported the different GTV-to-CTV margins, the variations in the dose prescription, and treatment schedules. The reasons for the heterogeneity of treatment details depend on institutional differences in the equipment and protocols, modifications by the attending physician based on the size of the tumor, and the distance between PTV and the organ at risk (OAR). The rarity of occurrence of this disease and the complexity of the OAR around the adrenal gland have prevented a formalization of the treatment protocols.
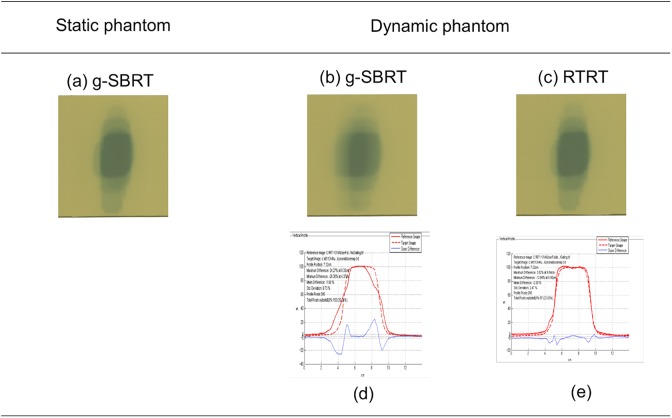
The adrenal gland changes position due to significant movement with respiration, so motion management is important to reduce the uncertainty in dosages.4 About the differences in dose distribution between RTRT and g-SBRT, it is useful to compare these with a moving phantom to imitate the target volume movement with respiratory motion. Figure 5 shows the dose distributions of RTRT and g-SBRT using phantoms. It is well demonstrated that the dose difference between the static phantom and the dynamic phantom was smaller in RTRT and there was a more condensed dose distribution obtained with RTRT than with g-SBRT.
Figure 5.
Dose distribution on Gafchromic films using phantoms for a target volume with a lateral diameter about 4-cm on this figure. Two-dimensional dose distributions of g-SBRT using (A) the static phantom and (B) the dynamic phantom, and (C) that with real-time tumor-tracking radiotherapy (RTRT) using the dynamic phantom. Dose profiles at the center of the target volume of (D) g-SBRT and (e) RTRT using the dynamic phantom. The dynamic phantom was moving in a cos4 manner with a period of 3 seconds and an amplitude of 20 mm along the lateral direction. The red dotted lines in (D) and (E) are the dose profiles of g-SBRT using the static phantom. The red solid lines in (D) and (E) are the dose profiles of g-SBRT and RTRT using the dynamic phantom, respectively. The dose difference between the static phantom and the dynamic phantom are shown as blue solid lines in (D) and (E) for g-SBRT and RTRT, respectively.
The Dmax and Dmean to GTV and PTV in g-SBRT in Table 2 are calculated with static CT data for the patients assuming that the target volume is not moving during g-SBRT. It implies that if we could have used precise 4D-CT planning accounting for tumor motion, the Dmax and Dmean to GTV and PTV in g-SBRT would have been lower than the values in Table 2. The Dmax and Dmean to OARs in g-SBRT in Table 3 may be higher or lower depending on the actual motion of the OAR during irradiation but the dose–volume histogram of the OAR would be smoothed-out by the general organ motion.
Another potential source of serious adverse reactions in the bowel is attachment of the GI tract to the adrenal glands. Dose constraints for the GI tract are based on its adverse reactions. The GI tract changes its shape and position freely during the treatment period in fractionated radiotherapy. However, Onishi et al reported fatal gastric bleeding after SBRT in a patient in whom the position of the posterior wall of the stomach bordering the left metastatic adrenal tumor did not change much, even though the dose constraints had been fulfilled in the planning.19 Plichta, et al also reported serious GI bleeding after SBRT with a maximum bowel radiation dose of 52.8 Gy delivered over 3 fractions; they suggested that dose constraints for the GI tract are important in the treatment of metastatic adrenal tumors.20 In the present study, we observed doses to 1.0 cm3 of the bowel, other than the stomach and duodenum, which exceeded 35 Gy in 8 fractions in 5 tumors in the RTRT series. It was fortunate that there were no serious adverse reactions in these patients; in these cases real-time monitoring of the GI tract would have been advisable. This suggesting that precise imaging in the treatment room may well be useful.
The results of this study highlights the difficulties in dosimetric comparisons between gated and nongated irradiation of an intrafractionally moving target. It is well known that a simple comparison can be misleading because dosimetric parameters using static CT data are inadequate surrogates for the actual dose deposited in the moving organs.26 Conversely, the differences in the parameters in this study suggest that the dose constraints on gated and nongated irradiation may not be simply transferable because of the significant differences in dosimetric parameters. The local tumor control rates with RTRT and g-SBRT in this series were sufficiently high for most of the patients with metastatic adrenal tumors. This is consistent with the findings of a recent review of various advanced radiotherapy technologies.21 Metastatic adrenal tumors can be large and can cause uncomfortable symptoms; when the tumor is small enough, it is reasonable to use these technologies to reduce the dose to the surrounding critical organs. It has been reported that surgical resection does not prolong OS, even for patients with oligometastatic non-small cell lung cancer27; these patients should therefore be informed about RTRT or g-SBRT as an alternative treatment.
An important question is whether the implantation of surrogate fiducial markers around the adrenal gland for RTRT is beneficial. Some patients survived for more than 2 years, so serious late adverse reactions need to be considered in decision-making for these patients. Although we observed no statistical differences in survival or adverse reaction rates between the RTRT and g-SBRT treatment groups, we noted some interesting results for LC. Complete response was obtained in 3 patients with relatively large tumors who underwent RTRT, but not at all in patients treated with g-SBRT. The LC rate was higher with RTRT even though the nominal dose to the target volume was smaller. Conversely, Grade 2 acute toxicity was observed in 4 patients treated with RTRT but not in any of the patients treated with g-SBRT, with no difference in the mean nominal dose to the organs at risk. These differences may be due to the more condensed dose distribution of RTRT compared to g-SBRT, and a prospective comparison between RTRT and the nongated g-SBRT is warranted. Because of the low toxicity, these technologies may become a potential treatment for nonmetastatic primary adrenal tumors in the future.28
The present study has several limitations. First, the small number of patients and retrospective design does not allow a confirmation of the differences between RTRT and nongated g-SBRT. Second, the variability of the margins, dose fractionation schedules, and total doses would have had an effect on the results. Third, we analyzed patients with adrenal gland metastasis from different primary tumor sites, and the OS can be affected by the percentage of primary tumor sites. Fourth, we did not take chemotherapy after RTRT or g-SBRT into account in this analysis. Chemotherapy would have had an impact on local tumor responses and may have influenced the results of the present study.
In conclusion, this study further strengthened our previous observation that precise radiotherapy methods, such as RTRT and g-SBRT, can provide safe and effective treatment for selected patients with adrenal metastasis while meeting the dose constraints for critical organs around the adrenal glands. For most oligometastatic patients with adrenal metastatic tumors, these treatments should be regarded as an alternative to surgical resection. It should be noted that RTRT showed significantly higher LC rates than g-SBRT, and a few tumors demonstrated a complete response after the RTRT without serious adverse reactions.
Acknowledgment
The authors would like to thank Enago (www.enago.jp) for the English language review.
Abbreviations
- 4DCT
4-dimensional CT
- CT
computed tomography
- BED
biologically effective dose
- CTV
clinical target volume
- GI
gastrointestinal
- g-SBRT
general stereotactic body radiotherapy
- GTV
gross tumor volume
- LC
local control
- OS
overall survival
- PTV
planning target volume
- RTRT
real-time tumor-tracking radiotherapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Onishi reports grants from the Japan Radiological Society during the study. Dr Shimizu reports grants from Hitachi Ltd, outside the submitted work. Dr Shirato reports personal fees from Astra Zeneca and grants from Ministry of Health, Labour and Welfare, Japan; Japan Agency for Medical Research and Development (AMED); and Ministry of Education, Culture, Sports, Science and Technology, Japan, during the study and grants from Shimadzu Corporation and Hitachi Ltd after the submitted work. In addition, Dr Shirato has a patent US6307914 B1 licensed to Hitachi Ltd, and a patent US6307914 B1 with royalties paid to Mitsubishi heavy industries and Milestone payment from Olympus Co Ltd for the development of a medical appliance through endoscope, EP 1588670 A4 and from Medikit Co Ltd for the development of a medical appliance through a needle.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI Grant Number JP16H05389 and the Global Institution for Collaborative Research and Education (GI-CoRE), Hokkaido University, founded by the Ministry of Education, Culture, Sports, Science and Technology MEXT, Japan.
ORCID iD: Norio Katoh, MD, PhD  https://orcid.org/0000-0003-3959-2114
https://orcid.org/0000-0003-3959-2114
Hiroshi Onishi, MD, PhD  https://orcid.org/0000-0002-3512-1166
https://orcid.org/0000-0002-3512-1166
References
- 1. Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sazawa A, Shinohara N, Harabayashi T, Abe T, Shirato H, Nonomura K. Alternative approach in the treatment of adrenal metastasis with a real-time tracking radiotherapy in patients with hormone refractory prostate cancer. Int J Urol. 2009;16(4):410–412. [DOI] [PubMed] [Google Scholar]
- 3. Katoh N, Onimaru R, Sakuhara Y, et al. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol. 2008;87(3):418–424. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Li F, Dong Y, Song Y, Yuan Z. Clinical study on the influence of motion and other factors on stereotactic radiotherapy in the treatment of adrenal gland tumor. Onco Targets Ther. 2016;9:4295–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75(1):71–75. [DOI] [PubMed] [Google Scholar]
- 6. Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82(2):919–923. [DOI] [PubMed] [Google Scholar]
- 7. Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187(4):245–251. [DOI] [PubMed] [Google Scholar]
- 8. Scorsetti M, Mancosu P, Navarria P, et al. Stereotactic body radiation therapy (SBRT) for adrenal metastases: a feasibility study of advanced techniques with modulated photons and protons. Strahlenther Onkol. 2011;187(4):238–244. [DOI] [PubMed] [Google Scholar]
- 9. Ahmed KA, Barney BM, Macdonald OK, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol. 2013;36(5):509–513. [DOI] [PubMed] [Google Scholar]
- 10. Desai A, Rai H, Haas J, Witten M, Blacksburg S, Schneider JG. A retrospective review of cyberknife stereotactic body radiotherapy for adrenal tumors (primary and metastatic): Winthrop University Hospital experience. Front Oncol. 2015;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: a retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011;7(1):145–151. [DOI] [PubMed] [Google Scholar]
- 12. Scorsetti M, Alongi F, Filippi AR, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: a retrospective analysis of 34 patients. Acta Oncol. 2012;51(5):618–623. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Shi Z, Wang Z, et al. Treating adrenal tumors in 26 patients with CyberKnife: a mono-institutional experience. PLoS One. 2013;8(11):e80654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudra S, Malik R, Ranck MC, et al. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat. 2013;12(3):217–224. [DOI] [PubMed] [Google Scholar]
- 15. Gamsiz H, Beyzadeoglu M, Sager O, et al. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori. 2015;101(1):98–103. [DOI] [PubMed] [Google Scholar]
- 16. Celik E, Semrau R, Baues C, et al. Robot-assisted extracranial stereotactic radiotherapy of adrenal metastases in oligometastatic non-small cell lung cancer. Anticancer Res. 2017;37(9):5285–5291. [DOI] [PubMed] [Google Scholar]
- 17. Franzese C, Franceschini D, Cozzi L, et al. Minimally invasive stereotactical radio-ablation of adrenal metastases as an alternative to surgery. Cancer Res Treat. 2017;49(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haidenberger A, Heidorn SC, Kremer N, Muacevic A, Furweger C. Robotic radiosurgery for adrenal gland metastases. Cureus. 2017;9(3):e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Onishi H, Ozaki M, Kuriyama K, et al. Serious gastric ulcer event after stereotactic body radiotherapy (SBRT) delivered with concomitant vinorelbine in a patient with left adrenal metastasis of lung cancer. Acta Oncol. 2012;51(5):624–628. [DOI] [PubMed] [Google Scholar]
- 20. Plichta K, Camden N, Furqan M, et al. SBRT to adrenal metastases provides high local control with minimal toxicity. Adv Radiat Oncol. 2017;2(4):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’—A systematic review. Cancer Treat Rev. 2014;40(7):838–846. [DOI] [PubMed] [Google Scholar]
- 22. Takao S, Miyamoto N, Matsuura T, et al. Intrafractional baseline shift or drift of lung tumor motion during gated radiation therapy with a real-time tumor-tracking system. Int J Radiat Oncol Biol Phys. 2016;94(1):172–180. [DOI] [PubMed] [Google Scholar]
- 23. Harada K, Katoh N, Suzuki R, et al. Evaluation of the motion of lung tumors during stereotactic body radiation therapy (SBRT) with four-dimensional computed tomography (4DCT) using real-time tumor-tracking radiotherapy system (RTRT). Phys Med. 2016;32(2):305–311. [DOI] [PubMed] [Google Scholar]
- 24. Onishi H, Kawakami H, Marino K, et al. A simple respiratory indicator for irradiation during voluntary breath holding: a one-touch device without electronic materials. Radiology. 2010;255(3):917–923. [DOI] [PubMed] [Google Scholar]
- 25. Matsuo Y, Onishi H, Nakagawa K, et al. Japan Conformal External Beam Radiotherapy Group; Japan Society of Medical Physics; Japan Society of Medical Physics; Japanese Society of Radiological Technology. Guidelines for respiratory motion management in radiation therapy. J Radiat Res. 2013;54(3):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao B, Yang Y, Li T, Li X, Heron DE, Huq MS. Dosimetric effect of intrafraction tumor motion in phase gated lung stereotactic body radiotherapy. Med Phys. 2012;39(11):6629–6637. [DOI] [PubMed] [Google Scholar]
- 27. Huang SH, Kong QL, Chen XX, He JY, Qin J, Chen ZG. Adrenalectomy does not improve survival rates of patients with solitary adrenal metastasis from non-small cell lung cancer. Ther Clin Risk Manag. 2017;13:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stigliano A, Cerquetti L, Lardo P, Petrangeli E, Toscano V. New insights and future perspectives in the therapeutic strategy of adrenocortical carcinoma (Review). Oncol Rep. 2017;37(3):1301–1311. [DOI] [PubMed] [Google Scholar]