Abstract
Different allopathic drugs are being used for the treatment of diabetes mellitus but more emphasis are being placed on the use of medicinal plants, herbs, and natural extracts of fruits and vegetables due to their easy availability, easy consummation with low cost, and with no well-reported side effects. White skinned sweet potato (WSSP; Ipomoea batatas L.) peel-off was selected to find out its antidiabetic potential as well as to explore the effects on selected biochemical parameters in diabetes-induced Wistar rats. In young (3–4 months) and old (up to 1 year) diabetic Wistar rats, it was found that WSSP (I. batatas L.) peel-off significantly (P < 0.05) decreased blood glucose level, protein glycation level, total cholesterol, triglycerides, and low-density lipoprotein (LDL)-cholesterol. A significant (P < 0.05) increase in high-density lipoprotein (HDL)-cholesterol level after treatment was also reported. Furthermore, it was also found that WSSP peel-off also had beneficial effects on total protein concentration, albumin, globulin, and liver enzymes (serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT)). It might be concluded that antidiabetic potential of WSSP extract is due to the presence of bioactive compounds like glycoprotein, anthocyanins, alkaloids, and flavonoids, which act as insulin-like molecules or insulin secretagogues constituents in sweet potatoes peel-off and these antidiabetic proteins were extracted out in more concentration in methanol due to its organic nature. Further research is needed to purify and quantify the antidiabetic components responsible for antidiabetic effects of WSSP and it should be available in compact dose form for the treatment of diabetic patients.
Keywords: biochemical parameters, diabetes mellitus, glycation, medicinal plants, Wistar rat
Introduction
Diabetes mellitus is a clinical syndrome due to relative or absolute deficiency of insulin or resistance to the action of insulin at the cellular level; as a result, hyperglycemia and glucosurea occurs.1 About 10% cases of this disease occur most often in the American population and 2 million populations affected by this disease are in Europe and North America.2 The formation of advanced glycation end products (AGEs) is a group of modified proteins and lipids which have potential to damage cells. AGEs are responsible for increased pathogenesis and diabetic complications in type 2 diabetes mellitus. Amplified activities of liver enzymes, for example, aspartate aminotransferase (AST; serum glutamic oxaloacetic transaminase (SGOT)) and alanine aminotransferase (ALT; serum glutamic pyruvic transaminase (SGPT)) are indicators of hepatocellular damage.3–5
Along with exercise, modern drugs such as pioglitazone, biguanides, meglitinides, thiazolidinedione, alpha glucosidase inhibitors, and sulphonylureas have better efficacy but lead to side effects like hypoglycemia, gastrointestinal tract disturbance, obesity, water intoxication, and hyponatremia with high cost.6 It has been reported that herbal medicines are gaining popularity to show better efficacy with no or very rare side effects proven by both in vivo and in vitro studies. Various chemical constituents or compounds obtained from medicinal plants have proven their efficacy and safety in the treatment of diabetes mellitus.7 Globally, potato is the fourth most crop cultivated, and due to high fiber contents especially skins left on and low glycemic index, sweet potatoes are a good food choice for diabetics. Pancreas does not face over burden on ingesting low-glycemic foods, and slow release of sugar also makes one feel satiated longer and could help to control blood sugar in diabetics. It was reported that antidiabetic effects of sweet potato were due to acylated anthocyanins, such as caffeoylsophorose, which acts as alpha-glucosidase inhibitor. Furthermore, adiponectin secreted by transgenic potato decreases the risk of type 2 diabetes mellitus, obesity, and hypertension. White skinned sweet potatoes (WSSPs) have hypoglycemic activity, and it increases the blood insulin level. WSSP increases the glucose tolerance and suppresses the glucose concentration after glucose loading in streptozotocin-induced diabetic rats.8,9 There are clear differences in insulin release between young and old Wistar rats. The acute insulin response was slower in older animals and did not reach as high as those obtained in young rats. Old rats appear to respond slowly as well as young ones to insulin. Pancreatic beta cells therefore retain response to the insulin-tropic effect with increasing age in Wistar rats. Therefore, this study was planned to explore the antidiabetic effect of WSSP (Ipomoea batatas L.) using diabetes-induced Wistar rats as experimental animal. Furthermore, it is well reported that young age population have more significant potential to recover as compared to old age population, so both young and old age rats were selected to explore the effect of herbal preparation.
Materials and methods
Preparation of plant extract
I. batatas was purchased from local market of Faisalabad (Pakistan). Plant was authenticated and specimen was kept in herbarium of Department of Physiology and Pharmacology, University of Agriculture, Faisalabad. Roots of I. batatas were used to prepare methanolic extract by following Metgud and Kore10 method. After each extraction, extract was filtered by Whattman filter paper no. 1 and evaporated to dryness via rotary evaporator under reduced pressure.
Experimental animals and induction of diabetes
Normal male Wistar rats (Laboratory bred) were used in this study. Both young and old rats are used in this study of WSSP peel-off on diabetic rats. Both young and old rats were used to confirm the effect of age in case of diabetes. Animals were acclimatized for 14 days. Animals were kept under standard animal housing conditions, that is, in room temperature facility with 12 h dark/light cycle. Throughout the study, animals were provided with unlimited access to standard diet and water ad libitum.
Diabetes was induced by administration of alloxan monohydrate (150 mg/kg (S/C)), after an overnight fasting for 12 h (had access only to water), to make them more prone to develop diabetes. Alloxan monohydrate was dissolved in 0.9% NaCl solution and administrated to male Wistar rats. After 3 days of alloxan monohydrate induction, glucose level was monitored. Wistar rats having blood glucose level of 200 ± 14.91 mg/dL after 3 days were selected for this study. It was monitored by rapid glucose estimation method (NIPRO blood diagnostic strips) that there is no significant (P < 0.05) difference in blood glucose level of all diabetic animal groups prior to conducting experiments.
Experimental group
A total of 36 rats including young (3–4 months; n = 18) and old (up to 1 year; n = 18) were selected for this study and animals were kept at standard housing conditions with 12 h light/dark cycle. These rats were kept in animal house situated at the Department of Physiology and Pharmacology, University of Agriculture, Faisalabad. Animals were given ad libitum access to water and standard diet. The animals were divided into six groups as Group 1 to Group 6. Group 1: Normal control young animals; Group 2: Normal control old animals; Group 3: Diabetic control young animals; Group 4: Diabetic control old animals; Group 5: Methanol extract–treated young diabetic rats; and Group 6: Methanol extract–treated old diabetic rats. Diabetic groups were given orally methanol extract with dose rate of 4 g/kg/day daily for 14 days on the basis of literature reviewed and primary dose optimization study having maximum antidiabetics affect. Feed usage and body weight of experimental rats were recorded before starting the dose and at 3rd, 6th, 9th, 12th, and 15th day of the experiment.
Physical and biochemical parameters analysis
Physical parameters including body weight, feed consumption, and water intake of each group were measured at 9:00 according to Pakistan standard time. Blood samples from all groups were collected after specific intervals in heparinized tubes. Plasma was separated after centrifugation and processed to find out blood glucose level, glycation level, total proteins, cholesterol level, liver enzyme, and cardiac enzymes by following standard protocols.
Statistical analysis
The obtained data subjected to statistical analysis for the determination of significance by using one-way analysis of variance (ANOVA). All data are expressed as means ± standard deviation. Statistical significance is expressed by P values less than 0.05.
Results
Physical parameters
Results of physical parameters are presented in Table 1 including body weight (grams) and feed consumption (grams) as mean ± standard error (SE) of young and old rats at various days in normal and diabetic group treated rats treated with methanol extract sweet potato peel-off. The interaction of body weight with days, age, and groups was not significant (P > 0.05). The overall mean of body weight with days and overall mean of body weight with age and groups was significantly (P < 0.05) different. Furthermore, the highest feed consumption was shown by old rats of the control group in eight day and diabetic treated rats of old groups in the 12th day. While the rats of the diabetic treated group were shown the lowest feed consumption in the 12th day. The highest interaction of the overall mean of the feed consumption with day was appeared in the 5th and 8th days, and lowest was shown on 12th day. The overall mean of the feed consumption with days and groups was appeared in old control rats group, while lowest was shown in the old rats of the diabetic treated group.
Table 1.
Mean body weight (g ± SE) of young and old rats at various days in normal and diabetic group treated rats treated with methanol extract sweet potato peel-off.
| Days | Young | Old | Overall Means | |||||
|---|---|---|---|---|---|---|---|---|
| C | D | DT | C | D | DT | |||
| 1 | Body weight | 222.56 ± 1.27 | 219.30 ± m1.59 | 186.60 ± 5.65 | 230.70 ± 1.91 | 225.66 ± 3.57 | 213.80 ± 4.54 | 216.44 ± 2.96FG |
| Feed usage | 15.48 ± 0.98i–s | 10.36 ± 0.48w–z{\} | 19.06 ± 1.06fg | 26.94 ± 2.09d | 16.54 ± 1.32g–o | 12.18 ± 0.63r–z{\ | 16.76 ± 1.09BC | |
| 2 | Body weight | 231.74 ± 1.51 | 221.70 ± 0.66 | 181.40 ± 5.32 | 234.02 ± 1.37 | 230.26 ± 1.09 | 213.80 ± 4.54 | 218.82 ± 3.54EF |
| Feed usage | 14.00 ± 0.63m-v | 8.36 ± 0.19A-‘a | 18.66 ± 2.98f-i | 26.74 ± 0.97 | 15.00 ± 0.32j-s | 17.24 ± 0.04f-n | 16.67 ± 1.14BC | |
| 3 | Body weight | 235.00 ± 0.63 | 224.34 ± 0.49 | 177.40 ± 4.98 | 244.54 ± 1.58 | 236.60 ± 0.35 | 211.34 ± 9.53 | 221.54 ± 4.28C-E |
| Feed usage | 16.58 ± 0.17g–o | 10.74 ± 0.66b–z{\} | 20.28 ± 0.44ef | 27.64 ± 0.92d | 12.26 ± 0.66r–z{\ | 14.14 ± 1.19m–u | 16.94 ± 1.09bc | |
| 4 | Body weight | 238.26 ± 0.94 | 226.34 ± 0.19 | 178.40 ± 5.21 | 248.34 ± 1.74 | 234.28 ± 0.31 | 209.76 ± 4.45 | 225.56 ± 4.41B–D |
| Feed usage | 17.26 ± 0.37f–n | 13.26 ± 0.97o–y | 15.45 ± 1.16i–s | 31.46 ± 0.74bc | 14.90 ± 1.45j–s | 9.32 ± 0.82{\}a- | 16.94 ± 1.34BC | |
| 5 | Body weight | 242.64 ± 0.80 | 229.00 ± 0.32 | 175.00 ± 4.85 | 250.94 ± 1.23 | 238.80 ± 0.93 | 207.56 ± 4.90 | 223.99 ± 4.91B–D |
| Feed usage | 18.74 ± 0.97f–i | 19.00 ± 0.71f–h | 12.64 ± 1.78o–z{ | 33.90 ± 0.84ab | 17.64 ± 0.80f–l | 9.52 ± 2.12{\}a- | 18.57 ± 1.50A | |
| 6 | Body weight | 247.30 ± 0.97 | 232.10 ± 0.56 | 174.20 ± 5.40 | 254.64 ± 0.80 | 243.12 ± 0.54 | 202.04 ± 4.71 | 225.57 ± 5.40AB |
| Feed usage | 17.36 ± 1.08f–m | 18.74 ± 0.37f–i | 16.10 ± 1.55g–q | 29.22 ± 2.18cd | 14.74 ± 0.37k–s | 10.89 ± 1.479u–z{\}A | 17.84 ± 1.16AB | |
| 7 | Body weight | 250.86 ± 0.33 | 233.94 ± 1.15 | 172.40 ± 6.73 | 262.66 ± 1.24 | 250.32 ± 1.24 | 199.04 ± 3.49 | 228.20 ± 6.08A |
| Feed usage | 19.00 ± 0.32f–h | 15.64 ± 0.48g–r | 14.42 ± 1.47l–t | 32.06 ± 1.00a–c | 14.26 ± 0.66l–u | 10.20 ± 2.05x–z{\}A– | 17.60 ± 1.36AB | |
| 8 | Body weight | 253.66 ± 0.48 | 233.94 ± 0.85 | 167.80 ± 6.51 | 269.34 ± 0.80 | 241.40 ± 1.29 | 202.80 ± 4.73 | 228.16 ± 0.39A |
| Feed usage | 18.00 ± 0.32f–k | 12.90 ± 0.84p–z | 16.06 ± 1.54g–q | 35.00 ± 0.63a | 14.64 ± 0.48k–s | 13.52 ± 1.37o–x | 18.35 ± 1.46A | |
| 9 | Body weight | 228.00 ± 2.21 | 221.20 ± 0.78 | 173.76 ± 0.37 | 232.94 ± 3.11 | 225.60 ± 3.38 | 202.44 ± 4.82 | 213.99 ± 3.94GH |
| Feed usage | 15.30 ± 0.40i–s | 9.64 ± 0.19z{\}a- | 13.58 ± 0.88o–w | 26.78 ± 0.66d | 14.26 ± 0.66l–u | 10.09 ± 0.65yz{\}a- | 14.94 ± 1.08DE | |
| 11 | Body weight | 243.24 ± 1.12 | 299.00 ± 0.95 | 168.50 ± 0.81 | 249.76 ± 1.33 | 238.46 ± 1.33 | 195.06 ± 5.49 | 220.67 ± 5.50DE |
| Feed usage | 18.00 ± 0.32f–k | 15.44 ± 0.88i–s | 9.82 ± 0.58z{\}a– | 31.90 ± 0.90bc | 15.56 ± 0.57h–r | 8.68 ± 0.82}a′- | 16.57 ± 1.44BC | |
| 12 | Body weight | 227.98 ± 2.51 | 221.42 ± 0.83 | 167.66 ± 1.49 | 235.70 ± 2.26 | 230.32 ± 1.78 | 189.14 ± 5.85 | 212.04 ± 4.76H |
| Feed usage | 13.58 ± 0.70o–w | 9.00 ± 0.32\}a-′ | 5.82 ± 0.25′a | 22.90 ± 0.84e | 12.00 ± 0.63s–z{\} | 5.22 ± 0.28 + | 11.42 ± 1.12F | |
| 13 | Body weight | 239.64 ± 1.43 | 227.80 ± 0.66 | 167.74 ± 0.97 | 247.64 ± 1.43 | 237.12 ± 1.37 | 189.66 ± 6.09 | 218.29 ± 5.53EF |
| Feed usage | 16.50 ± 0.16g–o | 15.44 ± 1.58i–s | 8.20 ± 0.20A–′a | 27.70 ± 1.41d | 12.90 ± 0.84p–z | 7.47 ± 0.17–′a | 14.70 ± 1.30E | |
| 14 | Body weight | 250.70 ± 0.87 | 232.30 ± 0.40 | 167.20 ± 1.59 | 265.02 ± 1.16 | 246.46 ± 1.72 | 193.14 ± 5.54 | 225.80 ± 6.47AB |
| Feed usage | 18.36 ± 0.48f–j | 13.80 ± 1.03n–v | 8.58 ± 0.27A-′ | 32.64 ± 1.11ab | 14.84 ± 1.21k–s | 7.65 ± 0.28A–′a | 15.98 ± 1.57CD | |
| Mean | Body weight | 240.11 ± 1.19B | 227.41 ± 0.62D | 173.48 ± 1.24F | 249.21 ± 12.76A | 237.24 ± 8.02C | 210.43 ± 1.59E | ±1.37 |
| Feed usage | 16.82 ± 0.24B | 13.53 ± 0.60D | 29.69 ± 0.48A | 14.69 ± 0.48A | 14.69 ± 0.27C | 10.30 ± 0.45E | 16.43 ± 0.35 | |
The letters in superscripts show statistical interaction and significance of results.
Results with P value below 0.05 were considered as significant.
Biochemical parameters
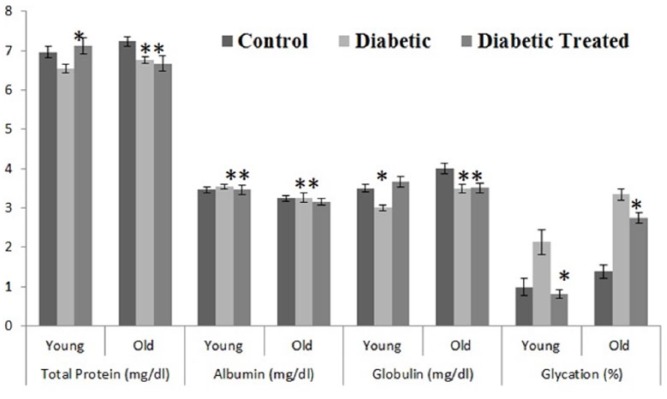
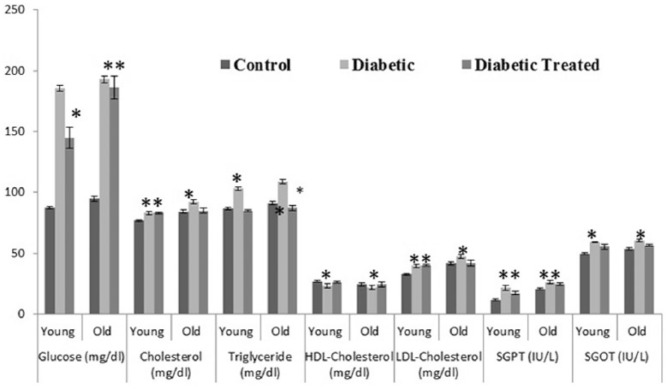
Results of different selected parameters like glucose (mg/dL), protein glycation level (%), total cholesterol (mg/dL), triglycerides (mg/dL), high-density lipoprotein (HDL) cholesterol (mg/dL), low-density lipoprotein (LDL) cholesterol (mg/dL), protein concentration (g/dL), albumin (g/dL), globulin (g/dL), and liver enzymes, namely, SGOT (U/L) and SGPT (U/L), after giving WSSP (I. batatas L.) peel-off methanolic extract both to young and old age Wistar rats for 14 days are represented as mean ± SE in Figures 1 and 2. It was found that WSSP (I. batatas L.) peel-off significantly (P < 0.05) decreased blood glucose level, protein glycation level, total cholesterol, triglycerides, and LDL-cholesterol. A significant (P < 0.05) increase in HDL-cholesterol level after treatment was also reported. Furthermore, it was also found that WSSP peel-off also had beneficial effects on total protein concentration, albumin, globulin, and liver enzymes (SGOT and SGPT).
Figure 1.
Mean ± SE plasma total protein, albumin, globulin, glycation concentration in all groups (control, diabetic, and methanol extract treated) of young and old rats (*significant P < 0.05; **non-significant P > 0.05).
Figure 2.
Mean ± SE plasma glucose, cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, SGPT, and SGOT in all groups (control, diabetic, and methanol extract treated) of young and old rats (*significant P < 0.05, **non-significant P > 0.05).
Discussion
The decrease in body weight in diabetes might be due to the less feed intake because sweet potatoes are rich in dietary fibers, have low glycemic index with reduced digestion, and provide the fullness of stomach requirement.11 Decrease in body weight might also be due to the improvement in glucose control after treatment with WSSP. The overall mean of feed intake with age and groups differs non-significantly in methanol extract treatment. Diabetic rats treated with methanol extract of white skin sweet potato peel-off had less feed intake than the control group rats irrespective of their days and age due to high fiber contents in WSSP, which satisfied the appetite and control the feed intake by slowing down the digestion.
Carotenoids in WSSP might decrease blood glucose level and insulin resistance in our body.12 The overall mean of young and old rats and the interaction of total protein with age and groups were non-significant. The decrease in protein in diabetic rats was the leading symptom of diabetes (type I and type II) due to decreased uptake of amino acid in peripheral tissues, and less production of adenosine triphosphate (ATP) causes decreased synthesis of protein; diabetes also contributed to catabolic reaction via destruction of structural protein.13 Increase in the albumin and globulin concentration indicated the effectiveness of dose and decrease in glucose concentration, which ultimately increases the protein production in liver acerbated by methanolic extract. Decrease in glycation level prevents formation of reactive oxygen species and reduces complications of type 2 diabetes. Elevated threat of atherosclerosis is due to alteration in lipid/ lipoprotein metabolism in diabetics. Our findings of cholesterol, triglyceride, and LDL-cholesterol were similar to Ludvik et al.14 findings, which might be due to increase in glucose metabolism.
HDL plays a defensive function against atherosclerosis because of its function in reverse cholesterol transport. HDL is also connected with metabolism of triglyceride-rich lipoprotein by stimulating lipoprotein lipase.
As diabetes is a metabolic disorder, it also affects the metabolic actions of liver. Amplified activities of liver enzymes, for example, AST and ALT, are indicators of hepatocellular damage. Elevated action of these markers is connected with insulin resistance, metabolic disorder, and type 2 diabetes mellitus and beneficial effect of methanol extract of WSSP peel-off in diabetic rats was reported.15 Finally, it could be concluded that administration of WSSP (I. batatas L.) in young (3–4 months) and old (up to 1 year) Wistar rats have significantly beneficial effects to regulate and control the glucose metabolism which ultimately influences the proteins and lipid metabolism in diabetics. Although results of this study revealed antidiabetic effects of methanolic I. batatas L extract in experimental Wistar rats, further research is needed to identify and quantify the phytochemical constituents responsible for these effects.
Acknowledgments
The authors acknowledge the cooperation of Department of Physiology and Pharmacology, University of Agriculture, Faisalabad.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Muhammad Daniyal  https://orcid.org/0000-0002-5663-5211
https://orcid.org/0000-0002-5663-5211
Saeed Ahmad  https://orcid.org/0000-0001-9291-7059
https://orcid.org/0000-0001-9291-7059
References
- 1. Al-Ali ZAJ. (2016) Some hematological and biochemical parameters in type 2 diabetic patients Missan/Iraq. International Journal of Advanced Research in Biological Sciences 3: 30–34. [Google Scholar]
- 2. Kumar P, Clark M. (2002) Diabetes mellitus and other disorders of metabolism. Clinical Medicine 2: 1069–1071. [Google Scholar]
- 3. Hyöty H. (2002) Enterovirus infections and type 1 diabetes. Annals of Medicine 34: 138–147. [PubMed] [Google Scholar]
- 4. Ludvik B, Neuffer B, Pacini G. (2004) Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care 27: 436–440. [DOI] [PubMed] [Google Scholar]
- 5. Hakim ZS, Patel BK, Goyal RK. (1997) Effects of chronic ramipril treatment in streptozotocin-induced diabetic rats. Indian Journal of Physiology and Pharmacology 41: 353–360. [PubMed] [Google Scholar]
- 6. Deepa VS, Rajaram K, Kumar PS. (2013) In vitro and in vivo antidiabetic effect of Andrographis lineata Wall. Ex.Nees and Andrographis serphyllifolia Wt.Ic leaf extracts. African Journal of Pharmacy and Pharmacology 7: 2112–2121. [Google Scholar]
- 7. King H, Aubert RE, Herman WH. (1998) Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 21: 1414–1431. [DOI] [PubMed] [Google Scholar]
- 8. Kusano S, Abe H. (2000) Antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.) in obese Zucker fatty rats. Biological & Pharmaceutical Bulletin 23: 23–26. [DOI] [PubMed] [Google Scholar]
- 9. Kusano S, Abe H, Tamura H. (2001) Isolation of antidiabetic components from white-skinned sweet potato (Ipomoea batatas L.). Bioscience, Biotechnology, and Biochemistry 65: 109–114. [DOI] [PubMed] [Google Scholar]
- 10. Metgud R, Kore S. (2014) Diabetes mellitus and periodontal disease. Indian Journal of Health Sciences and Biomedical Research 7: 6–11. [Google Scholar]
- 11. Rajkumar L, Srinivasan N, Balasubramanian K, et al. (1991) Increased degradation of dermal collagen in diabetic rats. Indian Journal of Experimental Biology 29(11): 1081–1083. [PubMed] [Google Scholar]
- 12. Bakala H, Verbeke P, Perichon M, et al. (1995) Glycation of albumin with aging and diabetes in rats: Changes in its renal handling. Mechanisms of Ageing and Development 78(1): 63–71. [DOI] [PubMed] [Google Scholar]
- 13. Lund ED. (1984) Cholesterol binding capacity of fiber from tropical fruits and vegetables. Lipids 19(2): 85–90. [DOI] [PubMed] [Google Scholar]
- 14. Ludvik BH, Mahdjoobian K, Waldhaeusl W, et al. (2002) The effect of Ipomoea batatas (Caiapo) on glucose metabolism and serum cholesterol in patients with type 2 diabetes: A randomized study. Diabetes Care 25(1): 239–240. [DOI] [PubMed] [Google Scholar]
- 15. Jaleel A, Halvatsiotis P, Williamson B, et al. (2005) Identification of Amadori-modified plasma proteins in type 2 diabetes and the effect of short-term intensive insulin treatment. Diabetes Care 28(3): 645–652. [DOI] [PubMed] [Google Scholar]