Short abstract
Tetrahydroxystilbene glucoside (THSG) is one of the active ingredients of Polygonum multiflorum. It has been shown to exert a variety of pharmacological effects, including antioxidant, anti-aging, and anti-atherosclerosis. Because of its prominent anti-inflammatory effect, we explored whether THSG had analgesic effect. In this study, we used a model of chronic inflammatory pain caused by injecting complete Freund’s adjuvant into the hind paw of mice. We found THSG relieved swelling and pain in the hind paw of mice on a dose-dependent manner. In the anterior cingulate cortex, THSG suppressed the upregulation of GluN2B-containing N-methyl-D-aspartate receptors and the downregulation of GluN2A-containing N-methyl-D-aspartate receptors caused by chronic inflammation. In addition, THSG increased Bcl-2 and decreased Bax and Caspase-3 expression by protecting neuronal survival. Furthermore, THSG inhibited the phosphorylation of p38 and the increase of nuclear factor κB (NF-κB) and tumor necrosis factor α (TNF-α). Immunohistochemical staining revealed that THSG blocked the activation of microglia and reduced the release of proinflammatory cytokines TNF-α, interleukin 1β (IL-1β), and interleukin 6 (IL-6). In conclusion, this study demonstrated that THSG had a certain effect on alleviating complete Freund’s adjuvant-induced chronic inflammatory pain.
Keywords: THSG, inflammation, NMDA receptor, neuronal apoptosis, microglia
Introduction
Pain is an unpleasant feeling and emotional sensation which not only protects the body from injury but also reflects many clinical diseases. But chronic persistent pain can greatly reduce the quality of life and is a major public health problem. Hence, pain relief is one of the main purposes of clinical drug therapy. Currently, only one-third of patients with chronic pain can be relieved with existing analgesics such as nonsteroidal anti-inflammatory drugs, local anesthetics, and opiates.1 Furthermore, some drugs have limited their widespread use due to adverse reactions. Therefore, it is necessary to develop new, safe, and effective drugs for chronic pain treatment.
Inflammation is an important cause of hypersensitivity to pain, usually accompanied by apoptosis. N-methyl-D-aspartate (NMDA) subtype of glutamate receptors (NMDARs) is a heterotetramer ion channel assembled from a combination of GluN1, GluN2, and GluN3 subunits.2 GluN2A and GluN2B are two of the best characterized regulatory subunits that play important but distinct roles in development and synaptic plasticity.3–5 Neuronal apoptosis is prevented when GluN2A subunit-containing NMDARs (GluN2ARs) is selectively activated,6,7 whereas the activation of GluN2B subunit-containing NMDARs (GluN2BRs) is associated with inflammatory pain. Previous study also demonstrated that intraperitoneal injection of GluN2B antagonists relieved the chronic inflammatory pain.8,9 Moreover, as immune effector cells resident in the central nervous system (CNS), microglia cells and their mediated inflammatory response also play an important role in the occurrence of pain. However, the interplay between microglial activation and NMDA receptor-mediated apoptosis remains unclear.
Polygonum multiflorum is one of the most widely used Chinese herbal medicines, and tetrahydroxystilbene glucoside (THSG) is one of the active ingredients extracted from the rhizome of P. multiflorum. THSG was reported to exert influence on anti-aging, anti-atherosclerosis, free radical scavenging, hypolipidemic, and hepatoprotective. Currently, the known functions include (i) exerting neuroprotection, antioxidation, cognition improvement, and anti-aging function by inhibiting acetylcholinesterase;10,11 (ii) anti-atherosclerosis;12 (iii) lowering lipid;13 (iv) liver protection;14 and (v) anti-inflammation.15 However, the effect of THSG on the chronic inflammatory pain has not yet been elucidated. In this study, based on the animal model of chronic inflammatory pain induced by injecting complete Freund’s adjuvant (CFA) into the hindpaw of mice, we determined whether THSG alleviated chronic inflammatory pain and explored the possible underlying molecular mechanism.
Materials and methods
Materials
All chemicals and reagents are of standard commercial biochemical quality. CFA, Hoechst 33258, and anti-β-actin antibody were obtained from Sigma (St. Louis, MO, USA). Cresyl violet stain was purchased from Leagene (Beijing, China). Anti-Iba-1 were purchased from Abcam (Cambridge, UK), anti-GluN2A and anti-GluN2B were purchased from Alomone Labs (Jerusalem, Israel), and anti-Caspase-3 and anti-Bax antibody were purchased from proteintech (Rosemont, IL, USA). The following antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA): anti-p-p38, anti-nuclear factor κB (NF-κB), anti-tumor necrosis factor α (TNF-α), and anti-Bcl-2. All secondary antibodies conjugated to horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). THSG were provided by Dr. Zhou Xuan-Xuan from the Department of Nature Medicine, Air Force Medical University.
Animals
We used adult male C57BL/6 mice aged 8–10 weeks and weighed 20–25 g that were purchased from Air Force Medical University Experimental Animal Center. They were kept in a 12-h light/dark room with a temperature of 24 ± 2°C and a relative humidity of 50%–60%. Food and water are freely available. All procedures were approved by the Animal Care and Use Committee of the Air Force Medical University. Prior to testing, all mice were accustomed to laboratory conditions for at least one week. All behavioral tests were conducted during lighting on the specified experiment day. The chronic inflammatory pain model was induced by injecting CFA (10 μl, 5μl CFA + 5μl saline) into the plantar surface skin of left hind paw mice. The equal volume of 0.9% saline was injected into the same place in the control animals.
Drug treatment and experimental designs
First of all, CFA (10 μl) and physiological saline were injected into the left hindpaw of 24 mice in experimental group and 6 mice in control group with a microsyringe, respectively. THSG was dissolved in saline to dilute it further. Two days after CFA injection, THSG (50, 100, 200 mg/kg) was perfused into three groups of experimental mice (n = 6 per group) by gavage needles. Both the blank control group and the model group were instilled with the same dose of physiological saline once a day for 10 consecutive days (from day 2 to day 11). This method is based on our preliminary estimate. To observe the analgesic effect of THSG, the mechanical allodynia was assessed at different time points (days 0, 1, 3, 7, 10, and 14) after CFA injection. Foot thickness, thermal hypersensitivity, and open field (OF) were measured on the 14th day after CFA injection. At last, half of the mice were sacrificed directly and half of the mice were treated with cerebral perfusion, and anterior cingulate cortex (ACC) specimens were taken for the further testing.
Mechanical allodynia
All mice were acclimated to the laboratory environment for one week. Prior to experiment, the mice were placed in a transparent cylindrical plastic housing on a wire mesh platform and allowed to acclimate for 30 min. The mechanical allodynia was assessed by using the up-down paradigm with a set of von Frey filaments (0.008–2 g) before (day 0) and after the CFA injection at day 1, 3, 7, and 14.16 The von Frey filaments were touched on the plantar surface of the left hindpaw of each mouse and repeated six times at intervals of 10 s. The paws between the left and right were tested with an interval of more than 3 min in the experiment. Positive responses included prolonged hindpaw withdrawal followed by licking or scratching. According to the result, the different force filaments were selected next time.
Thermal hyperalgesia
The mice were placed in a transparent cylindrical plastic housing on a glass platform and adapted to the environment for 30 min. According to the previous test,17 on the 14th day after CFA injection, the paw withdrawal latency (PWL) was measured by a commercially available plantar analgesia instrument (BME410A; Institute of Biological Medicine, Academy of Medical Science, China). The intensity of the thermal stimulus was adjusted to cause an average PWL of approximately 8–12 s in noninflammatory animals. The radiation source under the glass platform was placed on the plantar skin surface of the hindpaw of the mice when it was turned on and the radiation source was turned off automatically if the mice quickly lifted or licked its paw, this time was defined as PWL. Left paws were tested at 5-min intervals for a total of five trials. The paws between the left and right were tested with an interval of more than 10 min in the experiment. A 20-s cut off was used to prevent tissue damage.
Paw thickness
To evaluate the degree of paw edema of the mice, the thickness of the left and right hindpaw of the mice was measured by a Vernier caliper on the 14th day after CFA injection.18 The ability of THSG to attenuate inflammation-induced swelling was measured by comparing the thickness of mice hindpaw between groups.
Open field
OF was conducted as described previously19 on the 14th day. The apparatus (JLBehv-LAM-4; Shanghai Jiliang Software, China) was a square arena (30 × 30 × 30 cm) with clear Plexiglas walls and floor. It was placed inside an isolation chamber with dim illumination and a fan. Prior to experiment, the mice were placed in the center of the box for 15 min while keeping the surrounding environment quiet. The exploratory behaviors were videotaped using a video camera fixed above the floor for 15 min and analyzed using a video tracking system. We defined the “center” field as the central 15 × 15 cm2 area of the OF, which is a quarter of the total area.
Enzyme-linked immunosorbent assay
After behavior tests, the tissue samples of ACC were removed from the brain of mice that were dissected. According to the manufacturer’s instructions (R&D Systems), the content of inflammatory cytokines (TNF-α, interleukin 1β (IL-1β), and interleukin 6 (IL-6)) in ACC was detected by double-antibody sandwich method in this experiment.
Immunohistochemistry staining
After behavioral test, at first, the mice were anesthetized with excess chloral hydrate (5%, 0.5ml/30g) and then were perfused with physiological saline (20ml) and 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) through the ascending aorta cannulation. The brain was quickly removed from the mice and fixed overnight in 4% paraformaldehyde and dehydrated through an ascending sucrose series, 15% and 30% (w/v) sucrose in 0.1 M PBS at 4°C overnight, respectively.20 The specimen was embedded in paraffin and fixed after 48 h of dehydration. ACC segments of the frozen brain were cut into coronal sections (30 μm) by a cryogenic constant sectioning device (Leica), immediately soaked in 1% sodium azide liquid and stored at 4°C overnight. On the following day, all frozen brain sections were washed with 0.3% Triton X-100 PBS and blocked (10% goat serum, 0.1% Triton X-100 in PBS) for 2 h at 4°C. The brain slices were incubated with goat anti-Iba-1 (1:1000) in blocking solution for 24 h at 4°C, and then it were rinsed with PBS and incubated with mouse anti-rabbit IgG Alexa Fluor 594 (1:200) and mouse anti-goat IgG (1:200) at room temperature for 2 h at room temperature while being protected from light. All antibodies were diluted in PBS with 0.1% Triton X-100 and 2% bovine serum albumin. Nuclei were counterstained with Hoechst 33258. The brain slices were then moved to slides and coverslipped with 50% glycerine, and stained samples were photographed and analyzed with Olympus Fluoview FV100 microscope (Olympus, Japan).
Nissl staining
After CFA injection for 14 days, the brains were perfused with cold 4% paraformaldehyde in 0.01 M PBS (pH 7.4). Slices containing ACC were cut using a Leica CM1950 and then stained with 1% cresyl violet for 20 min. The images were captured with light microscope (Olympus BX60). Six sections from each animal were selected for Nissl staining.
Western blot analysis
To explore the molecular mechanism of the analgesic effect of THSG, we performed Western blot analysis of the relevant protein molecules as described previously.21 At the end of the behavioral test, ACC tissue samples were taken from the brain and placed in centrifuge tube, and the centrifuge tube was immediately placed on ice. Judging from the size of the tissue sample, total protein was extracted by using radio immunoprecipitation assay lysis buffer (Pierce Biotechnology), and the protease inhibitors were added immediately before use. Tissue proteins were quantified by a bicinchoninic acid assay kit, and then an equal amount of protein (30 μg) was separated by SDS-PAGE and electrotransferred onto an Immun-Blot polyvinylidene difluoride (PVDF) membrane with β-actin as a control. Each analysis was repeated for three times. PVDF membrane was cut out on the basis of molecular weight of a target protein and then blocked for 1 h with 5% nonfat milk in Tris-phosphate buffer containing 0.05% Tween 20 (TBST) at room temperature. Subsequently, the membrane was incubated with primary antibodies overnight including GluN2A (1:500), GluN2B (1:1000), phosphorylated-p38 (1:1000), NF-κB (1:1000), TNF-α (1:500), Caspase-3 (1:1000), Bcl-2 (1:1000), Bax (1:2000) at 4°C, and β-actin (1:10,000) served as a loading control. The next day, the membranes were washed three times with TBST for 10 min and then incubated with an HRP-conjugated secondary antibody (anti-rabbit/anti-mouse IgG for the primary antibody) for 1 h. After three washes with TBST for 10-min again, target protein signals were detected and digitized using enhanced chemiluminescent solution and Image J programs. The intensity of the band for each blot was calculated as a ratio to β-actin.
Statistical analysis
Data were presented as the mean and standard errors of the means (mean ± SEM). The statistical significance of differences between groups (SPSS version 19.0) was performed using one-way analysis of variance followed by least significant difference and S-N-K(s) t tests. In all cases, P < 0.05 was considered statistically significant.
Results
Effect of THSG on hindpaw edema, mechanical allodynia, and thermal hyperalgesia in mice
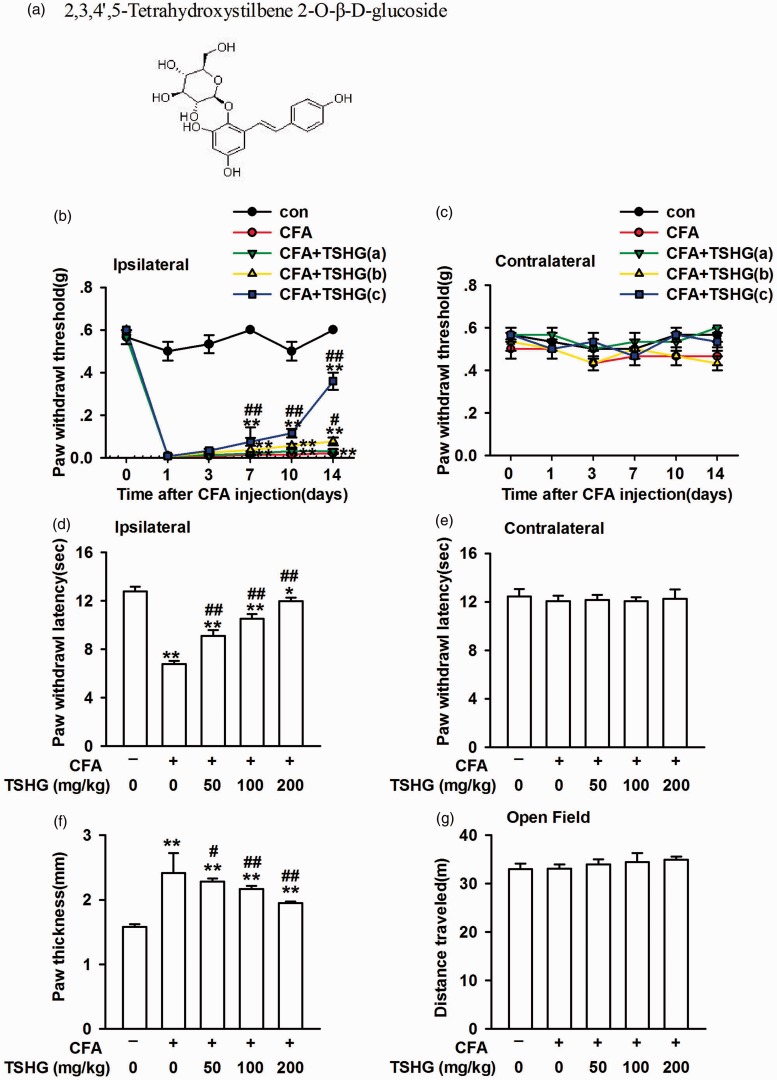
To investigate the effect and molecular mechanism of THSG (Figure 1(a)) on CFA-induced inflammatory pain, mice in the model group was injected of CFA into the plantar surface skin of the left hind paw and mice in the control group was injected with the same volume of saline. Following CFA injection, the paw of mice was swollen, whereas THSG reduced the degree of paw edema (Figure 1(f)). CFA injection caused mechanical allodynia and thermal hyperalgesia in the ipsilateral hindpaw (Figure 1(b) and (d)), whereas there was no difference observed in the contralateral hindpaw and the control group (Figure 1(c) and (e)). To evaluate the locomotor ability in mice after CFA injection and THSG treatment, the activity was observed in an OF test. Compared with the control group, the total travel distance was not changed following CFA injection or THSG treatment (Figure 1(g)). These results reveal that THSG plays a positive role in CFA-induced inflammatory pain, and it can alleviate the paw edema in mice. The motor function did not change after CFA injection or THSG treatment.
Figure 1.
THSG relieved the chronic inflammatory pain. (a) Chemical structures of THSG. (b and c) Mechanical allodynia was detected on days 0, 1, 3, 7, 10, and 14 after CFA injection. THSG (50, 100, and 200 mg/kg) attenuated mechanical allodynia in the ipsilateral hindpaw but had no effect on the contralateral hindpaw. (d and e) Thermal hyperalgesia was detected on day 14 after CFA injection. THSG reversed thermal hyperalgesia in the ipsilateral hindpaw but had no effect on the contralateral hindpaw. (f) THSG reduced the hindpaw edema compared to saline treated model mice. (g) No difference in the total distance traveled in each group in open field. Each value represents the mean ± SEM of three independent experiments (n = 6, *P < 0.05 vs. control group, **P < 0.01 vs. control group; #P < 0.05 vs. CFA-injected group, ##P < 0.01 vs. CFA-injected group). CFA: complete Freund’s adjuvant; THSG: tetrahydroxystilbene glucoside.
Effect of THSG on neuron survival and apoptosis in ACC
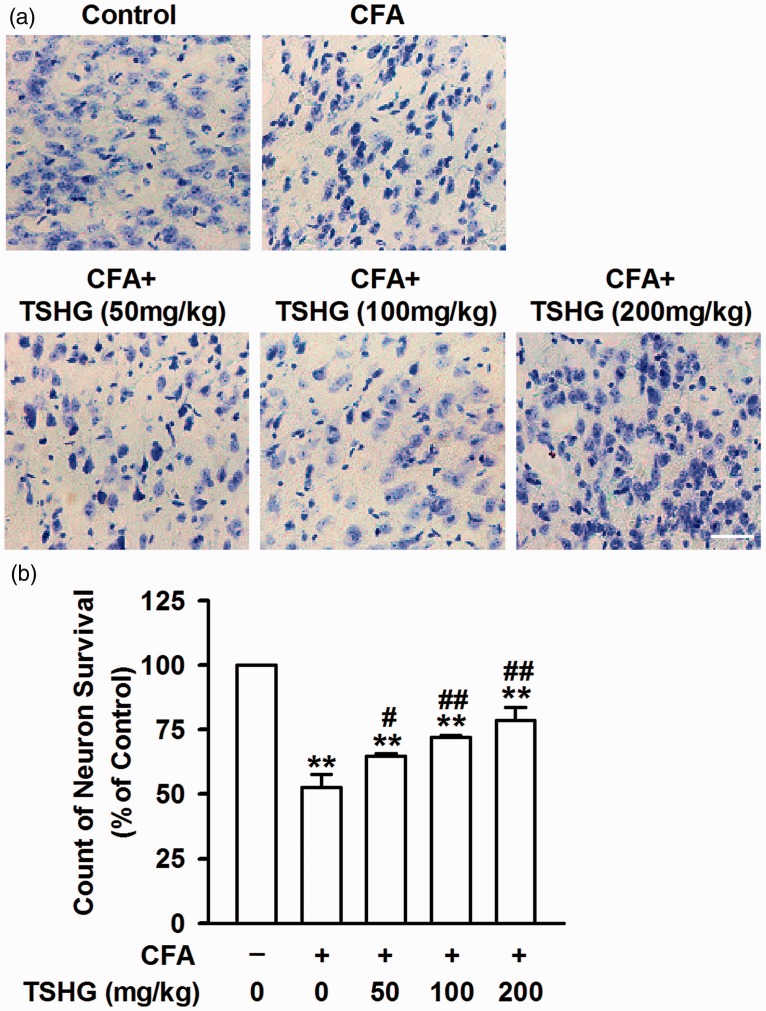
To determine the effect of THSG on neuron survival and apoptosis in ACC, Nissl staining (Figure 2(a)) was performed on the brain slices on the day 14. The number of neurons was significantly reduced in mice with CFA injection, meanwhile a large number of shrinking Nissl bodies was observed. But it has notably picked up after THSG treatment compared to the model group without THSG treatment, demonstrating the protective effect of THSG on neurons.
Figure 2.
THSG reversed the apoptosis of neuron. (a) The neuronal morphology was evaluated by Nissl staining the ACC slices on day 14 after CFA injection. Scale bar = 20 μm. (b) THSG administration significantly reduced the number of shrinking Nissl bodies. THSG increased the count of neuron survival after CFA injection for 14 days. Each value represents the mean ± SEM of three independent experiments (n = 6, **P < 0.01 vs. control group; #P < 0.05 vs. CFA-injected group, ##P < 0.01 vs. CFA-injected group). CFA: complete Freund’s adjuvant; THSG: tetrahydroxystilbene glucoside.
Effect of THSG on the expression of NMDARs and apoptosis-related protein in neurons of ACC
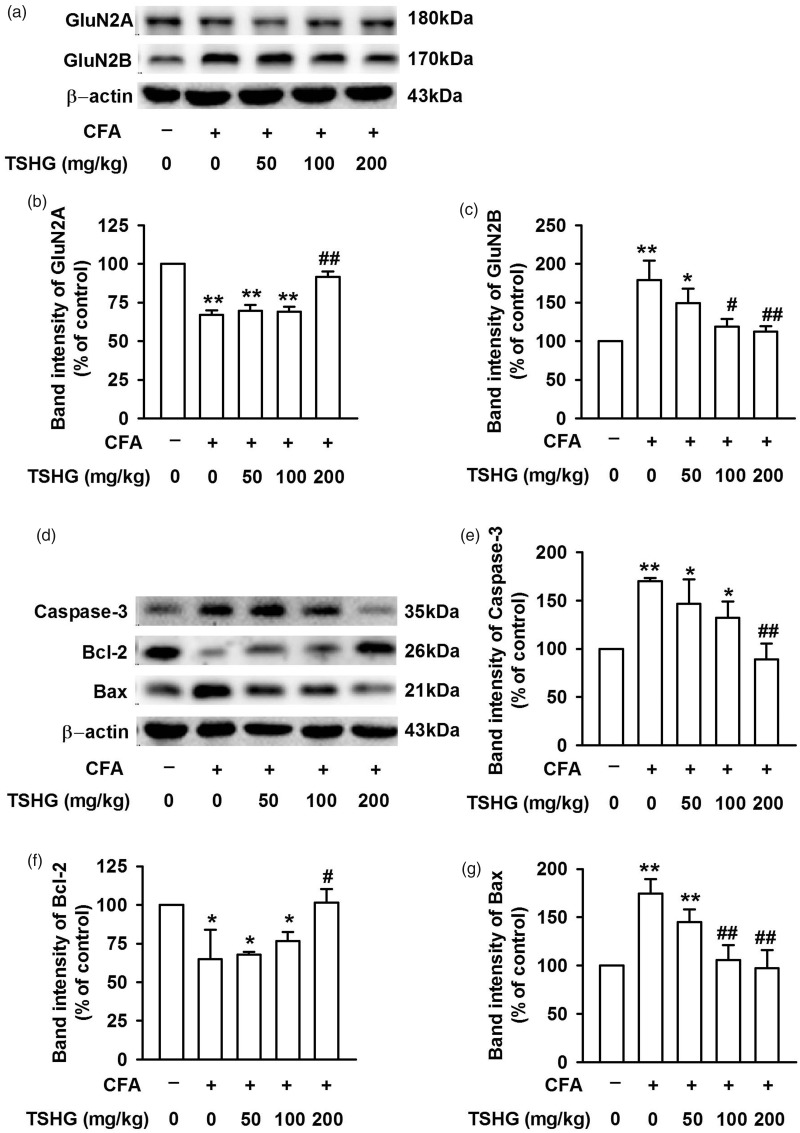
GluN2ARs and GluN2BRs are closely related to the survival of neurons, while Caspase-3, Bax, and Bcl-2 have been shown to participate in apoptosis-related activities (Figure 3(a) and (d)). After CFA injection in the model groups, the expression of GluN2ARs in ACC was decreased obviously compared to the control group (Figure 3(b)). In contrast, with CFA infection, the expression of GluN2BRs was increased significantly (Figure 3(c)) with no change observed in the control group, indicating that different subtypes of NMDARs in ACC exhibit different changes after CFA injection. Furthermore, Bcl-2 is an anti-apoptotic protein and Bax is a pro-apoptotic protein, respectively. As for Caspase-3, it serves as the most prominent terminal cleavage enzyme during apoptosis.22 In the model groups, Bcl-2 expression was depressed remarkably (Figure 3(f)), while Bax and Caspase-3 expression got enhanced evidently after CFA injection (Figure 3(e) and (g)). These results make it clear that CFA injection promoted the apoptosis of neurons in ACC. With THSG treatment in a series of doses (50, 100, and 200 mg/kg) on day 14, the above phenomena were reversed (Figure 3). All these data suggest that THSG enhances the expression of GluN2ARs and Bcl-2 and suppresses the expression of GluN2BRs, Bax, and Caspase-3, thereby inhibiting neuronal apoptosis in ACC.
Figure 3.
Effects of THSG on protein expression in ACC. (a) Representative results of Western blot analysis showed the expression levels of GluN2A and GluN2B. (b) THSG (200 mg/kg) significantly increased the downregulated expression of GluN2A after CFA treatment. (c) THSG (200 mg/kg) significantly decreased the upregulated expression of GluN2B after CFA treatment. (d) Representative results of Western blot analysis showed the expression levels of Caspase-3, Bcl-2, and Bax. (e–g) THSG (200 mg/kg) notably reversed the expression of the above protein in ACC of CFA-treated mice. Each value represents the mean ± SEM of three independent experiments (n = 6, *P < 0.05 vs. control group, **P < 0.01 vs. control group; #P < 0.05 vs. CFA-injected group, ##P < 0.01 vs. CFA-injected group). CFA: complete Freund’s adjuvant; THSG: tetrahydroxystilbene glucoside.
Effect of THSG on the activation of microglia in ACC
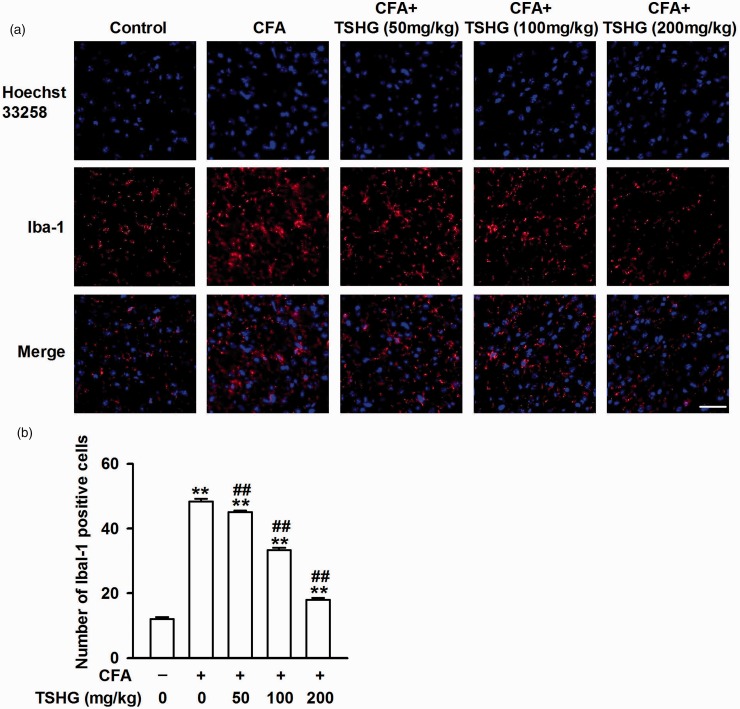
Microglia, a type of glial cell, is the major immune effector in the CNS.23 Microglia activation was increased in the process of inflammatory stimulation. ACC is a specific brain area responsible for pain regulation. Brain sections of ACC were collected and subjected to immunofluorescence staining against actin-binding protein (Iba-1) for microglia for two weeks after CFA injection. Compared with the control group, the fluorescence intensity of Iba-1 was improved notably in the model group, and the expression of Iba-1 was repressed in the brain sections of the mice treated with THSG (50, 100, and 200 mg/kg) (Figure 4(a)). This result shows that CFA injection can induce the activation of microglia in ACC, while THSG may reverse this change.
Figure 4.
Effects of THSG on the activation of microglia in ACC. (a) After CFA injection for 14 days, ACC slices were immunostained with microglial marker Iba-1 antibody (red), and nuclei were stained with Hoechst33258 (Blue). Scale bar = 20 μm. (b) THSG inhibited the activation of microglia in ACC of CFA-injected mice and had a dose-dependent effect. Each value represents the mean ± SEM of three independent experiments (n = 6, *P < 0.05 vs. control group, **P < 0.01 vs. control group; #P < 0.05 vs. CFA-injected group, ##P < 0.01 vs. CFA-injected group). CFA: complete Freund’s adjuvant; THSG: tetrahydroxystilbene glucoside.
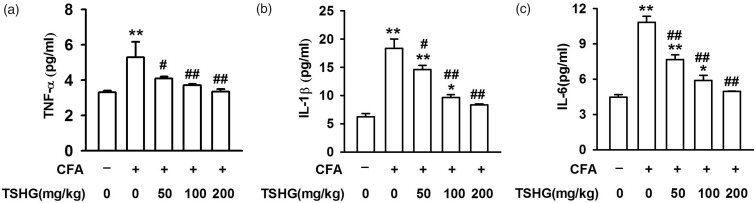
Effect of THSG on changes in inflammatory cytokines after CFA injection
To further determine the effects of THSG on inflammation, a sample of ACC tissue in the brain was harvested on day 14 after the CFA injection, and the concentrations of inflammatory cytokines in ACC were checked by enzyme-linked immuno-sorbent assay. CFA injection led to an upregulation in the concentrations of proinflammatory cytokines, such as TNF-α (5.294 ± 0.875 pg/ml), IL-1β (18.338 ± 1.643 pg/ml), and IL-6 (10.824 ± 0.519 pg/ml). The consequence was reversed with THSG treatment (50, 100, and 200 mg/kg), as these data show: TNF-α (4.090 ± 0.117, 3.701 ± 0.086, 3.346 ± 0.148 pg/ml), IL-1β (14.565 ± 0.781, 9.656 ± 0.497, 8.349 ± 0.141 pg/ml), and IL-6 (7.652 ± 0.399, 5.878 ± 0.428, 4.944 ± 0.047 pg/ml) (Figure 5(a) to (c)). It can be revealed that THSG has a positive effect on anti-inflammation, which may be one of the ways in which it inhibits the chronic inflammatory pain.
Figure 5.
Effects of THSG on the production of proinflammatory cytokines by ELISA detection. THSG reduced the elevated levels of TNF-α (a), IL-1β (b), and IL-6 (c) in ACC on day 14 after CFA injection. Each value represents the mean ± SEM of three independent experiments (n = 6, *P < 0.05 vs. control group, **P < 0.01 vs. control group; #P < 0.05 vs. CFA-injected group, ##P < 0.01 vs. CFA-injected group). CFA: complete Freund’s adjuvant; THSG: tetrahydroxystilbene glucoside; TNF: tumor necrosis factor; IL: interleukin.
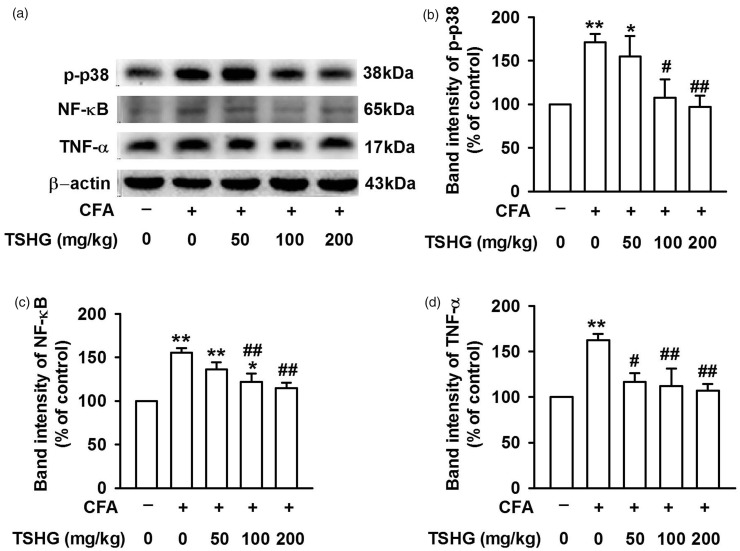
THSG blocks p38/NF-κB signaling pathway in CFA-induced inflammatory pain
Through behavioral tests, THSG was detected to effect for pain relief, but the specific molecular mechanism is not yet clear. P38 is one of the members of mitogen-activated protein kinase (MAPK) family and is closely associated with the development of inflammatory pain.24 Fourteen days after CFA injection, ACC tissue samples were collected and Western blot analysis was performed to analyze the expression levels of p-p38, NF-κB, and TNF-α (Figure 6(a)). In the model of inflammatory pain induced by CFA injection, phosphorylation of p38 (Figure 6(b)) and downstream signal molecule NF-κB (Figure 6(c)) were increased with inflammatory cytokines TNF-α also elevated evidently (Figure 6(d)). After THSG (50, 100, and 200 mg/kg) treatment, the expression of p-p38, NF-κB, and TNF-α in the model groups was inhibited remarkably, showing that THSG may exert its analgesic effect by inhibiting p38/NF-κB signaling pathway.
Figure 6.
Effects of THSG on the expression level of signaling pathway molecules involved in inflammation response. (a) Representative results of Western blot analysis showed the expression levels of phospho-P38, NF-κB, and TNF-α in ACC on day 14 after CFA injection. (b–d) THSG notably reversed the expression of the above protein in ACC of CFA-treated mice. Each value represents the mean ± SEM of three independent experiments (n = 6, *P < 0.05 vs. control group, **P < 0.01 vs. control group; #P < 0.05 vs. CFA-injected group, ##P < 0.01 vs. CFA-injected group). CFA: complete Freund’s adjuvant; THSG: tetrahydroxystilbene glucoside; TNF: tumor necrosis factor; NF: nuclear factor.
Discussion
In this study, it was found that THSG relieved CFA-induced chronic inflammatory pain on mechanical allodynia, thermal hyperalgesia, and paw edema in mice. Our results demonstrated that THSG exerts analgesic effect by protecting neuron, decreasing the activation of microglia and the release of several proinflammatory cytokines (TNF-α, IL-1β, and IL-6), and inhibiting the expression of phosphorylation of p38 and downstream signal molecule (NF-κB). These findings suggest that THSG was effective in the treatment of the chronic inflammatory pain.
A large number of studies have confirmed that the long-term synaptic plasticity of the ACC is a key pivot of the chronic inflammatory pain. ACC could collect the nociceptive information from thalamus, amygdala, and other pain-related areas in the cortex.25,26 Besides, peripheral nociceptive stimulation and/or injury models produce excitatory postsynaptic potentials in ACC. And related mechanism of excitatory synaptic transmission has been demonstrated. Glutamate is the main excitatory neurotransmitter of the CNS and plays an important role in brain function. The inflammatory pain enhances glutamatergic transmitter releases in layer II/III of ACC. An excessive release of glutamate can lead to excitotoxicity, which disable mitochondrial functions, rapidly increases the concentration of reactive oxygen species, and eventually cause neuronal apoptosis and development of hypersensitivity.27–29 However, gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the CNS. In animal studies, GABAergic synaptic transmission enhancement, by a muscarinic receptor agonist injected into the ACC, produces antinociceptive behaviors.30 In addition, coapplication of these two GABA agonists’ muscimol and baclofen can protect neurons by downregulating the function of NMDARs in animal model of cerebral ischemia. On the contrary to glutamatergic effect, enhanced GABAergic activity could balance excessive glutamatergic excitation which is the pivotal event leading to cell death.31
NMDARs are a heterotetramer consisting of three subunits: GluN1, GluN2, and GluN3.32 GluN2ARs and GluN2BRs are the most common NMDARs subtypes found in mammalian CNS. GluN2ARs function neuroprotection against ischemia by regulating cyclic adenosine monophosphate (cAMP) response element binding protein phosphorylation.33 Besides, glycine confers neuroprotection through nonionotropic activation of GluN2ARs and subsequent enhancement of Akt activation. Hu found that the activation of Akt is inhibited by knockdown of GluN2ARs, and then the neuronal apoptosis is increased.34 On the other hand, the activation of GluN2BRs plays an important role in the development and maintenance of chronic inflammatory pain. It is shown that after CFA injection, cAMP-dependent protein kinase A is enriched, which activate Src-family protein tyrosine kinases member Fyn. The active Fyn then promoted GluN2B activation, which evokes pain sensitization.8,9 Zhang et al. reported that GluN2BRs causes mitochondrial Ca2+ overload, releases cytochrome c, changes the level of apoptosis-related proteins Bcl-2, Bax and Caspase-3, which ultimately leads to neuronal apoptosis.7
The damaged neurons produce the neurotoxic soluble factors and, in turn, induce microglia activation.35 Minocycline provides neuroprotection against NMDA neurotoxicity by inhibiting microglia.36 Jeong et al. showed zymosan induces thermal and mechanical hyperalgesia as well as neuronal activation covering enhanced immunoreactivity of Fos, protein kinase C (PKC), and PKC-dependent phosphorylation of the NMDA receptor.37 Specially, zymosan also led to activated microglia activities. Microglia is the resident immune cells in CNS and microglia exist in a quiescent stage. Activation of microglia in ACC and secretion of inflammatory cytokines are closely associated with the chronic inflammatory pain. In neuropathic pain model, it has been observed that microglial activation is apparent at day 3 and peaked at day 14.38 Moreover, it has been reported that microglial response was initiated early (days 3–14), followed by delayed astrocyte activation (days 7–28).39 During autoimmune inflammation of the nervous system, microglia release and respond to several cytokines, including IL-1, IL-6, TNF-α, and IFN-γ, which contribute to the maintenance of persistent pain states.40
MAPK is a family of serine/threonine protein kinases, which are critical signaling molecules in glia cells. Activation of MAPKs in the primary afferent nerve and spinal cord may be involved in the development of pain hypersensitivity through a dependent or an independent transcriptional manner.41 As one of the members of MAPK family, p38 is a tyrosine phosphoprotein kinase which can be activated by inflammation, heat stress, osmotic shock, ultraviolet light, and cytotoxic chemicals.42 Liu et al. find that phosphorylation of p38 in microglia is increased through double immunofluorescence staining performed for p-p38 and Iba-1(microglia cell). The discovery demonstrates that p38 in microglia plays a critical role in pain.43 The application of minocycline both alleviates microglial activation and phosphorylation of p38.44 Our result reveals that 14 days after CFA injection, the expression of phosphorylation of p38 and NF-κB is increased in the model group. NF-κB serves as a key transcription factor that has been implicated in the regulation of proinflammatory cytokines and inflammatory pain. Quite a few studies have shown a notable increase in NF-κB expression after CFA-induced inflammatory pain, which is activated by inflammatory stimuli via p38 activation in proliferating cells. 45,46 Treatment of proliferating cells with the p38 specific inhibitor SB203580 inhibited the inflammation that induced the synthesis of NF-κB which confirms the above signaling pathway.24,47 Furthermore, our study suggested an increase of TNF-α following the activation of phosphorylation of p38 and NF-κB. The underlying mechanism may as follows: NF-κB positively regulates genes encoding cytokines (TNF-α, IL-1β, and IL-6), which play a role in immunity, anti-apoptosis, cell proliferation, and inflammatory pain.48 Meanwhile, NF-κB activity can also be induced by a broad range of stimuli, including inflammatory cytokines (TNF-α, IL-1β, and IL-6) and bacterial and viral products that show a positive feedback mechanism between NF-κB and TNF-α.49 To sum up, our result indicated that THSG repressed p38/NF-κB signaling pathway and reduced the expression of TNF-α. It may be the main molecular mechanism by which THSG exerts its analgesic effect.
In conclusion, our study demonstrated that THSG alleviated the chronic inflammatory pain induced by CFA and reduced paw edema in mice. It also revealed the possible molecular mechanism underlying the analgesic effect of THSG. This study found a new pharmacological effect of THSG, which provided a theoretical basis for further clinical research on analgesia and THSG is expected to be a new analgesic drug.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (No. 31500820 and 81703727) and the Shaanxi Science and Technology Coordination innovation project (No. 512675164038).
References
- 1.Corasaniti MT, Amantea D, Russo R, Bagetta G. The crucial role of neuronal plasticity in pain and cell death. Cell Death Differ 2006; 13: 534–536. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Bai H-H, Zhang Z-Y, Liu J-P, Suo Z-W, Yang X, Hu X-D. Disruption of SHP1/NMDA receptor signaling in spinal cord dorsal horn alleviated inflammatory pain. Neuropharmacology 2018; 137: 104–113. [DOI] [PubMed] [Google Scholar]
- 3.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 2005; 48: 289–301. [DOI] [PubMed] [Google Scholar]
- 4.Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 2007; 55: 779–785. [DOI] [PubMed] [Google Scholar]
- 5.Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JTR. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 2011; 70: 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Liu J, Zhang X, Guo Y, Xu Z, Cao W, Sun X, Sun W, Zhao M. Down-regulation of NR2B receptors partially contributes to analgesic effects of Gentiopicroside in persistent inflammatory pain. Neuropharmacology 2008; 54: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Liu J, Fan C, Mao L, Xie R, Wang S, Yang M, Yuan H, Yang X, Sun J, Wang J, Kong J, Huang S, Sun B. The GluN1/GluN2B NMDA receptor and metabotropic glutamate receptor 1 negative allosteric modulator has enhanced neuroprotection in a rat subarachnoid hemorrhage model. Exp Neurol 2018; 301: 13–25. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J-Y, Duan X-L, Yang L, Liu J-P, He Y-T, Guo Z, Hu X-D, Suo Z-W. Activity-dependent synaptic recruitment of neuroligin 1 in spinal dorsal horn contributed to inflammatory pain. Neuroscience 2018; 388: 1–10. [DOI] [PubMed] [Google Scholar]
- 9.Yang H-B, Yang X, Cao J, Li S, Liu Y-N, Suo Z-W, Cui H-B, Guo Z, Hu X-D. cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J Neurochem 2011; 116: 93–104. [DOI] [PubMed] [Google Scholar]
- 10.Su Y, Wang Q, Wang C, Chan K, Sun Y, Kuang H. The treatment of Alzheimer’s disease using Chinese medicinal plants: from disease models to potential clinical applications. J Ethnopharmacol 2014; 152: 403–423. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Xing Y, Ye C, Ai H, Wei H, Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer’s disease and intervention by using tetrahydroxystilbene glucoside. Behav Brain Res 2006; 173: 246–254. [DOI] [PubMed] [Google Scholar]
- 12.Yao WJ, Fan WJ, Huang C, Zhong H, Chen XFan, Zhang W. Proteomic analysis for anti-atherosclerotic effect of tetrahydroxystilbene glucoside in rats. Biomed Pharmacother 2013; 67: 140–145. [DOI] [PubMed] [Google Scholar]
- 13.Yang P-Y, Almofti MR, Lu L, Kang H, Zhang J, Li T-J, Rui Y-C, Sun L-N, Chen W-S. Reduction of atherosclerosis in cholesterol-fed rabbits and decrease of expressions of intracellular adhesion molecule-1 and vascular endothelial growth factor in foam cells by a water-soluble fraction of Polygonum multiflorum. J Pharmacol Sci 2005; 99: 294–300. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y, Ohminami H, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stilbene components of roots of Polygonum ssp. on liver injury in peroxidized oil-fed rats. Planta Med 1983; 49: 51–54. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Wang Y-Y, Yang J, Lu Y-F, Liu J, Shi J-S. Tetrahydroxystilbene glucoside attenuates neuroinflammation through the inhibition of microglia activation. Oxid Med Cell Longev 2013; 2013: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feehan AK. Novel endomorphin analogs are more potent and longer-lasting analgesics in neuropathic, inflammatory, postoperative, and visceral pain relative to morphine. J Pain 2017; 18: 1526–1541. [DOI] [PubMed] [Google Scholar]
- 17.Sun T, Wang J, Li X, Li Y-J, Feng D, Shi W-L, Zhao M-G, Wang J-B, Wu Y-M. Gastrodin relieved complete Freund’s adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int Immunopharmacol 2016; 41: 66–73. [DOI] [PubMed] [Google Scholar]
- 18.Wang D-S, Tian Z, Guo Y-Y, Guo H-L, Kang W-B, Li S, Den Y-T, Li X-B, Feng B, Feng D, Zhao J-N, Liu G, Zhao M-G. Anxiolytic-like effects of translocator protein (TSPO) ligand ZBD-2 in an animal model of chronic pain. Mol Pain 2015; 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Han J, Wang D-S, Yang Q, Feng B, Kang W-B, Yang L, Liu G, Zhao M-G. Sinomenine attenuates chronic inflammatory pain in mice. Metab Brain Dis 2017; 32: 211–219. [DOI] [PubMed] [Google Scholar]
- 20.Tian Z, Wang Y, Zhang N, Guo Y-y, Feng B, Liu S-b, Zhao M-g. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology 2013; 38: 2218–2233. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang Q, Zhang K, Liu SB, Zhao MG, Wu YM. Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun 2016; 56: 352–362. [DOI] [PubMed] [Google Scholar]
- 22.D’Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 2010; 17: 1104–1114. [DOI] [PubMed] [Google Scholar]
- 23.Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res 1987; 18: 155–171. [DOI] [PubMed] [Google Scholar]
- 24.Ulivi V, Giannoni P, Gentili C, Cancedda R, Descalzi F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J Cell Biochem 2008; 104: 1393–1406. [DOI] [PubMed] [Google Scholar]
- 25.Guo B. Chronic inflammatory pain impairs mGluR5-mediated depolarization-induced suppression of excitation in the anterior cingulate cortex. Cereb Cortex 2017; 28: 2118–2130. [DOI] [PubMed] [Google Scholar]
- 26.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016; 3: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda H, Zhao MG, Zhuo M. Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol Pain 2009; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz A, Alberdi E, Matute C. Mitochondrial division inhibitor 1 (mdivi-1) protects neurons against excitotoxicity through the modulation of mitochondrial function and intracellular Ca(2+) signaling. Front Mol Neurosci 2018; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding R, Jiang H, Sun B, Wu X, Li W, Zhu S, Liao C, Zhong Z, Chen J. Advanced oxidation protein products sensitized the transient receptor potential vanilloid 1 via NADPH oxidase 1 and 4 to cause mechanical hyperalgesia. Redox Biol 2016; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga K, Matsuzaki Y, Honda K, Eto F, Furukawa T, Migita K, Irie K, Mishima K, Ueno S. Activations of muscarinic M1 receptors in the anterior cingulate cortex contribute to the antinociceptive effect via GABAergic transmission. Mol Pain 2017; 13: 174480691769233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Li C, Wang R, Han D, Zhang Q-G, Zhou C, Yu H-M, Zhang G-Y. Activation of GABA receptors attenuates neuronal apoptosis through inhibiting the tyrosine phosphorylation of NR2A by Src after cerebral ischemia and reperfusion. Neuroscience 2007; 150: 938–949. [DOI] [PubMed] [Google Scholar]
- 32.Wu L-J, Zhuo M. Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics 2009; 6: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XM, Luo JH. GluN2A versus GluN2B: twins, but quite different. Neurosci Bull 2013; 29: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu R. Glycine triggers a non-ionotropic activity of GluN2A-containing NMDA receptors to confer neuroprotection. Sci Rep 2016; 6: 34459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 2005; 76: 77–98. [DOI] [PubMed] [Google Scholar]
- 36.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol 2001; 166: 7527–7533. [DOI] [PubMed] [Google Scholar]
- 37.Jeong YC, Son JS, Kwon YB. The spinal antinociceptive mechanism determined by systemic administration of BD1047 in zymosan-induced hyperalgesia in rats. Brain Res Bull 2015; 119: 93–100. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 2006; 97: 772–783. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Zhao YQ, Ribeiro-da-Silva A, Zhang J. Distinctive response of CNS glial cells in oro-facial pain associated with injury, infection and inflammation. Mol Pain 2010; 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero-Sandoval EA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs 2008; 9: 726–734. [PMC free article] [PubMed] [Google Scholar]
- 41.Edelmayer RM, Brederson J-D, Jarvis MF, Bitner RS. Biochemical and pharmacological assessment of MAP-kinase signaling along pain pathways in experimental rodent models: a potential tool for the discovery of novel antinociceptive therapeutics. Biochem Pharmacol 2014; 87: 390–398. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994; 265: 808–811. [DOI] [PubMed] [Google Scholar]
- 43.Liu R, Xu X, Xu Y. Pulsed radiofrequency on dorsal root ganglion relieved neuropathic pain associated with downregulation of the spinal interferon regulatory factor 8, microglia, p38MAPK expression in a CCI rat model. Pain Physician 2018; 21: E307–E322. [PubMed] [Google Scholar]
- 44.Huang Q, Mao X-F, Wu H-Y, Liu H, Sun M-L, Wang X, Wang Y-X. Cynandione A attenuates neuropathic pain through p38β MAPK-mediated spinal microglial expression of β-endorphin. Brain Behav Immun 2017; 62: 64–77. [DOI] [PubMed] [Google Scholar]
- 45.Hu B, Xu G, Zhang X, Xu L, Zhou H, Ma Z, Shen X, Zhu J, Shen R. Paeoniflorin attenuates inflammatory pain by inhibiting microglial activation and Akt-NF-κB signaling in the central nervous system. Cell Physiol Biochem 2018; 47: 842–850. [DOI] [PubMed] [Google Scholar]
- 46.Pinho-Ribeiro FA, Hohmann MSN, Borghi SM, Zarpelon AC, Guazelli CFS, Manchope MF, Casagrande R, Verri WA. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF-kappaB. Chem Biol Interact 2015; 228: 88–99. [DOI] [PubMed] [Google Scholar]
- 47.Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, Borghi SM, Bordignon J, Silva RL, Cunha TM, Alves-Filho JC, Cunha FQ, Casagrande R, Verri WA. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem 2016; 33: 8–14. [DOI] [PubMed] [Google Scholar]
- 48.Bowles RD, Mata BA, Bell RD, Mwangi TK, Huebner JL, Kraus VB, Setton LA. In vivo luminescence imaging of NF-kappaB activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol 2014; 66: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2006; 14: 839–848. [DOI] [PubMed] [Google Scholar]