Abstract
Background:
Painful dysfunctional shoulders with irreparable rotator cuff tears (IRCTs) in active patients are a challenge. Arthroscopic superior capsular reconstruction (ASCR) is a new treatment option originally described using a fascia lata autograft harvested through an open approach. However, concerns about donor site morbidity have discouraged surgeons from using this type of graft.
Hypothesis:
ASCR using a minimally invasive harvested fascia lata autograft produces good 6-month and 2-year shoulder outcomes in IRCTs, with low-impact thigh morbidity at 2 years.
Study Design:
Case series; Level of evidence, 4.
Methods:
From 2015 to 2016, a total of 22 consecutive patients (mean age, 64.8 ± 8.6 years) with chronic IRCTs (Hamada grade 1-2; Goutallier cumulative grade ≥3; Patte stage 1: 2 patients; Patte stage 2: 6 patients; Patte stage 3: 14 patients) underwent ASCR using a minimally invasive harvested fascia lata autograft. All patients completed preoperative and 6-month evaluations consisting of the Simple Shoulder Test (SST), subjective shoulder value (SSV), Constant score (CS), range of motion (ROM), acromiohumeral interval (AHI), and magnetic resonance imaging. Twenty-one patients completed the 2-year shoulder and donor site morbidity assessments.
Results:
The mean active ROMs improved significantly (P < .001): elevation, from 74.8° ± 55.5° to 104.5° ± 41.9° (6 months) and 143.8° ± 31.7° (2 years); abduction, from 53.2° ± 43.3° to 86.6° ± 32.9° (6 months) and 120.7° ± 37.7° (2 years); external rotation, from 13.2° ± 18.4° to 27.0° ± 16.1° (6 months) and 35.6° ± 17.3° (2 years); and internal rotation, from 1.2 ± 1.5 points to 2.6 ± 1.5 points (6 months) and 3.8 ± 1.2 points (2 years). The mean functional shoulder scores improved significantly (P < .001): SST, from 2.1 ± 2.9 to 6.8 ± 3.5 (6 months) and 8.6 ± 3.5 (2 years); SSV, from 33.0% ± 17.4% to 55.7% ± 25.6% (6 months) and 70.0% ± 23.0% (2 years); CS, from 17.5 ± 13.4 to 42.5 ± 14.9 (6 months) and 64.9 ± 18.0 (2 years). The mean shoulder abduction strength improved significantly (P < .001) from 0.0 to 1.1 ± 1.4 kg (6 months) and 2.8 ± 2.6 kg (2 years). The mean AHI improved from 6.4 ± 3.3 mm to 8.0 ± 2.5 mm (6 months) and decreased to 7.1 ± 2.5 mm (2 years). This 0.7 ± 1.5–mm overall decrease was statistically significant (P = .042). At 6 months, 20 of 22 patients (90.9%) had no graft tears. At 2 years, 12 of 21 patients (57.1%) were bothered by their harvested thigh, 16 (76.2%) noticed donor site changes, 16 (76.2%) considered that the shoulder surgery’s end result compensated for the thigh’s changes, and 18 (85.7%) would undergo the same surgery again.
Conclusion:
ASCR using a minimally invasive harvested fascia lata autograft produced good 6-month and 2-year shoulder outcomes in IRCTs, with low-impact thigh morbidity at 2 years.
Keywords: rotator cuff tear, superior capsular reconstruction, donor site morbidity, shoulder arthroscopic surgery, fascia lata autograft, minimally invasive
Painful and dysfunctional shoulders in active patients with irreparable rotator cuff tears (IRCTs) are a challenge. Reverse total shoulder arthroplasty is a valuable treatment option for chronic IRCTs.34,40 However, in the younger and more active patient without arthritis, reverse total shoulder arthroplasty’s limited survivorship and the potential for complex revisions after infections or instability raise concerns.5,15,16,48 In this setting, alternative treatment options, such as arthroscopic subacromial debridement, long head of the biceps tendon (LHBT) tenotomy, balloon plasty, partial rotator cuff tear (RCT) repair, rotator cuff interposition grafting, patch augmentation, and tendon transfer, are favored. Nevertheless, none of these are exempt from limitations, risks, or complications.# Thus, the treatment algorithm for IRCTs remains controversial.
Arthroscopic superior capsular reconstruction (ASCR) was recently added to this algorithm as a novel option to treat IRCTs in patients without arthritis. In the original technique described by Mihata et al,32 the superior capsule was reconstructed using a fascia lata autograft that was harvested through an open approach. However, despite the reportedly promising clinical results30–32 of ASCR with a fascia lata autograft, concerns about donor site morbidity have discouraged orthopaedic surgeons from using this type of graft.22 Despite the lack of evidence presented to support these concerns, this theoretical setback has led to the development of technical variations, such as ASCR with a dermal allograft and ASCR with an LHBT autograft.6,7,9,13,24,35,37,38,43 Indeed, no harvest site dysfunction has been reported in patients undergoing ASCR with a fascia lata autograft,30 and ASCR with an allograft13,37 and ASCR with an LHBT autograft9 have yet to achieve such promising shoulder outcomes as ASCR with a fascia lata autograft as originally reported by Mihata et al.32
Aiming to reproduce the promising clinical results of ASCR using a fascia lata autograft while attempting to reduce potential donor site morbidity, the first author (C.I.d.C.A.) introduced modifications to the technique for ASCR with a fascia lata autograft originally described by Mihata et al32 and replaced the open harvesting approach with a minimally invasive fascia lata autograft harvesting technique. Angelo and de Campos Azevedo1 reported the early donor site morbidity results (at 1 week, 6 months, and 18 months postoperatively) of the minimally invasive harvesting technique and concluded that it did not produce significant donor site morbidity or hip dysfunction.
The purpose of the current study was to evaluate the 2-year postoperative results on shoulder outcomes and donor site morbidity in patients with IRCTs treated with ASCR using a fascia lata autograft with minimally invasive graft harvesting. It was hypothesized that this combined technique would produce good 6-month and 2-year shoulder outcomes in IRCTs, with low-impact thigh morbidity at 2 years.
Methods
Study Design
Patients admitted with primary or recurrent RCTs who had failed conservative treatment, with no evidence of significant glenohumeral articular cartilage degeneration on true anteroposterior (AP) radiographs (Hamada grade 1 or 2),21 were offered arthroscopic surgery by a single surgeon (C.I.d.C.A.). If reparability was intraoperatively confirmed (ie, if after adequate release of adhesions, the torn tendons passed the grasper or suture tests, therefore successfully reaching their native footprint without undue tension), the patients underwent arthroscopic RCT repair and thus were not be enrolled in the study. If instead an IRCT was confirmed (ie, if the torn tendons were frail and did not pass the grasper or suture tests, therefore not reaching their native footprint without undue tension or further tearing), the patients underwent ASCR with a minimally invasive harvested fascia lata autograft and additional repair of the remaining (if viable) rotator cuff over the superior capsular graft (onlay partial RCT repair incorporated into the superior capsular reconstruction procedure) and thus were enrolled in the study.
This prospective clinical study was designed by the first author and was approved by an institutional review board, and each patient signed an informed consent form.
Shoulder Clinical Assessment
The patients were systematically assessed preoperatively and at 6 months and 2 years postoperatively by an experienced shoulder surgeon (C.I.d.C.A.). A goniometer was used to measure active painless shoulder range of motion (ROM) in elevation, abduction, and external rotation with the arm at the side. Active internal rotation was defined as the highest vertebral body that the patient’s thumb could reach without sustaining pain, and it was converted to a scale of 1 to 5 points: lateral thigh = 0, buttock = 1, sacrum = 2, lumbar = 3, 12th thoracic vertebra = 4, and 7th thoracic vertebra = 5. Painless shoulder abduction strength in full pronation, with the elbow in full extension and the shoulder in 90° of abduction in the scapular plane, was measured (in kg) using a digital dynamometer strapped to the forearm. The patients were assessed with functional shoulder scores: the Simple Shoulder Test (SST; 1-12 points),29 the subjective shoulder value (SSV; 0%-100%),19 and the Constant score (CS; 1-100 points).12
Donor Site Morbidity and Overall Subjective Outcome Assessment
At the 2-year evaluation, the patients were asked 3 closed-ended questions by the first author to assess the impact of donor site morbidity on the patient’s overall satisfaction with the surgical procedure: (1) “Does the harvested thigh bother you?” (2) “Does your shoulder surgery’s end result compensate for your thigh’s changes?” and (3) “Would you undergo the same surgery again?” Additionally, the patients were asked, in a closed-ended questionnaire, if they noticed any of the following donor site changes: deformity, pain, numbness, and specific donor site–related claudication.
Radiological Shoulder Assessment
True AP radiographs were obtained preoperatively and at 6 months and 2 years postoperatively. The acromiohumeral interval (AHI), which is the distance between the top of the humeral head and the undersurface of the acromion, was measured using a software measurement tool (PACS; Agfa HealthCare). Arthritis of the RCT was graded according to the revised radiographic classification of Hamada et al21 by a single experienced clinician (C.I.d.C.A.), who recorded all measurements.
Shoulder Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed preoperatively and at 6 months postoperatively with a 1.5-T closed-type scanner (GE Healthcare or Siemens [Magnetom]), and images were acquired in the sagittal (proton density fat-saturated and T1-weighted), coronal (proton density fat-saturated, T2-weighted fat-saturated, and T1-weighted), and axial (proton density–weighted and T2-weighted) planes.
The MRI scans were preoperatively evaluated for supraspinatus, infraspinatus, teres minor, and subscapularis muscle fatty degeneration and graded according to the classification by Goutallier et al.20 The Goutallier cumulative grade (the sum of the fatty infiltration stages of the 4 muscles)47 and the global fatty muscle degeneration index (GFMDI; the mean value of the grades for the supraspinatus, infraspinatus, and subscapularis)10 were calculated for each patient. RCT tendon retraction in the MRI coronal plane was classified according to Patte.36 At 6 months postoperatively, the coronal MRI scans were assessed for discontinuity of the graft to determine the fascia lata graft’s tearing rate. The MRI evaluations were performed by an experienced clinician (C.I.d.C.A).
Surgical Technique
Patients underwent surgery under general anesthesia and in the beach-chair position. Shoulder passive ROM was assessed, and stiffness was addressed under general anesthesia by manipulation maneuvers. The shoulder and ipsilateral thigh were surgically draped for shoulder arthroscopic surgery and for minimally invasive fascia lata harvesting. This technique has been previously described in detail,1 and it is therefore summarized here (see online Video Supplement 1).
The forearm was placed in 3-kg forward traction at 70° of elevation and 10° of abduction and in neutral shoulder rotation. ASCR was always performed through a 3-portal technique: a posterior (first) shoulder portal was established 2 cm medial to the posterolateral corner of the acromion, immediately under it, aiming the 4-mm and 30° arthroscope at the coracoid process; an anterior (second) portal was established in the rotator interval under direct glenohumeral arthroscopic vision, and a working cannula (8 × 85–mm Hex Flex; Conmed) with an outflow connection (attached to a closed-system arthroscopic pump: Double Pump RF; Medical Vision) was placed through it; and a lateral (third) portal was established directly under the lateral acromion (usually 1 cm long and digitally tested to ensure an adequate dimension with no obstacles to graft shuttling). A gauged probe and an arthroscopic grasper were used to confirm the poor quality of the supraspinatus and infraspinatus tendons and inability to reach the native footprint without undue tension (Figure 1).
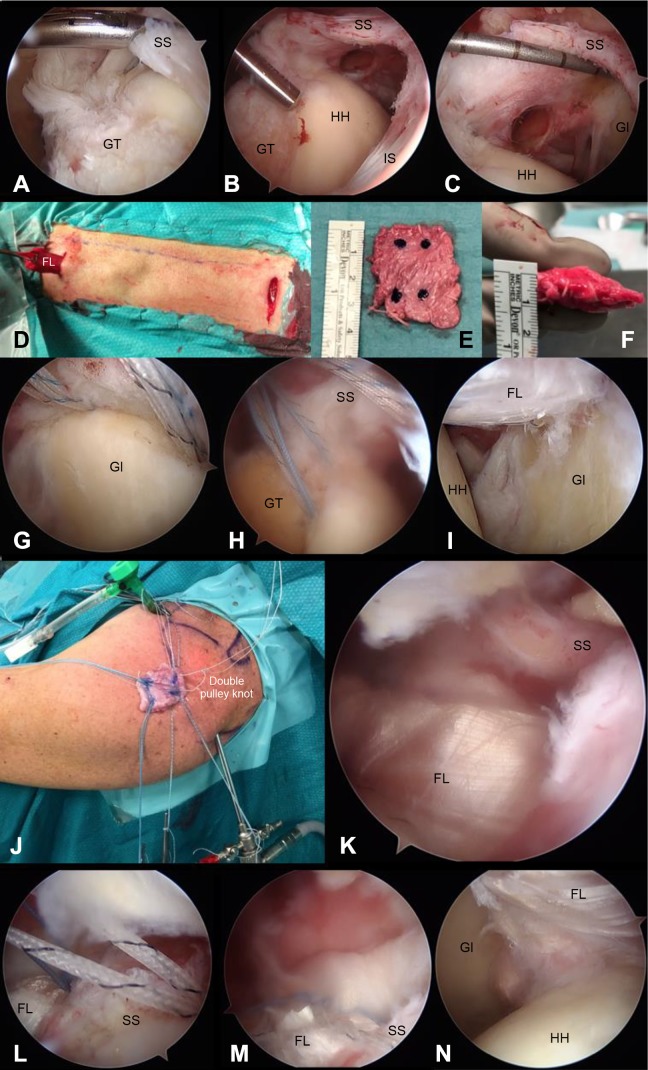
Figure 1.
Arthroscopic images of the left shoulder and thigh harvest. Posterior portal view: (A) grasper test with a large tendon tear under traction, (B) debrided footprint of the supraspinatus and infraspinatus tendons, (C) measurement of the irreparable defect, (D) minimally invasive fascia lata graft harvest in the left thigh, (E) folded and peripherally sutured graft with suture anchor markings, (F) graft width, (G) superior glenoid anchors, (H) humeral footprint anchors, and (J) sutures passing through the graft and the double-pulley knot and graft ready to be shuttled intra-articularly through the lateral portal. Posterior portal: (I) intra-articular view of the graft, (K) subacromial view of the graft and overlying rotator cuff remnants, (L) subacromial view of the sutures passing through the supraspinatus after the knots were tied, and (M) subacromial view of onlay partial rotator cuff tear repair after arming the lateral humeral anchors with the limbs of the sutures passing through the supraspinatus and infraspinatus. Anterior portal: (N) final intra-articular view of superior capsular reconstruction. FL, fascia lata graft; Gl, glenoid; GT, greater tuberosity; HH, humeral head; IS, infraspinatus tendon; SS, supraspinatus tendon.
In recurrent RCTs, all previous sutures were removed. The fascia lata autograft was harvested through 2 horizontal (transverse) 2 cm–long skin incisions on the ipsilateral thigh, both 4 cm anterior to the lateral intermuscular septum: one 15 cm distal to the anterior iliac spine and the other 10 cm proximal to the lateral femoral condyle. Intra-articular LHBT tenotomy was always performed, except when the LHBT was intra-articularly absent. Subscapularis tendon tears were repaired to their native footprint with mattress sutures using 2.8-mm all-suture double-loaded anchors (Y-Knot RC; Conmed). The supraspinatus and infraspinatus tendon footprints and the superior glenoid rim underneath the superior labrum were debrided using a 4 × 125–mm automated shaver (Formula Aggressive Plus Cutter; Stryker) and a 3.5 × 135–mm radiofrequency ablator probe (SERFAS Energy 90-S; Stryker).
For initial graft preparation, the superior capsular defect was measured from anterior to posterior and from medial to lateral using a gauged probe. The 15 to 20 × 3–cm harvested fascia lata graft was folded 4 to 5 times, depending on the intra-articular measurements, with at least 5 mm in excess medially and laterally. This resulted in a 5 to 8 mm–thick final superior capsular graft, which was typically 3.5 cm long and 2.5 cm wide. This graft was peripherally sutured in a continuous fashion with 1 nonabsorbable suture (No. 2 Hi-Fi; Conmed). Through the lateral portal, two 1.8-mm all-suture double-loaded anchors (Y-Knot Flex; Conmed) were implanted on the superior glenoid rim (approximately 1 cm apart) underneath the superior labrum. Additionally, two 2.8-mm all-suture double-loaded anchors (Y-Knot RC) were implanted on the supraspinatus footprint (approximately 1 cm apart).
The distances between the anchors were measured using the gauged probe. Using a dermographic pen (Devon; Covidien), the corresponding glenoid and humeral anchor placements were marked on the graft. After passing all suture limbs from the glenoid and humeral anchors through the graft and with the suture passer (Spectrum; Conmed) ex vivo, the graft was shuttled through the lateral portal into the glenohumeral joint using the double-pulley technique. All of the glenoid and humeral anchors’ sutures were tied. Subsequently, two 4.5-mm knotless anchors (PopLok; Conmed) were loaded with all of the suture limbs from the humeral footprint anchors and were implanted lateral to the humeral footprint in a transosseous-equivalent configuration. When feasible, the limbs of the sutures from the humeral footprint anchors were passed through the supraspinatus and/or infraspinatus remnants with the suture passer (Spectrum) before being loaded into the knotless lateral anchors and used in an onlay partial RCT repair to the superior capsular graft (see online Video Supplement 2). Otherwise, 2 sutures (No. 2 Hi-Fi) were passed from the superior margin of the teres minor to the posterior margin of the superior capsular graft.
All knots were tied with the shoulder at 70° of elevation and 10° of abduction and in neutral rotation. A dynamic subacromial arthroscopic examination was performed to exclude any subacromial conflict with the graft and knots throughout shoulder ROM. Whenever the subacromial space was considered to be in conflict with the graft or knots, anterior acromioplasty was performed using a 4 × 125–mm automated shaver blade (Formula 6-Flute Barrel Bur; Stryker).
Postoperative Protocol for Shoulder
For the first 3 weeks, the patients wore a sling and were instructed to remove it several times a day to perform active assisted shoulder elevation and elbow flexion exercises. Use of the sling was subsequently diminished, and all patients underwent the same shoulder rehabilitation protocol with progressive passive and active ROM exercises. Until 6 weeks postoperatively, active resistant elbow exercises were not allowed. Until 6 months postoperatively, active resistant shoulder exercises were not allowed. After 6 months, a return to full activity was progressively allowed.
Postoperative Protocol for Donor Site
A compressive dressing was applied to the donor site for 24 hours. All patients had an overnight postoperative hospital stay. The use of a compression stocking was advised for 6 weeks. No specific lower limb physical therapy was recommended. From 4 to 6 weeks, patients were instructed to avoid strenuous lower limb activities.
Statistical Analysis
A paired-samples t test (2-tailed) was used to compare the ROM, abduction strength, SSV, SST, CS, and AHI from preoperatively to 6 months postoperatively, from preoperatively to 2 years postoperatively, and from 6 months postoperatively to 2 years postoperatively. The Mann-Whitney U test was used to compare all continuous variables between the groups of patients who underwent ASCR with versus without onlay partial RCT repair, between the patients who underwent ASCR and anterior acromioplasty versus those who did not undergo anterior acromioplasty, between the ASCR and LHBT tenotomy versus ASCR with no LHBT patients, between the preoperative and postoperative AHI<7 mm versus and AHI≥7 mm patients, and between the intact graft versus graft tear patients. The Fisher exact test was used to compare all categorical variables between the groups, including donor site morbidity and overall subjective outcome results. A significant difference was defined as P < .05. For the power analysis, an alpha of 0.05 (95% CI) and a power of 0.8 (beta of 20%) were considered. The chosen variable was the CS, and the paired-samples t test was used for the sample size calculation. The sample needed to obtain a significant difference in functional outcomes was n = 12. SPSS Statistics 22 software (IBM) was used for statistical analyses.
Results
Study Population
From 2015 to 2016, 100 consecutive patients with RCTs underwent arthroscopic surgery by the same surgeon. Overall, 78 patients had reparable full-thickness (54 patients) or partial-thickness (24 patients) RCTs and underwent arthroscopic RCT repair; 22 patients had IRCTs and underwent ASCR with a minimally invasive harvested fascia lata autograft and were consequently enrolled in this study. The demographics of these 22 patients are summarized in Table 1.
TABLE 1.
Patient Demographics
| Patient | Sex | Age, y | Job Status | Job Type | Duration of Symptoms, mo | Side | Dominant-Sided Lesion? | Workers’ Compensation Claim? |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 72 | Active | Manual work | 4.7 | Right | Yes | No |
| 2 | Male | 76 | Retired | Manual work | 8.2 | Left | No | No |
| 3 | Female | 56 | Active | Manual work | 8.0 | Right | Yes | No |
| 4 | Female | 64 | Active | Manual work | 178.5 | Right | Yes | No |
| 5 | Female | 47 | Active | Manual work | 7.6 | Right | Yes | Yes |
| 6 | Female | 60 | Active | Manual work | 1.7 | Right | Yes | Yes |
| 7 | Female | 66 | Retired | Office work | 47.1 | Right | Yes | No |
| 8 | Male | 67 | Retired | Manual work | 13.3 | Right | Yes | No |
| 9 | Male | 74 | Retired | Manual work | 6.0 | Left | No | No |
| 10 | Male | 69 | Retired | Manual work | 9.2 | Left | No | No |
| 11 | Male | 54 | Active | Manual work | 6.1 | Right | Yes | Yes |
| 12 | Female | 73 | Retired | Manual work | 12.0 | Right | Yes | No |
| 13 | Male | 65 | Active | Manual work | 7.2 | Right | No | Yes |
| 14 | Female | 77 | Retired | Manual work | 181.2 | Left | No | No |
| 15 | Female | 49 | Active | Manual work | 2.9 | Right | Yes | Yes |
| 16 | Female | 67 | Retired | Manual work | 14.3 | Right | Yes | No |
| 17 | Female | 53 | Active | Manual work | 26.1 | Right | Yes | No |
| 18 | Female | 65 | Active | Manual work | 1.2 | Left | Yes | Yes |
| 19 | Female | 74 | Retired | Manual work | 19.0 | Left | No | No |
| 20 | Male | 65 | Active | Manual work | 11.6 | Left | No | No |
| 21 | Female | 62 | Active | Manual work | 1.3 | Right | Yes | Yes |
| 22 | Female | 71 | Retired | Office work | 26.5 | Right | Yes | No |
| Mean ± SD | 64.8 ± 8.6 | 27.0 ± 50.6 |
Overall, 13 patients (59.1%) were Hamada grade 1, and 9 patients (40.9%) were Hamada grade 2. The RCT tendon retraction was Patte stage 1 in 2 patients (9.1%), Patte stage 2 in 6 patients (27.3%), and Patte stage 3 in 14 patients (63.6%).
Patient 6 (1/22 patients, 4.5%), who had a history of diabetes mellitus and preobesity, presented with an early (2 weeks postoperatively) superficial anterior portal infection, which did not respond to oral antibiotics and therefore underwent arthroscopic lavage at 4 weeks postoperatively. The synovial liquid culture and biopsy findings were negative, including for Propionibacterium acnes, and the fascia lata autograft and superior capsular reconstruction site were intact. After receiving a course of intravenous antibiotic therapy, the infection resolved; therefore, this patient was not excluded from the study. Patient 18 (1/22 patients, 4.5%) completed the 6-month clinical, radiological, and MRI evaluations but could not be reached afterward and was thus lost to 2-year follow-up.
Intraoperative Findings
The types of RCTs and ASCR-associated variables are summarized in Table 2. A mean of 2.1 ± 1 (range, 0-5) total sutures were passed in the rotator cuff. There were significant differences in shoulder outcomes between the ASCR with LHBT tenotomy versus without LHBT groups regarding the CS at 2 years postoperatively (P = .013). There were no significant differences in outcomes between the onlay partial RCT repair versus non–onlay partial RCT repair groups or between the anterior acromioplasty and nonacromioplasty groups.
TABLE 2.
Types of RCTs and Arthroscopic Superior Capsular Reconstruction–Associated Variablesa
| Patient | Type of Tear | Torn Tendons | No. of Torn Tendons | Hamadab | Pattec | SC Repair? | Onlay Partial RCT Repair? | LHBT Procedure | Anterior Acromioplasty? |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Primary | SC, SS, IS | 3 | 1 | 3 | Yes | Yes | Tenotomy | No |
| 2 | Primary | SS | 1 | 1 | 2 | No | Yes | Tenotomy | Yes |
| 3 | Primary | SC, SS, IS | 3 | 2 | 3 | Yes | No | Tenotomy | No |
| 4 | Primary | SC, SS, IS | 3 | 2 | 1 | Yes | No | Tenotomy | Yes |
| 5 | Recurrent | SS | 1 | 1 | 2 | No | Yes | Absent LHBT | Yes |
| 6 | Primary | SC, SS, IS | 3 | 1 | 3 | Yes | Yes | Absent LHBT | No |
| 7 | Recurrent | SS | 1 | 1 | 2 | No | Yes | Tenotomy | Yes |
| 8 | Primary | SS, IS | 2 | 1 | 3 | No | Yes | Tenotomy | Yes |
| 9 | Primary | SC, SS, IS | 3 | 2 | 3 | Yes | Yes | Absent LHBT | Yes |
| 10 | Recurrent | SS | 1 | 1 | 1 | No | Yes | Absent LHBT | No |
| 11 | Primary | SC, SS, IS | 3 | 2 | 3 | No | No | Absent LHBT | Yes |
| 12 | Primary | SC, SS, IS | 3 | 2 | 3 | Yes | No | Absent LHBT | Yes |
| 13 | Recurrent | SS, IS | 2 | 2 | 3 | No | No | Absent LHBT | Yes |
| 14 | Primary | SC, SS, IS | 3 | 2 | 2 | Yes | No | Tenotomy | Yes |
| 15 | Recurrent | SS, IS | 2 | 1 | 3 | No | No | Absent LHBT | No |
| 16 | Primary | SS | 1 | 1 | 2 | No | No | Tenotomy | Yes |
| 17 | Primary | SS, IS | 2 | 1 | 3 | No | Yes | Tenotomy | Yes |
| 18 | Recurrent | SS | 1 | 1 | 3 | No | Yes | Absent LHBT | Yes |
| 19 | Primary | SS, IS | 2 | 2 | 3 | No | Yes | Absent LHBT | Yes |
| 20 | Primary | SS, IS | 2 | 2 | 2 | No | Yes | Tenotomy | Yes |
| 21 | Primary | SS, IS | 2 | 1 | 3 | No | Yes | Absent LHBT | No |
| 22 | Primary | SS | 1 | 1 | 3 | No | Yes | Tenotomy | Yes |
| Mean ± SD | 2.0 ± 0.8 | 1.4 ± 0.5 | 2.5 ± 0.7 |
aIS, infraspinatus; LHBT, long head of the biceps tendon; RCT, rotator cuff tear; SC, subscapularis; SS, supraspinatus;
bHamada classification: grade 1, acromiohumeral interval (AHI) ≥6 mm; grade 2, AHI ≤5 mm; grade 3, AHI ≤5 mm and acetabulization of the acromion; grade 4A, AHI ≤5 mm and glenohumeral narrowing without acetabulization of the acromion; grade 4B, AHI ≤5 mm and glenohumeral narrowing with acetabulization of the acromion; and grade 5, humeral head collapse.
cPatte classification: stage 1, tendon close to the bony insertion; stage 2, tendon at the level of the humeral head; and stage 3, tendon at the level of the glenoid.
Clinical Results
The mean preoperative and 2-year postoperative ROMs and functional shoulder scores are summarized in Table 3. All mean scores improved significantly after ASCR with a fascia lata autograft at 6 months (SST, 6.8; SSV, 55.7%; CS, 42.5) and 2 years (SST, 8.6; SSV, 70.0%; CS, 64.9), with mean preoperative to 6-month increases of 4.7 points for the SST (95% CI, 2.5-6.8; P = .00018), 22.7% for the SSV (95% CI, 11.4-34.1; P = .0004), and 24.9 points for the CS (95% CI, 20.1-29.7; P < .001) and with mean preoperative to 2-year increases of 6.5 points for the SST, 35.5% for the SSV, and 47.1 points for the CS, taking into account the average of the paired differences (2-year postoperatively to preoperatively) (P < .001).
TABLE 3.
Shoulder Range of Motion, Abduction Strength, and Functional Scoresa
| Patient | Elevation, deg | Abduction, deg | External Rotation, deg | Abduction Strength, kg | Internal Rotationb | CS (1-100) | SST (1-12) | SSV, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | 2 y | Preop | 2 y | Preop | 2 y | 6 mo | 2 y | Preop | 2 y | Preop | 2 y | Preop | 2 y | Preop | 2 y | |
| 1 | 45 | 180 | 30 | 180 | 20 | 20 | 1.0 | 1.5 | 2 | 4 | 19 | 75 | 3 | 11 | 30 | 70 |
| 2 | 60 | 145 | 30 | 115 | 0 | 27 | 4.0 | 6.0 | 0 | 4 | 4 | 81 | 0 | 12 | 50 | 95 |
| 3 | 0 | 80 | 0 | 60 | 0 | 0 | 0.0 | 1.0 | 4 | 4 | 14 | 29 | 5 | 4 | 40 | 50 |
| 4 | 170 | 180 | 45 | 90 | 0 | 50 | 0.0 | 1.0 | 0 | 4 | 22 | 67 | 5 | 11 | 50 | 70 |
| 5 | 60 | 180 | 50 | 110 | 20 | 50 | 2.0 | 2.0 | 0 | 1 | 13 | 58 | 1 | 7 | 50 | 70 |
| 6 | 90 | 130 | 90 | 75 | 20 | 30 | 0.0 | 0.5 | 0 | 1 | 12 | 34 | 0 | 1 | 50 | 0 |
| 7 | 90 | 160 | 30 | 120 | 30 | 40 | 4.0 | 5.0 | 2 | 4 | 21 | 82 | 3 | 12 | 60 | 90 |
| 8 | 45 | 180 | 45 | 180 | 0 | 25 | 3.0 | 12.0 | 2 | 5 | 12 | 100 | 0 | 12 | 20 | 100 |
| 9 | 160 | 145 | 130 | 145 | 0 | 0 | 2.5 | 2.0 | 0 | 4 | 36 | 74 | 0 | 11 | 30 | 90 |
| 10 | 80 | 100 | 80 | 90 | 0 | 30 | 3.0 | 4.0 | 1 | 3 | 16 | 45 | 0 | 3 | 20 | 80 |
| 11 | 10 | 100 | 20 | 70 | 10 | 25 | 0.0 | 0.5 | 0 | 2 | 0 | 38 | 12 | 4 | 10 | 60 |
| 12 | 180 | 175 | 180 | 100 | 70 | 50 | 1.5 | 2.0 | 4 | 4 | 48 | 74 | 4 | 11 | 20 | 70 |
| 13 | 45 | 135 | 45 | 125 | 0 | 25 | 0.0 | 4.5 | 4 | 5 | 23 | 69 | 2 | 12 | 25 | 70 |
| 14 | 50 | 170 | 30 | 160 | 0 | 60 | 0.0 | 1.0 | 2 | 5 | 10 | 77 | 0 | 11 | 40 | 100 |
| 15 | 70 | 110 | 60 | 115 | 10 | 45 | 0.0 | 0.5 | 0 | 4 | 14 | 43 | 0 | 4 | 50 | 50 |
| 16 | 30 | 110 | 0 | 80 | 15 | 60 | 1.0 | 3.3 | 0 | 4 | 8 | 67 | 0 | 8 | 30 | 60 |
| 17 | 120 | 180 | 90 | 180 | 0 | 40 | 0.0 | 3.8 | 0 | 5 | 16 | 75 | 2 | 11 | 30 | 90 |
| 18 | 80 | 45 | 5 | 0.0 | 1 | 12 | 2 | 0 | ||||||||
| 19 | 30 | 140 | 30 | 110 | 10 | 30 | 2.0 | 2.0 | 1 | 5 | 17 | 69 | 0 | 10 | 20 | 75 |
| 20 | 180 | 160 | 90 | 145 | 50 | 55 | 1.0 | 2.1 | 4 | 5 | 55 | 73 | 5 | 10 | 50 | 50 |
| 21 | 0 | 115 | 0 | 115 | 0 | 55 | 0.0 | 1.0 | 0 | 4 | 4 | 57 | 2 | 7 | 0 | 50 |
| 22 | 50 | 145 | 50 | 170 | 30 | 30 | 0.0 | 2.4 | 0 | 3 | 10 | 76 | 1 | 9 | 50 | 80 |
| Mean ± SD | 74.8 ± 55.5 | 143.8 ± 31.7 | 53.2 ± 43.3 | 120.7 ± 37.7 | 13.2 ± 18.4 | 35.6 ± 17.3 | 1.1 ± 1.4 | 2.8 ± 2.6 | 1.2 ± 1.5 | 3.8 ± 1.2 | 17.5 ± 13.4 | 64.9 ± 18.0 | 2.1 ± 2.9 | 8.6 ± 3.5 | 33.0 ± 17.4 | 70.0 ± 23.0 |
aCS, Constant score; preop, preoperative; SST, Simple Shoulder Test; SSV, subjective shoulder value.
bMeasured as the highest vertebral body that the patient's thumb could reach without pain: lateral thigh = 0, buttock = 1, sacrum = 2, lumbar = 3, T12 = 4, and T7 = 5.
The mean preoperative painless active shoulder ROMs were the following: elevation, 74.8°; abduction, 53.2°; external rotation, 13.2°; and internal rotation, 1.2 points. ROM also improved significantly at 6 months (elevation, 104.5°; abduction, 86.6°; external rotation, 27.0°; internal rotation, 2.6 points) and 2 years (elevation, 143.8°; abduction, 120.7°; external rotation, 35.6°; internal rotation, 3.8 points), with mean preoperative to 2-year increases of 69.3° in elevation, 22.0° in external rotation, and 2.6 points in internal rotation (P < .001). Preoperatively, all patients had 0-kg painless abduction strength. This improved postoperatively to 1.1 kg (range, 0-4 kg) at 6 months and 2.8 kg (range, 0.5-12 kg) at 2 years postoperatively, with a mean preoperative to 2-year increase of 2.8 kg (P < .001).
Donor Site Morbidity and Overall Subjective Outcome Results
At the 2-year evaluation, 18 of 21 patients (85.7%) underwent the same surgery again. Sixteen of 21 patients (76.2%) considered that the shoulder surgery’s end result compensated for the thigh’s changes, while 3 patients (14.3%) considered that it did not; the remaining 2 patients (9.5%) did not know how to answer. Twelve patients (57.1%) stated that their harvested thigh bothered them. Sixteen patients (76.2%) noticed local donor site changes: deformity (2/21, 9.5%), pain (8/21, 38.1%), numbness (8/21, 38.1%), or donor site–related claudication (1/21, 4.7%) (Table 4).
TABLE 4.
Subjective Questionnaire Responses at 2 Years Postoperativelya
| Patient | Would You Undergo the Same Surgery Again? | Does Your Shoulder Surgery’s End Result Compensate for Your Thigh’s Changes? | Does the Harvested Thigh Bother You? | Thigh Deformity? | Thigh Pain? | Thigh Numbness? | Claudication? |
|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | No | No | No | No | No |
| 2 | Yes | Yes | Yes | No | Yes | No | No |
| 3 | Yes | Yes | Yes | No | Yes | No | No |
| 4 | Yes | DNK | Yes | No | Yes | No | No |
| 5 | No | No | Yes | Yes | No | No | No |
| 6 | No | No | Yes | No | Yes | No | No |
| 7 | Yes | Yes | No | No | No | Yes | No |
| 8 | Yes | Yes | No | No | No | Yes | No |
| 9 | Yes | Yes | No | No | No | No | No |
| 10 | Yes | Yes | No | No | No | No | No |
| 11 | Yes | Yes | No | Yes | No | No | No |
| 12 | Yes | DNK | Yes | No | Yes | No | No |
| 13 | Yes | Yes | No | No | No | No | No |
| 14 | Yes | Yes | No | No | No | Yes | No |
| 15 | No | No | Yes | No | Yes | Yes | Yes |
| 16 | Yes | Yes | Yes | No | No | Yes | No |
| 17 | Yes | Yes | Yes | No | No | Yes | No |
| 18b | |||||||
| 19 | Yes | Yes | Yes | No | Yes | No | No |
| 20 | Yes | Yes | No | No | No | Yes | No |
| 21 | Yes | Yes | Yes | No | No | No | No |
| 22 | Yes | Yes | Yes | No | Yes | Yes | No |
aDNK, does not know how to answer.
bLost to 2-year follow-up.
Radiological Results
In 14 of 22 patients (63.6%), the preoperative AHI was less than 7 mm. In 6 of 22 patients (27.3%), the 6-month AHI was less than 7 mm. In 6 of 21 patients (28.6%), the 2-year AHI was less than 7 mm. The mean AHI decrease from 6 months to 2 years postoperatively (–0.7 ± 1.5 mm) was statistically significant (95% CI, –1.35 to –0.03; P = .042) (Table 5).
TABLE 5.
Radiological AHI and Magnetic Resonance Imaging Resultsa
| Patient | AHI, mm | Preoperative Goutallierb | Preoperative Cumulative Goutallier | GFMDI | Graft Tear at 6 mo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | 6 mo | 2 y | SS | IS | SC | TM | ||||
| 1 | 8 | 10 | 7 | 2 | 3 | 2 | 0 | 7 | 2.3 | No |
| 2 | 7 | 10 | 8 | 1 | 1 | 2 | 0 | 4 | 1.3 | No |
| 3 | 3 | 4 | 3 | 4 | 4 | 1 | 0 | 9 | 3.0 | Yes |
| 4 | 2 | 10 | 6 | 2 | 2 | 3 | 1 | 8 | 2.3 | No |
| 5 | 7 | 7 | 7 | 3 | 4 | 1 | 0 | 8 | 2.7 | No |
| 6 | 6 | 10 | 7 | 3 | 2 | 3 | 0 | 8 | 2.7 | No |
| 7 | 12 | 10 | 9 | 3 | 2 | 1 | 0 | 6 | 2.0 | No |
| 8 | 6 | 10 | 10 | 3 | 0 | 3 | 0 | 6 | 2.0 | No |
| 9 | 2 | 2 | 1 | 3 | 2 | 3 | 1 | 9 | 2.7 | Yes |
| 10 | 6 | 7 | 9 | 1 | 1 | 1 | 0 | 3 | 1.0 | No |
| 11 | 3 | 8 | 7 | 4 | 4 | 3 | 1 | 12 | 3.7 | No |
| 12 | 5 | 5 | 5 | 4 | 1 | 3 | 1 | 9 | 2.7 | No |
| 13 | 4 | 6 | 7 | 3 | 3 | 1 | 0 | 7 | 2.3 | No |
| 14 | 5 | 9 | 9 | 2 | 2 | 2 | 0 | 6 | 2.0 | No |
| 15 | 10 | 11 | 12 | 3 | 2 | 1 | 0 | 6 | 2.0 | No |
| 16 | 8 | 8 | 8 | 3 | 3 | 2 | 0 | 8 | 2.7 | No |
| 17 | 6 | 5 | 6 | 2 | 1 | 1 | 0 | 4 | 1.3 | No |
| 18 | 10 | 10 | 4 | 1 | 0 | 0 | 5 | 1.7 | No | |
| 19 | 5 | 10 | 9 | 3 | 3 | 1 | 0 | 7 | 2.3 | No |
| 20 | 4 | 8 | 7 | 4 | 4 | 2 | 0 | 10 | 3.3 | No |
| 21 | 16 | 5 | 4 | 4 | 4 | 0 | 0 | 8 | 2.7 | No |
| 22 | 6 | 10 | 9 | 2 | 2 | 2 | 0 | 6 | 2.0 | No |
| Mean ± SD | 6.4 ± 3.3 | 8.0 ± 2.5 | 7.1 ± 2.5 | 2.9 ± 0.9 | 2.3 ± 1.2 | 1.7 ± 1.0 | 0.2 ± 0.4 | 7.1 ± 2.1 | 2.3 ± 0.7 | |
aAHI, acromiohumeral interval; GFMDI, global fatty muscle degeneration index; IS, infraspinatus; SC, subscapularis; SS, supraspinatus; TM, teres minor.
bGoutallier classification: grade 0, no fatty streaks; grade 1, some fatty streaks; grade 2, more muscle than fat; grade 3, as much muscle as fat; and grade 4, less muscle than fat.
MRI Results
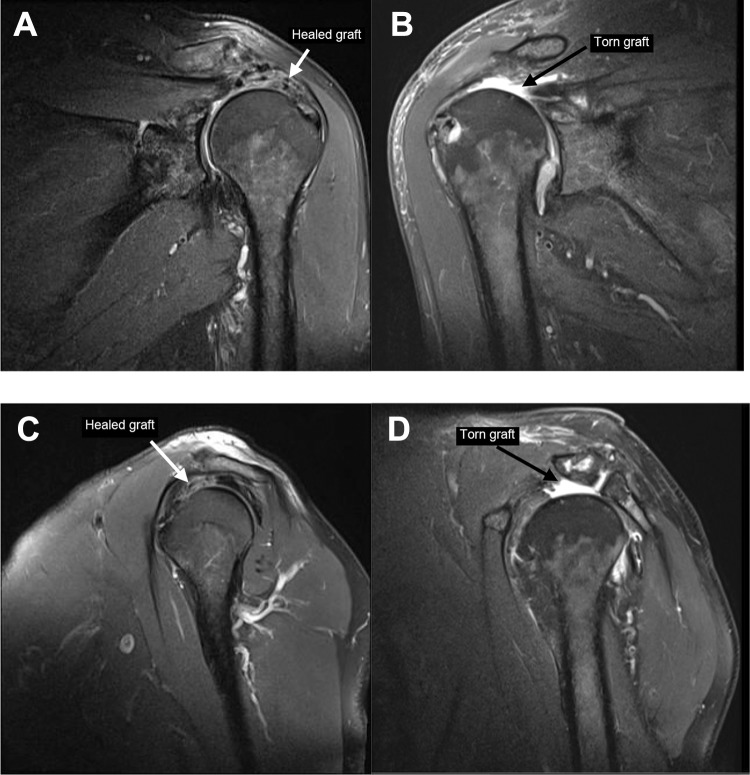
In our study, no statistically significant correlation was found between the preoperative Goutallier grade20 and the shoulder outcome scores or subjective overall assessment results. All patients had a preoperative cumulative Goutallier grade of ≥3.47 Nineteen of 22 patients (86.4%) had a preoperative GFMDI of ≥2.10 On 6-month MRI, 2 of 22 patients (9.1%) had a superior capsular graft tear (Figure 2).
Figure 2.
Six-month postoperative magnetic resonance imaging: a healed graft on proton density fat-saturated (A) coronal and (C) sagittal images of patient 2 and a torn graft on proton density fat-saturated (B) coronal and (D) sagittal images of patient 9.
Discussion
Overall Clinical Results
In this study of ASCR with a fascia lata autograft, a uniform, consistent surgical technique performed by a single surgeon produced statistically significant improvements in functional scores, active painless ROM, and abduction strength of the shoulder, with significant progressive increases from preoperatively to 6 months postoperatively and from 6 months to 2 years postoperatively.
3-Portal Technique of ASCR With a Fascia Lata Autograft
The 3-portal technique systematically used in our series allowed for the successful completion of ASCR with a fascia lata autograft in all patients. A technical variation of the procedure originally described by Mihata et al32 was introduced: the superior capsular graft was attached to the superior glenoid rim, underneath the superior glenoid labrum, preserving it; this allowed for both the glenoid and humeral suture anchors to be implanted through the lateral portal with a good attack angle, without the need to use additional accessory portals (eg, the Neviaser portal) that have been used by other authors to implant the glenoid anchors medial to the superior labrum.7,22,35,38,43 There was no need to use a cannula in the lateral portal: the 5 to 8 mm–thick final folded fascia lata graft was flexible, easy to handle, easy to suture, and easy to shuttle intra-articularly in the direction of the angle of the implanted anchors, as long as the suture limbs were kept tensioned and thus unentangled throughout the double-pulley shuttling process. The reported techniques of ASCR with an allograft used 1 to 3.5 mm–thick allografts. According to the biomechanical study by Mihata et al,33 5 to 8 mm–thick grafts may improve glenohumeral stability and reduce the graft tear rate. However, using a thicker allograft/xenograft may mean that a more technically demanding surgical technique of ASCR with an allograft will have to be devised because a thicker allograft/xenograft is less flexible and less easy to shuttle than a fascia lata graft.
Fascia Lata Autograft and Donor Site Morbidity
Our minimally invasive fascia lata harvesting technique did not produce subjectively important donor site dysfunction, probably because damage to the tensor fascia lata muscle superiorly (hip) and the distal iliotibial band inferiorly (knee) was avoided.42 Furthermore, we attempted to achieve good cosmesis through the use of 2 small skin incisions. Mihata et al32 used an open superior approach. In subsequent studies, using the same open approach, Mihata et al30,31 argued that harvesting the intermuscular septum was needed to obtain a thicker final graft and reported that no donor site dysfunction occurred. However, these studies provided limited information about donor site morbidity. In a study by Angelo and de Campos Azevedo,1 the same first 15 participants who were included in our study were specifically observed for donor site morbidity, and each patient completed a nonarthritic hip score assessment. The authors reported that daily activity limitation, subjective loss of strength, and local complications decreased from 1 week to 6 months and from 6 to 18 months postoperatively, and it was concluded that the minimally invasive technique did not produce significant donor site morbidity or hip dysfunction at 18 months.1
Nevertheless, our minimally invasive technique, despite favoring good cosmesis by avoiding a long scar, did require harvesting a 15 to 20 × 3–cm fascia lata graft to obtain at least a 5 mm–thick final graft after folding. Despite this relatively large resultant donor fascia lata defect, the study results show that existing donor site morbidity was well tolerated and that most of our patients considered it a good trade-off to regain painless shoulder function. This result is clinically relevant because avoiding donor site morbidity is one of the main goals of advocates for ASCR with an allograft and ASCR with an LHBT autograft.9,13,22,37 Furthermore, fascia lata autografts have presented some advantages, such as a higher availability than allografts46 and LHBT autografts, with the latter being even more unavailable in the setting of IRCTs.8 Indeed, half of the patients in our series had an absent LHBT, which supports the importance of discussing the availability of the autograft with the patients preoperatively whenever the native LHBT is being considered as a superior capsular graft in ASCR. Moreover, existing studies on ASCR with an LHBT autograft are limited because of preliminary retrospective technical notes,6,9,24 small sample sizes (16,24 to 99 shoulders), or short-term limited outcomes (6-month evaluations using the visual analog scale9).
Another reported advantage of the fascia lata autograft is its lower tear rate.46 Studies on ASCR with an allograft13 have reported a graft tear rate of 55%. In a clinical study on ASCR in 36 patients by Lee and Min,26 a high (36.1%) graft tear rate was reported. However, in their study with a small sample size, different types of grafts were used (dermal allografts and fascia lata autografts), and the single-row humeral-sided graft fixation technique used might have influenced the rate of failures, which occurred mainly through the humeral side. In a clinical study by Lim et al28 on ASCR with a fascia lata autograft in 31 patients, a 29% graft tear rate was reported at a mean follow-up of 15 months (range, 12-24 months). Nevertheless, our 6-month fascia lata autograft tear rate of 9.1% was higher than the final follow-up tear rates of 4.2% to 5% reported by Mihata et al30,32 (1/24 and 5/100 shoulders, respectively).
ROM Results
In the Mihata et al32 clinical study of ASCR with a fascia lata autograft in 23 patients with minimum 2-year follow-up (mean, 34.1 months), the mean preoperative active elevation of 24 shoulders was 84° ± 52.2°, and it increased to 156° (mean increase, 72°) in those who healed. In a clinical study of ASCR with an allograft by Denard et al,13 which included 59 patients with a minimum 1-year follow-up (mean, 17.7 months), the mean preoperative active elevation was 130° ± 48°, and it increased to 158° ± 32° (mean increase, 28°). In a clinical study of ASCR with an allograft by Pennington et al,37 36 patients with a minimum 2-year follow-up (after excluding 2 failures from the analysis) had a mean preoperative active elevation of 123°, which increased to 162° (mean increase, 40°). In our study of ASCR with a fascia lata autograft in 21 patients, the mean preoperative active elevation (74.8° ± 55.5°), which was lower than the preoperative elevations reported in the clinical studies of ASCR with an allograft,13,37 increased to 143.8° ± 31.7°, and to 147° in the intact graft group, at 2-year follow-up. Indeed, in our study, 14 of 21 patients (66.7%) had preoperative pseudoparalytic shoulders (active anterior elevation <90°), which reverted in all patients at 2 years, except in 1 (patient 3, who had a graft tear on 6-month MRI). These lower mean preoperative ROMs and increased mean improvements (mean increase in elevation of 72.2° in the intact graft group), with a reversal of 92% of the pseudoparalytic shoulders (13/14 patients), suggest that our ROM outcomes of ASCR with a fascia lata autograft may be more comparable with those of Mihata et al31,32 than those reported in the clinical studies of ASCR with an allograft. The type of graft might explain this fact.
Nevertheless, we cannot rule out that the technical variations used in our procedure of ASCR with a fascia lata autograft did not influence our study’s outcomes, which were inferior to the results presented by Mihata et al.32 In our technique of ASCR with a fascia lata autograft, we used all-suture anchors, the arm positioning was different, and the medial-lateral graft size was shorter. However, the use of all-suture anchors in arthroscopic RCT repair has been shown to have similar biomechanics compared with conventional anchors,2 and it has been shown to reduce the risk of loose body complications and joint damage, lead to improved bone preservation, and avoid distortions on MRI assessments.44
Functional Shoulder Scores
Denard et al13 used the SSV19 to assess shoulder function, reporting an increase from 35.0% ± 19.9% preoperatively to 76.3% ± 25.2% at 17.7 months postoperatively, with a mean increase of 41.3%, which was comparable with the mean SSV increase in our study (35.5%). Mihata et al32 and Pennington et al37 used different functional outcome scores.
In a study on arthroscopic repair of chronic full-thickness tears of the supraspinatus by Boileau et al,4 the mean CS in 65 shoulders increased from 51.6 ± 10.6 preoperatively to 83.8 ± 10.3 postoperatively (mean follow-up, 29 months), with a mean increase of 32.2 points. In a clinical study by Walch et al45 on arthroscopic LHBT tenotomy for the treatment of full-thickness RCTs in 307 shoulders, the mean CS increased from 48.4 ± 13.6 preoperatively to 67.6 ± 14.7 postoperatively (mean follow-up, 57 months), with a mean increase of 19.2 points. In our study, the mean preoperative and postoperative CSs were both lower (17.5 ± 13.4 and 64.9 ± 18.0, respectively) than the scores reported in these 2 studies.4,45 However, the mean CS increase in our study was higher (47.1 ± 19.6 points), suggesting that ASCR with a fascia lata autograft may be a successful treatment option for patients with RCTs presenting with a severely reduced preoperative CS.
Radiological Results
Mihata et al32 reported a significantly increased AHI (4.1 ± 1.7 mm) at a minimum 2-year follow-up after ASCR with a fascia lata autograft. In the study by Denard et al,13 the mean AHI did not improve significantly after 17.7 months (from 6.6 ± 3.0 mm preoperatively to 6.7 ± 3.0 mm at final follow-up). Pennington et al37 reported a 2.6-mm increase in the mean AHI at 2 years after ASCR with an allograft (from 7.3 mm to 9.9 mm in the patients without failures; n = 36). In our study, the AHI did not significantly increase from preoperatively to 2 years postoperatively (0.9 ± 3.6 mm). Moreover, it decreased significantly from 6 months to 2 years postoperatively (–0.7 ± 1.5 mm). However, this did not correlate with a decrease in ROM (not significant) or shoulder function (not significant).
MRI Results and RCT Irreparability
Our criteria of RCT irreparability may explain why there was a high frequency in the use of ASCR (22.0%) versus RCT repair in our study. Indeed, 8 patients in our study with Patte stage 1 or 2 RCTs, which usually are reparable tears, did not meet our study’s reparability criteria and underwent ASCR instead of RCT repair. These patients had clinical and imaging predictors of irreparability: 3 patients had previously undergone ≥1 RCT repairs with double-row transosseous-equivalent constructs, which failed with a retear of the supraspinatus tendon (patients 5, 7, and 10), and 2 patients had a massive RCT (supraspinatus, infraspinatus, and subscapularis tendons) with a mean duration of symptoms of over 178 months (patients 4 and 14). Furthermore, 2 patients underwent onlay partial RCT repair in addition to ASCR (patients 2 and 20). In fact, 5 of these 8 patients who had Patte stage 1 or 2 RCTs underwent rotator cuff repair: they underwent onlay partial RCT repair in addition to ASCR.
Several authors have attempted to establish a definition of an IRCT.25 Some consider an RCT irreparable if the existing defect cannot be closed intraoperatively or if it has been empirically determined that a successful intraoperative closure will almost certainly lead to structural failure of the repair site.18 Clinical and imaging predictors of irreparability have been proposed, such as an AHI of < 7 mm and stage 3 or 4 fatty infiltration.18 A cumulative Goutallier grade of ≥3 has been considered a predictive factor for RCT nonhealing,47 and a GFMDI of ≥2 reportedly has been associated with a 100% rotator cuff retear rate.10 In our series, 19 of 22 patients (86.4%) had a preoperative GFMDI of ≥2. Nevertheless, the tendon’s poor quality as evaluated intraoperatively was our ultimate criterion of irreparability. Every attempt was made to successfully repair the RCT in each patient before deciding to proceed to ASCR with a fascia lata autograft; this decision was straightforward in all patients because the grasper test was usually enough to determine poor tendon quality. However, any RCT repair ultimately may fail intraoperatively. Thus, surgeons should have an immediately available alternative solution to offer to these (previously informed) patients. In this circumstance, a fascia lata autograft is always available to perform ASCR through the same arthroscopic portals used for RCT repair.
Limitations
This study has some limitations. First, all evaluations were undertaken by the surgeon, increasing the risk for patient- and surgeon-related outcome reporting bias. However, an indirect-type satisfaction questionnaire was used in addition to the 3 validated shoulder outcome scores. This subjective outcome questionnaire was designed to reduce patient-related outcome reporting bias and the patient's sense of reporting dissatisfaction with the surgical procedure, thus avoiding the patient's fear of disappointing the surgeon.
Second, whenever the LHBT was present, LHBT tenotomy was concurrently performed. LHBT tenotomy is one of the treatment options for IRCTs,3,45 as are anterior acromioplasty and partial RCT repair. Additionally performed procedures may be considered confounding outcome factors because they might be co-responsible for the patients’ postoperative pain relief. Indeed, there were significant differences in patient outcomes between the groups that underwent ASCR with LHBT tenotomy versus without LHBT, but there were no statistical differences in outcomes between the acromioplasty and nonacromioplasty groups or between the onlay partial RCT repair and non–onlay partial RCT repair groups. However, the sample size did not allow us to exclude the possibility that anterior acromioplasty or onlay partial RCT repair may have an impact on outcomes. Therefore, further studies with larger sample sizes should be performed.
Third, in this study, there were no control group results with which to compare the ASCR results. Considering the previously discussed role of LHBT tenotomy in pain reduction in this setting, future randomized controlled studies comparing ASCR with a fascia lata autograft with LHBT tenotomy for IRCTs should be designed. A control group with tendon transfer, partial RCT repair, or (as suggested by Mihata et al32) complete RCT repair should also be considered in future studies, as well as control groups with other types of grafts (ASCR with allografts, xenografts, LHBT autografts, etc).
Fourth, besides having a small sample size, the present study had a short 2-year final follow-up. However, it has been stated that ASCR outcomes remain relatively constant beyond the 2-year evaluation.32 Moreover, this was a prospective study with the same follow-up period for each patient, enabling surgeons to inform their patients on what might be expected after this technique of ASCR with a fascia lata autograft at the 6-month and 2-year postoperative time points. Nevertheless, this is an ongoing study (the same participants were enrolled in a 3-year prospective study to test if the results remain constant), and we are committed to report the 3-year follow-up results.
Fifth, in this study, superior capsular graft integrity was analyzed at 6 months; therefore, we cannot exclude the possibility of graft tear occurrences afterward, which might correlate with the statistically significant decrease we found in the AHI from 6 months to 2 years postoperatively. However, this AHI decrease did not correlate with any decrease in ROM or shoulder function, which improved significantly in our study. Nevertheless, further prospective studies with an MRI analysis of graft integrity at 2 years should be designed.
Conclusion
ASCR using a minimally invasive harvested fascia lata autograft produced good 6-month and 2-year shoulder outcomes in IRCTs, with low-impact thigh morbidity at 2 years.
A Video Supplement for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/2325967118808242.
Supplementary Material
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Clínica GIGA Saúde (No. 02-2015-06).
References
- 1. Angelo A, de Campos Azevedo CI. Minimally invasive fascia lata harvesting in ASCR does not produce significant donor site morbidity [published online August 1, 2018]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-018-5085-1 [DOI] [PubMed] [Google Scholar]
- 2. Bernardoni E, Frank RM, Veera SS, et al. Biomechanical analysis of all-suture anchor fixation for rotator cuff repair. Orthop J Sports Med. 2018;6(7 suppl 4):2325967118S00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747–757. [DOI] [PubMed] [Google Scholar]
- 4. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229–1240. [DOI] [PubMed] [Google Scholar]
- 5. Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):1359–1370. [DOI] [PubMed] [Google Scholar]
- 6. Boutsiadis A, Chen S, Jiang C, Lenoir H, Delsol P, Barth J. Long head of the biceps as a suitable available local tissue autograft for superior capsular reconstruction: “the Chinese way”. Arthrosc Tech. 2017;6(5):e1559–e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burkhart SS, Denard PJ, Adams CR, Brady PC, Hartzler RU. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff repair. Arthrosc Tech. 2016;5(6):e1407–e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen CH, Chen CH, Chang CH, et al. Classification and analysis of pathology of the long head of the biceps tendon in complete rotator cuff tears. Chang Gung Med J. 2012;35(3):263–270. [DOI] [PubMed] [Google Scholar]
- 9. Chillemi C, Mantovani M, Gigante A. Superior capsular reconstruction of the shoulder: the ABC (Arthroscopic Biceps Chillemi) technique. Eur J Orthop Surg Traumatol. 2018;28(6):1215–1223. [DOI] [PubMed] [Google Scholar]
- 10. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clark NJ, Elhassan BT. The role of tendon transfers for irreparable rotator cuff tears. Curr Rev Musculoskelet Med. 2018;11(1):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160–164. [PubMed] [Google Scholar]
- 13. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93–99. [DOI] [PubMed] [Google Scholar]
- 14. Elhassan BT, Wagner ER, Werthel JD. Outcome of lower trapezius transfer to reconstruct massive irreparable posterior-superior rotator cuff tear. J Shoulder Elbow Surg. 2016;25(8):1346–1353. [DOI] [PubMed] [Google Scholar]
- 15. Farshad M, Gerber C. Reverse total shoulder arthroplasty: from the most to the least common complication. Int Orthop. 2010;34(8):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerber C, Canonica S, Catanzaro S, Ernstbrunner L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: results after 15 years. J Shoulder Elbow Surg. 2018;27(5):831–838. [DOI] [PubMed] [Google Scholar]
- 17. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920–1926. [DOI] [PubMed] [Google Scholar]
- 18. Gerber C, Wirth SH, Farshad M. Treatment options for massive rotator cuff tears. J Shoulder Elbow Surg. 2011;20(2):S20–S29. [DOI] [PubMed] [Google Scholar]
- 19. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717–721. [DOI] [PubMed] [Google Scholar]
- 20. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 21. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kany J, Grimberg J, Amaravathi RS, Sekaran P, Scorpie D, Werthel JD. Arthroscopically-assisted latissimus dorsi transfer for irreparable rotator cuff insufficiency: modes of failure and clinical correlation. Arthroscopy. 2018;34(4):1139–1150. [DOI] [PubMed] [Google Scholar]
- 24. Kim YS, Lee HJ, Park I, Sung GY, Kim DJ, Kim JH. Arthroscopic in situ superior capsular reconstruction using the long head of the biceps tendon. Arthrosc Tech. 2018;7(2):e97–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lädermann A, Denard PJ, Collin P. Massive rotator cuff tears: definition and treatment. Int Orthop. 2015;39(12):2403–2414. [DOI] [PubMed] [Google Scholar]
- 26. Lee S-J, Min Y-K. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? Preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2205–2213. [DOI] [PubMed] [Google Scholar]
- 27. Lewington MR, Ferguson DP, Smith TD, Burks R, Coady C, Wong IH. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45(13):3149–3157. [DOI] [PubMed] [Google Scholar]
- 28. Lim S, AlRamadhan H, Kwak JM, Hong H, Jeon IH. Graft tears after arthroscopic superior capsule reconstruction (ASCR): pattern of failure and its correlation with clinical outcome [published online August 24, 2018]. Arch Orthop Trauma Surg. doi:10.1007/s00402-018-3025-7 [DOI] [PubMed] [Google Scholar]
- 29. Lippitt S, Harryman DT, Matsen F. A pratical tool for function evaluation: the “simple shoulder test” In: Matsen F, Fu F, Hawkins R, eds. The Shoulder: A Balance of Mobility and Stability. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1993:501–518. [Google Scholar]
- 30. Mihata T, Lee TQ, Fukunishi K, et al. Return to sports and physical work after arthroscopic superior capsule reconstruction among patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46(5):1077–1083. [DOI] [PubMed] [Google Scholar]
- 31. Mihata T, Lee TQ, Hasegawa A, et al. Arthroscopic superior capsule reconstruction can eliminate pseudoparalysis in patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46(11):2707–2716. [DOI] [PubMed] [Google Scholar]
- 32. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459–470. [DOI] [PubMed] [Google Scholar]
- 33. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical effect of thickness and tension of fascia lata graft on glenohumeral stability for superior capsule reconstruction in irreparable supraspinatus tears. Arthroscopy. 2016;32(3):418–426. [DOI] [PubMed] [Google Scholar]
- 34. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544–2556. [DOI] [PubMed] [Google Scholar]
- 35. Narvani AA, Consigliere P, Polyzois I, Sarkhel T, Gupta R, Levy O. The “pull-over” technique for arthroscopic superior capsular reconstruction. Arthrosc Tech. 2016;5(6):e1441–e1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res. 1990;(254):81–86. [PubMed] [Google Scholar]
- 37. Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34(6):1764–1773. [DOI] [PubMed] [Google Scholar]
- 38. Petri M, Greenspoon JA, Millett PJ. Arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e751–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg. 2017;137(1):95–103. [DOI] [PubMed] [Google Scholar]
- 40. Sevivas N, Ferreira N, Andrade R, et al. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg. 2017;26(9):e265–e277. [DOI] [PubMed] [Google Scholar]
- 41. Shon MS, Koh KH, Lim TK, Kim WJ, Kim KC, Yoo JC. Arthroscopic partial repair of irreparable rotator cuff tears: preoperative factors associated with outcome deterioration over 2 years. Am J Sports Med. 2015;43(8):1965–1975. [DOI] [PubMed] [Google Scholar]
- 42. Tay VS-L, Tan KS, Loh ICY. Minimally invasive fascia lata harvest: a new method. Plast Reconstr Surg Glob Open. 2013;1(1):e7–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tokish JM, Beicker C. Superior capsule reconstruction technique using an acellular dermal allograft. Arthrosc Tech. 2015;4(6):e833–e839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van der Bracht H, Van den Langenbergh T, Pouillon M, Verhasselt S, Verniers P, Stoffelen D. Rotator cuff repair with all-suture anchors: a midterm magnetic resonance imaging evaluation of repair integrity and cyst formation. J Shoulder Elbow Surg. 2018;27(11):2006–2012. [DOI] [PubMed] [Google Scholar]
- 45. Walch G, Edwards TB, Boulahia A, Nove-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg. 2005;14(3):238–246. [DOI] [PubMed] [Google Scholar]
- 46. Wall KC, Toth AP, Garrigues GE. How to use a graft in irreparable rotator cuff tears: a literature review update of interposition and superior capsule reconstruction techniques. Curr Rev Musculoskelet Med. 2018;11(1):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wylie JD, Baran S, Granger EK, Tashjian RZ. A comprehensive evaluation of factors affecting healing, range of motion, strength, and patient-reported outcomes after arthroscopic rotator cuff repair. Orthop J Sports Med. 2018;6(1):2325967117750104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.