Abstract
Catastrophic antiphospholipid syndrome is a rare multisystem autoimmune condition characterised by rapid development of widespread thrombotic disease and subsequent multi-organ failure. It is the most severe complication of antiphospholipid syndrome, carrying significant morbidity and mortality. We report a patient with post-partum catastrophic antiphospholipid syndrome with cardiac, hepatic, renal and cutaneous manifestations. The diagnostic challenges in establishing a definitive diagnosis in catastrophic antiphospholipid syndrome is discussed, along with the difficulties in managing these patients in the intensive care unit.
Keywords: Catastrophic antiphospholipid syndrome, pregnancy, shock, thrombosis, multi-organ failure
Case report
A 26-year-old female, 33 weeks of gestation, second gravida, was admitted from an antenatal clinic due to worsening thrombocytopaenia (70 × 109/L). She had a haemoglobin level of 104 g/L, elevated liver enzymes ALT 111U/L (normal range (NR) <35) (NR<35), AST 73U/L (NR<30) and a urinary protein/creatinine ratio of 56 mg/mmol. There was a medical history of confirmed triple antibody positive primary antiphospholipid syndrome, previous pregnancy loss at 21 weeks of gestation, obesity (BMI 47.12) and non-alcoholic fatty liver disease. She was monitored throughout the current pregnancy in the high-risk obstetric clinic and received prophylactic enoxaparin 60 mg daily and aspirin 100 mg daily. In view of the perceived risk of developing HELLP (haemolysis, elevated liver enzymes, low platelet count) syndrome and her medical history, she underwent an emergency lower segment caesarean section (LSCS) at 33 + 3 weeks with no immediate complications. Aspirin was ceased and she was discharged home five days later with prophylactic enoxaparin 60 mg and cephalexin 500 mg for a mild caesarean wound infection.
On day 1 post-discharge, she presented to the Emergency Department with a 12 hour history of abdominal pain, associated with nausea and periodic episodes of sweating. On clinical examination, she appeared unwell with a new malar rash, left-upper-quadrant abdominal tenderness and evidence of poor healing of the LSCS wound. Her vital signs were heart rate (HR) 89 bpm, blood pressure (BP) 128/85 mm Hg, respiratory rate (RR) 18 bpm, SaO2 95% room air (RA) and temperature 37.1℃. Initial laboratory results at admission were haemoglobin 102 g/L, white cell count 12.4 × 109/L (neutrophils 10.5 × 109/L), platelet count 104 × 109/L, creatinine 50 µmol/L, ALT 70 U/L, AST 33 U/L, GGT 57 U/L and CRP of 180 mg/L. A pelvic ultrasound excluded any retained products of conception and an abdominal/pelvis CT scan revealed distended large bowel loops with no indication for surgical management. She was commenced on cephazolin, gentamicin and metronidazole for presumed sepsis.
On day 2 of her re-admission, she developed sinus tachycardia (HR 115–130), tachypnoea (RR 30, SaO2 98% on supplementary oxygen via nasal prongs) and fevers up to 39.5℃. Her antibiotic regimen was revised to tazocin and she received therapeutic enoxaparin prior to a CT pulmonary angiogram (CTPA) that excluded pulmonary emboli. On day 5, the patient was admitted to intensive care unit (ICU) due to concerns associated with persistent sinus tachycardia (regularly up to 140 bpm), hypoxia (SaO2 89% on RA) and temperatures to 39℃ despite resolution of the wound erythema. On days 5–10, there was persistent tachycardia despite broadening of antibiotic regime to cefepime and vancomycin. Whilst microbial cultures returned negative, she continued to spike daily temperatures >38℃. There was suspicion of a potential flare of her underlying antiphospholipid syndrome (APS) requiring immunosuppressive therapy; however, a concomitant septic source had yet to be excluded.
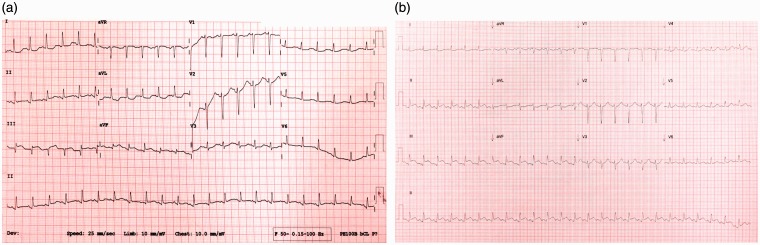
On day 10 of hospital admission, she developed digital ischaemia in the left great and second toe with increasing tachypnoea, tachycardia and hypotension (BP 69/48 mmHg, mean arterial pressure (MAP) 50 mmHg) requiring vasopressor support. ECG demonstrated ST elevation in leads II, III and aVF, with reciprocal ST depression in lead aVL and QT prolongation (Figure 1(a)) with no associated chest pain. The serum troponin I level was 28,860 ng/L (normal < 35) and a bedside transthoracic echocardiogram (TTE) illustrated inferolateral, inferoseptal and anteroseptal hypokinesia (Reference Echo video 1). The left ventricular ejection fraction (LVEF) was estimated at 40% with normal right ventricular function. No valvular abnormalities were identified. Spontaneous coronary artery dissection (previously reported in pregnancy) was excluded with an urgent coronary angiogram which demonstrated normal coronary arteries. The left ventriculogram showed moderate LV-dysfunction with regional wall motion abnormalities consistent with the TTE. Post-angiogram, there was further inferior ST elevation (Figure 1(b)) and in order to maintain a MAP of >65 mmHg, escalating doses of vasopressors were required including noradrenaline 4 mg/h, adrenaline 180 mcg/h and vasopressin 2.4 U/h. There was subsequent oliguria and deteriorating renal function, while sepsis remained a primary differential, it was later excluded with a hysteroscopy and an exploratory laparotomy.
Figure 1.
(a) ST elevation in leads II, III and aVF, with reciprocal ST depression in avL and (b) postangiogram.
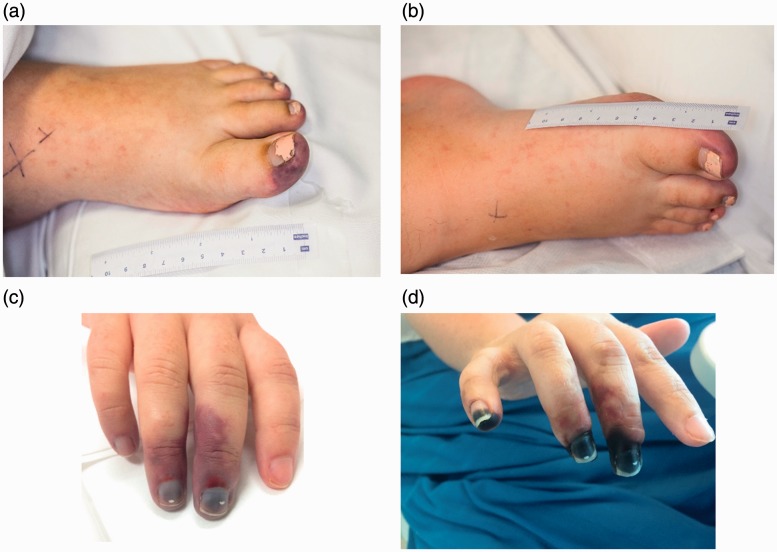
In view of her cardiac dysfunction, post-partum cardiomyopathy was considered; however, the ECG changes were uncharacteristic. The rapid development of definitive cutaneous thrombotic events (Figure 2(a) and (b)) along with the suspicion of known underlying pathology causing the multi-organ failure led to the consideration of catastrophic antiphospholipid syndrome (CAPS) as the most likely diagnosis.
Figure 2.
Day 15: Moderate ischaemia to the left first, third, fourth and fifth distal toes (a) and right first toe (b). Right hand, Day 15 (c) and Day 45 (d): demarcation around the distal interphalangeal joint of third, fourth and fifth fingers.
Treatment for CAPS was initiated with unfractionated heparin and methylprednisolone; however, she continued to deteriorate throughout the evening following the laparotomy with a rising serum lactate to 16 mmol/l, worsening digital ischaemia in the right foot and hand (Figure 2(c)) and development of acute kidney injury (creatinine increasing from 57 umol/L to 226 umol/L). Her vasopressor and inotropic support continued to increase, and in the context of her worsening hypotension with significant cardiac involvement, the decision was made to insert an intra-aortic balloon pump (IABP) (40cc balloon) via the left femoral artery. Continual renal replacement therapy was commenced secondary to anuria and worsening metabolic acidosis. Further immunosuppression was commenced with five courses of plasma exchange (2000 ml fresh frozen plasma and 2000 ml 4% albumin), followed by five days of intravenous immunoglobulin (IVIG) therapy (2 mg/kg).
On day 1 post-insertion of the IABP, vasopressor requirements were halved. TTE with IABP in-situ on 1:2 augmentation showed ongoing segmental wall defects with a further reduction in ejection fraction to 30–35%. The IABP was successfully weaned and subsequently removed after 72 h. Metoprolol was commenced at 25 mg twice daily as she continued to experience marked sinus tachycardia (HR 100–120 bpm).
A liver ultrasound was performed in view of a disproportionate rise in liver transaminases (AST 9 139 U/L, ALT 2 306U/L), excluding portal vein thrombosis. Digital ischaemia was treated with unfractionated heparin with a targeted activated partial thromboplastin times (aPTT) of 80–90 s, yet there was further progression of digital ischaemia with demarcation of the fingers of the right hand and modest ischaemia to the left and right toes (Figure 2).
Her ICU admission was complicated with a large haematoma in the left groin and a pseudoaneurysm (3 cm AP, 6 cm TVR) resulting from removal of the IABP, which was injected with thrombin, debrided and managed with a VAC dressing. Over the following weeks, her clinical condition improved with successful weaning of dialysis and oxygen therapy.
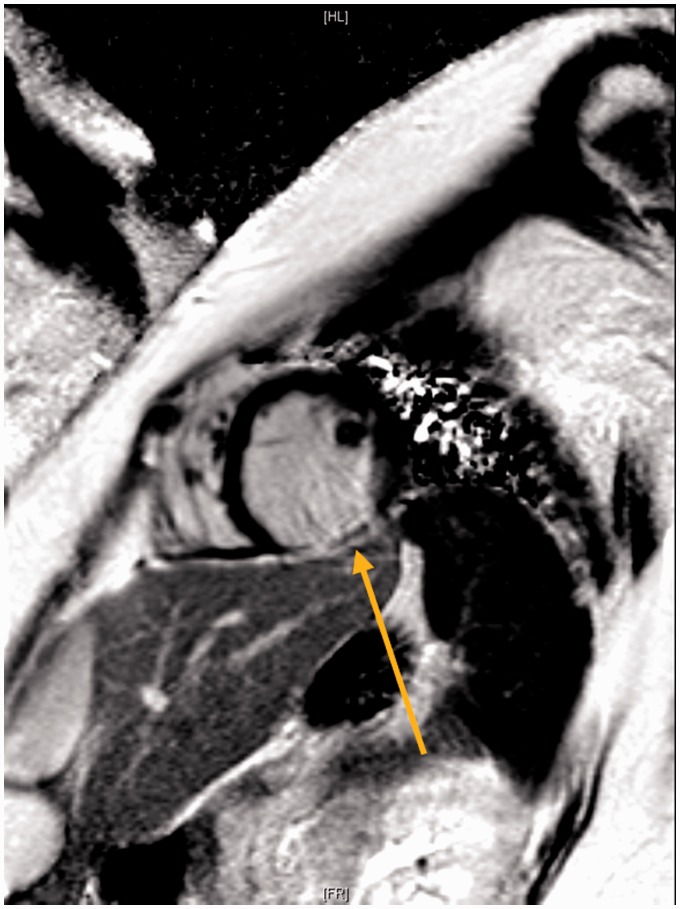
Progress echocardiograms continued to demonstrate moderate left ventricular, systolic dysfunction with an LVEF of approximately 40% (reference echo video 2). A cardiac magnetic resonance imaging (MRI) confirmed the possibility of inferior wall infarct with delayed gadolinium enhancement and dyskinesis in the inferolateral wall (Figure 3).
Figure 3.
Cardiac MRI, delayed PSIR sequence demonstrating cross-section of left ventricle with delayed gadolinium enhancement in the inferior wall (arrow).
Four weeks after the initiation of plasma exchange and IVIG, and 5 days following the transition to warfarin, new digital infarcts were noted on the left third and fourth toe and right second toe. This prompted further use of IVIG, conversion back to enoxaparin with recovery of renal function and a four-week period of weekly Rituximab. Ten weeks post-admission, progress TTE demonstrated a deterioration in systolic dysfunction with an LVEF of 25–30%. The patient was treated with a levosimendan infusion and then discharged home with carvedilol 25 mg TDS, enoxaparin 80 mg BD, aspirin 100 mg daily, hydroxychloroquine 200 mg and prednisolone 50 mg. The patient had normalising laboratory results and no further thrombi formation.
Discussion
Antiphospholipid syndrome and catastrophic antiphospholipid syndrome
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterised by an increased risk of vascular thromboses and/or obstetric complications with the presence of antiphospholipid antibodies. Definitive APS is based on the revised Sapporo criteria1 and requires one clinical and at least one laboratory criteria to be fulfilled (Figure 4).
Figure 4.
Updated Sapporo APS Classification Criteria.1
Catastrophic antiphospholipid syndrome (CAPS) is the most severe complication of APS affecting <1% of patients and characterised predominantly by multiple vascular occlusive events leading to multi-organ failure over a short period.2 The validated classification criteria for definitive CAPS is outlined in Figure 5 with reported mortality rates ranging from 30 to 50% despite therapy.3 To generate a greater understanding of this infrequent syndrome, an international CAPS registry in Europe was developed in 2000, documenting all clinical, laboratory and therapeutic data of patients with CAPS.4
Figure 5.
Validated classification criteria for definitive CAPS.5
The triple positive antiphospholipid antibodies (anti-B2-glycoprotein-I antibody, lupus anticoagulant (LA) and anticardiolipin antibody) have been found to correspond to a high-risk profile with higher rates of CAPS.6 A strong association has also been found with a positive LA in patients with CAPS (92.3%) vs. APS (53.6%) patients (p<0.001).7 Based on the Sappora criteria,1 the patient presented had a definitive prior diagnosis of APS following the delivery of a stillborn at 21 weeks of gestation in 2015 with pre-eclampsia and persistently triple positive antiphospholipid antibodies (Table 1). Frequent monitoring of antibodies was limited in view of unavailability to access serial titers.
Table 1.
Antiphospholipid antibody titer levels and results following first pregnancy (2015) and second pregnancy (2017).
| 2015–2016 | November 2015 | February 2016 | ||
|---|---|---|---|---|
| ACA IgG (GPL) | 63 | Strongly positive | 125 | Strongly positive |
| ACA IgM (MPL) | 15 | Equivocal | 23 | Positive |
| LA | 2.1 | Strongly positive | 2.2 | Strongly positive |
| B2 glycoprotein (U/ml) | 47 | Positive | 67 | Positive |
|
2017
|
Pre-exchange
|
Post-exchange | ||
| ACA IgG (GPL) | 20 | Equivocal | 16 | Equivocal |
| ACA IgM (MPL) | 19 | Equivocal | 6 | Negative |
| LA | 1.7 | Moderately positive | 1.2 | Weak positive |
| B2 glycoprotein (U/ml) | 21 | Positive | 4 | Negative |
Note: ACA IgG – strongly positive: > 60 GPL units, equivocal: 11–20 GPL units. ACA IgM – positive: 21–60 MPL units, equivocal: 11–20 MPL units, negative: 0–10 MPL units. B2 glycoprotein – normal range: < 7 U/ml. LA strongly and moderately positive ratio: 1.6–2.0, weak positive ratio: 1.2-1.5. ACA: anticardiolipin antibody; LA: lupus anticoagulant.
The diagnostic challenge
The acute thrombotic microangiopathies and multi-organ dysfunction with CAPS is commonly seen in many other conditions including thrombotic thrombocytopenic purpura (TTP), haemolytic uraemic syndrome (HUS) and disseminated intravascular coagulation (DIC). This generates diagnostic challenges in the definitive diagnosis of CAPS, especially in the acute period.
In contrast to CAPS, HUS and TTP are characterised by microangiopathic haemolytic anaemia with schistocytes on blood film and thrombocytopaenia with associated organ ischaemia.8 The patient's platelet count was normalised on admission to ICU and blood films never identified any schistocytes. With a normal ADAMTS13 level and lack of neurological manifestations, TTP was excluded. Absence of complement dysregulation, toxicogenic bacteria and AKI on presentation precluded HUS. The activated partial thromboplastin times (aPTT) normalises with HUS and TTP and is prolonged in CAPS due to the presence of the LA.9 The aPTT in this case was consistently between 41 and 58 s (NR: 24–38 s) prior to the commencement of therapeutic heparin.
Sepsis associated with DIC can present simultaneously with thrombocytopaenia, disseminated microvascular thrombosis and a haemorrhagic diathesis, the latter of which was not prevalent in this case. The lowest platelet count was 93 × 109/L (NR: 150–400) and while fibrinogen ranged from 4 to 7.4 g/L (NR: 1.8–4.4), levels would increase with sepsis and other inflammatory conditions as an acute phase reactant. A normalised fibrinogen level may therefore still indicate substantial fibrinogen consumption. Nonetheless, apart from scant candida growth from her left groin wound post-IABP removal, all other cultures remained negative.
Pregnancy-related CAPS
Throughout the literature, it is well-documented that a precipitating factor is required, such as infection, surgery, anticoagulant withdrawal, obstetric complications or neoplasia for the clinical manifestations of CAPS. A concurrent autoimmune disorder such as systemic lupus erythematosus (SLE) may be present in about 40% of cases (secondary APS).4,9–11
While this patient has some signs and symptoms suggestive of SLE, she previously had testing in November 2015 which returned negative ANA 1:160 and anti-dsDNA 11 IU/ml (negative) with normal complement levels. Anti-dsDNA was negative on her re-admission this year and as she did not fulfil the SLICC (Systemic Lupus Erythematosus International Collaborating Clinics) classification criteria for SLE, further testing while she was acutely unwell was not carried out. C3 and C4 levels, however, were checked at the next available opportunity, which were within normal range.
Pregnancy has been recognised as a potential trigger for CAPS, particularly with LA-positive patients. The PROMISSE trial, a multicentre prospective observational study examining the pregnancy complications in patients with APS and/or SLE, found the greatest significant risk factor for adverse obstetric outcomes was the presence of LA, 39% vs. 3%, when absent (P<0.0001).12 Hanouna et al.10 carried out a retrospective review on 13 pregnancy-related CAPS, occurring during pregnancy or 6-weeks post-partum between 2002 and 2012 and noted a significant correlation between HELLP syndrome and development of CAPS in 12/13 patients. Gomez-Puerta et al.11 reviewed 15 pregnancy-related CAPS from the CAPS Registry and reported 8/15 patients with HELLP syndrome prior to development of CAPS.
Two predictive factors inferred by Hanouna et al.10 in the development of CAPS were pre-eclampsia/eclampsia associated with HELLP syndrome and modifications in anticoagulation during the period around delivery. In this case, there was elevated liver transaminases and thrombocytopenia during both pregnancies. Aspirin and enoxaparin were temporarily ceased around the time of delivery in the second pregnancy and only recommenced once the platelet count was >100 × 109/L. It is noteworthy in this patient that there was a period of clinical improvement following delivery prior to the development of persistent tachycardia and high temperatures that were out of keeping to a local wound infection. This period of improvement was similarly observed in Hanouna et al.10 who reviewed 15 related pregnancy CAPS and proposed that high-risk patients who develop HELLP syndrome should be followed up closely in the post-partum period. Treatment should be resumed with aspirin and heparin in the hours following delivery, even in the setting of thrombocytopaenia secondary to HELLP.10
CAPS clinical features
Koniari et al.13 conveyed that given the long-term hypercoagulable nature of APS patients, thrombotic events remain infrequent. It is postulated that thrombotic events would occur more often in the microvasculature causing impaired multi-organ dysfunction from recurrent micro-thrombosis/micro-emboli. The underlying histopathology of microvascular thrombosis in CAPS remains poorly understood.
The primary confounding factor in this case was the significant cardiac compromise associated with the diagnosis of CAPS and the possible presence of peripartum cardiomyopathy. Myocardial infarcts in young patients with APS has a reported incidence of 2.8% in a cohort of 1000 patients in a prospective multicentre study.2 Most case reports in the literature document findings of coronary artery stenosis or intracardiac thrombi.14,15 The coronary angiogram in this case revealed normal epicardial coronary arteries with regional wall abnormalities and moderate LV global dysfunction. A cardiac MRI illustrated delayed gadolinium enhancement in the inferolateral wall with associated dyskinesis indicative of myocardial scarring and damage. It is of interest that this correlates with more inferior ST elevation post-angiography. The use of viscous, non-oxygenated contrast is likely to aggravate the microvascular obstruction consistent with the pathophysiology of CAPS. Hucker et al.16 and Rosenbaum et al.17 reported similar findings on two CAPS cases presenting with cardiogenic shock attributable to myocardial microthrombi and microinfarcts.
Cutaneous manifestations are common with Frances et al.18 reporting an incidence of 49% in 200 patients with APS and as the presenting manifestation in 30% of cases. In CAPS, dermatological features are present in up to 70% and include livedo reticularis (most common), cutaneous necrosis, digital gangrene, sublingual splinter haemorrhage and pseudovasculitis lesions.19 Digital ischaemia of the great toe was the presenting feature in this case, a clear thrombotic event 6 hours prior to development of shock and almost concomitant multi-organ failure. It is inferred that the digital ischaemia was a combination of the autoimmune process and poor perfusion from depressed cardiac output. Despite therapeutic heparin and plasma exchange therapy, the patient's digital ischaemia progressed to dry gangrene.
Shortly after the onset of shock, our patient developed rapid renal and hepatic dysfunction. Renal involvement is a prominent feature of APS and defined by the International Consensus Statement of CAPS as ≥50% increase in serum creatinine concentration, proteinuria >0.5 g/day and severe hypertension (BP > 180/100 mmHg). Our patient's serum creatinine concentration increased from 86 to 226 umol/L over 72 h, a measured 24-h urine protein of 680 mg in 2.94 L and subsequent development of oligo-anuria.
Hepatic involvement in CAPS is not widely reported in the literature. In this case, there was a disproportionate rise in liver transaminases (AST>ALT), elevated LDH (3977U/L) and coagulopathy (INR: 3.1), which may have resulted from the severe acute cardiac failure. Biopsies of either the kidney or liver in the acute setting was not feasible, illustrating the difficulty in fulfilling the classification criteria for CAPS in the clinical setting with predominant involvement of rapid vascular occlusion of smaller vessels, and larger vessels associated more with APS.2,5
The aetiology of shock in this patient is likely to be multifactorial. The moderate left ventricular dysfunction is almost certainly due to microvascular obstruction resulting in significant myocardial damage as evident by the large troponin elevation. The role of vasoplegia cannot be discounted in this instance prior to the escalating doses of inotropes and vasopressin given there was no measurement of systemic vascular resistance; however, the patient continued to deteriorate whilst on these vasoactive medications. The cardiac involvement likely played a significant role in the development of shock illustrated by the rapid improvement in her haemodynamic status with IABP counter-pulsation. Metabolic acidosis and generalised inflammation would have also contributed to the development of shock in this patient.
CAPS treatment
This patient could not fulfil the definitive criteria for CAPS given the inability to obtain histological confirmation, yet there is no alternative comprehensive diagnosis. She was treated for CAPS with triple therapy – a treatment regime of anticoagulation, glucocorticoids and plasma exchange that has demonstrated a recovery rate of 78%.20 The beneficial effects of plasma exchange are based on randomised control trials on treatment with other microangiopathic conditions such as TTP.21 It is recommended as grade 2c evidence by the American Society of Apheresis (ASFA) 22 and indicated when CAPS develops life-threatening clinical manifestations. There is no unanimity in the type of replacement fluid for plasma exchange; however, ASFA recommends albumin over FFP to reduce the side effects from excessive plasma. Treatment with plasma exchange is generally 3–5 days, based on retrospective analysis of the CAPS registry, but again, there is no consensus on the optimal duration of treatment.
IVIG therapy in addition to triple therapy is included in the International consensus guidelines for CAPS. There are no absolute guidelines in the dose or duration of IVIG, inferenced from the treatment of other autoimmune conditions and presumed to be given after the last day of plasma exchange to prevent removal. Two common regimes in recent case reports is 2 g/kg of body weight over 2–5 days or a lower dose of 400 mg/kg for 5 days23,24 Following 2001, the increasing use of combination therapy has led to a 20% reduction in mortality with Grade B recommendation by the Task Force on CAPS for triple therapy (anticoagulation + glucocorticoid + plasma exchange ± IVIG).25 No meta-analysis or RCTs have been conducted to demonstrate any differences in survival rates whether IVIG is given in addition to triple therapy. Sciascia et al.26 and Tenti et al.24 have exhibited a reduction in antiphospholipid titer levels with IVIG treatment in patients with primary APS and subsequently postulated to have similar beneficial effects in CAPS.
Rituximab, a monoclonal antibody against CD20, has demonstrated some efficacy with refractory CAPS in 12/20 patients included in the CAPS Registry.27 Further studies in its utility as a single agent is limited given the infrequent number of CAPS cases, and its use often in conjunction with other first line immunosuppressive therapy. Nonetheless, it is postulated to have synergistic effects with other immunosuppressive agents in the treatment for CAPS.28
Conclusion
This is the first reported case of post-partum CAPS with significant cardiac involvement requiring management with an IABP. Microvascular thrombosis appears more characteristic with CAPS in comparison to APS, contributing to the difficulties in fulfilling the definitive criteria for CAPS. With the expeditious multi-organ failure, identifiable CAPS trigger factors, prior HELLP syndrome and confirmed APS, this patient had a clinically significant APS profile for catastrophic APS. Cardiac MRI is an underutilised and alternative tool that could affirm the pathophysiology associated with this diffuse thrombotic process. Whilst the occurrence of this syndrome is rare, it should be recognised as a potential diagnostic entity especially in the context of significant multi-organ dysfunction with no identifiable septic source.
Supplementary Material
Acknowledgements
The authors thank the patient for written informed consent for the publication of this study.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Piette J-C, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002; 46: 1019–1027. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Pinto I, Espinosa G, Cervera R. Catastrophic antiphospholipid syndrome - 20 years later. Curr Rheumatol Rev 2013; 9: 73–80. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Pinto I, Moitinho M, Santacreu I, et al. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun Rev 2016; 15: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 5.Asherson RA, Cervera R, de Groot PG, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus 2003; 12: 530–534. [DOI] [PubMed] [Google Scholar]
- 6.Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118: 4714–4718. [DOI] [PubMed] [Google Scholar]
- 7.Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2015; 74: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 8.Asherson RA, Espinosa G, Cervera R, et al. Catastrophic antiphospholipid syndrome: proposed guidelines for diagnosis and treatment. J Clin Rheumatol 2002; 8: 157–165. [PubMed] [Google Scholar]
- 9.Nayer A, Ortega LM. Catastrophic antiphospholipid syndrome: a clinical review. J Nephropathol 2014; 3: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanouna G, Morel N, Le Thi Huong D, et al. Catastrophic antiphospholipid syndrome and pregnancy: an experience of 13 cases. Rheumatology 2013; 52: 1635–1641. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Puerta JA, Cervera R, Espinosa G, et al. Catastrophic antiphospholipid syndrome during pregnancy and puerperium: maternal and fetal characteristics of 15 cases. Ann Rheum Dis 2007; 66: 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmon JE. PROMISSE: progress in understanding pregnancy complications in patients with SLE. Arthritis Res Ther 2012; 14: A39. [Google Scholar]
- 13.Koniari I, Siminelakis SN, Baikoussis NG, et al. Antiphospholipid syndrome; its implication in cardiovascular diseases: a review. J Cardiothorac Surg 2010; 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prashanth P, Mukhaini M, Riyami A. A rare presentation of primary antiphospholipid syndrome. Oman Med J 2009; 24: 300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosove MH, Brewer PM. Antiphospholipid thrombosis: clinical course after the first thrombotic event in 70 patients. Ann Intern Med 1992; 117: 303–308. [DOI] [PubMed] [Google Scholar]
- 16.Hucker WJ, Chatzizisis YS, Steigner ML, et al. Myocardial catastrophe: a case of sudden, severe myocardial dysfunction. Circulation 2014; 130: 854–862. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum AN, Anavekar NS, Ernste FC, et al. A case of catastrophic antiphospholipid syndrome: first report with advanced cardiac imaging using MRI. Lupus 2015; 24: 1338–1341. [DOI] [PubMed] [Google Scholar]
- 18.Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of the antiphospholipid syndrome: Two hundred consecutive cases. Arthritis Rheum 2005; 52: 1785–1793. [DOI] [PubMed] [Google Scholar]
- 19.Stojanovich L, Marisavljevic D, Rovensky J, et al. Clinical and laboratory features of the catastrophic antiphospholipid syndrome. Clin Rev Allergy Immunol 2009; 36: 74–79. [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Puerta JA, Espinosa G, Cervera R. Catastrophic antiphospholipid syndrome: diagnosis and management in pregnancy. Clin Lab Med 2013; 33: 391–400. [DOI] [PubMed] [Google Scholar]
- 21.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 1991; 325: 393–397. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz J, Winters JL, Padmanabhan A, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher 2013; 28: 145–284. [DOI] [PubMed] [Google Scholar]
- 23.El-Shereef RR, El-Abedin Z, Abdel Aziz R, et al. Catastrophic antiphospholipid syndrome. Case Rep Rheumatol 2016; 2016: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenti S, Guidelli GM, Bellisai F, et al. Long-term treatment of antiphospholipid syndrome with intravenous immunoglobulin in addition to conventional therapy. Clin Exp Rheumatol 2013; 31: 877–882. [PubMed] [Google Scholar]
- 25.Cervera R, Rodríguez-Pintó I. Catastrophic antiphospholipid syndrome: task force report summary. Lupus 2014; 23: 1283–1285. [DOI] [PubMed] [Google Scholar]
- 26.Sciascia S, Giachino O, Roccatello D. Prevention of thrombosis relapse in antiphospholipid syndrome patients refractory to conventional therapy using intravenous immunoglobulin. Clin Exp Rheumatol 2012; 30: 409–413. [PubMed] [Google Scholar]
- 27.Berman H, Rodriguez-Pinto I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev 2013; 12: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Pinto I, Cervera R, Espinosa G. Rituximab and its therapeutic potential in catastrophic antiphospolipid syndrome. Ther Adv Musculoskelet Dis 2015; 7: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.