Abstract
Suosuo grape (Vitis vinifer L) is traditionally used as a therapeutic agent for measles and hepatitis by the ethnic Uighurs. This work aimed to investigate the anti-HBV effect of total triterpene (VTT), total flavonoids (VTF) and total polysaccharides (VTP) from Suosuo grape, and their synergistic effects were also tested. The viral antigens of cellular secretion, HBsAg and HBeAg, were determined by enzyme linked immunosorbent assay (ELISA).The quantity of HBV-DNA released in the supernatant was assayed by real-time PCR. It was found that it effectively suppressed the secretion of HBsAg and HBeAg from HepG2.2.15 cells in a dose-dependent manner, as well as the HBV DNA. The results of orthogonal design experiment showed that the combination of VTT 20 μg/mL, VTF 50 μg/mL and VTP 50 μg/mL had the best optimistic inhibitory effects on HBeAg secretion.
Keywords: Vitis vinifer L, anti-hepatitis viral activity, orthogonal design
1. Introduction
Hepatitis B virus (HBV) infections remain a major public health problem for nearly 350 million people. HBV infections frequently results in acute and chronic hepatitis and may develop into liver cirrhosis and hepatocellular carcinoma [1]. Although immunization against HBV has been widely used in preventing viral infections, current therapies for these diseases have limited efficacy, therefore there is still an urgent need for the development of effective antiviral agents to eliminate HBV or to inhibit HBV proliferation in carriers [2]. In recent years, people have paid close attention to active anti-viral compounds from traditional medicine or natural products such as oleanolic acid, silymarin, and schizandrin [3,4] because of their better effects.
Suosuo grape (Vitis vinifera L) is widely grown in Xinjiang Province in China, and its fruit has been used as a folk medicine for hepatitis and pedo-measles [5]. Previous studies showed that total triterpene (VTT), total flavonoids (VTF) and total polysaccharides (VTP) from Suosuo grape had significantly protective effects against immunological liver damage induced by Bacille Calmette- Guerin (BCG) plus lipopolysaccharide (LPS) in vitro and in vivo, and its mechanism might be related to clearance of free radicals, decrease of lipid peroxidation products, protection of hepatocytes membranes, improvement of hepatocyte function and regulation of the secretion of immune cytokines and expressions of apoptosis genes [6,7,8,9]. The aim of this work was to investigate the synergistic inhibitory effects of VTT, VTP and VTF from Suosuo grape on HBV in vitro.
2. Results and Discussion
2.1. Toxic effect of extracts from suosuo grape on Hep G2.2.15 cells
The cytoxicity test results showed that the TC50 of VTT, VTP, VTF and 3-TC were 160.61, 1412.85, 284.91 and 244.97 μg/mL, respectively.
2.2. Inhibitory effect of extracts from suosuo grape on the expression of HBsAg, HBeAg and HBV DNA replication
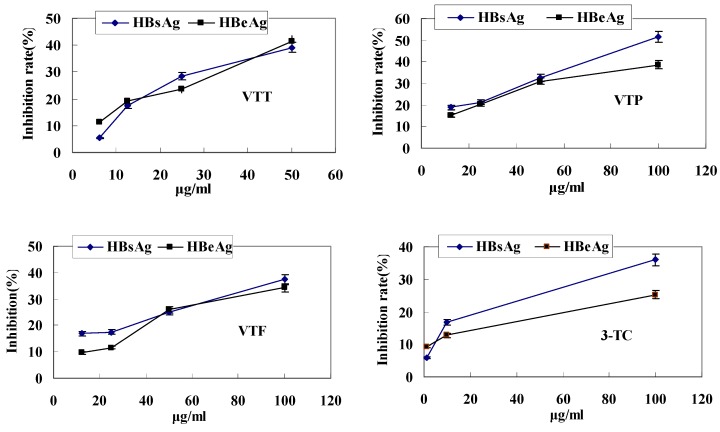
In this work, the inhibitory effect of extracts from suosuo grape on the expression of HBsAg, HBeAg and HBV DNA replication were investigated, and the results are illustrated in Figure 1. The VTT, VTF and VTP from suosuo grape were able to inhibit the expression of HBsAg, HBeAg, and HBV DNA replication in vitro in a dose-dependent manner. The median effective dose (IC50) of inhibition HBsAg were 62.54, 112.69 and 327.56 µg/mL, the individual therapeutic indexes of HBsAg were 2.57, 12.54 and 0.87. The median effective doses (IC50) of HBeAg inhibition were 89.28, 204.46 and 215.34 µg/mL, the individual therapeutic indexes of HBeAg were 1.80, 6.91 and 1.32 (Table 1). The inhibitory effect on HBV DNA was enhanced with the increasing concentration of extracts (Table 2).
Figure 1.
Inhibitory effect of VTP, VTT, VTF and 3-TC on HBsAg and HBeAg secretion.
Table 1.
Therapeutic index.
| Sample | TC50(μg/mL) | HBsAg | HBeAg | ||
|---|---|---|---|---|---|
| IC50(μg/mL) | TI | IC50(μg/mL) | TI | ||
| VTT | 160.61 | 62.54 | 2.57 | 89.28 | 1.80 |
| VTP | 1412.85 | 112.69 | 12.54 | 204.46 | 6.91 |
| VTF | 284.91 | 327.56 | 0.87 | 215.34 | 1.32 |
| 3-TC | 244.77 | 315.37 | 0.78 | 9051.01 | 0.03 |
Table 2.
The effect of extracts from Vitis vinifer L on HBV DNA replication
| Sample | Concentration(μg/mL) | HBV DNA(copies/mL) | HBV DNA inhibition ratio (%) |
|---|---|---|---|
| VTT | 50 | (4.842 ± 4.457) × 105 | 59.17 |
| 25 | (8.269 ± 2.002) × 105 | 30.28 | |
| 12.5 | (0.944 ± 1.460) × 106 | 20.40 | |
| 6.25 | (1.015 ± 5.230) × 106 | 14.42 | |
| VTP | 100 | (4.823 ± 3.394) × 105 | 59.33 |
| 50 | (7.525 ± 5.883) × 105 | 36.55 | |
| 25 | (0.873 ± 5.335) × 106 | 26.39 | |
| 12.5 | (1.158 ± 2.027) × 106 | 2.36 | |
| VTF | 100 | (6.211 ± 1.910) × 105 | 47.63 |
| 50 | (0.970 ± 0.432) × 106 | 18.21 | |
| 25 | (0.892 ± 2.872) × 106 | 24.79 | |
| 12.5 | (1.162 ± 0.201) × 106 | 2.02 | |
| 3-TC | 100 | < 103 | > 99.99 |
| 10 | < 103 | > 99.99 | |
| 1 | < 103 | > 99.99 | |
| Cell group | (1.186 ± 0.226)×106 |
2.3. Inhibitory effect of extracts from suosuo grape on the expression of HBsAg, HBeAg and HBV DNA replication
The synergistic effects of suosuo grape extract (A-VTT, B-VTP and C-VTF) inhibition of secretion of HBeAg by Hep G 2.2.15 cells could be examined using an experimental design. In the present study, all selected factors were examined using an orthogonal L 8(2)7 test design. The total evaluation index was used to analysis by statistical methods. The results of the orthogonal test and extreme difference analysis are presented in Table 3. The analysis of variance was performed using the statistical software SPSS 13.0 and the results are listed in Table 4. The results showed that there had synergistic effect among VTT, VTP and VTF on inhibition of HBeAg secretion by Hep G 2.2.15 cells, and the strengths of inhibition were A×B×C > B×C > B > C, in turn. The optimal combination of extracts was A2B1C1: using VTT 20 μg/mL, VTF 50 μg/mL and VTP 50 μg/mL together on HepG2.2.15.
Table 3.
Analysis of L8 (2)7 test results.
| NO | A | B | A×B | C | A×C | B×C | A×B×C | HBeAg inhibition ratio(%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | X1 | X2 | X3 | ΣX | |||
| 1 | 1(50) | 1(50) | 1 | 1(50) | 1 | 1 | 1 | 65.55 | 60.24 | 45.88 | 171.67 | ||
| 2 | 1(50) | 1(50) | 1 | 2(25) | 2 | 2 | 2 | 46.29 | 49.39 | 54.45 | 150.13 | ||
| 3 | 1(50) | 2(25) | 2 | 1(50) | 1 | 2 | 2 | 51.18 | 46.37 | 46.86 | 144.41 | ||
| 4 | 1(50) | 2(25) | 2 | 2(25) | 2 | 1 | 1 | 38.53 | 29.96 | 41.22 | 109.71 | ||
| 5 | 2(25) | 1(50) | 2 | 1(50) | 2 | 1 | 2 | 59.84 | 64.33 | 69.06 | 193.23 | ||
| 6 | 2(25) | 1(50) | 2 | 2(25) | 1 | 2 | 1 | 48.08 | 37.31 | 35.59 | 120.98 | ||
| 7 | 2(25) | 2(25) | 1 | 1(50) | 2 | 2 | 1 | 46.12 | 48.08 | 29.80 | 124.00 | ||
| 8 | 2(25) | 2(25) | 1 | 2(25) | 1 | 1 | 2 | 64.08 | 58.94 | 47.58 | 170.60 | ||
| Ⅰ | 575.92 | 636.01 | 616.40 | 633.31 | 607.66 | 645.21 | 526.36 | ||||||
| Ⅱ | 608.81 | 548.72 | 568.33 | 551.42 | 577.07 | 539.52 | 658.37 | ||||||
| R | 32.89 | 87.29 | 48.07 | 81.89 | 30.59 | 105.69 | 132.01 | ||||||
A, VTT; B, VTP; C, VTF.
Table 4.
The analysis of variance of test results.
| The source of variation | SS | υ | MS | F | Sig. |
|---|---|---|---|---|---|
| Total variation | 2771.173 | 23 | |||
| Total inter-treatment | 1968.782 | 7 | |||
| A | 45.073 | 1 | 45.073 | 0.899 | 0.357 |
| B | 317.481 | 1 | 317.481 | 6.331 | 0.023 |
| C | 279.416 | 1 | 279.416 | 5.572 | 0.031 |
| Frist interaction A×B | 96.280 | 1 | 96.280 | 1.920 | 0.185 |
| A×C | 38.990 | 1 | 38.990 | 0.777 | 0.391 |
| B×C | 465.432 | 1 | 465.432 | 9.281 | 0.008 |
| Second interaction A×B×C | 726.110 | 1 | 726.110 | 14.479 | 0.002 |
| Error | 802.392 | 16 | 50.149 |
A, VTT; B, VTP; C, VTF.
3. Experimental
3.1. Plant material
Suosuo grapes were collected from Tulufan, Xinjiang Urghur autonomous region, in China, in May 2006. The plant materials were identified as the fruits of Vitis vinifer L by Yan Fu Zhang, Institute of Materia Medica of Xingjiang. A voucher specimen was deposited at the Institute of Materia Medica of Xinjiang in China.
3.2. Extraction and isolation
Five kilogram of the plant material was extracted with deionized water under reflux at 80 ºC for 6 h in several batches. The combined water extracts was evaporated under vacuum to specific gravity of 1.1-1.2, and ethanol about five times the original volume was added and kept at 4 for 24 h. After being filtered and being washed by ethanol, acetone, and diethyl ether, the precipitates was collected and redissolved in distilled water and treated with Sevag’s reagent several times to remove protein and dialyzed against deionised water for 24 h at 4 ºC. The polysaccharides (VTP) were obtained though further precipitated with ethanol. The gruffs was extracted with 95% ethanol under reflux at 80 ºC for 6 h in several batches. The extracts were combined, filtered and evaporated under vacuum. The concentrated extracts was diluted with water and successively treated with petroleum ether and EtOAc. Total triterpenes (VTT) was prepared by the method of 95% ethanol recrystallized from the EtOAc fraction. The mother liquor of VTT crystalline powder combined with supernatant of ethanol precipitation to be separated by AB-8 resin. After elution with deionized water and cleaning of impurities, the 50% ethanol eluent was collected to afford total flavonoids (VTF).
3.3. Oleanolic acid content of VTT
Chromatographic separation was achieved by using HPLC system consisting of a Shimadzu LC-10AD and YMC-Pack ODS-A column (150 × 4.6 mm, 5μm, with pre-column), the mobile phase consisted of methanol and H2O (86:14). Detection wavelength was 215 nm. The flow rate was 1.0 mL/min. Oleanolic acid content of VTT was 65.52 mg/100 mg.
3.4. Flavonoid content of VTF
Total flavonoid content in the extracts was determined using the method described in the Chinese Pharmacopeia [10]. Quantitation was based on the standard curve of rutin (0.2–1.0 mg/mL), dissolved in 95% ethanol. Flavonoids content was calculated with rutin as the standard and total flavonoid content of VTF was 35.28 mg/100 mg
3.5. Experiment design
The group design method was used to observe the inhibitory effects of VTT (6.25, 12.5, 25, 50 μg/mL), VTF (12.5, 25, 50, 100 μg/mL), VTP (6.25, 12.5, 25, 50 μg/mL) and 3-TC (1, 10, 100 μg/mL) on HepG2.2.15 cells secreting HBsAg, HBVeAg and HBV DNA. The synergistic effect of three extracts from Suosuo grape on HBV was studied by orthogonal experiments designed as followed: three factors were determined according to screening samples (A-VTT, B-VTP and C-VTF); every factor was divided with two levels (Table 5). Interactions of the three factors and their main effects were examined, and errors obtained though re-experiments.
Table 5.
Factors and levels for orthogonal test.
| Variable | Level | |
|---|---|---|
| 1 | 2 | |
| A, VTT | 50 | 25 |
| B, VTP | 50 | 25 |
| C, VTF | 50 | 25 |
3.6. Cell culture
The HepG2.2.15 cell line which is stably transfected with HBV DNA, was grown in Dulbecco’s modified Eagle’ medium nutrient mixture containing geneticin (G418) 100 μg mL-1 with 10% (v/v) heat-inactivited fetal bovine serum (FBS), 1 × 105 U mL-1 penicillin, 1.0 mg mL-1 streptomycin. Cells were incubated at 37 ºC in a humidified 5% CO2 atmosphere.
3.7. Cytotoxicity assays
The cytotoxic effects of VTT, VTP, VTF and 3-TC were evaluated by MTT essay [11]. Cells were seeded in 96-well culture plates at a density of 1 × 106 cell mL-1 and cultured at 37 ºC for 48 h. Then the culture medium was removed and replaced with fresh medium supplemented with various concentrations of VTT, VTP, VTF and 3-TC every other day. After eight days of culture, the medium was discarded and 10 μL of a solution of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was added and the cultures were incubated for an additional 4 h. The supernatant was then removed and the cells were dissolved in DMSO (150 μL/well). The relative cell viability was quantitatively measured by scanning at 490 nm on an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tek ELX800; Bio-Tek Instruments Inc., Winooski, VT, USA) and the 50% inhibitory concentration (TC50) on cells was calculated by MTT assay as follows: cell inhibition (%) = (Acell control – Asample) / (Acell control – Acontrol) ×100%.
3.8. HBsAg and HBeAg analysis
The HepG2.2.15 cells were plated at a density of 1 × 106 cell mL-1 on 96-well cell culture plates and routinely cultured. Different concentrations of test samples were supplemented to the medium in triplicate after cells were plated at 37 ºC for 48 h. After incubation with test smples for eight days, the supernatants were collected and clarified by centrifugation at 5000×g for 5 min, HBsAg and HBeAg were determined by ELISA kits, performed according to the protocol provided with the kit (Ke Hua Biological Corp, Shanghai, China). The results were read at 450 nm by an ELISA reader (Bio-Tek ELX800; Bio-Tek Instruments Inc., Winooski, VT, USA). The inhibition of samples on HBsAg and HBeAg were calculated by formula as follows:
HBsAg (or HBeAg) inhibition (%) = (Acell control – Asample) / (Acell control – Acontrol) × 100%, and the 50% inhibitory concentration (IC50) on cells was calculated.
The therapeutic index (TI) was calculated by IC50 and TC50 (TI= TC50 / IC50) to judge the effect of test samples. TI > 2 as effective and low-toxicity; 1 < TI < 2 as low-effective and toxicity and TI < 1 expressed that sample have bigger toxicity and cannot be used as anti-HBV agent.
3.9. Detection of HBV DNA
The quantity of HBV DNA in the supernatant was detected by real-time PCR (ABI PRISM 7300 Sequence Detector, PE Biosystems) based on the TaqMan technology. Viral DNA was extracted from the culture supernatant and the amount of hepatitis B viral DNA was quantified using a diagnostic kit (Shenyou bio technology Co. Ltd., Shanghai, China). A series dilution of known amounts of HBV DNA was used as a control. The inhibitor rate of sample on HBV DNA was calculated by formula as following: the inhibitory rate (%) = (Copiescell control –Copiessample) /Copiescell control×100%.
3.10. Statistical analysis
Data were expressed as mean±SD and were carried out using SPSS Version 13.0 software. Analysis of variance was performed by ANOVA procedures and P < 0.05 was considered to be statistically significant.
4. Conclusions
The VTT, VTF and VTP from suosuo grape had antiviral effect in vitro. These active extracts could play an antiviral effect alone or by synergistic use. Further in vivo investigation to clarify their anti-HBV activities and the mechanisms of these bioactivities is required.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 30660157).
Footnotes
Sample Availability: Samples are available from the authors.
References and Notes
- 1.Yeh S.F., Gupta M., Sarma D.N.K., Mitra S.K. Downregulation of hepatitis B surface antigen expression in human hepatocellular carcinoma cell lines by HD-30, a polyherbal formulation. Phytother. Res. 2003;17:89–91. doi: 10.1002/ptr.864. [DOI] [PubMed] [Google Scholar]
- 2.Niederau C., Heintges T., Lange S., Goldmann G., Niedrau C.M., Mohr L.H. Long-term follow-up of HBeAg postive patients treated with interferon alfa for chronic hepatitis B. N. Engl. J. Med. 1996;334:1422–1427. doi: 10.1056/NEJM199605303342202. [DOI] [PubMed] [Google Scholar]
- 3.Liu J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharm. 2005;100:92–94. doi: 10.1016/j.jep.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Wang R.B., Kong J., Wang D.L., Lien L.L., Eric J.L. A survey of chinese herbal ingredients with liver protection activities. Chinese Med. 2007;2:1–8. doi: 10.1186/1749-8546-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y.M. Pharmacography of Uighur, Part One. Xinjiang Science & Technology & Hygiene Publishing House; Urumuqi, China: 1999. pp. 541–542. [Google Scholar]
- 6.Liu T., Ma L., Zhao J., Su D.Q. Effect of polysaccharide from vitis viniferal on immunological liver injury in vitro. Lishizhen Med. Mater. Med. Res. 2008;19:107–109. [Google Scholar]
- 7.Liu T., Ma L., Zhao J., Ma Q. The protective effects of polysaccharide from vitis viniferal on immunological liver injury in mice and its mechanism. Acta Nutr. Sin. 2008;30:596–601. [Google Scholar]
- 8.Liu T., Ma L., Zhao J., Ma Q. Protective effects of triterpenoids from vitis viniferal L. on immunological liver injury in vitro. J. Xinjiang Med. Univ. 2007;30:1222–1225. [Google Scholar]
- 9.Liu T., Ma L., Zhao J., Li T. Protective effects of total flavones extracted from vitis viniferal L. on immunological liver injury in mice. J. Xinjiang Med.Univ. 2007;30:1226–1229. [Google Scholar]
- 10.Chinese Pharmacopoeia Commission . Chinese Pharmacopoeia. Volume 1. Chemical Industry Press; Beijing, China: 2005. p. 247. [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation in cytoxicity assay. J. Immunol. Meth. 1983;65:55–65. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]